Form CMS-10193 The Medicare Clinical Laboratory Competitive Biddin
Medicare Clinical Laboratory Services Competitive Bidding Demonstration Project : Bidding Form
CMS 10193 LAB Application Form (without Track Changes) 07 06 06 -- jk edits 7-13-06
Medicare Clinical Laboratory Services Competitive Bidding Demonstration Project: Bidding Form
OMB: 0938-1008
 Department
of Health and Human Services Form Approved
Department
of Health and Human Services Form Approved
Centers for Medicare & Medicaid Services OMB No #####
THE MEDICARE CLINICAL LABORATORY COMPETITIVE BIDDING DEMONSTRATION PROJECT APPLICATION FORM |
|
For CMS Use Only |
|
Application Number |
Date Application Received
|
A. BIDDING STATUS
|
|||
ALL organizations currently supplying, or planning to supply, more than $1,000 in demonstration tests annually are required to complete this application. Bidders should complete all sections of this application. Non-bidders only need to complete sections A, B (items 1-6, 10,11) and G. The rules of the demonstration are found in the APPLICATION FORM: INSTRUCTIONS FOR COMPLETION. Check either 1 or 2 and indicate whether or not you are bidding:
1. The applicant is required to bid under the rules of the demonstration and is: bidding on the demonstration tests not bidding on the demonstration tests (and therefore will not receive Medicare Part B payment for demonstration tests)
2. The applicant is not required to bid under the rules of the demonstration and is: bidding on the demonstration tests not bidding on the demonstration tests (and therefore will receive Medicare Part B payment for demonstration tests)
|
|||
B. APPLICANT INFORMATION
|
|||
B1. Business and Ownership Information |
|||
1. Applicant’s Business Information |
|||
Applicant’s Legal Business Name
|
|||
Mailing Address (Number, Street)
|
|||
City
|
State |
Zip Code
|
|
Telephone Number (Include Area Code) |
Fax Number (Include Area Code)
|
||
Indicate the length of time the applicant completing this form has been doing business in the CBA. _________years, ______________months |
|||
2. Federal Tax Identification Number (TIN) _________________________________________________________________________________ |
|||
3. “Doing Business As” Name ________________________________________________________________________________________ |
|||
4. Type of Business |
|||
Type of Healthcare Organization
Independent Laboratory Hospital Physician Office Outpatient/Ambulatory Surgery Center or Clinic Nursing Home Dialysis Facility Home Health Agency Other (please specify) __________________________________________ |
Type of Ownership
Government (local or state) Private non-profit Proprietary, individual Proprietary, partnership Proprietary, corporate (privately held) Proprietary, corporate (publicly traded) Other (please specify) __________________________________________ |
||
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938- . The time required to complete this information collection is estimated to average 1-100 hours per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have comments concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to: CMS, 7500 Security Boulevard, Attn: PRA Reports Clearance Officer, Mail Stop C4-26-05, Baltimore, Maryland 21244-1850.
|
|||
5. Ownership Read the instructions for completion carefully. List individually each owner, partner, or managing organization of the applicant. If additional space is needed, check here and attach the additional information using the same format. |
|||||||||||||||||
Owner #1 Legal Name as Reported to the IRS
|
|||||||||||||||||
Mailing Address (Number, Street)
|
|||||||||||||||||
City |
State |
Zip Code
|
|||||||||||||||
Telephone Number (Include Area Code) |
Fax Number (Include Area Code)
|
||||||||||||||||
Federal Tax Identification Number (TIN)
|
Fiscal Intermediary (FI) Medicare Provider Number (if applicable) |
||||||||||||||||
“Doing Business As” Name
|
|||||||||||||||||
Check all that apply and provide the relevant dates and percent ownership where applicable:
5% or more ownership interest (Effective date of ownership ___________________________ % ownership___________________________________________) Managing Organization (Effective date of control of Managing Organization______________________________________________________________________) Partner (Effective date of partnership _____________________________________________________________________________________________________)
|
|||||||||||||||||
Owner #2 Legal Name as Reported to the IRS
|
|||||||||||||||||
Mailing Address (Number, Street)
|
|||||||||||||||||
City |
State |
Zip Code
|
|||||||||||||||
Telephone Number (Include Area Code) |
Fax Number (Include Area Code)
|
||||||||||||||||
Federal Tax Identification Number (TIN)
|
Fiscal Intermediary (FI) Medicare Provider Number (if applicable) |
||||||||||||||||
“Doing Business As” Name
|
|||||||||||||||||
Check all that apply and provide the relevant dates and percent ownership where applicable:
5% or more ownership interest (Effective date of ownership ___________________________ % ownership___________________________________________) Managing Organization (Effective date of control of Managing Organization______________________________________________________________________) Partner (Effective date of partnership _____________________________________________________________________________________________________)
|
|||||||||||||||||
6. Business Establishment Information |
|||||||||||||||||
(Current) Establishment/Incorporated State Date (mm/dd/yyyy) |
|||||||||||||||||
Additional Information |
|||||||||||||||||
|
|||||||||||||||||
(Historic) Previously Established/Incorporated State Date (mm/dd/yyyy) |
|||||||||||||||||
Additional Information |
|||||||||||||||||
|
|||||||||||||||||
B2. Quality and Medicare Information |
|||||||||||||||||
7. Quality Assurance Contact
|
|||||||||||||||||
Name
|
|||||||||||||||||
Title
|
|||||||||||||||||
Mailing Address
|
|||||||||||||||||
City |
State
|
Zip Code |
|||||||||||||||
Telephone Number (Include Area Code) |
Fax Number (Include Area Code)
|
||||||||||||||||
E-mail Address
|
|||||||||||||||||
8. Laboratory Registry Have any of the applicant’s laboratories ever appeared on the annual Laboratory Registry under CLIA? YES NO If yes, please provide the laboratory name, laboratory director, address, CLIA identification number and date. |
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
If yes, was the CLIA certificate Suspended Limited Revoked Other |
|||||||||||||||||
9. Proficiency Testing Check all programs the applicant’s laboratories currently participate in: |
|||||||||||||||||
Accutest |
AAB |
CTS |
EXCEL |
MLE |
New Jersey |
CAP |
AAFP |
||||||||||
API |
Pennsylvania |
Puerto Rico |
WSLH |
Maryland |
ASCP |
New York State |
|||||||||||
May we contact the proficiency testing program(s)? YES NO (please explain below) |
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
10. Laboratory(ies) Serving the CBA If additional space is needed, check here and attach the additional information using the same format. |
|||||||||||||||||
Laboratory #1 Legal Business Name
|
|||||||||||||||||
Mailing Address (Number, Street)
|
|||||||||||||||||
City |
State |
Zip Code
|
|||||||||||||||
Laboratory Director (name)
|
|||||||||||||||||
Does this person direct other laboratories? YES NO If yes, please list the name(s), address(es), and the CLIA Identification Number of the additional laboratory(ies).
|
|||||||||||||||||
|
|||||||||||||||||
Is this a Medicare certified facility? YES NO If yes, please indicate the Fiscal Intermediary (FI) Medicare Provider Number ________________________________ |
|||||||||||||||||
Provider Number Assigned by Medicare Part B Carrier (indicate “n/a” if not applicable) |
National Provider Identification (NPI) number
|
||||||||||||||||
CLIA Identification Number |
Hospital or Part A Medicare Provider Number (indicate “n/a” if not applicable)
|
||||||||||||||||
Indicate the type of CLIA certificate held by the laboratory and the expiration date of the certificate. |
|||||||||||||||||
Certificate of Compliance __________________________(expiration date) |
Certificate of Accreditation __________________________(expiration date) |
||||||||||||||||
If the laboratory holds a Certificate of Accreditation under CLIA, please indicate the accrediting organization(s). |
|||||||||||||||||
JCAHO |
AOA |
AABB |
CAP |
COLA |
ASHI |
||||||||||||
May we contact the accrediting organization(s)? YES NO |
|||||||||||||||||
Laboratory #2 Legal Business Name
|
|||||||||||||||||
Mailing Address (Number, Street)
|
|||||||||||||||||
City |
State |
Zip Code
|
|||||||||||||||
Laboratory Director (name)
|
|||||||||||||||||
Does this person direct other laboratories? YES NO If yes, please list the names and addresses of the additional laboratories. |
|||||||||||||||||
|
|||||||||||||||||
Is this a Medicare certified facility? YES NO If yes, indicate the Fiscal Intermediary (FI) Medicare Provider Number ________________________________ |
|||||||||||||||||
Provider Number Assigned by Medicare Part B Carrier (indicate “n/a” if not applicable) |
National Provider Identification (NPI) number
|
||||||||||||||||
CLIA Identification Number |
Hospital or Part A Medicare Provider Number (indicate “n/a” if not applicable)
|
||||||||||||||||
10. Laboratory (ies) Serving the CBA (continued) |
|||||||||||||||||
Laboratory #2 (continued) Indicate the type of CLIA certificate held by the laboratory and the expiration date of the certificate. |
|||||||||||||||||
Certificate of Compliance __________________________(expiration date) |
Certificate of Accreditation __________________________(expiration date) |
||||||||||||||||
If the laboratory holds a Certificate of Accreditation under CLIA, please indicate the accrediting organization(s). |
|||||||||||||||||
JCAHO |
AOA |
AABB |
CAP |
COLA |
ASHI |
||||||||||||
May we contact the accrediting organization(s)? YES NO |
|||||||||||||||||
Laboratory #3 Legal Business Name
|
|||||||||||||||||
Mailing Address (Number, Street)
|
|||||||||||||||||
City |
State |
Zip Code
|
|||||||||||||||
Laboratory Director (name)
|
|||||||||||||||||
Does this person direct other laboratories? YES NO If yes, please list the names and addresses of the additional laboratories.
|
|||||||||||||||||
|
|||||||||||||||||
|
|||||||||||||||||
Is this a Medicare certified facility? YES NO If yes, indicate the Fiscal Intermediary (FI) Medicare Provider Number ________________________________ |
|||||||||||||||||
Provider Number Assigned by Medicare Part B Carrier (indicate “n/a” if not applicable) |
National Provider Identification (NPI) number
|
||||||||||||||||
CLIA Identification Number |
Hospital or Part A Medicare Provider Number (indicate “n/a” if not applicable)
|
||||||||||||||||
Indicate the type of CLIA certificate held by the laboratory and the expiration date of the certificate. |
|||||||||||||||||
Certificate of Compliance __________________________(expiration date) |
Certificate of Accreditation __________________________(expiration date) |
||||||||||||||||
If the laboratory holds a Certificate of Accreditation under CLIA, please indicate the accrediting organization(s). |
|||||||||||||||||
JCAHO |
AOA |
AABB |
CAP |
COLA |
ASHI |
||||||||||||
May we contact the accrediting organization(s)? YES NO |
|||||||||||||||||
B3. Financial and Legal Information |
|||||
11. Authorized Official(s) |
|||||
Authorized Official(s) First Name |
Last Name |
Title
|
|||
Telephone Number (Include Area Code) |
E-mail Address
|
||||
Authorized Official(s) First Name |
Last Name |
Title
|
|||
Telephone Number (Include Area Code) |
E-mail Address
|
||||
12. Bank References |
|||||
Reference #1 Institution Name
|
Line of Credit (if any, in dollars) |
||||
Account Number(s) |
Contact Person |
Telephone Number (Include Area Code)
|
|||
Reference #2 Institution Name |
Line of Credit (if any, in dollars)
|
||||
Account Number(s) |
Contact Person |
Telephone Number (Include Area Code)
|
|||
13. Financial Information Please submit the financial information requested in the instructions for this question. An authorized official of the applicant should sign below to certify the submitted financial information. |
|||||
I HEREBY CERTIFY that I have examined the accompanying financial statement and that to the best of my knowledge and belief, it is a true, correct and complete statement prepared from books and records that we have prepared in accordance with the Generally Accepted Accounting Principles. |
|||||
Authorized Official (Print) |
Title |
Date
|
|||
Authorized Official (Signature)
|
|||||
14. Adverse Legal Actions Have any of the adverse legal actions listed in Table A (see instructions) been imposed against the applicant, any of the applicant’s subcontractors or any of the applicant’s owners? If yes, report each adverse legal action, when it occurred, the law enforcement authority/court/administrative body that imposed the action and the resolution. Attach a copy of the adverse legal action documentation(s) and resolution(s). |
|||||
|
|||||
|
|||||
|
|||||
|
|||||
Is the applicant, any of the applicant’s subcontractors or any of the applicant’s owners currently the subject of an investigation that could potentially result in imposition of an adverse legal action listed in Table A (see instructions)? If yes, report the circumstances and status of the investigation. |
|||||
|
|||||
|
|||||
C. GEOGRAPHIC COVERAGE, TEST MENU, AND SUBCONTRACTING
|
||||
1. Geographic Coverage Indicate the zip codes that you currently serve within the CBA. If you serve all of the zip codes in a particular county, you may enter the name of the county. |
||||
|
||||
|
||||
Are there any specific tests provided by the applicant that are not available for all of the zip codes and counties listed above? YES NO If yes, please provide the HCPCS codes for these tests as well as a brief explanation for why they can not be provided to all of the zip codes and counties you serve in the CBA. |
||||
|
||||
|
||||
Do you plan to expand your service area under the competitive bidding demonstration project? YES NO If yes, indicate the additional zip codes or counties you will serve within the CBA: |
||||
|
||||
|
||||
|
||||
2. Specimen Transport and Logistics Check all that apply
Specimens are collected by client and transported via courier service (e.g., local courier, FedEx) Applicant provides specimen collection at client location and transports specimen to testing laboratory Applicant provides specimen pick-up service for routine and STAT collection Applicant provides specimen collection on-site at laboratory (primary address) Applicant provides specimen collection sites within the demonstration area (addresses to be listed below)
Provide a copy of your current requisition or test request form. If not available, provide an explanation.
|
||||
3. Specimen Collection Locations
|
||||
Location #1 Name
|
||||
Mailing Address (Street)
|
||||
City |
State |
Zip Code
|
||
Function (check all that apply) |
||||
Only Specimen Drop Off |
Venipuncture |
Limited Laboratory Testing (please specify) __________________________________________________ |
||
3. Specimen Collection Locations (continued) |
||||||||||
Location #2 Name
|
||||||||||
Mailing Address (Street)
|
||||||||||
City |
State |
Zip Code
|
||||||||
Function (check all that apply) |
||||||||||
Only Specimen Drop Off |
Venipuncture |
Limited Laboratory Testing (please specify) __________________________________________________ |
||||||||
Location #3 Name
|
||||||||||
Mailing Address (Street)
|
||||||||||
City |
State |
Zip Code
|
||||||||
Function (check all that apply) |
||||||||||
Only Specimen Drop Off |
Venipuncture |
Limited Laboratory Testing (please specify) __________________________________________________ |
||||||||
4. Test Menu Indicate the CLIA specialty(ies) of testing performed in-house. |
||||||||||
Histocompatibility |
Microbiology |
Diagnostic Immunology |
Chemistry |
Hematology |
||||||
Immunohematology |
Pathology |
Radiobioassay |
Clinical Cytogenetics |
Other (specify) _________ |
||||||
How will your laboratory provide a comprehensive demonstration test menu (for Medicare beneficiaries) under the Competitive Bidding Demonstration Project? Check all that apply. |
||||||||||
Laboratory currently offers demonstration test menu (in-house testing) Laboratory plans to expand (in-house testing, provide additional information in question 6) Laboratory currently subcontracts/refers to provide demonstration test menu (provide additional information in question 5) Laboratory plans to subcontract/refer to provide demonstration test menu (provide additional information in question 5) Other (explain) |
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
5. Subcontracting/Referred Tests Do you “send out” or refer laboratory tests to another laboratory, or plan to do so under the demonstration? YES NO |
||||||||||
If yes, please identify the legal entities you currently or anticipate subcontracting or referring tests to, specify what tests will be subcontracted/referred, and specify the prices charged to the applicant for subcontracted/referred tests.
|
||||||||||
Subcontractor/Reference Laboratory Legal Name |
|
Demonstration Tests or Specialties to be Subcontracted/Referred |
Copies of Subcontractor/Reference Laboratory Prices Attached? |
|||||||
|
|
|
YES NO Pending |
|||||||
|
|
|
YES NO Pending |
|||||||
|
|
|
YES NO Pending |
|||||||
|
|
|
YES NO Pending |
|||||||
|
|
|
YES NO Pending |
|||||||
|
|
|
YES NO Pending |
|||||||
|
|
|
|
|||||||
5. Subcontracting/Referred Tests (continued) |
If subcontractor/reference laboratory prices charged to the applicant are not attached or are pending, please explain. If necessary, attach additional pages to explain subcontractor/reference laboratory relationships, tests, and prices. |
|
|
|
6. Expansion Do you plan to expand if awarded a competitive bid contract? YES NO If yes, describe your expansion plan: |
|
|
|
|
In what month/year do you anticipate that the added capacity from your expansion plan will become available?____________________ (month/year)
|
D. BID PRICES, VOLUME AND CAPACITY
|
|||||||
1. Test Volume What was the total number of tests provided for residents of this CBA by the applicant during calendar year 2005? |
|||||||
0-50,000 |
50,001-100,000 |
100,001 – 250,000 |
250,001 – 500,000 |
||||
500,001-750,000 |
750,001- less than 1 million |
1 million – 5 million |
More than 5 million |
||||
What percentage was for Medicare beneficiaries? |
|||||||
0% - 10% |
11%-20% |
21%-30% |
31%-40% |
41%-50% |
|||
51%-60% |
61%-70% |
71%-80% |
81%-90% |
91%-100% |
|||
2. Revenue What was the total revenue collected from tests provided for residents of this CBA by the applicant during calendar year 2005? |
|||||||
$0-$250,000 |
$250,001 - $500,000 |
$500,001 - $750,000 |
$750,001 - less than $1 million |
||||
$1 million - less than $3 million |
$3 million - less than $6 million |
$6 million - $10 million |
More than $10 million |
||||
What percentage was collected from Medicare? |
|||||||
0% - 10% |
11%-20% |
21%-30% |
31%-40% |
41%-50% |
|||
51%-60% |
61%-70% |
71%-80% |
81%-90% |
91%-100% |
|||
3. Non-patient Test Percentage If you are a hospital or physician office laboratory (or other organization with patients), what percentage of your total test volume in the CBA is provided to non-patients? For example, if you are a hospital providing 15% of your tests as “outreach” business to persons who are not inpatients or outpatients of your organization, check the 11-20% box.
If you are an independent clinical laboratory, check here. |
|||||||
0% - 10% |
11%-20% |
21%-30% |
31%-40% |
41%-50% |
|||
51%-60% |
61%-70% |
71%-80% |
81%-90% |
91%-100% |
|||
4
Provide your Medicare bid price in column D for each HCPCS code. |
||||
5. Current Volume and Maximum Annual Capacity
Indicate the applicant’s current total (all payers) annual test volume and estimated maximum annual test capacity by CLIA specialty for all residents of the CBA.
|
||||
CLIA Specialty |
|
Current Volume |
|
Capacity |
Histocompatability |
|
|
|
|
Immunohematology |
|
|
|
|
Microbiology |
|
|
|
|
Pathology |
|
|
|
|
Diagnostic Immunology |
|
|
|
|
Radiobioassay |
|
|
|
|
Chemistry |
|
|
|
|
Clinical Cytogenetics |
|
|
|
|
Hematology |
|
|
|
|
Other (specify)__________________________ |
|
|
|
|
|
||||
Explain any extra capacity you reported above. Check all that apply. Attach additional sheets to explain if necessary.
Extra capacity in current configuration Expansion plan reported in Section C, question 6 Subcontracting/Referrals Other (explain) ___________________________________________________________________________________________________________________
|
||||
Will all of the extra capacity reported above, if any, be available to provide demonstration tests? YES NO (explain) |
||||
If necessary, attach additional sheets to explain your capacity to expand demonstration test volume.. |
||||
E. Additional Information (Optional) (Specialized testing services provided, etc.--see instructions) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F. CERTIFYING STATEMENT |
|
I, the undersigned, certify to the following:
|
|
Authorized Official Name (First, Middle, Last)
|
Title/Position |
Signature
|
Date |
Form
CMS – 10193 Page
| File Type | application/msword |
| File Title | MEDICARE CLINICAL LABORATORY COMPETITIVE BIDDING PROGRAM |
| Author | Jyoti Aggarwal |
| Last Modified By | CMS |
| File Modified | 2006-07-13 |
| File Created | 2006-07-13 |
© 2026 OMB.report | Privacy Policy
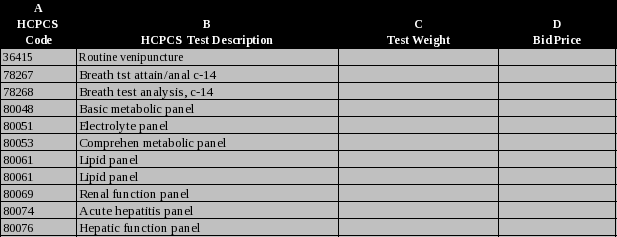 .
Medicare Bid Price by HCPCS Code
.
Medicare Bid Price by HCPCS Code