Nci
NCI.doc
National Network of Tobacco Censation Quitlines Evaluation
OMB: 0925-0576
REQUEST FOR CLEARANCE FOR THE EVALUATION OF THE NATIONAL
NETWORK OF TOBACCO CESSATION QUITLINES INITIATIVE
(NNTCQ INITIATIVE)
July 31, 2006
Linda Squiers, Ph.D.
Project Officer for Research
National Cancer Institute, Cancer Information Service
6116 Executive Blvd.
Rockville, MD 20850
301-594-9075 (voice)
301-402-0555 (fax)
TABLE OF CONTENTS
Chapter Page
A.1 Circumstances Making the Collection of Information Necessary 1
A.2 Purpose and Use of the Information 3
A.2.2 Audiences for Data and Results 4
A.3 Use of Information Technology and Burden Reduction 5
A.4 Efforts to Identify Duplication and Use of Similar Information 5
A.5 Impact on Small Businesses and Other Small Entities 6
A.6 Consequences of Collecting the Information Less Frequently 7
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 7
A.8 Comments
in Response to the Federal Register Notice and Efforts
to
Consult Outside Agency 7
A.9 Explanation of Any Payment or Gift to Respondents 9
A.10 Assurance of Confidentiality Provided to Respondents 9
A.11 Justification for Sensitive Questions 10
A.12 Estimates of Hour Burden Including Annualized Hourly Costs 10
A.13 Estimates
of Other Total Annual Burden to Respondents
or
Recordkeepers 17
A.14 Annualized Cost to the Federal Government 17
A.15 Explanation for Program Changes or Adjustments 18
A.16 Plans for Tabulation and Publication and Project Time Schedule 18
A.16.1 Key Informant Surveys 18
A.17 Reasons(s) Display of OMB Expiration Date is Inappropriate 20
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions 20
19 Certification for Paperwork Reduction Act Submissions 22
B. COLLECTIONS
OF INFORMATION EMPLOYING STATISTICAL
METHODS 23
B.1 Respondent Universe and Sampling Methods 23
B.2 Procedures for the Collection of Information 25
B.3 Methods to Maximize Response Rates and Address Non-Response 27
B.4 Tests of Procedures or Methods to be Undertaken 27
B.5 Individuals
Consulted on Statistical Aspects and Individuals Collecting
and/or
Analyzing Data 28
List of Tables
Table Page
A.12-1 Estimate of respondent hour burden for the Process Evaluation
of the
NNTCQ Initiative 11
TABLE OF CONTENTS (Cont’d)
List of Tables (Cont’d)
Table Page
A.12-2 Estimate average interview time for State Tobacco Control Manager 13
A.12-3 Estimated average interview time for State Quitline Administrator 14
A.12-4 Estimated average interview time for State Quitline Service Provider 15
A.12-5 Estimated average interview time for State Quitline Partner 15
A.12-6 Estimated average interview time for NAQC 16
A.12-7 Annualized costs to respondents 17
A.16-1 Project time schedule 20
TABLE OF CONTENTS (Cont’d)
List of Attachments
Attachment Page
1 LEGISLATIVE AUTHORITY A1-1
2A STATE TOBACCO CONTROL MANAGER DATA COLLECTION
INSTRUMENT A2A-1
2B STATE QUITLINE ADMINISTRATOR DATA COLLECTION
INSTRUMENT A2B-1
2C STATE QUITLINE SERVICE PROVIDER DATA COLLECTION
INSTRUMENT A2C-1
2D STATE QUITLINE PARTNER DATA COLLECTION INSTRUMENT A2D-1
2E NAQC REPRESENTATIVE DATA COLLECTION INSTRUMENT A2E-1
3 INTRODUCTORY LETTER A3-1
4 IRB EXCEPTION MEMO A4-1
5 60-DAY FEDERAL REGISTER NOTICE A5-1
6A WESTAT CONFIDENTIALITY PLEDGE A6A-1
6B NCI SYSTEM OF RECORDS FEDERAL REGISTER NOTICE A6B-1
A.1 Circumstances Making the Collection of Information Necessary
Cigarette smoking is the leading preventable cause of death and disease in the United States. Each year smoking causes about 440,000 premature deaths and costs the nation approximately $75 billion in direct health care expenses. About three out of four U.S. smokers say they want to quit, but fewer than 5 percent of smokers who quit for at least one day are able to stay tobacco-free for 3 to 12 months. The rate of successful quitting increases dramatically when smokers use evidence-based treatments such as physician advice, FDA-approved medications, or telephone counseling (CDC, 2004). In recent years, there has been growing evidence that telephone counseling is an effective strategy for increasing cessation (Stead, Lancaster, & Perera, 2003; Fiore, Bailey, and Cohen, 2000). The types of services offered by quitlines vary considerably among the states but usually include the provision of tobacco-related health information, advice to quit and assistance in a quit attempt, and referrals to health care providers or community resources.
In August 2002, the Secretary of the U.S. Department of Health and Human Services established a Subcommittee on Cessation of the Interagency Committee on Smoking and Health. The Subcommittee was charged with creating a set of evidence-based recommendations for reducing tobacco use in the United States. One of their recommendations was the establishment of a federally funded National Network of Tobacco Cessation Quitlines to provide universal access to evidence-based counseling and medications for tobacco cessation (Fiore et al., 2004). In February 2004, the U.S. Department of Health and Human Services announced plans for a national network of tobacco cessation quitlines to provide all smokers in the United States access to the support and latest information to help them quit.
The National Cancer Institute’s (NCI’s) Cancer Information Service (CIS) and the Centers for Disease Control and Prevention (CDC) Office of Smoking and Health (OSH) are collaborating with other partners to implement the National Network of Tobacco Cessation Quitlines (NNTCQ) Initiative (the Initiative). The Initiative provides funding to states to enhance or establish state quitlines, makes CIS’s Smoking Quitline services available to states until they have a quitline and—in keeping with the Subcommittee’s recommendation to provide a national portal to available state or regionally managed quitlines—establishes a single, national access telephone number that routes callers to their own state’s quitline. To provide the highest level of assistance to smokers across the country who want to quit, NCI established a new toll-free telephone number (1–800–QUIT–NOW) on November 8, 2004. A key element of this telephone number is that it is a single, national portal that routes the caller to the quitline operated by the state from which he or she is calling. Thus, a key element of the Initiative is that it seeks to create capacity for each state to provide quitline services to its citizens while simultaneously promoting knowledge of a single, national telephone number accessible by all U.S. residents.
The aim of the National Network of Tobacco Cessation Quitlines (NNTCQ) initiative (the Initiative) is to strengthen service delivery; provide a mechanism for integration and implementation of state, regional, and national campaigns; and increase healthcare utilization by minority and medically underserved populations. NCI, CDC, and other state, private industry, and partner organizations (the North American Quitline Consortium) have created the infrastructure and a coordinated mechanism to offer cessation services to the American public. The Initiative seeks to enhance existing state-managed quitlines and to encourage the establishment of quitlines in states without them. It is expected that successful implementation of the Initiative will foster partnerships across state quitlines for technology transfer, sharing of effective practices, and understanding patterns of use and reach to special populations, thereby ensuring a sustained level of effectiveness over time.
At approximately the same time as the 1-800-QUIT-NOW number was established, NCI and CDC developed a draft plan to evaluate the Initiative. This plan encompassed both a process evaluation of the planning and initial implementation of the Initiative and an outcome evaluation of the effects of the Initiative. The goal of this evaluation is to monitor the implementation of the Initiative, assess its impact on key stakeholders, and examine its implications for public health. At this point in time, NCI has funded the process evaluation and has developed the final evaluation plan. In addition to documentary, evidentiary, and secondary data sources of information, the process evaluation plan calls for a series of in-depth telephone interviews (see Attachments 2a-e) with key informants and stakeholders who were directly or indirectly involved in or affected by the implementation of the Initiative. These informants and stakeholders include Federal staff, state tobacco control mangers, state tobacco quitline administrators, and representatives of national organizations, quitline service providers, and representatives of organizations that partner with individual state quitlines, such as community health organizations and anti-tobacco coalitions. These interviews will provide information not otherwise available from other existing sources.
The Public Health Services Act outlines the research and information dissemination mission of the National Cancer Institute. Attachment 1 contains the full text of 42 USC, Sections 285a, 1-3.
Section 285a of 42 USC states that:
“The National Cancer Program shall consist of … an expanded, intensified, and coordinated cancer research program encompassing the research programs conducted and supported by the Institute and the related research programs of the other national research institutes …”
Section 285a-1 further states that:
“The Director of the Institute shall establish and support demonstration, education, and other programs for the detection, diagnosis, prevention, and treatment of cancer…” Programs established and supported under this section shall include {among others}: “…the demonstration of new methods for the dissemination of information to the general public concerning the prevention, early detection, diagnosis, and treatment and control of cancer and information concerning unapproved and ineffective methods, drugs, and devices for the diagnosis, prevention, treatment, and control of cancer.”
This evaluation is specifically designed to support this mission by providing a means to evaluate a new public health initiative that has not previously been studied through other data collection efforts. Since this young Initiative is the first to combine national access to telephone tobacco cessation services with capacity-building of such services across all States, evaluation of the Initiative is essential to determining how its implementation proceeded and how the Federal, State, and private forces affected and were affected by the initial process of implementing it. NCI and CDC are also in the process of planning an outcome evaluation, which will look at the immediate effects of the Initiative; that will of necessity take place after the Initiative has been established long enough for its effects to take hold and be detectable; the current timeline anticipates that the first outcome evaluation will begin in 2007.
A.2 Purpose and Use of the Information
The overall purpose of the evaluation is to assess the implementation of the Initiative and monitor its public health impact. The evaluation will provide valuable practical information to NCI, CDC and their partners concerning the development and implementation of the NNTQC initiative as a potential model for Federal-State partnerships and the impact of the Initiative on enhancing state quitline capacity. Findings from the evaluation will be used to: 1) strengthen key partnerships within cessation research and practice, 2) build and enhance states’ capacity to provide quality quitline services, 3) increase the public use of quitline services, and 4) sustain quality quitline services.
A.2.1 Research Questions
The evaluation of the Initiative will provide the only source of data available to answer the following major research questions:
What are the primary goals of the Initiative? What facilitated the implementation of the Initiative? What were the barriers to implementing the Initiative?
What collaborations/partnerships occurred within the federal government? What collaborations/partnerships occurred between federal and state governments? What partnerships developed within states around their quitlines?
What telecommunications infrastructure did 1-800-QUIT-NOW add? What new or additional capabilities were enabled by this technology?
What level of funding was provided to new and existing quitlines?
To what extent did federal support facilitate and/or hinder state quitlines? To what extent was state capacity to deliver quitline services enhanced or maintained?
How did state quitlines use the resources provided by the federal government?
When and to what extent were promotion efforts planned for 1-800-QUIT-NOW? What promotion efforts were actually implemented for 1-800-QUIT-NOW?
To what extent did states engage in partnership activities? How are the Initiative-facilitated collaborations valued by the quitline staff?
A.2.2 Audiences for Data and Results
Primary users of the evaluation data include stakeholders who will make decisions about the Initiative based on evaluation findings. Stakeholders for the NNTCQ evaluation include federal program managers, departments and agencies, (specifically DHHS, NCI, and CDC), Congress, state tobacco control managers, state quitline administrators, partner organizations (e.g., the North American Quitline Consortium), researchers, and health care providers, public health professionals, and cessation service providers. In addition to primary evaluation stakeholders, stakeholders of the Initiative include a larger group of interested parties who are served and affected by the Initiative including the American public, smokers, and nonsmokers who are exposed to environmental tobacco smoke.
A.3 Use of Information Technology and Burden Reduction
The data collected from respondents will utilize semi-structured telephone interview guides. As detailed in Section A.12, the number of respondents for a given version of the interview is relatively small (50-100) and the interviewer will collect much of the information using the guide to stimulate open-ended responses and probes to foster an interactive dialogue around a number of broad issues. This approach will be combined where feasible with some closed-ended factual questions and scaled items related to opinions and beliefs. Given this approach, use of an automated data collection system such as Computer-Assisted Telephone Interviewing would not add meaningfully to the effectiveness of the data collection or reduce the burden. In addition, the cost of developing such a system would not be balanced by any cost savings or efficiencies over the course of the data collection, because of the relatively small number of respondents. Moreover, given the expert role the executive interviewers will play, it is preferable for them to focus on the content and flow of the semi-structured interview, and on making recording responses and side notes, rather than on the mechanics of keying responses into a computer.
With the permission of the respondents, the telephone interviews will be digitally recorded. Use of recording technology is likely to improve the ultimate quality of the analytical data, since the analysts can review the qualitative responses in full when preparing for and conducting analysis. If desirable, transcriptions of the audio recordings can be analyzed using content analysis software. Finally, knowledge that an interview is being recorded may permit an interviewer to focus on the key elements of a response and on side notes, rather than on extensive verbatim recording; this, in turn, may somewhat reduce the time length of a given interview, and the concomitant burden for the respondent.
A.4 Efforts to Identify Duplication and Use of Similar Information
Because much of the information being collected relates to a new Initiative, the needed information has not been previously collected or assembled and does not exist elsewhere. However, based on discussions between NCI and CDC, with partners such as the North American Quitline Association, and with the external consultants and Expert Panel members, NCI has adopted the following approaches to avoid duplication.
The North American Quitline Consortium has inaugurated an annual survey of the State quitlines that comprise its membership. This survey collects operational, budgetary, and cessation service information about the quitlines as well as summary information characterizing the service users; it collects no individual clinical data. Currently, NAQC has data collected in 2004 and 2005. NCI has determined that a number of the data elements collected by the survey can serve the analysis needs of the evaluation. NCI is currently arranging to obtain access to these NAQC data, limited to use in the evaluation of the Initiative.
Because the current evaluation is a process evaluation, a substantial portion of the data can be obtained from documentary sources. These include planning documents, meeting minutes and reports, guidance documents, technical documents, and so forth. The analytical and data collection designers have identified many data items that can be gleaned from such documents and will not need to be collected from respondents.
A specific, rich collection of documentary sources is the set of funding applications and semi-annual progress reports that each State has submitted to CDC in relation to supplemental Federal funding for Initiative-related activities. Information available from these State submissions will not be collected from respondents.
Some data, such as those used to characterize and group States for analysis, will be obtained from existing Federal data sources, especially CDC databases. These cover items such as smoking prevalence and per capita spending on tobacco control. These will not be collected from respondents.
In some instances, it will be necessary to extend the information available from file or documentary sources by obtaining the informant’s opinion or judgment in regard to the constructs covered by secondary sources; that is, no similar information exists and it will be collected from respondents.
A.5 Impact on Small Businesses and Other Small Entities
It is likely that quitline service providers and partner organizations can be characterized as small businesses or small entities. Note that, while physicians or dentists may be among the respondents for these organizational entities, it will not be in their capacity as individual health care providers but as managers or representatives of organizations such as managed care organizations, public health organizations, or health coalitions, which themselves are small entities. The data collection for the quitline service providers and partners will collect less information than will that for State informants. It will consist of a parsimonious set of topics about which the respondent will be asked for open-ended responses regarding experiences, opinions, and judgments. No extensive, detailed data will be collected from these two groups of respondents. The State Quitline Service Provider and State Quitline Partner instruments included as Attachment 2 demonstrate this point.
A.6 Consequences of Collecting the Information Less Frequently
The process evaluation for which clearance is being sought constitutes a one-time data collection. Respondents will be asked to respond to the data collection one time only.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This project fully complies with all guidelines of 5 CFR 1320.5. In addition, key informant interview response times will not exceed 1 hour.
.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agency
Notice of this study was published in the Federal Register on January 27, 2006 (Volume 71, Number 18, Pg. 4595-4596). A copy of the 60-day notice is provided as Attachment 4. No public comments were received.
This evaluation builds on the input of many experts in the field of tobacco cessation research, interventions, program implementation, policy, and the provision of cessation and quitline services. Substantial efforts were made to consult with additional content experts and experts on issues related to telephone cessation evaluation methodology and data collection procedures. The individuals consulted as external consultants and expert panel members for this evaluation are listed below, along with their respective areas of expertise.
EXTERNAL CONSULTANTS: 2005-2006 |
EXPERTISE |
Susan J. Curry, PhD Director Institute for Health Research and Policy University of Illinois at Chicago Phone: 312.355.4438 |
Health Research and Policy |
Michael C. Fiore, MD, MPH Professor of Medicine Director, Center for Tobacco Research and Intervention University of Wisconsin School of Medicine and Public Health Phone: 608.262.8673 |
Tobacco Research, Intervention, and Policy |
Paula Keller, MPH Senior Policy Advisor Center for Tobacco Research and Intervention University of Wisconsin School of Medicine and Public Health Phone: 608.262.4094 |
Tobacco Research, Intervention, and Policy |
EXPERT PANEL MEMBERS |
EXPERTISE |
Lourdes Baezconde-Garbanati, PhD Institute for Health Promotion and Keck School of Medicine, USC Assistant Professor - Institute for Prevention Research Phone: 626.457.6606 |
Tobacco Research, Technical Assistance, Training, Communication, Minority Health Care |
Karen DeLeeuw Colorado State Tobacco Control Program Colorado Department of Public Health and Environment Emergency Medical Services and Prevention Division Phone: 303.692.2515 |
State Tobacco Control Manager
|
Gary Giovino, PhD Director, Tobacco Control Research Program, Roswell Park Cancer Institute Research Triangle Institute Department of Cancer Prevention and Population Sciences Phone: 716.845.4402 |
Tobacco Control Research |
Tim McAfee, MD Executive Medical Director, Free & Clear Phone: 206.876.2100 |
Quitline Service Provider, Cessation Treatment and Research |
Donna Vallone, PhD Ass't VP of Research American Legacy Foundation Phone: 202-454-5555 |
Tobacco Research, Training, and Technical Assistance |
Shu Hong Zhu, PhD Associate Professor of Family and Preventive Medicine UCSD School of Medicine Phone: 858.300.1056 |
Smoking Behavior and Cessation; Tobacco Quitline Research and Evaluation |
A.9 Explanation of Any Payment or Gift to Respondents
There will be no payment or gift to respondents.
A.10 Assurance of Confidentiality Provided to Respondents
All information gathered through the administration of the key informant telephone interviews focuses on organizational activities rather than information about individuals, and no questions of a sensitive nature will be asked of individuals. It will be necessary to obtain identifying information about the different individuals being interviewed (name, organizational affiliation, and title/position). Additionally, identifying information (phone number, email address) will be necessary to schedule interviews. Every attempt will be made to keep this information confidential, and it will not be released or used for any other purpose. No statements gathered during these interviews will be attributed to a specific individual in any reports prepared from this data.
With the permission of the respondent and to the extent feasible, key informant telephone interviews will be recorded to supplement the open-ended responses and qualitative notes recorded by the interviewers on the interview instrument. The audio recordings will be secured and handled in the same way as any other form of electronic data, whether stored on computer system central storage or on removable media (e.g., Audio CD) (see Attachment 6A, procedures 4 and 5).
Prior to the beginning of each interview, respondents will be told the following: the purpose of the interview; how the results will be used; participation is voluntary and they may refuse to answer any question at any time or end the interview at any time without penalties; the information they provide will be kept confidential, and will not be disclosed to anyone but the researchers conducting this study except as otherwise required by law; individual names and positions will not be connected with any responses in any reports prepared from the data; and all individual responses will either be combined with the responses of others in or reported anonymously (e.g., as the response of a “state tobacco control manager”); in the latter instance, no individual response will be reported if the content or context of the response would have the possibility of revealing the identity of the respondent. The interviewer will seek the respondent’s permission to record the interview and will honor a request not to do so.
Volunteers who participate in this study will be subject to assurances and safeguards as provided by the Privacy Act of 1974 (5 USC 552a), which requires the safeguarding of individuals against invasion of privacy. The Privacy Act also provides for the confidential treatment of records maintained by a Federal agency according to either the individual’s name or some other identifier. Westat, the study contractor, has its own policy and procedures regarding assurance of confidentiality and a pledge that all employees must sign (see Attachment 6a).
The NCI published a System of Records notice in the Federal Register on Thursday, September 26, 2002 (Vol. 67, No. 187, pp. 60776-60779). All members of the NCI/CIS and CDC/OHS and staff working with evaluation data will adhere to the provisions stipulated within that announcement (see Attachment 6b).
In October 2005, Westat’s IRB Chairman, Dr. Thomas W. McKenna, indicated that the project is exempt from IRB review under the provisions of 45 CFR §46.102 (f). IRB documentation is provided as an attachment (Attachment 5) to the clearance package.
A.11 Justification for Sensitive Questions
Other than personal identifying information necessary to conduct data collection (name, phone number, title, organization name), no individual data will be collected. No information will be collected about individuals. All responses will address aspects of the organization that the respondent represents; no personally sensitive questions will be asked.
A.12 Estimates of Hour Burden Including Annualized Hourly Costs
Estimates of hour burden are shown in Table A. 12-1 and are based on timed test interviews. The State Tobacco Control Manager interview will consist of a census of the 51 State and D.C. tobacco control managers participating in a 1-hour interview. The State Quitline Administrator interview will consist of a census of the 51 State and D.C. tobacco quitline administrators participating in a 1-hour interview. The State Quitline Service Provider interview will consist of a census of the 19 private not-for-profit, private for-profit, and public organizations that provide telephone cessation services to the State quitlines participating in a 45-minute interview. The State Quitline Partner interview will consist of a referral sample of 2 partner organizations in each state participating in a 30-minute interview (total n=102). The NAQC Representative interview will consist of 5 representatives of the administrative staff and organizational member workgroups participating in a 30 minute interview. Following Table A.12 -1 is an explanation of the basis of the hour burden estimates.
Table A.12-1. Estimate of respondent hour burden for the Process Evaluation of the NNTCQ Initiative
Type of respondent |
Estimated number of respondents |
Frequency of Response |
Average hours per response* |
Annual hour burden |
State Tobacco Control Manager |
51 |
1 |
1.00 |
51.00 |
State Quitline Administrator |
51 |
1 |
1.00 |
51.00 |
State Quitline Service Provider |
19 |
1 |
.75 |
14.25 |
State Quitline Partner |
102 |
1 |
.50 |
51.00 |
NAQC Representative |
5 |
1 |
. 50 |
2.50 |
Total |
|
|
|
169.75 |
The average hours per response are based on a formula that incorporates a combination of timings from live internal tests, allocations of average response times to different types of questions, and estimates of the effects of skip patterns. There are four factors in the formula:
1) timed live readings of the question text, question by question;
2) allocation of estimated average times for responses to standard types of questions: open-ended – 30-90 seconds, depending on interview type; brief answer - 10 seconds; closed ended - 5 seconds;
3) counts of each of the above three types of question in each instrument; and
4) analysis of major and minor skip patterns to estimate the actual number of each type of question that an average respondent would be asked.
The resulting estimates are produced by adding together the reading time and allocated response time for each question type, multiplying the resulting time by the number of such questions asked on average, and finally adding together the times for all three question types. Tables A. 12-2 through A.12 6 lay out this process in detail for each of the five questionnaires.
It is helpful to be aware of the following points when reviewing these tables. First, for stem-and-leaf closed-ended questions, every leaf is counted separately. Second, reading time means the actual live reading time required to read aloud to the respondent everything he or she is intended to hear, including each question, plus all response categories that are to be read aloud. Third, for the long-form open ended questions, a longer response time allowance is made in Provider, Partner, and NAQC interviews: the nature of their relationship to the quitlines and of the particular open-ended questions asked of them is expected to yield a more exploratory, discursive interview. Finally, to estimate the time required to respond to the State Tobacco Control Manager and State Quitline Administrator instruments, separate estimates are calculated for those States whose quitlines were in existence prior to 2005 (n=36) and those that inaugurated their quitlines in 2005 or 2006 (n=15), then an overall estimate is produced by a weighted average of the two estimates. The reason for handling these two instruments in this manner is that there is a large set of questions that are skipped for latter group, so the estimates are likely to be more precise if these known skips are explicitly accounted for. The other skips in these two instruments, and all skips in the other three instruments, are based on individual question responses; since there can be no a priori determination of such skips, the estimates use a 50% skip rate for the number of such questions. If anything, more than 50% will be skipped, since the preponderance of skips occur as follow-on questions in table-type questions listing a large superset of activities, situations, etc., that may apply to quitlines in the aggregate, but only a few of which will apply to any given quitline, thereby skipping the follow-on questions for most items in the list. |
|
|
|
Table A.12-2. Estimated average interview time for State Tobacco Control Manager
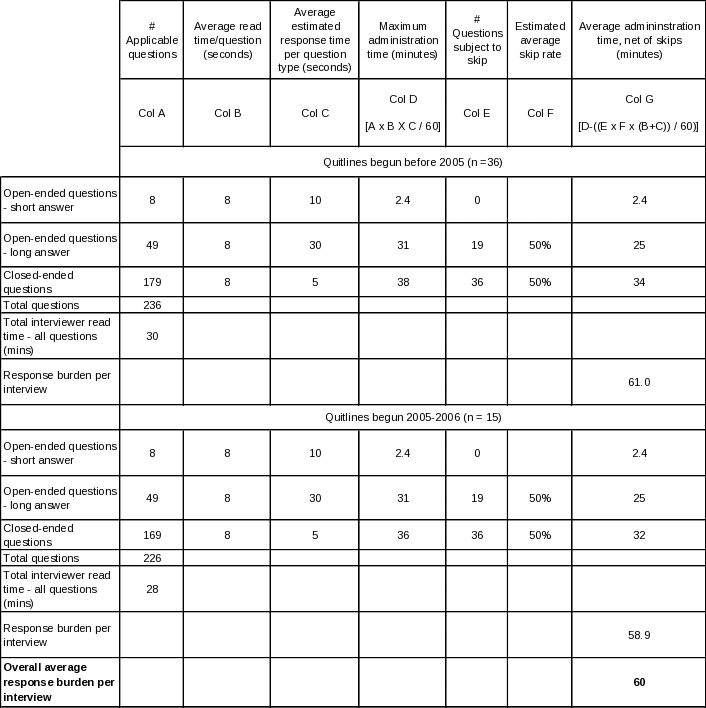
Table A.12-3. Estimated average interview time for State Quitline Administrator
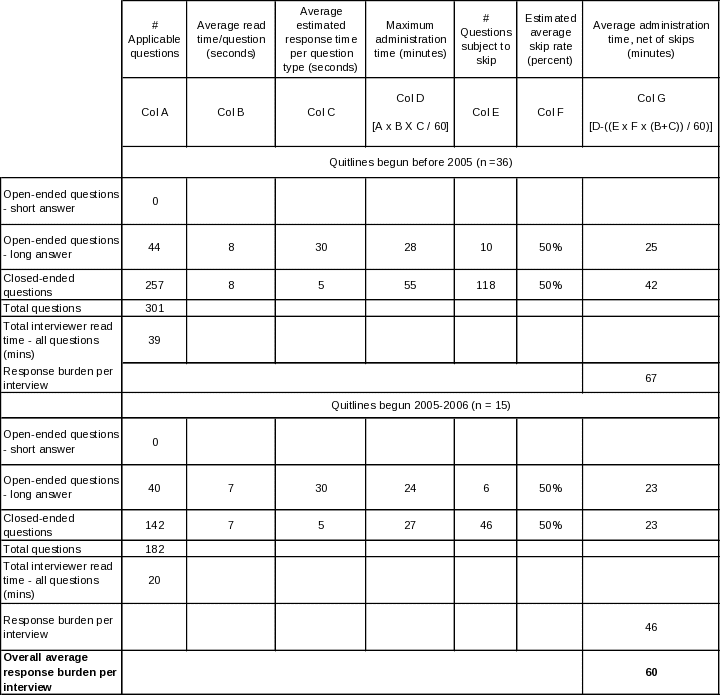
Table A.12-4. Estimated average interview time for State Quitline Service Provider
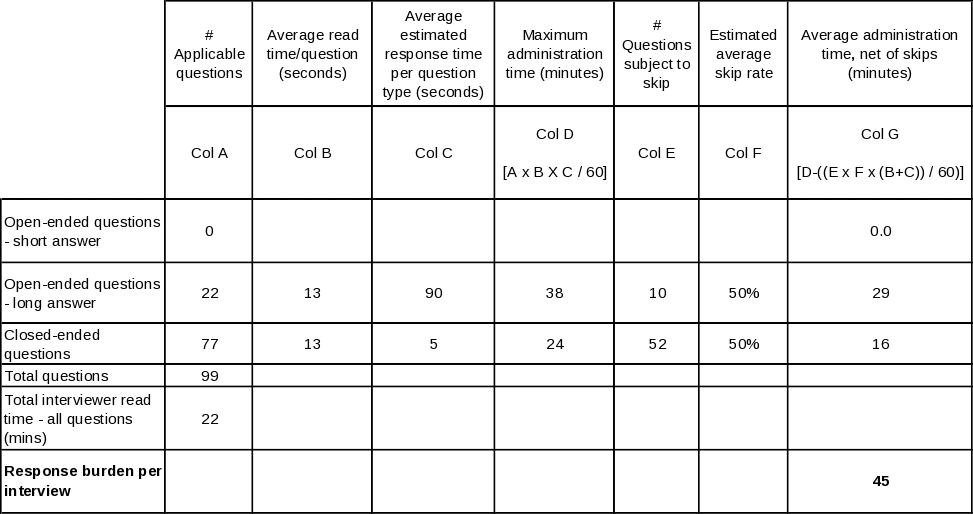
Table A.12-5. Estimated average interview time for State Quitline Partner
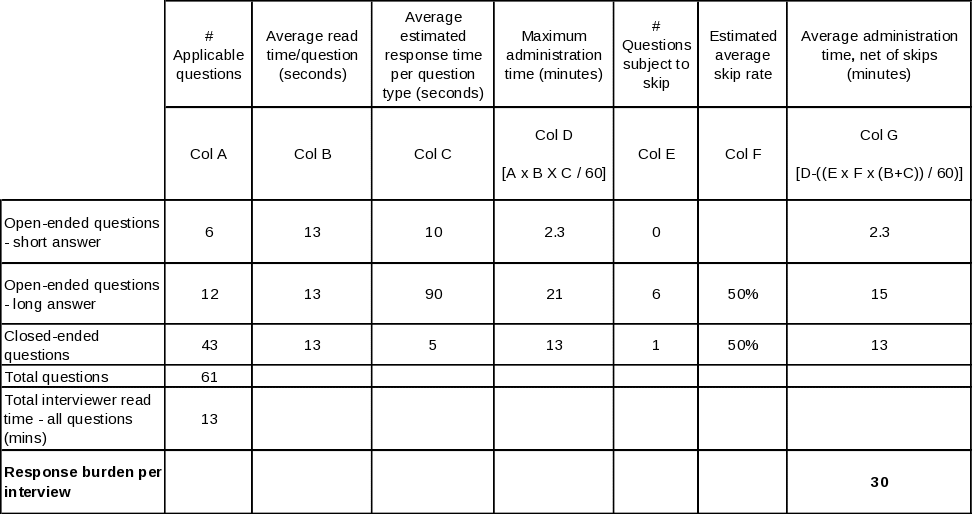
Table A.12-6. Estimated average interview time for NAQC Representative
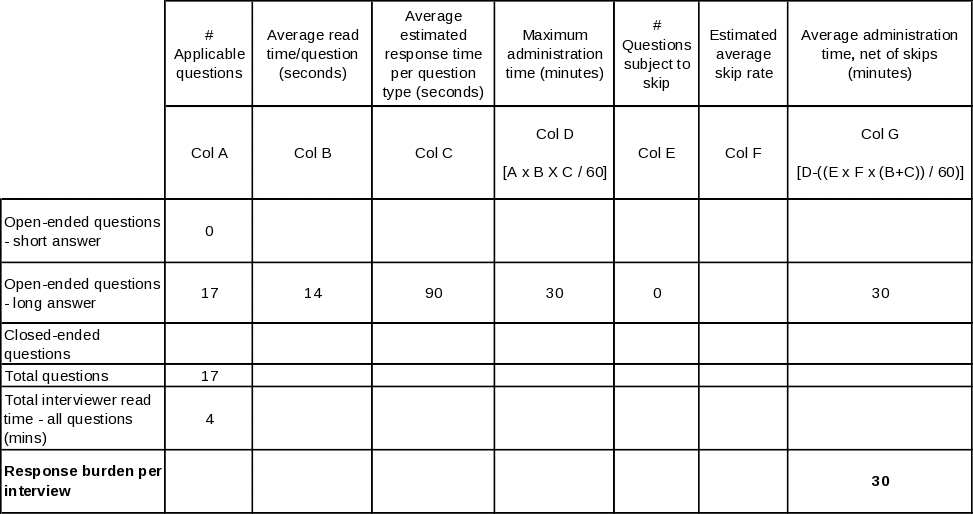
The interviewees will predominately be individuals from fairly high management level positions in the state, as well as Quitline partners from a variety of types of organizations. These individuals are expected to be largely in administrative and management positions. Service Providers to be interviewed are also expected to primarily be contracted professionals in their respective fields, and NAQC representatives are expected to be from a membership that may similarly include health care and other professionals as well as administrators. The cost to the respondents for the total burden is estimated to be $7,129.50, that is, $42 per hour for 169.75 burden hours. There are no other costs to respondents. These costs are summarized in Table A12-7.
Table A.12-7. Annualized costs to respondents
Type of respondent |
Estimated number of respondents |
Frequency of Response |
Average hours per response |
Hourly Wage Rate |
Respondent Cost |
State Tobacco Control Manager |
51 |
1 |
1.00 |
$42 |
$2142.00 |
State Quitline Administrator |
51 |
1 |
1.00 |
$42 |
$2142.00 |
State Quitline Service Provider |
19 |
1 |
.75 |
$42 |
$598.50 |
State Quitline Partner |
102 |
1 |
.50 |
$42 |
$2142.00 |
NAQC Representative |
5 |
1 |
.50 |
$42 |
$105.00 |
Total |
|
|
|
|
$7,129.50 |
A.13 Estimates of Other Total Annual Burden to Respondents or Recordkeepers
There are no costs to respondents beyond those present in Section A.12. There are no operating, maintenance, or capital costs associated with the collection.
A.14 Annualized Cost to the Federal Government
Based on the current HHS NNTCQ budget, the total cost to the Federal Government for the proposed evaluation is $1,397,700 for the 18-month period from September 29, 2005 to March 28, 2007. The annualized cost is approximately $931,707. This amount includes all direct and indirect costs of the design, data collection, analysis, and reporting phases of the evaluation. The annualized costs of Federal employees for monitoring the contract are estimated to be $33,580. These costs are based on 10 percent of the project officer’s time, 5 percent of the CIS research director’s time, as well as an additional .25 FTE, which includes various CIS staff who contribute to the design and report.
A.15 Explanation for Program Changes or Adjustments
This is a new collection of information.
A.16 Plans for Tabulation and Publication and Project Time Schedule
This study will include analysis of responses to five survey instruments, based on key informant telephone interviews conducted with state tobacco control managers, state quitline administrators, state quitline service providers, state quitline partners, and NAQC members. The analysis of these instruments is described in further detail below.
A.16.1 Key Informant Surveys
The five survey instruments will be composed of both common and unique sets of questions across the five stakeholder groups. Survey questions will include a mix of close-ended, open-ended and scaled items. The open-ended questions will be summarized based on common themes and compared across stakeholder groups. Descriptive statistics will be developed with the close-ended and scaled items.
The analysis of key informant interviews will be guided by the research questions articulated in Section A.2.1.
Key Informant Group Survey |
Research Questions |
Measures and Analysis |
NAQC |
How effectively was the Initiative developed and launched? How effective were the collaborations that occurred? |
Descriptive summaries of agencies involved, resources provided. Comparison of resources, collaborations provided by stakeholders. Mean effectiveness ratings of collaborations. |
State Quitline Service Provider, State Quitline Administrator |
How was the telecommunications infrastructure for 1-800-QUIT-NOW developed and implemented? Perceived benefits and barriers of telecommunications? |
Descriptive summaries of steps involved in planning and implementing. Comparison of perceived benefits and barriers pre and post Initiative and across stakeholder groups. |
Key Informant Group Survey |
Research Questions |
Measures and Analysis |
State Tobacco Control Manager, State Quitline Administrator |
How was the Request for Application (RFA) process developed and implemented? How did supplemental funding influence states with existing and non-existing quitlines? |
Comparison of state tobacco funding environment (total tobacco $, quitline $, etc.) pre and post Initiative funding. Descriptive summary of RFA process from both federal and state perspectives. |
State Tobacco Control Manager, State Quitline Administrator |
What promotion efforts were planned and implemented for 1-800-QUIT-NOW? To what extent did 1-800-QUIT-NOW appear in the media? What promotion efforts were planned and implemented for state quitline numbers? |
Descriptive summaries of promotion activities planned and implemented. Percent of Initiative funding used for promotions compared across states, for states with and without existing quitlines. . |
State Tobacco Control Manager |
What types of technical assistance, training and communications were provided to states relating to Initiative? How effective? |
Response distributions of the number and type of technical assistance (TA), training, & communications provided by federal government. Comparison of mean utility ratings for TA, training & communications provided to states. Descriptions of TA, training and communications. |
State Tobacco Control Manager, State Quitline Administrator |
To what extent was state capacity to deliver quitline services enhanced or maintained by the Initiative? |
Comparison of quitline services, programs and operations across three categories of states, 1) states with quitlines < $200,000; 2) states with quitlines >= $200, 000; and 3) states with no quitlines prior to the Initiative. |
State Tobacco Control Manager, State Quitline Partner, State Quitline Administrator |
How did the Initiative influence regional and state partnerships? |
Means, response distributions of the number and types of partners in existence as a result of the Initiative. Descriptions of the partnerships, including functions, financial relationships, and activities. |
The key informant telephone interviews will be completed in approximately three months after receiving OMB approval. The remaining contract period will be used to analyze and disseminate findings from the study. Table A.16.1 below provides the scheduled timeline for completing the study.
Table A.16-1. Project time schedule
Study Activity |
Time Schedule After OMB Approval |
Key Informant Interviews |
|
State Tobacco Control Managers |
1-3 Months after OMB approval |
State Quitline Administrators |
1-3 Months after OMB approval |
State Quitline Service Providers |
1-3 Months after OMB approval |
State Quitline Partners |
1-3 Months after OMB approval |
NAQC Representatives |
1-3 Months after OMB approval |
Coding and Analysis |
1-5 Months after OMB approval |
Final Report |
7 Months after OMB approval |
A.17 Reasons(s) Display of OMB Expiration Date is Inappropriate
NCI is not seeking an exception to the display of the OMB expiration date. The OMB expiration date will be displayed in the upper right-hand corner of the telephone interview protocols and all materials that are seen by respondents (e.g., advance mailings).
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
NCI is not requesting an exception to the certification requirements.
REFERENCES
Fiore MC, Croyle RT, Curry SJ, Cutler CM, Davis RM, Gordon C, Healton C, Koh HK, Orleans CT, Richling DE, Satcher D, Seffrin J, Williams C, Williams LN, Keller PA, Baker TB: Preventing 3 million premature deaths and helping 5 million smokers quit: A national action plan for tobacco cessation. American Journal of Public Health 2004, 94:205-210.
Centers for Disease Control and Prevention. Telephone Quitlines: A Resource for Development, Implementation, and Evaluation. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
Stead LF, Lancaster T, Perera R: Telephone counseling for smoking cessation. Cochrane Database Syst Rev 2003:CD002850.
Fiore MC, Bailey WC, Cohen SJ: Treating tobacco use and dependence: Clinical Practice Guideline. Rockville, MD, U.S. Department of Health and Human Services, U.S. Public Health Service, 2000.
19 Certification for Paperwork Reduction Act Submissions
Placeholder to Insert Signature Page from form 83i
| File Type | application/msword |
| File Title | REQUEST FOR CLEARANCE FOR THE EVALUATION OF THE NATIONAL |
| File Modified | 2006-10-16 |
| File Created | 2006-10-16 |
© 2026 OMB.report | Privacy Policy