2245ss01
2245ss01.doc
NESHAP for Hospital Ethylene Oxide Sterilization (40 CFR part 63, subpart WWWWW) (Proposed Rule)
OMB: 2060-0713
OMB-83I FORM SUPPORTING STATEMENT
FOR OMB REVIEW OF ICR NO. 2245.01
INFORMATION COLLECTION REQUEST FOR THE NATIONAL EMISSION STANDARDS FOR HAZARDOUS AIR POLLUTANTS (NESHAP) FOR THE HOSPITAL ETHYLENE OXIDE STERILIZATION SOURCE CATEGORY
U.S. ENVIRONMENTAL PROTECTION AGENCY
EMISSIONS, MONITORING, AND ANALYSIS DIVISION
RESEARCH TRIANGLE PARK, NORTH CAROLINA 27711
OCTOBER 2006
PART A OF THE SUPPORTING STATEMENT
1.0 Identification of the Information Collection
(a) Title and Number of the Information Collection.
"NESHAP for Hospital Ethylene Oxide Sterilization (40 CFR part 63, subpart WWWWW) (Renewal)." This is a new information collection request (ICR). The EPA tracking number is 2245.01.
(b) Short Characterization.
Potential respondents are owners or operators of existing and new hospital ethylene oxide (EO) sterilization facilities. The source category includes area source facilities that perform the operations necessary to sterilize medical items with ethylene oxide at hospitals.
Respondents are required to submit a one-time initial Notification of Compliance Status. General requirements applicable to all National Emission Standards for Hazardous Air Pollutants (NESHAP) require respondents to maintain records of all notifications; monitoring data; and supporting documentation for notifications and reports. Records and reports must be retained for a total of 5 years (2 years at the site; the remaining 3 years of records may be retained off-site). The files may be maintained in electronic form such as microfilm, computer disks, or magnetic tape.
For sources that are uncontrolled, the Hospital EO Sterilization NESHAP would require respondents to monitor and record for the management practice (MP) (i.e., whether sterilization units are operated with full loads). Sources that are controlled would be exempt from the MP and be required to certify that they are controlling sterilization emissions and follow manufacturer recommended procedure. These requirements are described in Attachment 1.
2. Need For and Use of the Collection
(a) Need/Authority for the Collection.
The U.S. Environmental Protection Agency (EPA) is charged under Section 112 of the Clean Air Act (CAA) to establish NESHAP for new or existing area sources of hazardous air pollutants ". . . which provide for the use of generally available control technologies or management practices by such sources to reduce emissions of hazardous air pollutants." Certain records and reports are necessary for the Administrator to confirm the compliance status of affected sources and identify new or reconstructed sources subject to the standards. Specific information needed by EPA for the proposed Hospital EO Sterilization NESHAP includes verification that the management practice is properly followed for uncontrolled sources, and for controlled sources, that control devices are operating and will continue to operate in accordance with applicable state and local laws or, if controls are voluntary, in accordance with manufacturers’ specifications. These recordkeeping and reporting requirements are specifically authorized by section 114 of the CAA (42 U.S.C. 7414) and are set out in the General Provisions to 40 CFR part 63.
(b) Practical Utility/Users of the Data.
The information will be used by Agency enforcement personnel to: (1) identify area sources; (2) ensure that the management practice is being properly applied; and (3) ensure that emission control devices are being properly operated and maintained. Based on the reported information, EPA can decide which plants, records, or processes should be inspected. The records that plants maintain will indicate to EPA whether sterilization units are operating properly.
3. Nonduplication, Consultation, and Other Collection Criteria
(a) Nonduplication.
A search of existing standards and outgoing ICRs revealed no duplication of information-gathering efforts. However, certain reports required by State or local agencies may duplicate information required by the standards. In such cases, a copy of the report submitted to the State or local agency may be provided to the Administrator in lieu of the report required by the standards. The EPA has issued guidance to State and local agencies encouraging them to consolidate duplicative requirements into a single element to be reported.
(b) Public Notice Required Prior to ICR Submission to OMB
This section is not applicable because this is a rule-related ICR.
(c) Consultations.
Participants in the development process for this proposed rulemaking included representatives from industry and other stakeholders. A 60-day public comment period will be provided after proposal, during which the public will be given the opportunity to comment on the proposed NESHAP. All comments received will be considered, and some may be reflected in the development of the final NESHAP.
(d) Effects of Less Frequent Collection.
If the relevant information were collected less frequently, EPA could not be reasonably assured that a plant is in compliance with the standards. In addition, our authority to take administrative action would be significantly reduced. Section 113(d) of the CAA limits the assessment of administrative penalties to violations which occur no more than 12 months before initiation of the administrative proceeding. Since administrative proceedings are less costly and require use of fewer resources than judicial proceedings, both EPA and the regulated community benefit from preservation of our administrative powers. Consequently, less frequent reports would not result in a reduced burden.
(e) General Guidelines.
The proposed Hospital EO Sterilization NESHAP would require that facilities retain records for a period of 5 years, which exceeds the 3‑year retention period specified in the general information collection guidelines in 5 CFR 1320.6(f) of the Office of Management and Budget (OMB) regulations implementing the Paperwork Reduction Act. However, the 5‑year retention period is consistent with the retention requirements in the General Provisions in subpart A of 40 CFR part 63 and the retention requirement in the operating permit program under 40 CFR part 70. At a minimum, respondents will be required to retain onsite the most recent 2 years of data. The remaining 3 years of data could be retained at a readily accessible onsite or offsite storage facility. None of the other guidelines in 5 CFR 1320.6 are being exceeded.
(f) Confidentiality
All information submitted to the Agency for which a claim of confidentiality is made will be safeguarded according to the Agency policies set forth in Title 40, Chapter 1, Part 2, Subpart B -- Confidentiality of Business Information (see 40 CFR 2; 41 FR 36902, September 1, 1976; amended by 43 FR 39999, September 28, 1978; 43 FR 42251, September 28, 1978; 44 FR 17674, March 23, 1979).
(g) Sensitive Questions.
The information to be reported consists of emissions data and other information that are not of a sensitive nature. Therefore, this section is not applicable because this ICR would not involve matters of a sensitive nature.
4. The Respondents and the Information Requested
(a) Respondents/NAICS Codes.
Respondents are owners or operators of Hospital EO Sterilization facilities that are area sources of hazardous air pollutants (HAP) emissions. A total of 1,900 existing facilities are estimated to be required to comply with initial reporting requirements of the proposed rule and 627 existing facilities are estimated to be required to comply with all of the requirements of the proposed rule by the third year of the rule. No new facilities are projected during the first 3 years of the proposed rule. The respondents are classified under the Standard Industrial Classification (SIC) codes and North American Industrial Classification System (NAICS) codes summarized in Table 1. Only certain processes classified in the SIC or NAICS codes listed in Table 1 would be regulated by the Hospital EO Sterilizers NESHAP.
(b) Information Requested.
(i) Data items, Including Recordkeeping Requirements. Attachment 1, Summary of Reporting and Recordkeeping Requirements, summarizes the recordkeeping and reporting requirements including the required retention time for all records.
(ii) Respondent activities. The respondent activities required by the standards are identified in Table 2 and introduced in Section 6(a). To the extent practicable, the activities required by respondents were designed to make use of or to be consistent with existing reporting and recordkeeping practices.
5. The Information Collected--Agency Activities, Collection Methodology, and Information Management
(a) Agency Activities.
A list of Agency activities is provided in Table 3 and introduced in Section 6(c).
(b) Collection Methodology and Management.
Information contained in the one-time only reports submitted to us will be reviewed for accuracy and completeness. Data obtained during periodic visits by our personnel from records maintained by the respondents will be tabulated and published for internal use in compliance and enforcement programs.
(c) Small Entity Flexibility.
The EPA estimates that 95 facilities of the 1,900 facilities that would be subject to the proposed NESHAP are owned by small businesses based on the definition used by the Small Business Administration (a small business whose parent company has less than $31.5 million in gross revenue, NAICS 62211 and 62231). The EPA does not expect a significant economic impact on a substantial number of small entities.
The NESHAP rule will allow the affected facilities 1 year from the effective date of the rule to comply. Under Section 112(i), the Administrator or the applicable regulatory authority also may grant one additional year if the owner or operator demonstrates that more time is needed to install controls for a source.
(d) Collection Schedule.
Collection of data will begin after the effective date of the Hospital EO Sterilization NESHAP. The compliance date for existing sources is 1 year after the effective date. The compliance date for new or reconstructed sources is the effective date if the source startup date is before the effective date or upon startup if the startup date is on or after the effective date. No new or reconstructed sources are anticipated during the 3-year ICR clearance period. The schedule for notifications required by the rule is summarized below.
For each initial compliance demonstration at uncontrolled and controlled sources, facilities must submit an initial notification of compliance status no later than 60 days following the completion of initial compliance period. Records at uncontrolled sources necessary to determine compliance with the management practice must be compiled on a daily basis. These data are not required to be reported but must be kept on site for inspection.
6. Estimating the Burden and Cost of the Collection
(a) Estimating Respondent Burden.
The annual burden estimates for reporting and recordkeeping are presented in Table 2. These numbers were derived from estimates based on EPA's experience with other standards.
(b) Estimating Respondent Costs.
The information collection activities for sources subject to the proposed rule are presented in Table 2. Labor costs for all relevant activities were estimated based on the most recently available labor rate data from the U.S. Bureau of Labor Statistics (BLS) (http://www.bls.gov/news.release/ecec.t11). Labor costs are divided into the following three categories: (1) technical, (2) management, and (3) clerical. The labor rates, including fringe benefits, reported by BLS for December 2005 (the most recent rates available) are $42.31 per hour ($42.31/hr) for technical personnel, $49.02/hr for managerial personnel, and $21.55/hr for clerical personnel. The base labor rates were adjusted by an overhead and profit rate of 167 percent. The final total labor rates are $71 for technical personnel, $82 for management, and $36 for clerical. In addition to labor costs, capital/startup costs include the costs purchasing file cabinets for storing records. Operation and maintenance (O&M) costs include the costs for photocopy and postage costs associated with reporting requirements. The capital/startup costs were estimated and annualized as described in the footnotes to Table 2.
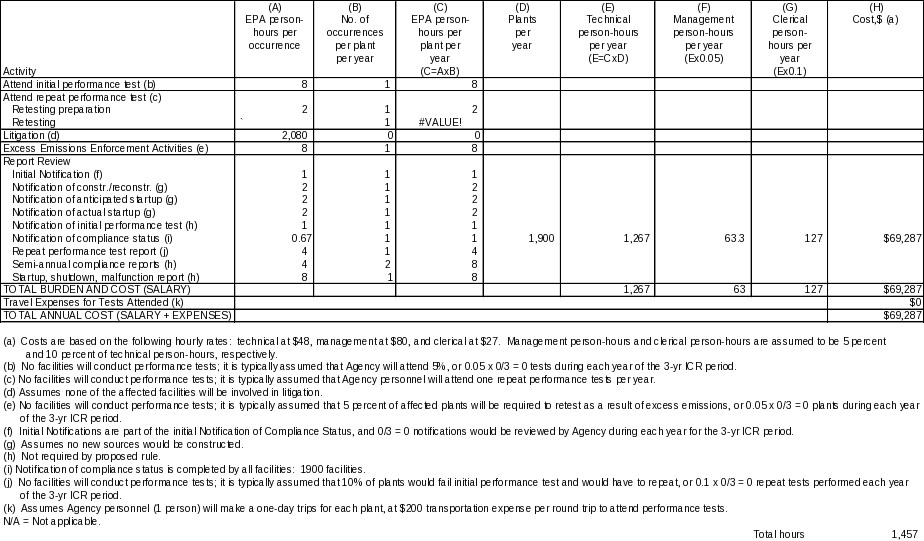
(c) Estimating Agency Burden and Cost.
No costs can be attributed to the development of the information collection requirements because the information collection requirements were developed as an incidental part of standards development. The recordkeeping requirements on the part of the respondents are required under the General Provisions to 40 CFR part 63. Because the respondent burden has already been applied under the General Provisions, no additional operational costs will be incurred by the Federal Government. Publication and distribution of the information are part of the Compliance Data System, with the result that no Federal costs can be directly attributed to the ICR. Examination of records to be maintained by the respondents would occur incidentally as part of the periodic inspection of sources that is part of EPA's overall compliance and enforcement program, and, therefore, would not attributable to the ICR. The only costs that the Federal government would incur are user costs associated with the analysis of the recorded information, as presented in Table 3. Labor rates for Federal employees are based on the January 2006, Office of Personnel Management labor rates for General Schedule employees (http://www.opm.gov/oca/06tables/indexGS.asp). The base labor rates are $30.06/hr for technical personnel (GS-12, step 5), $49.69 for management personnel (GS-15, step 5), and $16.95/hr for clerical personnel (GS-7, step 5). The base labor rates were multiplied by the standard government benefits multiplication factor of 1.6. The resulting average hourly labor costs are $48 for technical personnel, $80 for management, and $27 for clerical.
(d) Estimating the Respondent Universe and Total Burden and Costs.
The EPA has identified 1,900 existing facilities that would be subject to the proposed rule. All of these existing facilities would be required to submit an Initial Notification of Compliance Status. An estimated 627 facilities would be required to comply with all of the monitoring, recordkeeping, and reporting requirements of the rule. Details on the number of respondents affected by each individual burden item are provided in the footnotes of Table 2.
(e) Bottom Line Burden Hours and Costs/Master Tables.
(i) Respondent tally. The bottom line respondent burden hours and costs, presented in Table 2, are calculated by adding person-hours per year down each column for technical, managerial, and clerical staff, and by adding down the cost column. The total annual number of responses is 633. The estimated total annual hours are 23,694 at an annual labor cost of $1,621,478. The estimated total capital/startup costs that would be incurred during each of the 3 years following promulgation are $49,115. The annualized capital/startup costs are $5,393. The total annual O&M cost is estimated to be $3,167. The total annualized cost requested (including the annualized capital/startup and O&M costs) is $8,559.
(ii) The Agency tally. The bottom line Agency burden hours and costs, presented in Table 3, are calculated as in the respondent table, by adding person-hours per year down each column for technical, managerial, and clerical staff, and by adding down the cost column. In this case, total cost is the sum of this total salary cost and total travel expenses for tests attended. The total annual hours are 1,457. The total annual cost is $69,287.
(iii) Variations in the annual bottom line. This section does not apply since no significant variation is anticipated.
(f) Reasons for Change in Burden.
This section does not apply because this is a new collection.
(g) Burden Statement
The annual public reporting and recordkeeping burden for this collection of information is estimated to average 23,694 for the 1,900 affected hospital EO sterilization facilities.
Burden means the total time, effort, or financial resources expended by persons to generate, maintain, retain, or disclose or provide information to or for a Federal agency. This includes the time needed to review instructions; develop, acquire, install, and utilize technology and systems for the purposes of maintaining information, and disclosing and providing information; adjust the existing ways to comply with any previously applicable instructions and requirements; train personnel to be able to respond to a collection of information; search data sources; complete and review the collection of information; and transmit or otherwise disclose the information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The OMB control numbers for EPA’s regulations are listed in 40 CFR part 9 and 48 CFR chapter 15. To comment on the Agency's need for this information, the accuracy of the provided burden estimates, and any suggested methods for minimizing respondent burden, including the use of automated collection techniques, EPA has established a public docket for this ICR under Docket ID Number EPA-HQ-OAR-2005-0171, which is available for online viewing at www.regulations.gov, or in person viewing at the Air and Radiation Docket Information Center in the EPA Docket Center (EPA/DC), EPA West, Room B102, 1301 Constitution Avenue, NW, Washington, D.C. The EPA Docket Center Public Reading Room is open from 8:30 a.m. to 4:30 p.m., Monday through Friday, excluding legal holidays. The telephone number for the Reading Room is (202) 566-1744, and the telephone number for the Air and Radiation Docket and Information Center is (202) 566-1742. An electronic version of the public docket is available at www.regulations.gov. This site can be used to submit or view public comments, access the index listing of the contents of the public docket, and to access those documents in the public docket that are available electronically. When in the system, select “search,” then key in the Docket ID Number identified above. Also, you can send comments to the Office of Information and Regulatory Affairs, Office of Management and Budget, 725 17th Street, NW, Washington, D.C. 20503, Attention: Desk Officer for EPA. Please include the EPA Docket ID Number EPA-HQ-OAR-2005-0171 and OMB Control Number 2060-NEW in any correspondence.
NOTE: The EPA Docket Center suffered damage due to flooding during the last week of June 2006. The Docket Center is continuing to operate. However, during the cleanup, there will be temporary changes to Docket Center telephone numbers, addresses, and hours of operation for people who wish to make hand deliveries or visit the Public Reading Room to view documents. Consult EPA's Federal Register notice at 71 FR 38147 (July 5, 2006) or the EPA website at http://www.epa.gov/epahome/dockets.htm for current information on docket operations, locations and telephone numbers. The Docket Center’s mailing address for U.S. mail and the procedure for submitting comments to www.regulations.gov are not affected by the flooding and will remain the same.
PART B OF THE SUPPORTING STATEMENT
This section is not applicable because statistical methods are not used in data collection associated with this regulation.
TABLE 1. SIC AND NAICS CODES
SIC |
NAICS |
Examples of respondents |
NA |
62211 |
General Medical and Surgical Hospitals |
NA |
62231 |
Specialty (Except Psychiatric and Substance Abuse) Hospitals |
TABLE 2. ANNUAL RESPONDENT BURDEN AND COST OF REPORTING AND RECORDKEEPING REQUIREMENTS OF THE PROPOSED STANDARD
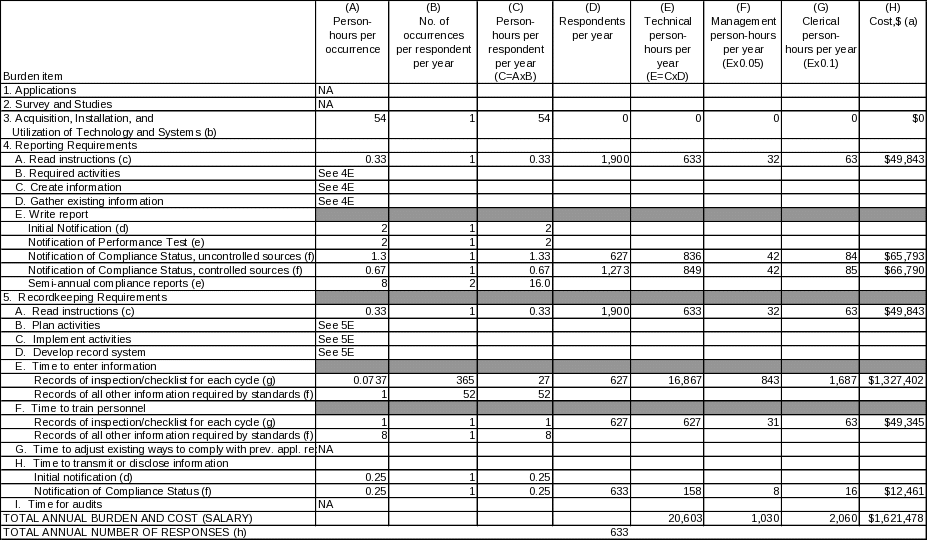

TABLE 3. ANNUAL BURDEN AND COST TO THE FEDERAL GOVERNMENT FOR THE PROPOSED RULE
ATTACHMENT 1
SUMMARY OF REPORTING AND RECORDKEEPING REQUIREMENTS
Summary of Recordkeeping and Reporting Requirements
Requirements |
Regulation Reference |
Notifications |
|
Notification of compliance status |
''63.12(d)(2) and 63.10430(b) |
Records |
|
Record retention |
''63.10(b)(1) and 63.10434(a)-(c) |
Records and documentation supporting initial notification of compliance status |
''63.10432(a)-(b) |
Reports |
None required by proposed rule |
| File Type | application/msword |
| File Title | OMB-83I FORM SUPPORTING STATEMENT |
| Author | mkicenhour |
| Last Modified By | ckerwin |
| File Modified | 2006-10-27 |
| File Created | 2006-10-26 |
© 2026 OMB.report | Privacy Policy