Part A-ADAM II Justification OMB Final
Part A-ADAM II Justification OMB Final.doc
Arrestee Drug Abuse Monitoring (ADAM II)
OMB: 3201-0013
Cambridge, MA Lexington, MA Hadley, MA Bethesda, MD Chicago, IL
Abt Associates Inc. 55 Wheeler Street Cambridge, MA 02138
Part A - Justification
Request for
OMB Review
January 12, 2007
Prepared by
Lisa Magged
Sarah Kuck
Dana Hunt
Prepared for
Robert L. Cohen
Executive Office of the President
Office of National Drug Control Policy (Planning and Budget)
Washington, DC 20503
Contents
Arrestee Drug Abuse Monitoring Program (ADAM) II 2
1. Circumstances That Make Collection of Data Necessary 2
2. Purposes and Uses of the Data 5
3. Use of Improved Information Technology to Reduce Burden 6
4. Efforts to Identify and Avoid Duplication 7
5. Efforts to Minimize Burden on Small Businesses or Other Entities 7
6. Consequences If the Information Is Not Collected or Is Collected Less Frequently 7
8. Public Comment Received on Federal Register Notice 8
9. Incentives to Respondents 8
10. Assurances of Confidentiality Provided to Respondents 8
11. Justification for Questions of a Sensitive Nature 9
12. Estimate of Information Collection Burden 9
13. Estimate of Total Annual Cost Burden 10
14. Estimates of Annualized Costs 10
15. Change in Annual Reporting Burden 10
16. Plans for Tabulation and Publication of Results 10
18. Exceptions to Certification Statement 11
Part B: Collection of Information Employing Statistical Methods 12
1. Respondent Universe and Sampling Methods 12
2. Information Collection Procedures 15
3. Methods to Maximize Response Rates 22
5. Individuals Consulted on Statistical Aspects of the Design 25
Arrestee Drug Abuse Monitoring Program (ADAM) II
The Office of National Drug Control Policy’s (ONDCP’s) mission, since its authorization in 1988, has been to establish the Nation’s drug control policy, to set priorities for advancing that policy, and to identify and monitor objective measures of that policy’s success. The original Arrestee Drug Abuse Monitoring program (ADAM) was a critical source of data for ONDCP in meeting its objectives, and its demise in 2003 left a serious gap in accurate and timely information on trends in drug use.
The first ADAM data collection was instituted in 2000 as a replacement for the Drug Use Forecasting program (DUF), which employed a non-scientific sampling procedure to select primarily felony arrestees in 23 urban areas throughout the country. The year 2000 revision of ADAM instituted a representative sampling strategy among booked male arrestees in an expanded network of 35 sites. The original OMB approval for this collection authorized 100,000 responses and a time burden of 62,500 hours (OMB control # 1121-0137). That authorization expired in 2005.
With ADAM II, ONDCP and its contractor, Abt Associates, Inc., will initiate a new data collection that will replicate the ADAM methodology in order to obtain data comparable to previously established trends. ADAM II will implement two quarters of data collection in ten sentinel ADAM sites to revive monitoring drug trends, with a particular focus on obtaining valid and reliable information on methamphetamine use. Representing minimal adjustments to the previously approved ADAM survey, the ADAM II survey will collect data about drug use, drug and alcohol dependency and treatment, and drug market participation among booked male1 arrestees within 48 hours of arrest. Data collection will take place across two back-to-back quarters in each of 10 counties from a county-based representative sample of 250 male arrestees per quarter for a total of 500 arrestees annually per site or a total of 5000 arrestees across sites annually. Collection will occur in two cycles at each site to provide estimates for two calendar quarters each year. One year of collection will occur beginning April 1, 2007 and ending March 31, 2008. If additional data collection periods are optioned by ONDCP, subsequent cycles of back-to-back data collection will occur beginning April 1, 2008. Participation is voluntary and confidential, and the procedures will include a personal interview (lasting approximately 20 minutes) and collection of a urine specimen. Though a convenience sample of female and juvenile arrestees was a part of the previous ADAM program, those groups will be excluded from this effort for the purposes of economy.
1. Circumstances That Make Collection of Data Necessary
Objective measures of progress in meeting the Nation’s drug strategy goals are an essential part of ONDCP’s work. Since its inception ONDCP has worked with federal, state and local agencies to create and improve data on the Nation’s drug problems. ONDCP also understands that arrestees can be a unique bellweather of drug use trends. They tend to be the first and the heaviest consumers of illegal drugs. In 1998 the National Institute of Justice began a multi-city data collection program called Drug Use Forecasting (DUF) designed to monitor trends in drug use among the arrestee population. While a landmark effort, the DUF model was based on a non-probability based, convenience sample of both booking facilities and respondents, limiting its utility for estimation. In 2000 NIJ commissioned a redesign of the program and created the Arrestee Drug Abuse Monitoring (ADAM ) data collection system which created sampling and data collection protocols that allowed scientifically sound prevalence estimation in 35 counties across the country.
From 2000–2003 the ADAM program provided a route to estimating drug use and examining drug market behaviors for a range of illegal drugs. It became the backbone of the ONDCP estimates of nationwide drug consumption and expenditure published in the National strategy and in the ONDCP publication, What America Spends on Drugs. Since the ADAM research program under the National Institute of Justice was terminated in 2003, the Director of ONDCP has determined that there is a critical need to resume the collection of trend information on drug abuse among booked arrestees. ADAM II is vital to estimating the magnitude and patterns of chronic drug use, to understanding its connection to crime, and to examining market trends. In particular, ADAM II will be essential for providing valid and reliable information on methamphetamine use to continue to determine the extent and possible spread of the problem since the cessation of the ADAM program in 2003.
Throughout the 1990s, data sources including DUF indicated that methamphetamine use was growing steadily in the West and Northwest. By the turn of the millennium, it had reappeared in many areas of the Midwest and South and surfaced to a lesser degree in the Northeast and Mid Atlantic. But data from the general population, as reflected in the National Survey on Drug Use and Health (NSDUH), showed a relatively minor increase nationwide rising from just under 2% of the adult population in 1994 to approximately 4.3% in 2005. The premier national survey of youth, Monitoring the Future (MTF), reports that lifetime methamphetamine use has dropped somewhat in the past several years from a high of 8.2% in 1999 to 4.5% in 2005. National treatment data from the Treatment Episode Data Set (TEDS) on admissions to treatment indicate a steady rise in the number of persons nationwide who enter treatment for methamphetamine abuse. From 1992 to 2002 the rate of treatment admissions for methamphetamine abuse in the U.S. increased fivefold, but still from less than 1% in 1992 to over 8% in 2004. The Drug Abuse Warning Network emergency room reports show a similar trend nationally: a slight rise nationally from just under 16,000 mentions in 1995 to 17,696 in 2002.
But National trend data are seriously misleading. While national data such as these show some changes in overall use, albeit at low levels, regional data on methamphetamine use provide a far more serious picture of the problem. TEDS data show that in 1992 only two states (Hawaii and California) reported more than 5% of total treatment admissions were for methamphetamine. In 2003, 26 states reported over 5%, 8 states reported over 20%, and 2 states (Hawaii and Idaho) reported over 40% methamphetamine admissions. The highest rates were reported in Hawaii and the West, where states like Idaho reported 42%, Nevada reported 28%, and California reported 31%. Midwestern states like Iowa (20%), and Southern states like Arkansas (22%) also report rates far higher than the national average. While the highest rates of use remain in the West and Midwest, there are increases in other new areas. In North Dakota, for example, in 1992 no admissions were for methamphetamine; in 2003, 12% of North Dakota admissions were for meth abuse.
City level data in DAWN emergency room mentions are similarly dramatic. While some cities with high numbers of ER mentions for meth have remained unchanged or even declined somewhat (Los Angeles, San Francisco, San Diego, Dallas, Denver), other areas have experienced enormous upswings in ER mentions since 1995: Seattle (109% increase), Minneapolis (243% increase), New Orleans (194% increase), St. Louis (97% increase). But even regional treatment and ER data present a limited segment of the drug use population, those who are seeking assistance. In fact, research indicates that a significant number of serious drug users do not access treatment, particularly in the early stages of their drug use careers. They do, however, intersect with the criminal justice system due to the very nature of their behavior—obtaining and using illegal drugs.
DUF and now ADAM have been the important supplementary source of data these users. As shown in Figure 1.1 below, both ADAM and its predecessor, DUF, detected the increased presence of methamphetamine among arrestees in the early to mid-1990’s. While the DUF data collected prior to 2000 cannot be used to estimate trends with known levels of confidence, this rise is worth noting. For example, Omaha, Nebraska urine screens of arrestees were virtually free of methamphetamine until the mid-1990s (Figure1.1), when meth-positive UAs began to increase. From 2000-20003 it almost doubled. Similarly, San Jose arrestees tested positive at rates under 20% throughout the 1990s; from 2000–2003 the percent positive rose from just over 20% to over 35%. While ADAM showed the rise of methamphetamine use in the West and Midwest, there was little to no use apparent in the Northeast and in some areas of the South. Three years have passed since last collection and we anticipate that this picture may have changed. Only by reinstituting collection in these sites will we be able to determine if the same movement that characterized the western half of the country over the past decade has moved east.
A national data collection that provides local and regional estimates in this population is a critical key to adequately and accurately tracking the changes in methamphetamine use and other changing patterns of drug use.
Figure 1.1
Arrestees
Testing Positive for Methamphetamine in Selected DUF/ADAM Sites
1989‑2003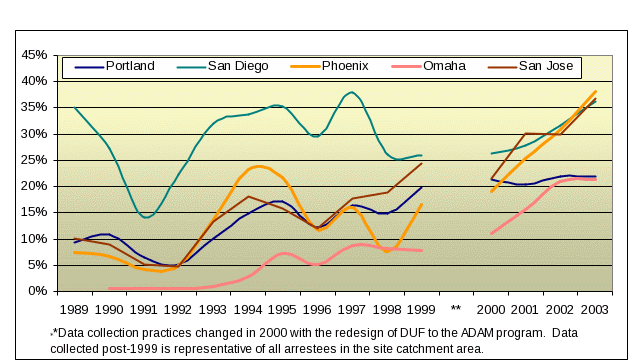
2. Purposes and Uses of the Data
With ADAM II, ONDCP will initiate a new data collection that will replicate the ADAM methodology to obtain data directly comparable to previously established trends. ADAM obtained data regarding drug use and abuse, drug markets, and treatment needs among booked arrestees. Working with ONDCP, Abt Associates developed chronic user estimates for ADAM sites and used ratio estimation procedures to extend site-based estimates to the Nation. ADAM-based estimates are used in a range of other contexts:
To convert System to Retrieve Information from Drug Evidence (STRIDE) data into reliable estimates of retail price/purity for heroin, cocaine, methamphetamine and marijuana;
To convert Domestic Monitor Program (DMP)/Heroin Signature Program (HSP) data into national estimates of the flow of heroin into the U.S. from South American, Mexico and the rest of the world;
To monitor how enforcement practices have affected local markets for crack and powder cocaine, heroin, methamphetamine and marijuana;
To monitor the emergence and spread of methamphetamine across the United States; and
To develop and execute quick-turnaround monitoring of emergent drugs across the ADAM sites.
With ADAM II ONDCP will initiate a new study that is in fewer sites (10) but methodologically equivalent to the ADAM model that was implemented in 2000–2003. The key goals of ADAM II are to support ONDCP’s efforts to monitor trends in drug use and drug markets by:
Providing data on the prevalence of drug use in 10 US counties;
Providing data to monitor the possible spread of methamphetamine use into new areas; and
Obtaining consistent data to support statistical trend analysis with 2000–2003 ADAM data in those 10 counties.
3. Use of Improved Information Technology to Reduce Burden
Imposing the least burden on ADAM II respondents and security personnel in jail facilities is a significant priority. Various electronic data capture strategies were considered, including supplying interviewers with notebook computers, PDAs, or tablet PCs that could run an electronic ADAM instrument and upload data through the Internet. Despite the efficiencies that these technologies afford, past experience with ADAM has shown that:
Paper copies are a more stable medium for confinement facilities where lack of power or Internet connectivity can translate technical problems into lost time and data.
Paper is a better medium for interacting with respondents who must review their previous calendar responses to recall information in context.
Technology can impede access to jail populations and create unnecessary tension for security personnel.
Even in criminal justice settings where evaluators have agreements to bring technology in, changing circumstances can often result in delays and missed interview shifts. Many booking facilities will not permit electronic equipment to be brought into their holding areas. Many facilities will see laptops, even the smaller notebooks, as intrusive or as security risks.
As noted above, there are also issues with accessing power for electronic equipment; that is, no electrical outlets. While interviewers can, and would, carry extra batteries, this approach raises other issues. For example, batteries may be removed from computers both on entering and leaving facilities to insure that no contraband enters or leaves the premises, and power and/or data can be lost with battery removal.
Electronic equipment may be perceived differently by respondents and impact their answers. Given the complexity and size of the ADAM instrument, existing PDAs might complicate data collection because the screens are too small to readily encompass the calendar that is central to ADAM II data collection. Further, PDAs are somewhat more fragile and more susceptible to data loss or damage as they are jostled through security procedures and screening devices.
Data for ADAM have always been collected with paper-and-pencil instruments and ADAM II will continue this methodology to maintain comparability with data from the earlier collections. However, the challenges in this environment remain to be: (1) increasing the accuracy of data collected via paper and pencil and (2) providing rapid turnaround of data both for analysis and for quality control checks on interviewer performance. To address these issues, scannable paper interviews will be implemented and uploaded via character recognition software. Very recent improvements in character recognition have made scanning a rapid and accurate process that provides interviewers with the convenience of paper data entry as well as with the transmission and storage advantages of software. This solution will result in a better data processing system than traditional data entry that can be subject to human error and adds more rapid turnaround.
4. Efforts to Identify and Avoid Duplication
The Office of National Drug Control Policy will submit 60-day and 30-day Federal Register notices intended to solicit public comment on the proposed information collection.
Like the original ADAM project, ADAM II is the only data collection effort that supports statistical trend analysis of drug use in this population. From 2000–2003 the ADAM system provided a unique route to estimating chronic drug use and examining market behaviors by capturing information on a critical segment of the user population–those users, often the most drug-involved, who interact with the criminal justice system. Many of the drug users identified at the time of booking are not found in the Nation’s other drug use monitoring efforts. Since they are often living in transient housing arrangements, they are not in the National Survey on Drug Use and Health. Many do not access treatment and, consequently, are not in the Substance Abuse and Mental Health Administration’s Treatment Episode Data Set. Interviewing this population represents a complementary, not duplicative, effort.
ADAM was also the only national drug study that utilized drug testing (urinalysis). ADAM II will replicate this methodology for ADAM II sites to monitor drug trends, with a particular focus on methamphetamine use and provide information on drug use and abuse, drug markets, and treatment needs among booked arrestees missing in these other studies.
5. Efforts to Minimize Burden on Small Businesses or Other Entities
No small businesses or other entities will be involved as respondents. All respondents will be booked male arrestees in police departments and/or county jails.
6. Consequences If the Information Is Not Collected or Is Collected Less Frequently
The Office of National Drug Control Policy has a need for the results of the ADAM II study in order to make informed decisions about policies, priorities, and objectives for the Nation's drug control program. ADAM II is the only source of data that adequately accesses the heaviest, most problematic users. Without data on chronic drug use and market behaviors, policymakers are not equipped to design policies and programs to reduce illicit drug use, manufacturing, trafficking, crime, violence, and drug-related health consequences. In particular, ONDCP has a pressing need to monitor methamphetamine use and ADAM data are particularly relevant to this task where little is known about the nature of the market and what may impact shifts in use.2
7. Special Circumstances Requiring Collection of Information in a Manner Inconsistent with Section 1320.5(d)(2) of the Code of Federal Regulations
None of the special circumstances listed apply to this data collection.
8. Public Comment Received on Federal Register Notice
No public comments were received during the 60-Day comment period.
As in the original ADAM study, a small food incentive such as a candy bar, potato chips, or sandwich will be provided to respondents either during or subsequent to the interview (depending on site regulations).
10. Assurances of Confidentiality Provided to Respondents
At each site, interviewers trained by Abt Associates will collect voluntary and confidential interviews and urine specimens from booked adult male arrestees. Names and other personal identifiers will not be collected. To preserve anonymity, a common ID number (barcode) will be attached to the interview form and urine specimen container so that self-reported data may be connected to urinalysis results.
Abt Associates frequently collects sensitive data from vulnerable populations and is familiar with the necessary protections that accompany this type of data collection, particularly with populations who may be involved in illegal behaviors. To protect respondents who share their personal information, as in the original ADAM work Abt will apply for and secure a National Institute of Health Certificate of Confidentiality to protect all data collected by Abt Associates and its subcontractors from subpoena. Abt Associates currently holds Certificates of Confidentiality for several studies, including studies involving children, offenders, and persons undergoing sensitive treatment protocols, and is familiar with the process. The final certificate will contain language that reinforces the protections of confidentiality to each arrestee and the absence of identifying information on all sample and survey data collection tools. Before each interview, the ADAM II interviewers will inform research participants of the Certificate of Confidentiality and the protection that the certificate provides.
As in the original ADAM work, IRB approval has been obtained for ADAM II for data collected on study participants. Abt Associates has a standing Institutional Review Board, which holds a Federal Wide Assurance (FWA) from the Office for Human Research Protections (OHRP). Abt Associates is firmly committed to protecting all human subjects involved in its research, and each Project Director or Principle Investigator at Abt is required to complete Human Subjects training. Training programs for all interviewers also include a discussion of human subjects issues, and all interviewers will read and sign a Certificate of Confidentiality for submission to the ADAM Data Center.
Prior to each interview, interviewers will read the consent and confidentiality information contained on the consent sheet and will ask the respondent if he wishes to participate in the interview and if he is willing to provide a urine sample (see attached consent script). All arrestees who provide data for this study will be assured, in writing, that the information they provide will not be released in a form that is identifiable. No identifying information will be attached to any data supplied to the Office of National Drug Control Policy, law enforcement, other researchers, or any other person or agency.
Unless an arrestee voluntarily agrees to participate, the interview cannot be done. Each study subject must voluntarily agree to participate prior to administering the ADAM questionnaire. The back of the ADAM II facesheet will provide a consent script to be read to potential respondents that has been approved by Abt’s IRB for reading to study subjects. It includes two separate consent agreements: one for the interview and one for providing a urine specimen. The subject may agree to the interview and not agree to provide a specimen and still be included in the sample. If the subject consents, the interview will be completed. If the subject is unwilling, the reason for refusal will be recorded, the subject will be returned to the holding area and all materials (interview form, facesheet and lab supplies) stored.
Individual-level databases and computer files will be protected by restricted use passwords or other techniques to limit access to staff involved in data analysis. No data will ever be reported by the contractor in any form where individual respondents can be identified.
11. Justification for Questions of a Sensitive Nature
The intent of the ADAM II survey is to support the Office of National Drug Control Policy’s efforts to estimate trends in drug use and examine drug market behaviors. Because drug use and illegal activities are potentially sensitive subjects, some questions will be sensitive for the respondents. Arrestees will be asked about drug use, drug and alcohol dependency and treatment, and drug market participation. Questions included in the ADAM II survey have been adapted from the NIJ methamphetamine addendum, and have been extensively used with arrestee populations in past iterations of the Arrestee Drug Abuse Monitoring Program (ADAM), approved by OMB in 1999 (OMB control # 1121-0137). Subjects may skip questions at any time or terminate the interview. Since the initiation of the redesigned ADAM instrument in 2000 of which this instrument is a minor adaptation, over 89,924 arrestees have been interviewed successfully.
12. Estimate of Information Collection Burden
Exhibit 1
Estimated Respondent Burden
|
|||||
Data Collection Activity |
Number of Respondents per Data Collection per Site per Quarter |
Number of Sites |
Number of Data Collections |
Time per Response (minutes) |
Total Hour Burden |
Arrestee Drug Abuse Monitoring (ADAM) II Survey Base+ Option 1 |
250 |
10 |
2 |
20.5 |
1667 |
ADAM II Option 2 |
250 |
10 |
2 |
20.5 |
1667 |
ADAM II Option 3 |
250 |
10 |
2 |
20.5 |
1667 |
Total request (all sites; all quarters) |
15,000 |
|
|
|
5,001 |
13. Estimate of Total Annual Cost Burden
There are no respondent costs associated with this data collection other than the hour burden estimated in item 12.
14. Estimates of Annualized Costs
The estimated annualized cost for the Arrestee Drug Abuse Monitoring (ADAM) Program II is $1,992,657. The total amount includes the following:
Task |
Labor Hours |
Labor Costs |
Operations Direct/Indirect Costs |
Total |
Review and adjust study protocol |
678 |
$23,843 |
$49,578 |
$73,421 |
Site selection and recruitment |
630 |
$26,654 |
$54,461 |
$81,115 |
Subcontract urinalysis of specimens |
290 |
$13,522 |
$27,453 |
$40,975 |
Prepare for Data Collection |
1,260 |
$47,498 |
$271,757 |
$319,255 |
Implement data collection |
926 |
$43,645 |
$708,900 |
$752,545 |
Data linkage and processing |
2,590 |
$75,231 |
$253,772 |
$329,003 |
Analysis and dissemination |
666 |
$34,992 |
$70,333 |
$105,325 |
Progress reporting and briefings |
1,514 |
$80,289 |
$210,729 |
$291,018 |
Total |
8,554 |
$345,674 |
$354,228 |
$1,992,657 |
15. Change in Annual Reporting Burden
This request is for a new information collection.
16. Plans for Tabulation and Publication of Results
Abt Associates will prepare ADAM II study findings for dissemination to a wide range of audiences, including ONDCP, the study sites, drug researchers, practitioners, policy makers, and the interested lay audience. Reporting activities will include:
Date(s) |
Report(s) |
Description |
Ongoing Throughout ADAM II Study, October 1, 2006 – March 31, 2008 |
Special Requests |
Special requests may include briefing materials and reports for senior government officials, presentations for research conferences, and short special topic monographs. |
Quarterly Throughout ADAM II Data Collection, April 1, 2007 – March 31, 2008 |
Quarterly Reports for ADAM II Sites |
Quarterly reports will be created for each site and consist of a simple one-page report on each quarter’s data collection. |
March 31, 2008 |
Final Camera-ready and Web-ready Report |
The final camera and web-ready report will present ADAM II study findings that practitioners and general audiences may digest for their own research purposes. |
March 31, 2008 |
Final Technical Design Report |
The final technical design report will describe in detail:
All instrumentation, training materials, protocols, data collection and data entry manuals will be included. The report will include a Technical Appendix describing the final statistical approaches for estimation and trend analysis. |
All data collection instruments will include the OMB expiration date.
18. Exceptions to Certification Statement
No exceptions are requested.
1 ADAM II will obtain data from male arrestees because the adult male component was the most robust segment of the ADAM study.
2 Hunt, D., Kuck, S. and Truitt, L. (2006). Methamphetamine: Lessons Learned, U.S. Department of Justice, NIJ Report, 20973, February.
Abt
Associates Inc. Request for OMB Review
| File Type | application/msword |
| File Title | Abt Single-Sided Body Template |
| Author | faheye |
| Last Modified By | cohen_r |
| File Modified | 2007-01-05 |
| File Created | 2006-12-12 |
© 2026 OMB.report | Privacy Policy