Case, Comparison, and Subcohort Ascertainment Methodology
Appendix K site specific ascert.doc
The National Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) Study
Case, Comparison, and Subcohort Ascertainment Methodology
OMB: 0920-0741
CADDRE Case, Comparison Group, and Subcohort Ascertainment Methodology
STUDY POPULATION
The study population for each site is described below using the 2000 year estimates. A description of the catchment area below is followed by a table describing the number of live births reported in 2000, the racial composition of the mothers, proportion of children under the age of 18 years of age living in poverty, and the urban/rural distribution of the catchment area. In 1989, the National Center for Health Statistics adopted the use of race/ethnicity of the mother for birth data, rather than race/ethnicity of the child, as a standard classification. Thus, the race/ethnic composition reported below is the race/ethnicity of the mother. Due to differences in reporting ethnicity among the states, race is categorized as White-Non-Hispanic and White-Hispanic and also as a general category of Hispanic and Non-Hispanic.
California
The catchment area will include a two county area: Alameda and Santa Clara counties.
Colorado
The catchment area will include the seven-county Denver metropolitan area: (Arapahoe, Adams, Boulder, Broomfield, Denver, Douglas, and Jefferson counties). Broomfield did not officially become a county until November 2001, and consisted of portions of Adams, Boulder, Jefferson, and Weld counties. The portion of Weld county that is now part of Broomfield was not included in the estimates since most sources did not report the demographics by zip code, however, we do not expect that these numbers would significantly change the proportions reported below.
Georgia (CDC)
The catchment area will include the five-county metropolitan Atlanta area: Clayton, Cobb, DeKalb, Fulton, and Gwinnett counties.
Maryland
The catchment area will include seven jurisdictions in northeastern Maryland: Anne Arundel, Baltimore, Carroll, Cecil, Harford and Howard Counties and Baltimore City.
North Carolina
The catchment area will include a ten county area: Alamance, Chatham, Davidson, Durham, Forsyth, Guilford, Johnston, Orange, Randolph, and Wake counties.
Pennsylvania
The catchment area will include three counties in Pennsylvania: Chester, Montgomery and Philadelphia counties.
Population Characteristics of Catchment Areas
|
California |
Colorado |
Georgia |
Maryland |
North Carolina |
Pennsylvania |
|
|
|
|
|
|
|
Number of Live Births |
47,454 |
38,342 |
49,966 |
35,037 |
34,000 |
37,217 |
|
|
|
|
|
|
|
Race/Ethnicity of Mother |
|
|
|
|
|
|
American Indian / Alaska Native |
0.34% |
0.84% |
- |
0.32% |
1.00% |
0.24% |
Asian/Pacific Islander |
26.32% |
4.02% |
- |
4.86% |
2.00% |
3.95% |
Black/African American |
7.10% |
5.78% |
41.01% |
33.20% |
31.00% |
28.08% |
White |
- |
- |
- |
- |
- |
64.62% |
White-Non-Hispanic |
34.39% |
60.23% |
39.40% |
55.19% |
61.00% |
- |
White-Hispanic |
29.55% |
29.07% |
13.90% |
6.48% |
5.00% |
- |
Unknown/Other |
30.64% |
0.07% |
6.63% |
0.32% |
- |
3.11% |
|
|
|
|
|
|
|
Non-Hispanic |
69.36% |
- |
- |
- |
- |
93.49% |
Hispanic |
30.64% |
- |
- |
- |
- |
6.51% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proportion of children under 18 years living in poverty |
14.75% (1998) |
10.67% (1999) |
16.3% |
10.1% (1999) |
33.0% (2002) |
24.75% |
|
|
|
|
|
|
|
Urban/Rural Distribution |
|
|
|
|
|
|
Urban |
98.59% |
95.35% |
93.90% |
86.07% |
78.0% |
97.00% |
Rural |
1.41% |
4.65% |
6.10% |
13.93% |
22.0% |
3.00% |
|
|
|
|
|
|
|
CASE AND COMPARISON GROUP ASCERTAINMENT
Ascertainment Methodology from Part C (0-3 years)
California
Background: The Regional Centers provide services to residents of the 6 county catchment
area (San Andreas Regional Center, Golden Gate Regional Center, Regional Center of the East Bay). The Early Start program serves kids 0-3 who have or are at risk of developmental disabilities. There are several ‘at risk’ categories, including very low birthweight/prematurity, parent has developmental disability, perinatal drug exposure etc. Children 3 and up who have one of 5 developmental disabilities are eligible to receive regional center services. The five conditions are: autism, mental retardation, cerebral palsy, epilepsy, and neurologic conditions like mental retardation. We estimate that there will be approximately 350 children in the Regional Center system in our catchment area who may be eligible for participation in this study. Children with an autism spectrum disorder who do not meet full DSM-IV criteria for autistic disorder and who do not have significant impairments may not be deemed eligible for services at the regional center.
Obtaining Approvals: California CADDRE will receive IRB approval from the state IRB. California CADDRE also has an MOU with the Department of Developmental Services. California CADDRE will also work with each of the three Regional Center directors to make sure that our protocol will be implemented by RC staff in the best way possible for each respective Regional Center.
Ascertaining Probable Cases:
Appropriate Regional Center staff will identify children in the study age range who have become eligible for RC services on the basis of autism.
Or, we will query the RC databases (CDER and Early Start) at each participating RC for children with the appropriate birth year and diagnosis (e.g., autism code=1,2,or 9). The RC databases will be queried every 3 months.
The following eligibility criteria will be used in the RC database query:
Born in study birth years
Diagnosis of autism (code=1, 2, or 9) on CDER or Early Start
Diagnosis of PDD under Major Medical Conditions
Ascertaining Comparison Group
Appropriate Regional Center staff will identify children in study age range who have become eligible for RC services on the basis of mental retardation/developmental delay BUT NOT AUTISM
Or, we will query the RC databases (CDER and Early Start) at each participating RC for children with the appropriate birth year and diagnosis. The RC databases will be queried every 3 months.
The following eligibility criteria will be used in the query:
Diagnosis of mental retardation (mild, moderate, severe, profound) but not autism on CDER; diagnosis of developmental delay on Early Start (waiting for recommendations from clinicians group regarding what conditions should be included in this group)
Contacting Families: RC staff give study informational packet to families. If the family does not respond to this letter, within 14 days, RC staff will send an additional packet to the family. If the Regional Center does not receive a response from the second mailing within 14 days, a RC staff member will call the family. Active consent (or a signed waiver) is needed for study staff to contact family directly.
Screening Method: Regional center medical records will be reviewed as the screener.
Colorado
Table 1 below provides an overview of Part C and Part B.
Background: The section of the Individuals with Disabilities Education Act (IDEA) dealing with infants and toddlers up to age 3 years with disabilities. Eligibility for Part C Services is defined as (i) developmental delay in the areas of cognitive, physical, communication, social adaptive OR (ii) has a diagnosed physical or mental condition which has a high probability of resulting in developmental delay. The term ‘developmental delay’ is defined by each state.
The Part C agency is Early Childhood Connections and is administered under the Colorado Department of Education (CDE). In Colorado, Part C serves approximately 1,000 children who are 2 years of age. Part C data in Colorado is not coded with ICD-9 diagnoses. Children with the following categories of conditions are eligible for Part C:
Children with Significant Delays in Development
Cognition
Communication
Physical including vision and hearing
Social or emotional development
Adaptive behavior
Children with Conditions Associated with Significant Delays in Development
Chromosomal syndromes
Congenital syndromes
Sensory Impairments
Metabolic Disorders
Prenatal/perinatal infections resulting in significant medical problems
Low birth weight (<1200 g)
Postnatal acquired problems resulting in delays in development
The specific diagnosis (i.e. Trisomy 21, autism, etc.) is not always indicated. In 2000-2001, there were 914 children with an indication of adaptive delays, cognitive delays, communication delays, physical delays, and social delays.
Obtaining Approvals:
CADDRE staff met with 4 of the 6 Denver Metropolitan area county-level Part C agencies in March 2004 about the feasibility of notifying their participating families about the study. It was determined that once IRB approval is obtained, more formal arrangements will be made with each agency or their Community Centered Board (CCBs), depending on the structure of each county. There are seven counties in the catchment area.
Note: Community Centered Boards (CCBs) are key providers of early intervention services in Colorado. CCBs manage and deliver the services for persons with developmental disabilities.
Ascertaining Probable Cases: Because Part C is not as reliable by eligibility category, we would likely ask that the agency only query by birth year. The county will be asked to send introductory letters to those families of children in the catchment area that meet the birth year criteria. Pending agreements, we will ask that the database be queried on a bi-annual basis by the Part C staff, or (if applicable) the CCBs will automatically send an invitation letter once the child is evaluated for services. Additionally, study flyers and contact information will be available to Part C staff to give to interested families.
Ascertaining Comparison Group: Same as above for Probable Cases.
Contacting Families:
A letter from the agency (on their letterhead) will be sent with the CADDRE Introductory Letter and response card. The agency letter will state that they have not provided the family’s information to CADDRE, however, if they want to participate then to send in the response card to the CADDRE Study Coordinator. The response cards will be addressed to CADDRE. If the potential participant returns the response card indicating interest, then the potential participant will receive a phone call from the CADDRE Study Coordinator. A second Introductory Letter to those who do not respond will be sent by those agencies with the resources available to do so. Some agencies may choose to automatically send a second letter and response card approximately 3 weeks from the date the first letter is mailed. The letter will include a statement, “if you are interested in receiving more information about the study and have already responded, please disregard”. The reason for this is that some agencies will not have the capability to track which cards were returned to CADDRE, so this process will reduce the burden on the source.
Screening Method: Families who indicate interest in the study by returning the response card will be contacted via telephone by the Study Coordinator. If the child meets the eligibility criteria and the family provides verbal consent to participate, then the SCQ will be administered to determine whether the child will be assigned to the neurocognitively impaired comparison group (Possible NIC) or the ASD group (Possible Case).
Georgia (CDC)
No plan to use Part C.
Maryland/Delaware
No plan to use Part C.
North Carolina
Background: In NC, the Child Development Service Agencies (CDSAs) of the Maternal and Child Health Division of the North Carolina DHHS is responsible for children under 5 years of age. There are four regional CDSAs within the study catchment area.
Obtaining Approvals: Once IRB approval has been obtained from UNC, a statement of support will be obtained from the state director of the CDSAs . We will obtain a written agreement from directors of individual CDSAs that acknowledges understanding of our study goals and recruitment strategy. This will not be a binding contract, but rather a tool to facilitate effective communication regarding study goals.
Ascertaining Probable Cases: A database maintained by the NC State Center for Health Statistics will be queried. The query will encompass all children of eligible ages in the catchment area who have an ASD diagnosis OR ICD-9 codes consistent with CDC criteria for surveillance case ascertainment. The database will be queried on a semiannual basis. Decisions will be made regarding a “waiting period” after diagnosis depending upon recommendations of CADDRE workgroups (this would be less feasible for children at the upper age limit of the study). See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group: The same database queried for probable cases will also be queried at the same time to ascertain the comparison group. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: We plan to employ a passive consent process. The agency will send the initial introductory letter briefly explaining our study and offering parents/guardians the opportunity to “opt out” of further contact regarding the study. This will be via a postcard provided along with a mailing envelope to return to the Dept of Epidemiology/State Center for Health Statistics. We will allow 3 weeks from the date the letter is mailed for parents to return the card.
If we do not hear back from parents by the specified date, and the letter is not returned as undeliverable, we will begin the recruitment process as outlined by CADDRE. We will call to begin recruitment and send the recruitment package once telephone contact and verbal participation consent has been secured. (We will develop procedures for dealing with situations where family does not have a home telephone).
For those families with an invalid mailing address in the CDSA record, we will conduct tracking/tracing to attempt to locate the family’s correct mailing address, and attempt to send the initial letter again. (For missing phone numbers only, we will not delay mailing the letters, but will attempt to obtain a phone number from directory assistance or on-line directories.) We will also provide families the opportunity to correct or supply their phone number with an enclosed card in the informational packet.
Screening Method: Initial screening to identify potentially eligible subjects will be through the agency database. Query results may be confirmed by existing surveillance data record review if IRB approval is granted for this pre-recruitment process. To confirm eligibility, telephone screening will be performed by study staff for parents who indicate that they would like to participate in the study.
Pennsylvania
Background: The Part C programs in Pennsylvania vary across counties. The Early Intervention Programs (EIP) of Chester, Montgomery and Philadelphia counties provide services to children ages 0-3 years. The agencies are: Elwyn, Inc. (Philadelphia), Chester County Early Intervention Program, and Montgomery County Early Intervention Program. The services are administered by the PA Office of Mental Retardation. All special education disability categories are eligible for Part C as well as certain ICD-9 diagnoses. A diagnostic code is not necessary to receive services, and many children in the 0-3 age range are identified as having a “developmental delay.” Eligibility criteria are a 25% delay and/or and established condition with a high probability of developmental delay. The centralized database contains: diagnosis, date of referral, referral sources, timelines, services and supports, costs, date of transition or exit. The database is called the Early Intervention Reporting System (EIPS). Once in the system the child’s diagnoses, disability categories etc are assigned EIPS codes.
Obtaining Approvals: PA CADDRE will obtain letters-of-approval from the Directors of the early intervention programs.
Ascertaining Probable Cases: The Directors of the EIPs will designate a staff member to review their records to identify children who meet the eligibility criteria. Elwyn Inc maintains a computerized database that identifies children as having “autism” and “pervasive developmental disability.” The records include date of birth, personal information, services provided, location of services, case status, and other information. A similar, but less comprehensive database/record system is available at the EIPs of Chester and Montgomery counties. Along with a database search, records may be reviewed by the designated staff member. The databases and records will be reviewed for new cases every 6 months. See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Groups: The Directors of the EIPs will designate a staff member to review their records to identify children who meet the eligibility criteria as having, mental retardation, cognitive, physical, communication, social/emotional or adaptive delays and other exceptionalities. Elwyn Inc maintains a computerized database that includes primary and secondary disabilities, personal information, date of birth, services provided, location of services, case status and other information. A similar, but less comprehensive database is available at the EIPs of Chester and Montgomery counties. Along with a database search, records may be reviewed by the designated staff member. The databases and records will be reviewed for new cases every 6 months. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Familes: The staff designee will prepare lists of eligible cases and comparison children. The agency will make the initial contact by sending the invitation packet to the parent/guardian of each eligible potential case and comparison on the list. The designee will record date of mailing, and disposition of responses. A tally of the number “Yes,” “No” and no reply responses will be kept by the agency. PA CADDRE investigators will collect positive response cards weekly from the agency and will make all subsequent contacts with the families that express interest in the study. Potential subjects will be contacted within the time frame designated in the protocol. The identities and contact information of individuals who respond negatively (e.g. they do not wish to be contacted), will not be given to the investigators. Individuals who do not return the response will be receive one additional mailing with receipt confirmation.
Screening Methods: Investigators will verify the contact information and residency eligibility of individuals who affirmatively respond to the initial mailing. If residency requirement is met the individual will be contacted by telephone to conduct a brief screening survey to further establish eligibility and receive more information about the project. The parent will be contacted by telephone and the telephone screener will ask selected questions to verify if child meets study inclusion criteria as a probable case or comparison subject.
B. Ascertainment Methodology from Part B/Special Education
California
Same methodology for the Regional Centers as for Part C.
Colorado
Background: The section of IDEA dealing with individuals with disabilities ages 3 – 21 years. Part B is intended to support early intervention and special education services children with disabilities and their families. The Part B agency is administered under the CDE and is called the Colorado Preschool Program. In Colorado, Part B serves approximately 5000 children 3-5 years of age. Part B data in Colorado is not coded with ICD-9 diagnoses. Children with the following categories of conditions are eligible for Part B: mental retardation, hearing impairments (including deafness), speech or language impairments, visual impairments (including blindness), serious emotional disturbances, orthopedic impairments, autism, traumatic brain injury, other impairments or specific learning disabilities. The specific diagnosis (i.e. autism, etc.) is not always indicated. In 2000-2001 there were 58 children with autism, 3425 with speech and language delays, and 86 with multiple delays in Part B.
The Part B counts are typically run once a year. The CDE maintains a database of all of the children, however, they do not have parent names and addresses. This information is maintained by the district level database managers.
The specific diagnosis (i.e. Trisomy 21, autism, etc.) is not always indicated. In 2000-2001 there were 58 children with autism, 3425 with speech and language delays, and 86 with multiple delays in Part B.
Obtaining Approvals: Once IRB approval is obtained, formal arrangements will be established with the Special Education directors at each school district.
Ascertaining Probable Cases: Because of the large number of school districts (15) in the catchment area, the data on the participants in Part B will be consolidated and organized at theCDE. The local data managers will be asked to send their data (including names and addresses) to the CDE Part B data manager. The Part B data manager will query the data for children meeting the study criteria and contact the families, as described below. Pending agreements, the database will be queried on an annual basis.
Ascertaining Comparison Group: Same as above for Probable Cases.
Contacting Families:
A letter from CDE (on their letterhead) will be sent with the CADDRE Introductory Letter and response card. The CDE letter will state that they have not provided the family’s information to CADDRE, however, if they want to participate then to send in the response card to the CADDRE Study Coordinator. The response cards will be addressed to CADDRE. If the potential participant returns the response card indicating interest, then the potential participant will receive a phone call from the CADDRE Study Coordinator. CDE will be asked to send a second letter with a response card approximately 3 weeks from the date the first letter is mailed to all families that have not responded to CADDRE. The letter will include a statement that “if you are interested in receiving more information about the study and have already responded, please disregard”.
Screening Method: Families who indicate interest in the study will be contacted via telephone by the Study Coordinator. If the child meets the eligibility criteria and the family provides verbal consent to participate then the SCQ will be administered to determine whether the child will be assigned to the neurocognitively impaired comparison group (Possible NIC) or the ASD group (Possible Case).
Table 1. Part B and Part C Comparisons
|
Part B (3-21 years) |
Part C (0-2 years) |
Name |
Colorado Preschool Program (3-5 years) |
Early Childhood Connections |
Administered Through |
Colorado Department of Education |
Colorado Department of Education |
Administered By |
School Districts (15) |
Counties (7) |
Data Managers |
Colorado Department of Education |
County-level Part C Agencies and Community Centered Boards (CCBs) |
Permissions |
Special Education directors at each school district. |
County-level Part C Coordinators and CCBs |
Methods of Sampling Existing Database |
|
Ask counties to query the database by DOB and send a letter to the families. |
Possible Methods After Initial Database Query |
Follow procedures above on a bi-annual or annual basis.
|
|
Letter Sent to Families |
CDE representative |
Part C representative |
Approximate Number Served |
~ 5000 (3-5 years) |
~ 1000 (2 years) |
Georgia (CDC)
Background: Potential case children and children in the neurodevelopmentally impaired group will be ascertained from school sources and clinic sources currently used by the Metropolitan Atlanta Developmental Disabilities Surveillance Program (MADDSP). The ascertainment methodology relies on the consequences of Part B of Public Law 94-142 as amended, the Individuals with Disabilities Education Act (IDEA)(6), which mandates that the public schools provide a free and appropriate education for all children with disabilities between the ages of 3 and 21. As a result, most children eligible for GA-CADDRE are either enrolled in special education programs at nine public school systems serving the study area or enrolled in other Georgia Department of Education programs for children who have developmental disabilities (e.g., state schools for children who are hearing or vision impaired and regional psychoeducational centers). The nine participating school systems are Atlanta City Schools, Buford City Schools, Decatur City Schools, Clayton County Schools, Cobb County Schools, DeKalb County Schools, Fulton County Schools, Gwinnett County Schools, and Marietta City Schools.
During 2000, there were 84,710 children, 3-4 years of age, in the five-county metro Atlanta area. Of that, there were 1,834 3-4 year old children in the special education database. A student or youth from 3 through 21 years of age is considered to have a disability under the Individuals with Disabilities Education Act (IDEA) if the student or youth meets the eligibility criteria in any of the following areas: 1) Autism, 2) Deaf/Blind, 3) Deaf/hard of hearing, 4) Emotional and behavioral disorder, 5) Intellectual disability (mild, moderate, severe, profound), 6) orthopedic impairment, 7) other health impairment, 8) significant developmental delay, 9) speech-language impairment, and 10) visual impairment.
Obtaining Approvals: IRB approval will be obtained from each of the nine participating school systems. A detailed study protocol is required to be submitted to the Research Guidelines Department of each system.
Ascertaining Probable Cases: Children who were served by the participating school systems at any time and who meet the study’s eligibility criteria will be considered potential cases. See Appendix E for the eligibility criteria that will be used in the query.
The Project Coordinator for the GA-CADDRE Study will make three data requests per year to each of the participating school systems. Data requests will be made October 1, December 1, and March 1. Data request dates are based on the dates when the school systems have to provide the Department of Education with the Full-time Equivalent (FTE) counts. The FTE count is reflective of the number of children enrolled in school at that point in time. The Project Coordinator will ask the Data Request Coordinator in each system to conduct a database query of all children 3 to 10 years of age who are in the select exceptionality categories (still to be determined but would be listed above). The Data Request Coordinator will provide the data tapes to the GA CADDRE Data Manager/Computer Programmer.
Children with an Autism Exceptionality Code: Parents of children with an autism exceptionality code will be contacted and screened for eligibility. If the child is eligible for participation and the parent consents, the child will be placed in the potential case group.
Children with a non-Autism Exceptionality Code: Parents of children with a non-autism exceptionality code will be contacted and screened for eligibility. If the child is eligible for participation, the parent will receive the Social Communication Questionnaire (SCQ) to screen for ASD. If the child is positive based on this screening tool they will be placed in the potential case group. If the child screens negative on the SCQ, they will be placed in the pool of children to be randomly sampled for the neurodevelopmentally impaired group.
Ascertaining Comparison Group: The ascertainment procedures for children in the neurodevelopmentally impaired group will be identical to the procedures followed for obtaining the potential cases through Part B. This group will be comprised of a random sample of the children who were in the non-autism exceptionality categories and screened negative on the SCQ.
Contacting Families: Potential study participants will be contacted by the Centers for Disease Control and Prevention (CDC) through an invitation packet. If the potential participant returns the response card indicating interest, then the potential participant will receive an invitation phone call from a member of the project staff. If there is no response from the potential participant within 14 days, project staff will place a follow up telephone call. In-person contact or an additional letter will be attempted if the potential participant is not available by telephone. If the potential participant returns the response card indicating no interest, no additional follow-up contact will be made.
Screening Method: The families will be contacted by telephone. If the child meets the eligibility criteria and the family provides verbal consent to participate, then the SCQ will be administered to determine whether the child is eligible to be assigned to the neurodevelopmentally impaired group or the ASD group.
Maryland
Background: The responsible agency for Part B is the Maryland State Department of Education. The Part C program in each local school system refers children they’ve been following to the Part B program. After initial assessments, the Individualized Education Plan Team, which includes the parents of the child, will determine the category of disability for eligibility. Roughly 20% of the children this age fall into the nonspecific Developmental Delay category (MD permits use of this category through age 6). On December 1, 2000, there were 4,385 children in the study catchment area in the MD Part B program, of which just 144 had the classification of autism.
Obtaining Approvals: In Maryland approval will come from the Director of Special Education Services for the Maryland State Department of Education. Once approved by the Director, the MSDE Special Education Services Division will request assistance from the local school districts in the recruitment area.
Ascertaining Probable Cases: MSDE will generate from their automated database lists of children potentially eligible based on classification and eligibility criteria. MSDE will contact local school districts to obtain contact info (MSDE files include no contact info). Contact letters will be mailed from MSDE. MSDE will work with local school districts to follow-up on returned undeliverables. The database will be queried once each year (three times during the study recruitment period) to pick up on children newly eligible or newly classified.
Ascertaining Comparison Group: We hope to draw subjects for the non-ASD-affected-comparison group from the same pool contacted above, limited to those who do not screen positive on the ASD screener. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: No passive consent is permitted in Maryland. Only those responding to initial mailings who indicate actively that they are interested in being contacted will be contacted by study investigators.
Screening Method: Those indicating interest will be contacted over the telephone by study staff within two-weeks of the receipt of the response card. Eligibility criteria will be confirmed and the SCQ will be administered as a case-group screen. Those screening positive on the SCQ as well as those with an autism exceptionality code in the Part B databases will be considered further as potential cases. Other children will be considered for comparison group membership.
North Carolina
Background: In NC, the agency responsible for Special Education is the Exceptional Children Division of the NC Department of Public Instruction. Children served by this agency must have been evaluated within the last three years of their classification in an exceptionality category. Preschool children become eligible for services upon reaching their third birthday.
Obtaining Approvals: Once IRB approval is obtained from UNC, a statement of support will be obtained from the state director of the Exceptional Children Division. We will obtain a written agreement from individual school system’s Special Education Directors to allow information about the study to be mailed from the school system to parents of potentially eligible children, and/or to allow study recruitment materials to be placed in locations that serve the eligible population.
Ascertaining Probable Cases: An agency database would be queried to identify potentially eligible children in the appropriate age range and with a classification of ASD, MR, or any classification that is consistent with CDC criteria for surveillance case ascertainment. The database will be queried on a semiannual basis, after exceptional children counts are reported in April and December. See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group: Same as above. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: For potentially eligible children identified through the DPI, we would use an active consent process rather than passive. Rather than sending an initial brief introductory letter, a complete packet of information about the study would be mailed to parents (by school personnel), with a response card and phone number for the study provided. If the study coordinators receive a response from parents within three weeks, a follow up letter with another response card would be mailed by school personnel
To facilitate the mailing of study packets from the school systems to the families, a special education teacher could be hired on a part-time basis during the summer months.
Screening Method: Once a response is received from a parent/guardian indicating interest in the study, he/she would be contacted by phone by study staff to do a telephone eligibility screener. Should information from the medical or educational record be required to confirm eligibility, the parent would be asked to sign the appropriate IRB approved waiver.
Pennsylvania
Background: The Intermediate Units (IU) of The Pennsylvania Department of Education oversee preschool and school age educational services. The Philadelphia county Intermediate Unit has a contract with Elwyn, Inc. to provide services to children ages 3-5. Children who are eligible for Part B services in Pennsylvania have been identified as having an exceptionality from the list of thirteen. The exceptionalities are:
Mental Retardation
Deafness or Hearing Impairment
Speech or Language Impairment
Blind or Visual Impairment
Serious Emotional Disturbance
Physical Disability
Other Health Impairment
Specific Learning Disability
Deaf-blind
Multiple Disability
Autism
Traumatic Brain Injury
Gifted
ICD-9 codes may be used to identify children who are eligible for special accommodations covered by Section 504 of the Americans with Disabilities Act.
Chester and Montgomery County IUs oversee pre-school and special education services for ages 3-5. The IUs serves the educational needs of children ages 3-18 (grades preschool to grade 12). Each county maintains the special education records of children in the county as well as socio-demographic information on children receiving special education services. Each IU functions independently and has a director who reports to the PA-Department of Education, and a Director of Special Education who reports to the Director of the I.U. Approximately 180 potential cases are expected from the I.U.s. The IUs are: Chester County Intermediate Unit #24, Montgomery County Intermediate Unit #23, and Philadelphia County Intermediate Unit #26.
Obtaining Approvals: Cases will be ascertained from the existing databases of the Division of Special Education of the Intermediate Unit (I.U.) of the PA-Department of Education of each County. A letter of approval will be sought from the Directors of the three IUs after they receive and review the completed study protocol. An application can be submitted to the IRBs of the Children’s Hospital of Philadelphia and the University of Pennsylvania once letters are obtained.
Ascertaining Probable Cases: The I.Us. maintain confidential and privacy-protected databases of children who receive special education services. The data is stored in the PENN DATA, student services, special education, and health information databases at the respective IUs and Elwyn Inc. Specific information includes: name, address, date of birth, primary, secondary and tertiary disability, services provided, location of services and other information. A designee of the Divisions of Special Education for each IU and Elwyn Inc. will query the database and prepare a list of children identified with autism and PDD according to the eligibility criteria. This list will be retained by the IUs. Databases will be reviewed every 6 months for potential cases. See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group: A staff designee from the Divisions of Special Education for each IU and Elwyn Inc will query the same databases to prepare a list of potential comparison children, including children identified with specific exceptionalities based on eligibility criteria. The query will be restricted by date of birth and primary and secondary exceptionalities, which may include mental retardation, speech and language impairment, hearing impairment, specific learning disability, and severe emotional disorder. Databases will be reviewed every 6 months for potential comparison children. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: To comply with FERPA regulations, the parent/guardian of each potential case or comparison child will receive a letter from the school district on behalf of PA-CADDRE asking for permission to be contacted by an investigator to describe the case-cohort study. PA-CADDRE will prepare the template letter which will be placed on IU stationary and sent by post. The designee will record date of mailing, and disposition of responses. A tally of the number “Yes,” “No” and no reply responses will be kept by the agency. The investigators will collect positive response cards weekly from the IU or Elwyn Inc and make all subsequent contacts only with the families that express interest in the study. Potential subjects will be contacted within one week of receipt of the response card. The identities and contact information of individuals who respond negatively, i.e. they do not wish to be contacted, will not be provided to the investigators. Individuals who do not return the response will receive one additional mailing with receipt confirmation.
Screening Methods: Investigators will verify the contact information and residency eligibility of individuals who positively respond to the initial mailing. If residency requirement is met the individual will be contacted by telephone to conduct a brief screening survey to further establish eligibility and receive more information about the project. The parent will be contacted by telephone and the telephone screener will ask selected questions to verify if child meets study inclusion criteria as a probable case or comparison subject. After category of eligibility is established the investigator will describe the study and group assignment and obtain verbal consent. A packet of study information will then be mailed to the parent/guardian.
C. Ascertainment Methodology from Clinics/Private Providers
California
Kaiser Clinics
Background: Kaiser Permanente (KP) clinics in Northern California Region. All KP members are eligible for services at Kaiser clinics. Potential biases are that only KP members and some Medicare patients will be ascertained from this source.
We estimate identifying approximately 125 children per birth year from Kaiser. NOTE: many of these children will also be enrolled at the RC.
Obtaining Approvals: California CADDRE will receive approval from the state IRB and from the Kaiser IRB to contact families in the Kaiser network.
Ascertaining Probable Cases:
Electronic outpatient database scanned for children in study age range who have an ASD diagnosis. This will be done periodically – every 3 months.
Review medical records to determine birth residence county, current residence county, home language, diagnostic info, guardianship etc.
Send informational letter to child’s primary care provider asking to let us know if child should not be contacted about the study
If don’t hear back from PCP, send introductory letter to family inviting participation
Ascertaining Comparison Group:
Electronic outpatient database scanned for children in study age range who have one of the diagnoses we choose BUT NOT an ASD diagnosis. This will be done periodically – every 3 months.
Review medical records to determine birth residence county, current residence county, home language, diagnostic info, guardianship etc.
Send informational letter to child’s primary care provider asking to let us know if child should not be contacted about the study
If don’t hear back from PCP, send introductory letter to family inviting participation.
See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: Invitations to families will be sent by Lisa Croen, Kaiser Division of Research. If the family does not respond to this letter after 14 days, Kaiser staff will send an additional packet to the family. If CADDRE does not receive a response from the second mailing within 14 days, a Kaiser staff member will call the family. No consent is required to contact family directly.
Screening Method: Kaiser families will be screened for eligibility by record review.
Other Clinics/Private Providers
Background: Private clinics and providers serving residents of the 6 county catchment area. Main clinics include Children’s Hospital Oakland, University of San Francisco, California Pacific Medical Center, Santa Clara Valley Medical Center, and Stanford University. All children referred to these clinics are eligible for assessment and diagnostic services.
Obtaining Approvals: California CADDRE will receive IRB approval from each individual clinic, in addition to receiving approval from the state IRB.
Ascertaining Probable Cases:
Appropriate clinic staff will identify children in study age range (meeting study birth year criteria) who have been diagnosed with an ASD.
All new patients to clinic in the study age range are asked to sign waiver/consent form at intake.
See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group:
Appropriate clinic staff identify children in study age range who have been diagnosed with one of the affected control diagnoses (TBD) BUT NOT ASD.
All new patients to clinic in the study age range are asked to sign waiver/consent form at intake.
See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: Clinic staff give study informational packet to families. If the family does not respond to this letter, within 14 days, clinic staff will send an additional packet to the family. If CADDRE does not receive a response from the second mailing within 14 days, a clinic staff member will call the family. Active consent (or a signed waiver is required for CADDRE study staff to contact family directly.
Screening Method: Medical record review will be used to identify potential children.
Colorado
Background: Hospitals, clinics, and private practices that are within the catchment area will be contacted to inform them of the study and elicit participation in identification of potentially eligible subjects. These sources include: JFK Partners at UCDHSC, The Child Development Unit at The Children’s Hospital, and private practices. The study will also be promoted generally to the broader pediatric clinical community so that clinical practitioners who are not ASD specialists will become aware of the study and can provide study contact information to potentially interested parents. Possible biases among these sources include under-representation by minorities and lower income families.
Obtaining Approvals: Once IRB approval has been received, we will establish arrangements with the individual facilities.
Ascertaining Probable Cases: We will request that each facility query their medical record/billing information systems and send initial contact letters to parents of children meeting study requirements who have had one or more of the ICD codes listed in Appendix E. The sources will be asked to run a query biannually.
Ascertaining Comparison Group: Same as above for Probable Cases.
Contacting Families: The site will be asked to send the Introductory Letter and response card to all potential families identified by the queries. The letter will state that they have not provided the family’s information to CADDRE, however, if they want to participate then to send in the response card to the CADDRE Study Coordinator. The response cards will be addressed to CADDRE. If the potential participant returns the response card indicating interest, then the potential participant will receive a phone call from the CADDRE Study Coordinator. For families who do not respond to CADDRE after 3 weeks, a second Introductory Letter will be sent by those facilities with the resources available to do so. Some facilities may choose to automatically send a second letter to their families.
Screening Method: Families who indicate interest in the study will be contacted via telephone by the Study Coordinator. If the child meets the eligibility criteria and the family provides verbal consent to participate then the SCQ will be administered to determine whether the child will be assigned to the neurocognitively impaired comparison group (Possible NIC) or the ASD group (Possible Case).
Georgia (CDC)
Background: In addition to school sources, non-educational sources (such as clinics) will be used to ascertain children for this study. These sources include: a) Georgia Department of Human Resources facilities that provide services for children who have developmental disabilities, b) metro-area pediatric tertiary care centers --Egleston Children’s Hospital, Grady Memorial Hospital and Scottish Rite Children’s Medical Center and their associated clinics, and c) select private sources that provide diagnostic or intervention services for children with developmental disabilities.
Georgia Department of Human Resources (DHR)-Division of Mental
Health/Developmental Disabilities/Autistic Disorder: DHR is a public agency which serves children from birth to 22 years at risk for developmental disabilities or with a diagnosed medical condition. Several facilities that fall under this agency will be used as abstraction sources. These facilities accept Medicaid and private insurance.
DHR- Children’s Medical Services (CMS): CMS is a public agency that serves children from birth to 21 years of age who are medically eligible. CMS includes state and federally funded clinics. Medicaid is an acceptable form of insurance.
Clinical Sources: These include hospital-affiliated sources, private clinicians’ practices, and diagnostic and treatment centers. Insurance varies on the type of facility.
Biases include underrepresentation of minorities at some sources and bias toward higher SES, especially at sources that only accept private insurance.
While the exact number of children identified at the non-school sources is not available, we expect to review approximately 1,200 source files at these facilities.
Obtaining Approvals: A formal agreement will be made with each non-school source.
Ascertaining Probable Cases: The Project Coordinator will initiate a data request with each non-school source. The data requests will be made at the same time the data requests are made with the school sources (October 1, December 1, and March 1). See Appendix E for the eligibility criteria that will be used in the query.
Children with a diagnosis of ASD: Children with an ICD-9 code of Autistic Disorder, Pervasive Developmental Disorder- Not Otherwise Specified, or Asperger’s syndrome will be contacted and screened for eligibility.
Children with a non-ASD diagnosis
Children with other ASD-related conditions will receive the SCQ to determine their potential group enrollment.
Ascertaining Comparison Group: The ascertainment procedures for children in the neurodevelopmentally impaired group will be identical to the procedures followed for obtaining the potential cases. This group will be comprised of a random sample of children who had a non-ASD diagnosis and screened negative based on the SCQ.
Contacting Families: Potential study participants will be contacted by the Centers for Disease Control and Prevention (CDC) through an introductory packet. If the potential participant returns the response card indicating interest, then the potential participant will receive an invitation phone call from a member of the project staff. If there is no response from the potential participant within 14 days, project staff will place a follow up telephone call. In-person contact or an additional letter will be attempted if the potential participant is not available by telephone. If the potential participant returns the response card indicating no interest, no additional follow-up contact will be made.
Screening Method: The families of children who had a non-ASD ICD-9 code will be contacted by telephone. If the child meets the eligibility criteria and the family provides verbal consent to participate, then the SCQ will be administered to determine whether the child is eligible to be assigned to the neurodevelopmentally impaired group or the ASD group.
Maryland
Background: Clinical ascertainment will involve clinics affiliated with three institutions in the catchment area: 1) Kennedy Krieger Institute (KKI); 2) University of Maryland Health System (UMD); and 3) Mt. Washington Pediatric Hospital. All are located in Baltimore, MD.
Obtaining Approvals: IRB approvals will be required at Kennedy Krieger and University of Maryland. Mt. Washington will accept an approval from either KKI or UMD.
Ascertaining Probable Cases: The planned approach to querying for potential cases will be to query billing databases for all age-eligible children with select ICD diagnosis for any encounter and last address within the study area. The query will be done three times during the study recruitment period. See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group: All those screening negative will be considered as potential DD comparison group members.
Contacting Families: Letters will be sent by the collaborating investigator at each institution with a response card that parents interested in participating can return directly to the CADDRE study site. Mailings will be done from the query list focusing on older children first. Letters to nonrespondants with valid addresses will be remailed once. Only those parents returning a response card will be contacted by the CADDRE study site.
Screening Method: Parents returning cards indicating interest will be contacted by telephone to have eligibility criteria confirmed and the SCQ administered. All those with a PDD diagnosis code, regardless of SCQ score, and those without PDD codes but with positive SCQ screens will be considered further for inclusion in the case group
North Carolina
Background: Hospitals, clinics, and pediatric practices of private providers within the catchment area who serve the target population will be catalogued and systematically contacted to inform them of the study and elicit participation in identification of potentially eligible subjects. These sites should be the same as those identified through the surveillance protocol, and therefore we would notify them of the probability that some of their patients are eligible for the study.
Obtaining Approvals: For some providers, approval of this study by the UNC IRB would be sufficient. Larger providers, such as hospitals, would likely have their own IRBs. Study staff at UNC would prepare and coordinate the appropriate IRB submission.
Ascertaining Probable Cases: If available, a clinical database would be queried using study eligibility criteria, as well as the ICD-9 or DSM codes consistent with CDC criteria for surveillance case ascertainment. If unavailable, clinical staff deemed to have legitimate patient access would be asked to identify potentially eligible subjects. See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group: Same as above, except that MR children would be identified. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: Whenever possible, a passive consent process would be followed, as outlined for the Child Development Service Agencies (Part C). If disallowed by the IRB or the provider, we would follow an active consent process as outlined for the DPI (Part B).
Screening Method: Same as for cases and comparisons ascertained by other sources.
Pennsylvania
Background: Diagnostic centers in the three county catchment areas provide diagnostic and referral services to children of all age ranges, races and family income levels. The age range of clients is pre-school though middle school and all developmental disabilities are identified. Confidential patient records are maintained in the files of the clinician who performs the diagnostic procedures. These records contain the information to be reviewed during the study. The patient files should contain developmental, health, and other histories, diagnostics tests reports and scores, referral information and disposition and recommended treatment plans. There are three types of sources in two categories. They may be in the residency area or outside of the residency area and are diagnostic centers and independent clinicians. Approximately 275 potential cases are expected to be identified from the clinical sources.
Diagnostic Centers
Children's Hospital of Philadelphia. Regional Autism Center. Philadelphia.
Thomas Jefferson University Hospital. Departments of Pediatric Neurology/Developmental Pediatrics. Philadelphia.
St. Christopher’s Hospital. Departments of Pediatric Neurology/Developmental Pediatrics. Philadelphia
Center for Autistic Children. Robert Naseef, Ph.D. Psychologist. Philadelphia.
Crozer-Keystone Health. Departments of CKHN \Pediatrics. Upton, PA
Reading Pediatrics Incorporated. Reading, Pa
B. Independent Clinicians
Steven A. Kossor, Licensed Psychologist. Exton, PA
James J. Stone, PsyD. Collegeville, Doylestown PA.
McHarg, Malcolm L., M.D. Montgomery Hospital. Norristown, PA
Obtaining Approvals: The investigators will obtain a cooperative agreement with the clinical source of potential case and comparison children. A letter of approval will be sought from the Medical Directors or private providers after they receive and review the completed study protocol. An application can be submitted to the IRBs of the Children’s Hospital of Philadelphia and the University of Pennsylvania once letters are obtained.
Ascertaining Probable Cases: The primary provider or staff designee from the clinical source will query existing medical records databases for children who meet eligibility criteria, i.e. have been assigned selected ICD-9 diagnostic codes. The staff will prepare a list of potential case children, including children identified with specific exceptionalities based on eligibility criteria. The query will be restricted by date of birth and ICD-9 codes, which will include but will not be limited to autism, PDD-NOS, and PDD. Databases will be reviewed every 6 months for potential case children. See Appendix E for the eligibility criteria that will be used in the query.
Ascertaining Comparison Group: The primary provider or staff designee from the clinical source will query existing medical records databases for children who meet eligibility criteria, i.e. have been assigned selected ICD-9 diagnostic codes. The staff will prepare a list of potential comparison children, including children identified with specific exceptionalities based on eligibility criteria. The query will be restricted by date of birth and ICD-9 codes, which will included but will not be limited to mental retardation, developmental disability, speech impairment, hearing impairment, behavioral disorders, hyperactivity, and severe emotional disorder. Databases will be reviewed every 6 months for potential comparison children. See Appendix E for the eligibility criteria that will be used in the query.
Contacting Families: The staff designee will prepare a list of eligible case and comparison child. The medical director/pediatrician/or designee will send an invitation packet to the parent/guardian on behalf of the Investigators. PA-CADDRE will prepare the template letter which will be placed on the stationary of the clinical source. The designee will record date of mailing, and disposition of responses. A tally of the number “Yes,” “No” and no reply responses will be kept by the clinical sourced. The investigators will collect positive response cards weekly from the sources and make all subsequent contacts only with the families that express interest in the study. Parents/guardians of potential cases and comparison children will be contacted within one week of receipt of the response card. The identities and contact information of individuals who respond negatively , i.e. they do not wish to be contacted, will not be provided to the investigators. Individuals who do not return the response will be receive one additional mailing with receipt confirmation.
Screening Methods: Investigators will verify the contact information and residency eligibility of individuals who positively respond to the initial mailing. If residency requirement is met the individual will be contacted by telephone to conduct a brief screening survey to further establish eligibility and receive more information about the project. The parent will be contacted by telephone and the telephone screener will ask selected questions to verify if child meets study inclusion criteria as a probable case or comparison subject. After category of eligibility is established the investigator will describe the study and group assignment and obtain verbal consent. A packet of study information will then be mailed to the parent/guardian.
SUBCOHORT ASCERTAINMENT
California
Background: These files are owned by the State of California Department of Health Services Vital Statistics Section. The estimated number of children born in our catchment area each year is 80,000.
Obtaining Approvals: California CADDRE must get IRB approval from the Committee for the Protection of Human Subjects and the State Registrar to obtain files containing personal identifiers. Once approval is obtained, we can directly sample potential controls from the electronic file and start the recruitment process. We can send letters directly to sampled controls to invite participation in the study.
Ascertainment: We will restrict file to CADDRE birth residence counties. Subcohort will be randomly sampled using algorithm developed and used by all CADDRE sites. We will obtain the AVSS file for each birth year included in our study. This file contains the all elements on the birth certificate, including names, birth street address and mother’s social security number.
Tracking: We will either contract tracking and tracing services out to a vendor that will provide this service for all CADDRE sites, or we will develop our own tracking and tracing protocol in-house. If in-house, it will consist of purchasing access to several online services that are designed to aid this process. Starting with the birth address, mother’s name and social security number, the tracker will attempt to find the current address and phone number. Once this has been accomplished, the introductory letter will be sent to this address.
Contacting Families: California CADDRE will select some number of sampled controls and send an initial packet of information to current address. Family will receive the packet from California CADDRE/California Department of Health Services. If the family does not respond to this letter, within 14 days, CADDRE staff will send an additional packet to the family. If CADDRE does not receive a response from the second mailing within 14 days, a CADDRE staff member will call the family. No consent is needed prior to CADDRE staff mailing or calling family.
Colorado
Background: The Vital Records Section in the Colorado Department of Public Health and Environment issues and maintains the birth certificates for the state of Colorado. In the seven-county Denver Metropolitan area there were an estimated 40,000 live births reported in 2002 and 2003.
Obtaining Approvals: A copy of the protocol, documentation of IRB approval, and signed confidentiality agreements by anyone who will have access to the data will be submitted to the Section Chiefs of the Vital Records Section and Health Statistics for approval.
Ascertainment: Once the request is approved, HSVR will sample a block of children in the birth cohort years.
Contacting Families: HSVR will send a letter (on their letterhead) with the CADDRE Introductory Letter and response card. The HSVR letter will state that they have not provided the family’s information to CADDRE, however, if they want to participate then to send in the response card to the CADDRE Study Coordinator. The response cards will be addressed to CADDRE. If the potential participant returns the response card indicating interest, then the potential participant will receive a phone call from the CADDRE Study Coordinator. HSVR will send a second letter with a response card approximately 3 weeks from the date the first letter is mailed to all families that have not responded to CADDRE. The letter will include a statement that “if you are interested in receiving more information about the study and have already responded, please disregard”.
Tracking: Letters that are returned as ‘not deliverable’ will be traced by HSVR for a current address using the same methods that are used by the other CADDRE sites (Appendix D).
Screening Method: Families who indicate interest in the study will be contacted via telephone by the Study Coordinator. If the child meets the eligibility criteria and the family provides verbal consent to participate then the SCQ will be administered. Children whose SCQ results indicate that the child is likely to have ASD will be invited for a developmental evaluation at the clinic visit.
Georgia (CDC)
Background: Subcohort children will be selected from Georgia vital records. The use of birth certificates as a mechanism for obtaining normal controls is possible because Georgia’s vital records are maintained electronically within the Department of Human Resources (DHR). Further, CDC has access to the electronic birth certificate files for linkages with MADDSP and MACDP for additional information on child and maternal characteristics. An estimated 40,000 children are born in the five-county metropolitan Atlanta area.
Obtaining Approvals: CDC will obtain a DHR sponsor and IRB approval from DHR.
Ascertainment: Potential subcohort members will be randomly selected from among all cohort children in a 1:1 case to control ratio. Subcohort members will be identified by Georgia state birth certificates on the basis of birth range and residence in the five-county metropolitan Atlanta area at the time of birth. Complete birth certificates will be provided to the CDC. The birth certificate files will be linked with Georgia death certificate files to remove any potential members who are deceased. The status of the family’s current residence will be determined by – fill in later.
Based on the previous steps, contact will take place with each identified child’s legal guardian to determine if the child meets the potential subcohort inclusion criteria.
Tracking: Approaches for tracking are currently being investigated.
Contacting Families: CDC will make the initial contact with the potential study participants through an introductory packet. If the potential participant returns the response card indicating interest, then the potential participant will receive a phone call from a member of the project staff. If the potential participant does not return the response card, follow-up contact will be made by project staff within 14 days to follow-up. The primary method for contact will be the telephone. Face-to-face contact or an additional letter will be attempted if the potential participant is not available by telephone. If the potential participant returns the response card indicating no interest, no additional follow-up contact will be made.
Maryland
Background: Birth certificates in Maryland are maintained by the Division of Vital Records within the Department of Health and Mental Hygiene (DHMH).
Obtaining Approvals: Once IRB approval is obtained from both CDC and JHU, an application will be made directly to the DHMH IRB.
Ascertainment: Birth certificate files will be used to define the eligible cohort born in the study region. Since birth certificate data cannot be released without consent, sampling of the eligible birth cohort will be done by DHMH. Birth cohort members born in the study area will be simple random sampled in batches for contact.
Contacting Families: Initial contact with potential participants will be done by DHMH. DHMH will send a letter with an enclosed pre-paid response card that can be returned directly to JHU from interested families. A reminder contact will be made six months after the initial mailing.
Tracking: Identifying information for all potential participants initially contacted, who do not indicate interest, will be put through the contact tracing/tracking procedure directly by DHMH personnel. Letters will be resent to potential participants showing different contact information after tracing procedures are run.
North Carolina
Background: Birth certificates are maintained by Vital Records within the State Center for Health Statistics. There are an estimated 34,000 live births per year in the catchment area.
Obtaining Approvals: Approval will be obtained from the UNC IRB. An agreement will be obtained from the Director of this agency.
Ascertainment: There will be a random sample of children eligible by geographic area and birth date.
Tracking: Tracking will be done by a social research assistant supported by this project, using public databases and directories or by centralized methods secured by CADDRE.
Contacting Families: A passive consent process will be followed, with an initial letter/packet coming directly from UNC. Similar procedures to those outlined for the CDSAs (Part C) would be followed.
Pennsylvania
Background: The source of subcohorts is the Pennsylvania State Department of Health, Bureau of Vital Statistics of the Pennsylvania. Children who may be enrolled as subcohorts will be identified from Birth Certificates of children born and residing in the three county catchment area and who meet the eligibility criteria.
Obtaining Approvals: The case-cohort study protocol will be sent to the University of Pennsylvania Office of Regulatory Affairs and The Children’s Hospital of Philadelphia (CHOP) Office of Research Affairs and IRB approval must be obtained prior to requesting Birth Certificates from the Department of Health. IRB approval typically takes 1 to 3 months.
While awaiting IRB approval PA-CADDRE will contact the Division of Vital Statistics of the Department of Health in PA to indicate the intention to apply for access to confidential information from Birth Certificates for recruiting population–based controls. An application will be sent to the Division of Vital Statistics of the PA Department of Health along with the full research protocol, all instruments and questionnaires, and IRB approval letters. The application will include all sample consents and subject correspondence letters. The protocol will describe in detail methods to protect the confidentiality of the subjects and disposition of confidential information once ascertained. The application may be sent by the Division of Vital Statistics to the Institutional Review Board of the PA Department of Health.
Ascertainment: PA CADDRE will request the names, addresses and telephone numbers of parents of all children: born in Chester, Montgomery and Philadelphia Counties and were born between the inclusion dates of: January 1, 2000 and February 1, 2002. Birth certificates contain the following information:
Place of birth
Date of birth
Sex
Name/mother/father
Name child
Hospital
County
Boro/city/township
Mailing address
Telephone number
Tracking: When this request is approved a designee in the Division of Vital Statistics will prepare a list of names and addresses of eligible residents. Once a list is prepared it will undergo an additional hand screening process at the Office of Vital Statistics in New Castle, PA. At this site the list is screened for adoption status and infant death. This process is not automated and will take several months to one year to complete. A final list of screened and eligible residents, sorted by zip code or county will be transferred to PA-CADDRE via PC diskette. PA CADDRE will match current zip codes with zip codes in three counties and begin the recruitment process.
Contacting Families: When the diskette of children is obtained from the Division of Vital Statistics (DVS), investigators will randomly select a number (n) that will receive the invitation packet. PA-CADDRE will send the initial contact letter to the parent(s) describing the study. The DVS requires that a consent for the full study be included with initial contact letter, however, the DVS will consider an intermediate step that requests permission to contact by telephone by the investigators to describe the case-cohort study in detail and conduct recruitment. The parent/guardian(s) will respond by post by signing a consent-to-contact form and return it in a self-addressed stamped envelope. The letter will indicate that study staff will contact the parent/guardian again by post if the response form is not returned. Approximately two weeks from mailing date, PA-CADDRE will follow-up with a post-card reminding the potential subject of the invitation packet. Once written consent-to-contact is obtained the PA-CADDRE will contact the potential control by telephone for initial screening. If the potential control passes the eligibility screening they will be recruited into the study as a subcohort and will receive the next set of study material.
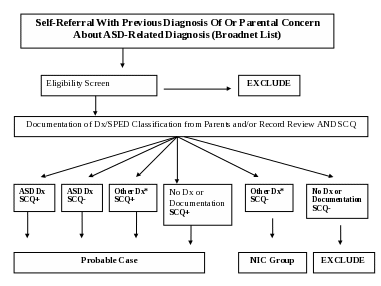
SELF-REFERRALS
CADDRE anticipates that caregivers with affected children may refer themselves to the study. They may express their interest directly to CADDRE study personnel by email or telephone, or request information about the study from their pediatrician or other healthcare provider. When the caregiver contacts the site, the study personnel will collect contact information (caregiver’s name, child's name, diagnosis, date of birth, address, telephone number or email, etc.) Please refer to the Self-Referral procedures below. Additionally, each child must meet the specific eligibility criteria outlined in section II. D. of the protocol. If the child is enrolled in the study, a notation will be made in the database that the child was self-referred.
Appendix K site specific ascertainment
Updated: November 24,
2006 Page
| File Type | application/msword |
| File Title | CADDRE Collaborative Case-Control Study |
| Author | Lisa A. Croen |
| Last Modified By | pax1 |
| File Modified | 2006-12-29 |
| File Created | 2006-12-29 |
© 2026 OMB.report | Privacy Policy