Clinic Visit - Case Child
The National Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) Study
appendix P Dysmorphology Exam
Clinic Visit - Case Child
OMB: 0920-0741
Appendix P: Dysmorphology Exam
Protocol for Conducting Exam
Data Collection form for Exam
PROTOCOL FOR PHYSICAL AND DYSMORPHOLOGY EXAMINATION
(revised 11-14-05)
Objective
The recommended format is intended to facilitate recording data from anthropometric and dysmorphology examination, and determine if a subject (or cohort control) is likely to have a genetic syndrome. The physical examination will include anthropometrics (height, weight, head circumference) and standardized dysmorphology examination.
Methods
Measures
Physical anthropometric measurements according to standards described in training manual.
Data will be recorded for physical and dysmorphology examination in the data recording form (see Appendix).
Measurement and description of specified features (including face, hands, feet and others) in standardized fashion by use of digital camera and measurement software. Measurements will be recorded in datasheets.
Procedures
Assessment Team
Qualified examiner (see below for qualifications) for dysmorphology examination and photography.
Pediatric clinician for supervision of physical measurements and recording data of dysmorphology examination
Each center will include an experienced pediatric clinician who will train and supervise the examiner.
Depending on the availability at each site, this clinician might be a Developmental or Behavioral Pediatrician, Child Neurologist, Child Psychiatrist, Pediatric Nurse Practitioner or Pediatric Geneticist.
Each site will have access to a Consulting Pediatric Geneticist for assessment and analysis of photographs to determine if a syndrome is likely. Please see Quality Assurance section below.
Photographs
Photogrammatic digital measurements:
Use of a digital camera (minimum 2.0 megapixels) and software program for measurement of specified dysmorphic features [web site is http://www.kuleuven.ac.be/bio/sys/carnoy/ ].
The centers will assure that the examiner will be trained in the use of the software program.
Photographs of child will include:
Views of face
Profile (both sides against dark background)
Full face portrait
Each hand with fingers spread
Feet (without shoes and socks, placed flat against a dark background)
Ear (length; from photograph – position, rotation morphology)
Standards for each photograph will be enumerated in the training manual.
Qualified Examiner:
Education: Master’s level (or equivalent degree) candidate preferred, with background in working with pediatric population(s) in a clinical department (e.g., genetics, other pediatric departments) or other research project involving children.
Experience:
Previous direct clinical experience in examining children, recording data, under supervision of pediatric clinician.
Completion of written and videotaped training curriculum according to published standards of measurement.
Experience observing in a pediatric clinical genetics clinic (e.g, craniofacial clinic or others), working with geneticist and/or genetics counselor for a minimum of 6 sessions.
Quality Assurance
To maintain quality and consistent data collection each of the CADDRE centers will:
Identify a clinician who will supervise and train the examiner(s), establish reliability, and oversee quality of data collection.
Identify a consulting Pediatric Geneticist who will be available to assist with training curriculum and evaluate photographs of subjects to confirm clinical impressions as needed.
Maintain a library of standard references (see reference list below).
Develop a written and videotaped curriculum for standards of physical measurements and data recording. Sample examinations will be videotaped as part of a curriculum for training examiners.
Measures of Quality assurance
Within the center – options to be considered will include (one or both of the following)
The clinical team will periodically compare direct clinical measurements by a geneticist or experienced examiner (using sliding anthropometric caliper) with photogrammetric digital measurements. The expectation of agreement (X% of measurement vs. number of trials to establish agreement) will be determined.
Duplicate photographs of the same child will be measured in sequence (by the same examiner) and/or by other examiners in the same program. The expectation of agreement X% of measurement vs. number of trials to establish agreement) will be determined.
Between centers – Interrater reliability measures (between centers) will be completed quarterly, with standards of agreement to be determined.
REFERENCES
Hall JG, Froster-Iskenius UG, Alanson JE. Handbook of Normal Physical Measurements. NY: Oxford University Press; Jones KL. Smith's Recognizable Patterns of Human Malformation 5th edition. Philadelphia, PA: WB Saunders and Company, 1989.
Jones KL. Smith’s Recognizable Patterns of Human Malformation, 5th Edition. Phildelphia: W.B. Saunders Company, 1997.
Miles JH, Hillman RE. Value of a clinical morphology examination in autism. American Journal of Medical Genetics 91:245-253 (2000).
Rodier
PM, Bryson SE, Welch JP. Minor
malformations and physical measurements in autism: data from Nova
Scotia. Teratology.
1997
May;55(5):319-25
http://download.interscience.wiley.com/cgi-bin/fulltext?ID=46014&PLACEBO=IE.pdf
Waldrop, M. & Halverson, C. Minor physical anomalies and hyperactive behaviour in young children. In J. Hellmuth (Eds.), Exceptional Infant. Studies in Abnormalities (pp. 343-380). New York: Brunner/Mazel, 1971
Dysmorphology Exam: Data Collection Form
STUDY ID# Date of examination:
G
Form Approved
OMB NO. __________
Exp. Date __________
Date of Birth: Reviewing Physician:
Chronological Age: Date of Review:
DYSMORPHOLOGY EXAMINATION
I. Growth parameters |
Measurement Note Units |
Percentile |
COMMENTS (From in person exam) |
COMMENTS (From photograph review) |
Head circumference (cm) |
|
|
|
n/a |
Height (cm) |
|
|
|
n/a |
Weight (kg) |
|
|
|
n/a |
Inner canthal distance (mm) |
|
|
|
|
Palpebral fissure length (mm) |
|
|
|
|
Hand Measurements |
Right |
Left |
COMMENTS (From in person exam) |
COMMENTS (From image review) |
||
Using copied image of palmar surface of hand |
Size (cm) |
%ile |
Size (cm) |
%ile |
|
|
Palm + middle finger |
|
|
|
|
|
|
Palm |
|
|
|
|
|
|
Middle finger |
|
|
|
|
|
|
2nd or Index finger |
|
|
|
|
|
|
4th or ring finger |
|
|
|
|
|
|
Public Reporting Burden
Statement
Public reporting burden of
this collection of information is estimated to average 15 minutes
per response, including the time for reviewing instructions,
searching existing data sources, gathering and maintaining the data
needed, and completing and reviewing the collection of information.
An agency may not conduct or sponsor, and a person is not required
to respond to a collection of information unless it displays a
currently valid OMB control number. Send comments regarding this
burden estimate or any other aspect of this collection of
information, including suggestions for reducing this burden to
CDC/ATSDR Reports Clearance
Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333;
ATTN: PRA (0920-XXXX)
II. Minor congenital anomalies |
COMMENTS (From in person exam) |
COMMENTS (From photograph review) |
|
HEAD |
|
||
Frontal Bossing
|
|
|
|
Widow's peak |
|
|
|
Low hairline (posterior) |
|
|
|
Double/multiple hair whorl(s) |
|
|
|
Frontal upsweep |
|
|
|
Nasolabial fold (at rest) |
|
|
|
Epicanthal folds |
|
|
|
Nose |
|
|
|
Mouth |
|
|
|
|
LEFT |
RIGHT |
COMMENTS (From in person exam) |
COMMENTS (From photograph review) |
|
EARS |
|
||||
Ear position (low +/=) |
□ Low set □ Normal |
□ Low set □ Normal |
|
|
|
Ear shape |
□ Simple □ Lop shape □ Normal |
□ Simple □ Lop shape □ Normal |
|
|
|
Ear shape - helix |
□ Folded helix □ Normal |
□ Folded helix □ Normal |
|
|
|
Ear shape - helix |
□ Notches in helix □ Normal |
□ Notches in helix □ Normal |
|
|
|
Ear lobes |
□ Adherent □ Normal |
□ Adherent □ Normal |
|
|
|
HANDS |
|
||||
Nails |
□ Abnormal – describe □ Normal |
□ Abnormal – describe □ Normal |
|
|
|
Index finger > middle finger |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
Single transverse crease |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
Curved 5th finger |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
FEET |
|
||||
Nails |
□ Abnormal –describe □ Normal |
□ Abnormal – describe □ Normal |
|
|
|
2nd & 3rd toes long as great toe |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
3rd toe longer than second |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
Syndactyly of toes |
# toes ________ □ Present (full) □ Partial □ Absent (normal) |
# toes ________ □ Present (full) □ Partial □ Absent (normal) |
|
|
|
Short toes |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
Toe spacing |
□ Normal □ Wide spaced |
□ Normal □ Wide spaced |
|
|
|
Toe walking |
□ Present □ Absent |
□ Present □ Absent |
|
|
|
SKIN |
|
||||
Cutaneous findings suggestive of neurocutaneous disorder - ambient light |
|
Record number, location and measurement(s): |
|
|
|
Cutaneous findings with Woods Lamp illumination |
|
Record number, location and measurement(s): |
|
|
|
Other Observations:
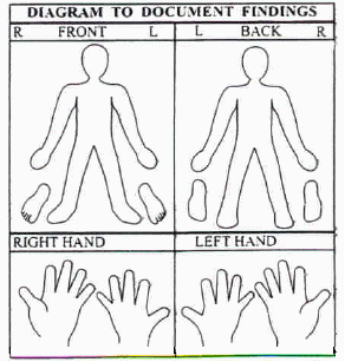
Photographs taken/ comments (Examiner please check which ones)
Face (frontal)
Back of head (for hairline)
Profile – left
Profile – right
Hand (volar or non-palm side) – left
Hand (volar or non-palm side) – right
Foot – left
Foot – right
Skin; note which parts of body: ____________________________________________
______________________________
Signature of Examiner Signature of Reviewer
Appendix P
| File Type | application/msword |
| File Title | PROTOCOL FOR SUBJECT MEASUREMENT AND PHOTOGRAPHY |
| Author | THE CHILDREN'S HOSPITAL OF PHILADELPHIA |
| Last Modified By | pax1 |
| File Modified | 2006-12-29 |
| File Created | 2006-12-29 |
© 2026 OMB.report | Privacy Policy