IRB Approvals
Attachments 4A-4C - IRB Approvals.doc
Generic Clearance to Collect Medical Outcome and Risk Factor Data from a Cohort of U.S. Radiologic Technologists
IRB Approvals
OMB: 0925-0405
A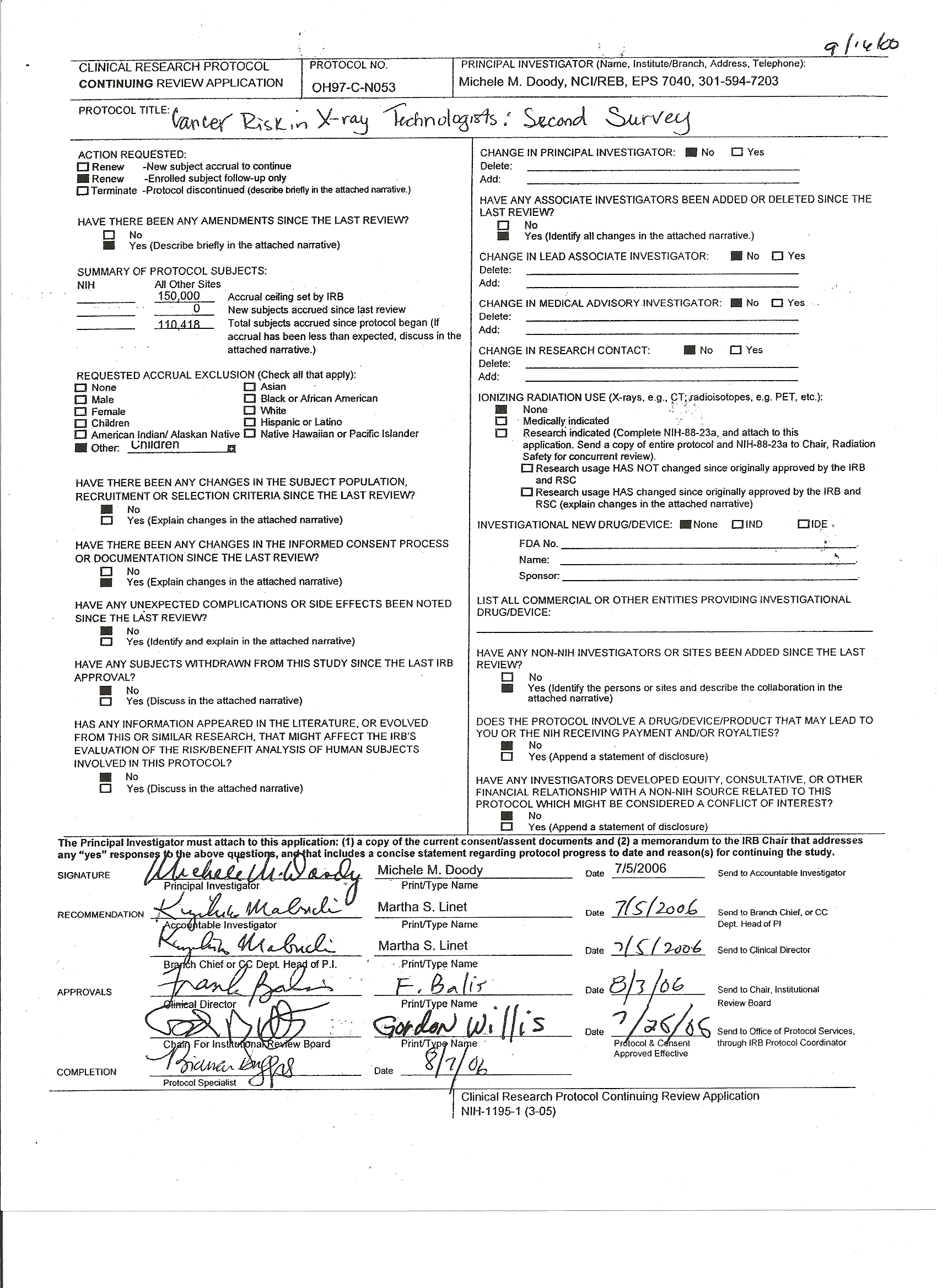 ttachment
4A – National Cancer Institute IRB Approval (OH97-C-N053)
ttachment
4A – National Cancer Institute IRB Approval (OH97-C-N053)
Attachment 4B – University of Minnesota IRB – Main Study (8005M02489)
Subject:
IRB Approval of Continuing Review
From:
Date:
Fri, 15 Sep 2006 23:10:08 -0500 (CDT)
To:
The IRB: Human Subjects Committee renewed its approval of the referenced study listed below:
Study Number: 8005M02489
Principal Investigator: Bruce Alexander
Expiration Date: 09/13/2007
Approval Date: 09/14/2006
Title(s):
Cancer Risk in X-Ray Technologists
U.S. Radiologic Technologists Cohort: New Strategies for Follow-up
The U.S. Radiologic Technologists Study
________________________________________________________
You may go to the View Completed section of http://eresearch.umn.edu/ to view or print your continuing review submission.
For grant certification purposes you will need this date and the Assurance of Compliance number, which is FWA00000312 (Fairview Health Systems Research FWA00000325, Gillette Childrens Specialty Healthcare FWA00004003). Approval will expire one year from that date. You will receive a report form two months before the expiration date.
In the event that you submitted a consent document with the continuing review form, it has also been reviewed and approved. If you provided a summary of subjects' experience to include non-UPIRTSO events, these are hereby acknowledged.
As Principal Investigator of this project, you are required by federal regulations to inform the IRB of any proposed changes in your research that will affect human subjects. Changes should not be initiated until written IRB approval is received. Unanticipated problems and adverse events should be reported to the IRB as they occur. Research projects are subject to continuing review.
If you have any questions, please call the IRB office at (612) 626-5654.
The IRB wishes you continuing success with your research.
Attachment 4C – University of Minnesota IRB – Genetic Studies (9312M07534)
Subject:
IRB Approval of Continuing Review
From:
Date:
Thu, 5 Oct 2006 23:10:11 -0500 (CDT)
To:
The IRB: Human Subjects Committee renewed its approval of the referenced study listed below:
Study Number: 9312M07534
Principal Investigator: Bruce Alexander
Expiration Date: 10/03/2007
Approval Date: 10/04/2006
Title(s):
Cancer Risks in X-Ray Technologists: Early-Onset Breast Cancer among Radiation Technologists
Genetics Studies among Radiologic Technologists
________________________________________________________
This e-mail confirmation is your official University of Minnesota RSPP notification of continuing review approval. You will not receive a hard copy or letter.
This secure electronic notification between password protected authentications has been deemed by the University of Minnesota to constitute a legal signature.
You may go to the View Completed section of http://eresearch.umn.edu/ to view or print your continuing review submission.
For grant certification purposes you will need this date and the Assurance of Compliance number, which is FWA00000312 (Fairview Health Systems Research FWA00000325, Gillette Childrens Specialty Healthcare FWA00004003). Approval will expire one year from that date. You will receive a report form two months before the expiration date.
In the event that you submitted a consent document with the continuing review form, it has also been reviewed and approved. If you provided a summary of subjects' experience to include non-UPIRTSO events, these are hereby acknowledged.
As Principal Investigator of this project, you are required by federal regulations to inform the IRB of any proposed changes in your research that will affect human subjects. Changes should not be initiated until written IRB approval is received. Unanticipated problems and adverse events should be reported to the IRB as they occur. Research projects are subject to continuing review.
If you have any questions, please call the IRB office at (612) 626-5654.
The IRB wishes you continuing success with your research.
| File Type | application/msword |
| File Title | Attachment 6A – National Cancer Institute IRB Approval (OH97-C-N053) |
| Author | Michelle Doody |
| Last Modified By | anisz |
| File Modified | 2007-03-01 |
| File Created | 2007-03-01 |
© 2026 OMB.report | Privacy Policy