Guidance for Industry: Chemistry Manufacturing and Control Changes for Approved NADA or ANADA
Guidance FDAMA 116.doc
Supplements and Other Changes to Approved New Animal Drug Applications - Final Rule
Guidance for Industry: Chemistry Manufacturing and Control Changes for Approved NADA or ANADA
OMB: 0910-0600
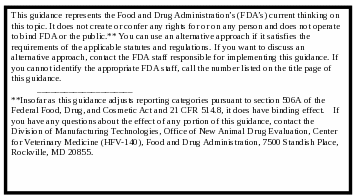
CONTAINS NON-BINDING RECOMMENDATIONS
83
Guidance for Industry
Chemistry, Manufacturing, and Controls Changes to an Approved NADA or ANADA
Comments and suggestions regarding this guidance should be sent to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Room 1061, Rockville, MD 20852. Comments may also be submitted electronically on the Internet at http://www.fda.gov/dockets/ecomments. Once on this Internet site, select “Docket # 99D-1651 Chemistry, Manufacturing and Controls Changes to an Approved NADA or ANADA" and follow the directions. All written comments should be identified with Docket No. 99D-1651.
For questions regarding this guidance document, contact Dennis Bensley, Jr., Center for Veterinary Medicine (HFV-140), Food and Drug Administration, 7519 Standish Place, Rockville, MD 20855, 301-827-6956. E-mail: [email protected].
Additional copies of this guidance document may be requested from the Communications Staff, HFV-12, Center for Veterinary Medicine, Food and Drug Administration, 7519 Standish Place, Rockville, MD 20855, and may be viewed on the Internet at http://www.fda.gov/cvm.
_____________________________________________________________________________
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Veterinary Medicine (CVM)
Month 2007
___________________________________________________________________
TABLE OF CONTENTS
I. INTRODUCTION AND BACKGROUND ..............................................................................................4
II. REPORTING CATEGORIES ……………………………………………………………………………6
III. GENERAL REQUIREMENTS …………………………………………………………………………..7
IV. ASSESSING THE EFFECT OF MANUFACTURING CHANGES……………………………………8
A. ASSESSMENT OF THE EFFECTS OF THE CHANGE…………………………………………………..8
1. Conformance to Specifications………………………………………………………………………………………..8
2. Additional Testing……………………………………………………………………………………………………. 9
B. EQUIVALENCE……………………………………………………………………………………………10
C. ADVERSE EFFECT………………………………………………………………………………………..10
V. COMPONENTS AND COMPOSITION…………………………………………………………….…..11
VI. MANUFACTURING SITES………………………………………………………………………….…..11
A. GENERAL CONSIDERATIONS…………………………………………………………………………..11
B. MAJOR CHANGES (PRIOR APPROVAL SUPPLEMENT)……………………………………………..13
C. MODERATE CHANGES (SUPPLEMENT - CHANGES BEING EFFECTED)………………………....14
1. Supplement - Changes Being Effected in 30 Days………………………………………………………………...14
2. Supplement - Changes Being Effected……………………………………………………………………………....15
D. MINOR CHANGES (MINOR CHANGES AND STABILITY REPORT)…………………….………….15
VII. MANUFACTURING PROCESS…………………………………………………………………………15
A. GENERAL CONSIDERATIONS…………………………………………………………………………..15
B. MAJOR CHANGES (PRIOR APPROVAL SUPPLEMENT)……………………………………………..16
C. MODERATE CHANGES (SUPPLEMENT - CHANGES BEING EFFECTED)…………………………18
1. Supplement - Changes Being Effected in 30 Days………………………………………………………………...18
2. Supplement - Changes Being Effected………………………………………………………………………………19
D. MINOR CHANGES (MINOR CHANGES AND STABILITY REPORT)……………….……………….19
VIII. SPECIFICATIONS………………………………………………………………………………………..20
A. GENERAL CONSIDERATIONS…………………………………………………………………………..20
B. MAJOR CHANGES (PRIOR APPROVAL SUPPLEMENT)……………………………………………..21
C. MODERATE CHANGES (SUPPLEMENT - CHANGES BEING EFFECTED)…………………………22
1. Supplement - Changes Being Effected in 30 Days………………………………………………………………...22
2. Supplement - Changes Being Effected………………………………………………………………………………23
D. MINOR CHANGES (MINOR CHANGES AND STABILITY REPORT)……………………….……….23
IX. CONTAINER CLOSURE SYSTEM…………………………………………………………………….24
A. GENERAL CONSIDERATIONS………………………………………………………………………….24
B. MAJOR CHANGES (PRIOR APPROVAL SUPPLEMENT)…………………………………………….24
C. MODERATE CHANGES (SUPPLEMENT - CHANGES BEING EFFECTED)………………………...26
1. Supplement - Changes Being Effected in 30 Days………………………………………………………………..26
2. Supplement - Changes Being Effected……………………………………………………………………………...26
D. MINOR CHANGES (MINOR CHANGES AND STABILITY REPORT)……… ……………………….26
X. MISCELLANEOUS CHANGES…………………………………………………………………………28
A. MAJOR CHANGES (PRIOR APPROVAL SUPPLEMENT)……………………………………………..28
B. MODERATE CHANGES (SUPPLEMENT - CHANGES BEING EFFECTED)…………………………29
1. Supplement - Changes Being Effected in 30 Days………………………………………………………………...29
2. Supplement - Changes Being Effected………………………………………………………………………………29
C. MINOR CHANGES (MINOR CHANGES AND STABILITY REPORT)…………………………………29
XI. MULTIPLE RELATED CHANGES……………………………………………………………………..30
ATTACHMENT: TYPE OF OPERATION AND CGMP INSPECTIONS………………………………...33
GLOSSARY………………………………………………………………………………………………………….36
GUIDANCE FOR INDUSTRY
Chemistry, Manufacturing and Controls Changes to an Approved NADA or ANADA
I. INTRODUCTION AND BACKGROUND
This guidance provides recommendations to holders of new animal drug applications (NADAs) and abbreviated new animal drug applications (ANADAs) who intend to make post-approval chemistry, manufacturing, and controls (CMC) changes in accordance with section 506A of the Federal Food, Drug, and Cosmetic Act (the Act) and 21 CFR 514.8. The guidance covers recommended reporting categories for post-approval changes for animal drugs. Recommendations are provided for post-approval changes in: (1) components and composition, (2) manufacturing sites, (3) manufacturing process, (4) specifications, (5) container closure system, as well as (6) miscellaneous changes and (7) multiple related changes.
On November 21, 1997, the President signed the Food and Drug Administration Modernization Act of 1997 (the Modernization Act). 1 Section 116 of the Modernization Act amended the Act by adding section 506A, which provides requirements for making and reporting manufacturing changes to an approved application and for distributing a drug made with such change. FDA has revised its regulations on supplements and other changes to an approved application (21 CFR 514.8) to conform to section 506A of the Act.
This guidance does not provide recommendations on the specific information that should be developed by an applicant to assess the effect of the change on the identity, strength (e.g., assay, content uniformity), quality (e.g., physical, chemical, and biological properties), purity (e.g., impurities and degradation products), or potency (e.g., biological activity, bioavailability, bioequivalence) of a drug as these factors may relate to the safety or effectiveness of the drug. An applicant should consider all relevant FDA guidance documents for recommendations on the information that should be submitted to support a given change.
FDA has published guidances, including the SUPAC (scale-up and postapproval changes) guidances, that provide recommendations on reporting categories. To the extent that the recommendations on reporting categories in this guidance are found to be inconsistent with guidances published before this guidance was finalized, the recommended reporting categories in such previously published guidances are superseded by this guidance. This guidance does not provide extensive recommendations on reporting categories for components and composition changes (see section V). Therefore, recommended reporting categories for components and composition changes provided in previously published guidances, such as the SUPAC guidances, still apply. Section 506A of the Act and 21 CFR 514.8(b)(3) provide for two types of changes-being-effected supplements (see section II), while previously there was only one type. It is important for applicants to use this guidance to determine which type of changes-being-effected supplement is recommended. FDA intends to update the previously published guidances to make them consistent with this guidance.
If guidance for either recommended filing categories or information that should be submitted to support a particular change is not available, CVM’s Division of Manufacturing Technologies, HFV-140, should be consulted.
FDA’s guidance documents, in general, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required. Insofar as this guidance adjusts reporting categories pursuant to section 506A of the Federal Food, Drug, and Cosmetic Act and 21 CFR 514.8, it does have binding effect. If you have any questions about the effect of any portion of this guidance, contact the Division of Manufacturing Technologies, Office of New Animal Drug Evaluation, Center for Veterinary Medicine (HFV-140), Food and Drug Administration, 7500 Standish Place, Rockville, MD 20855.
II. REPORTING CATEGORIES
Section 506A of the Act and FDA's regulation at 21 CFR 514.8 provide for four reporting categories that are distinguished in the following paragraphs.
A major change is a change that has a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug. A major change requires the submission of a supplement and approval by FDA prior to distribution of the drug made using the change. This type of supplement is called, and must be clearly labeled, a Prior Approval Supplement (21 CFR 514.8(b)(2)). An applicant may ask FDA to expedite its review of a prior approval supplement for public health reasons (e.g., drug shortage) or if a delay in making the change described in the supplement would impose an extraordinary hardship on the applicant. This type of supplement is called, and must be clearly labeled, a Prior Approval Supplement-Expedited Review Requested (21 CFR 514.8(b)(2)(iv)). Requests for expedited review based on extraordinary hardship should be reserved for manufacturing changes made necessary by catastrophic events (e.g., fire) or by events that could not be reasonably foreseen and for which the applicant therefore could not plan.2
A moderate change is a change that has a moderate potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug. There are two types of moderate change. One type of moderate change requires the submission of a supplement to FDA at least 30 days before the distribution of the drug made using the change. This type of supplement is called, and must be clearly labeled, a Supplement--Changes Being Effected in 30 Days (21 CFR 514.8(b)(3)). The drug made using a moderate change cannot be distributed if FDA informs the applicant within 30 days of receipt of the supplement that a prior approval supplement is required (21 CFR 514.8(b)(3)(v)). For each change, the supplement must contain information determined by FDA to be appropriate and must include the information developed by the applicant in assessing the effects of the change (21 CFR 514.8(b)(1)(ii) and (b)(3)(iv)). If FDA informs the applicant within 30 days of receipt of the supplement that information is missing, distribution must be delayed until the supplement has been amended to provide the missing information (21 CFR 514.8(b)(3)(v)(B)). FDA may identify certain moderate changes for which distribution can occur when FDA receives the supplement. This type of supplement is called, and must be clearly labeled, Supplement--Changes Being Effected (21 CFR 514.8(b)(3)(vi)). If, after review, FDA disapproves a changes being effected in a 30- days supplement or changes-being-effected supplement, FDA may order the manufacturer to cease distribution of the drugs that have been made using the disapproved change (21 CFR 514.8(b)(3)(vii)).
A minor change is a change that has minimal potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug. The applicant must describe minor changes in its next annual report to the application and the annual report must be clearly labeled, Minor Changes and Stability Report (21 CFR 514.8(b)(4)).
Under 21 CFR 514.8(b)(2)(v), an applicant may submit one or more protocols (i.e., comparability protocols) describing tests, studies, and acceptance criteria to be achieved to demonstrate the absence of an adverse effect from specified types of changes. A comparability protocol can be used to reduce the reporting category for specified changes. A proposed comparability protocol must be submitted as a prior approval supplement, if not approved as part of the original application (21 CFR 514.8(b)(2)(v)). On February 25, 2003, FDA issued a draft guidance on comparability protocols entitled Comparability protocols - Chemistry, Manufacturing, and Controls Information.
III. GENERAL REQUIREMENTS
Other than for editorial changes in previously submitted information (e.g., correction of spelling or typographical errors, reformatting of batch records), an applicant must notify FDA about each change in each condition established in an approved application beyond the variations already provided for in the application (21 CFR 514.8(b)(1)(i)).
A supplement or annual report must include a list of all changes contained in the supplement or annual report (21 CFR 514.8(b)(1)(v)). On the list, FDA recommends that the applicant describe each change in enough detail to allow FDA to quickly determine whether the appropriate reporting category has been used. For supplements, this list must be provided in the cover letter (21 CFR 514.8(b)(1)(v)). In annual reports, the list should be included in the summary section. The supplement or annual report also is required to describe the change fully (21 CFR 514.8(b)(1)(i)).
An applicant making a change to an approved application under section 506A of the Act also must conform to other applicable laws and regulations, including current good manufacturing practice (CGMP) requirements of the Act (21 U.S.C. § 351(a)(2)(B)) and applicable regulations in Title 21 of the Code of Federal Regulations (e.g., 21 CFR parts 210, 211, 225, 226, and 514). For example, manufacturers must comply with relevant CGMP validation and recordkeeping requirements (21 CFR parts 210, 211, 225, 226) and ensure that relevant records are readily available for examination by authorized FDA personnel during an inspection.
An applicant must include a statement in each supplement and amendment to a supplement certifying that a field copy has been provided to the appropriate FDA district office (21 CFR 514.8(b)(1)(iv)).3
IV. ASSESSING THE EFFECT OF MANUFACTURING CHANGES
A. Assessment of the Effects of the Change
The holder of an approved application under section 512 of the Act must assess the effects of the change before distributing a drug made with a manufacturing change (21 CFR 514.8(b)(1)(ii)).4 For each change, the supplement or annual report must contain information determined by FDA to be appropriate and include the information developed by the applicant in assessing the effects of the change (section 506A(b), (c)(1), (d)(2)(A), and (d)(3)(A) of the Act). The type of information that must be included in a supplemental application or annual report is specified in 21 CFR 514.8(b)(2)(iii), (b)(3)(iv), (b)(3)(vi), and (b)(4)(iii).
1. Conformance to Specifications
An assessment of the effects of a change on the identity, strength, quality, purity, and potency of the drug should include a determination that the drug substance intermediates, drug substance, in-process materials, and/or drug product affected by the change conform to the approved specifications.5 A specification is a quality standard (i.e., tests, analytical procedures, and acceptance criteria) provided in an approved application to confirm the quality of drugs including, for example, drug substances, Type A medicated articles, drug products, intermediates, raw materials, reagents, components, in-process materials, container closure systems, and other materials used in the production of a drug. (21 CFR 514.8(a)(2)(iv)). Acceptance criteria are numerical limits, ranges, or other criteria for the tests described (21 CFR 514.8(a)(2)(iv)). Conformance to a specification means that the material, when tested according to the analytical procedures listed in the specification, will meet the listed acceptance criteria.
2. Additional Testing
In addition to confirming that the material affected by manufacturing changes continues to meet its specification, the applicant should perform additional testing, when appropriate, to assess whether the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug have been or will be affected. The assessment should include, as appropriate, evaluation of any changes in the chemical, physical, microbiological, biological, bioavailability, and/or stability profiles. This additional assessment could involve testing of the post-change drug itself or, if appropriate, the material directly affected by the change. The type of additional testing that an applicant should perform would depend on the type of manufacturing change, the type of drug, and the effect of the change on the quality of the drug. For example:
Evaluation of changes in the impurity or degradant profile could first involve profiling using appropriate chromatographic techniques and then, depending on the observed changes in the impurity profile, toxicology tests to qualify a new impurity or degradant or to qualify an impurity that is above a previously qualified level.6
Evaluation of the hardness or friability of a tablet after certain changes.
Assessment of the effect of a change on bioequivalence could include, for example, multipoint and/or multimedia dissolution profiling and/or an in vivo bioequivalence study.
Evaluation of extractables from new packaging components or moisture permeability of a new container closure system.
An applicant should refer to all relevant FDA guidance documents for recommendations on the information that should be submitted to support a given change. If guidance for information that should be submitted to support a particular change is not available, CVM's Division of Manufacturing Technologies, HFV-140, should be consulted.
B. Equivalence
When testing is performed, the applicant should assess the extent to which the manufacturing change has affected the identity, strength, quality, purity, and potency of the drug product. Typically, this is accomplished by comparing test results from pre- and post-change material and determining if the test results are equivalent. Simply stated: Is the drug made after the change equivalent to the drug made before the change? Equivalence comparisons frequently have a criterion for comparison with calculation of confidence intervals relative to a predetermined equivalence interval. For this, as well as for other reasons, equivalent does not necessarily mean identical. Equivalence also may relate to maintenance of a quality characteristic (e.g., stability) rather than a single performance of a test.
C. Adverse Effect
Some manufacturing changes have an adverse effect on the identity, strength, quality, purity, or potency of the drug. In many cases, the applicant chooses not to implement these manufacturing changes, but sometimes the applicant wishes to do so. If an assessment indicates that a change has adversely affected the identity, strength, quality, purity, or potency of the drug, FDA recommends that the change be submitted in a prior approval supplement, regardless of the recommended reporting category for the change. For example, a process change recommended for a changes-being-effected-in-30-days supplement could cause the formation of a new degradant that requires qualification and/or identification. The applicant's degradation qualification procedures may indicate that there are no safety concerns relating to the new degradant. Even so, the applicant should submit this change in a prior approval supplement with appropriate information to support the continued safety and effectiveness of the drug. During the review of the prior approval supplement, FDA will assess the impact of any adverse effect on the drug as this change may relate to the safety or effectiveness of the drug.
Applicants are encouraged to consult with CVM's Division of Manufacturing Technologies, HFV-140, if there are any questions on whether a change in a characteristic would be viewed by CVM as adversely affecting the identity, strength, quality, purity, or potency of the drug.
V. COMPONENTS AND COMPOSITION
Changes in the qualitative or quantitative formulation, including inactive ingredients, as provided in the approved application are considered major changes and must be submitted in a prior approval supplement, unless exempted by regulation or guidance (21 CFR 514.8(b)(2)(ii)(A)). The deletion or reduction of an ingredient intended to affect only the color of the drug product may be reported in an annual report (21 CFR 514.8(b)(4)(ii)(B)). Guidance on changes in components and composition that may be submitted in a changes-being-effected supplement or annual report is not included in this document because of the complexity of these recommendations, but may be covered in one or more guidance documents describing post-approval changes (e.g., SUPAC documents).
VI. MANUFACTURING SITES
A. General Considerations
CVM must be notified when a manufacturer changes to a manufacturing site that is different from those specified in the approved application (21 CFR 514.8(b)(1)(i)). Manufacturing sites can include those used by an applicant to, for example, (1) manufacture or process drug products,7 in-process materials, Type A medicated articles, drug substances or drug substance intermediates, (2) package drug products, (3) label drug products, and (4) test components, drug product containers, closures, packaging materials, in-process materials, Type A medicated articles, or drug products. Sites include those owned by the applicant or contract sites used by an applicant. Testing sites include those performing physical, chemical, biological, and microbiological testing to monitor, accept, or reject materials as well as those performing stability testing. Sites used to label drug products are considered those that perform labeling of the drug product's primary or secondary packaging components. Sites performing operations that place identifying information on the dosage form itself (e.g., ink imprint on a filled capsule) are considered to be facilities that manufacture or process the drug product. FDA recommends that the supplement or annual report should identify whether the proposed manufacturing site is an alternative to or replacement for the site or sites provided for in the approved application.
FDA recommends that a move to a different manufacturing site, when it is a type of site routinely subjected to FDA inspection, be submitted as a prior approval supplement if the site does not have a satisfactory CGMP inspection8 for the type of operation9 being moved (see sections VI.B.1 and 2).
For labeling, secondary packaging, and testing site changes, the potential for adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug is considered to be independent of the type of drug or specific type of operation being performed. Therefore, the recommended reporting category for any one of these manufacturing site changes will be the same for all types of drugs and operations. For manufacturing sites used to (1) manufacture or process drugs including, for example, drug products, Type A medicated articles, in-process materials, drug substances, or drug substance intermediates or (2) perform primary packaging operations, the potential for adverse effect depends on factors such as the type of drug and operation being performed. Therefore, recommended reporting categories may differ depending on the type of drug and operation.
Except for the situations described in sections VI.B.4, VI.C.1.b, and VI.D.5, construction activities at a manufacturing site or moving production operations within a building or between buildings at the same manufacturing site, do not have to be reported to CVM.
We recommend that a move to a manufacturing site that involves other changes (e.g., process, equipment) should be evaluated as a multiple related change (see section XI) to determine the appropriate reporting category.
A change from one drug substance manufacturer to another typically involves more than simply a site change. In the majority of cases, there will be additional differences (e.g., route of synthesis, process, solvents, equipment). Without extensive knowledge of both sources (i.e., old and new), an applicant cannot adequately describe the differences between the sources, or evaluate the multiple change. Therefore, in the case where the applicant does not have the extensive knowledge to provide this information and evaluation, the site change should be reported in a prior approval supplement. When an applicant does have the extensive knowledge to describe the differences between the sources, it can report the change as appropriate after evaluation as a multiple change. If the change is not reported in a prior approval supplement, the applicant should include a statement in the submission that the change involves no changes that should be reported in a prior approval supplement.
B. Major Changes (Prior Approval Supplement)
The following are examples of changes considered to have a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. A move to a different manufacturing site, except one used to manufacture or process a drug substance intermediate, when the new manufacturing site has never been inspected by FDA for the type of operation that is being moved, or the move results in a restart at the new manufacturing site of a type of operation that has been discontinued for more than two years.
2. A move to a different manufacturing site, when the new manufacturing site does not have a satisfactory CGMP inspection for the type of operation being moved.
3. A move to a different manufacturing site for (1) the manufacture, processing, or primary packaging of drug products when the primary packaging components control the dose delivered to the patient or the formulation modifies the rate or extent of availability of the drug, or (2) the manufacture or processing of in-process materials with modified-release characteristics. Examples of these types of drug products include modified-release solid oral dosage forms,10 transdermal systems, liposomal drug products, depot drug products, oral and nasal metered-dose inhalers (MDIs), dry powder inhalers (DPIs), and nasal spray pumps.
4. Transfer of the manufacture
of an aseptically processed sterile drug substance or aseptically
processed sterile drug product to (1) a newly constructed or
refurbished aseptic processing facility or area, or (2) an existing
aseptic processing facility or area that does not manufacture
similar (including container types and sizes) approved drug
products. Once this change has been approved, subsequent site
changes to the facility for similar drug product types and processes
may be submitted as a changes-being-effected-in-30-days supplement
(see section VI.C.1.b).
5. Transfer of the manufacture of a drug product sterilized by terminal processes to a newly constructed facility at a different manufacturing site. Once this change has been approved, subsequent site changes to the facility for similar drug product types and processes may be submitted as a changes-being-effected-in-30-days supplement (see section VI.C.1.a).
C. Moderate Changes (Supplement-Changes Being Effected)
The following are examples of changes considered to have a moderate potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug. If the new site does not have a satisfactory CGMP inspection for the type of operation being moved (see sections VI.B.1 and 2), then FDA recommends that the changes listed below (excluding changes relating to drug substance intermediate manufacturing sites) be submitted in a prior approval supplement.
1. Supplement-Changes Being Effected in 30 Days
a. A move to a different manufacturing site for the manufacture or processing of any drug including, for example, drug product, Type A medicated article, in-process material, or drug substance that is not otherwise provided for in this guidance.
b. For aseptically processed sterile drug substance or aseptically processed sterile drug product, a move to an aseptic processing facility or area at the same or different manufacturing site, except as provided for in section VI.B.4.
c. A move to a different manufacturing site for the primary packaging of (1) any drug that is not otherwise listed as a major change, and (2) modified-release solid oral dosage form drug products.
d. A move to a different manufacturing site for testing if (1) the test procedures approved in the application or procedures that have been implemented via an annual report11 are used, (2) all post-approval commitments made by the applicant relating to the test procedure(s) have been fulfilled (e.g., providing methods validation samples), and (3) the new testing facility has the capability to perform the intended testing.
2. Supplement-Changes Being Effected
A move to a different manufacturing site for the manufacture or processing of the final intermediate.
D. Minor Changes (Annual Report)
The following are examples of changes considered to have a minimal potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug. If the new site does not have a satisfactory CGMP inspection for the type of operation being moved, then FDA recommends that the changes listed below (excluding changes relating to drug substance intermediate manufacturing sites) be submitted in a prior approval supplement (see sections VI.B.1 and 2).
1. A move to a different manufacturing site for secondary packaging.
2. A move to a different manufacturing site for labeling.
3. A move to a different manufacturing site for the manufacture or processing of drug substance intermediates, other than the final intermediate.
4. A change in the contract sterilization site for packaging components when the process is not materially different from that provided for in the approved application.
5. A transfer of the manufacture of a finished product sterilized by terminal processes to a newly constructed building or existing building at the same manufacturing site.
6. A move to a different manufacturing site for the ink imprinting of solid oral dosage form drug products.
VII. MANUFACTURING PROCESS
A. General Considerations
The potential for adverse effects on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug depends on the type of manufacturing process and the changes being instituted for the drug. In some cases, there may be a substantial potential for adverse effect, regardless of direct testing of the drug for conformance with the approved specification. When there is a substantial potential for adverse effects, a change must be submitted in a prior approval supplement (section 506A(c) of the Act).
B. Major Changes (Prior Approval Supplement)
The following are examples of changes considered to have a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Changes that may affect the controlled (or modified) release, metering, or other characteristics (e.g., particle size) of the dose delivered to the animal, including the addition or deletion of a code imprint by embossing, debossing, or engraving on a modified-release solid oral dosage form.
2. Changes that may affect drug product sterility assurance including, where appropriate, process changes for sterile drug substances and sterile packaging components. These include:
Changes in the sterilization method (e.g., gas, dry heat, irradiation). These include changes from sterile filtered or aseptic processing to terminal sterilization, or vice versa.
Addition, deletion, or substitution of sterilization steps or procedures for handling sterile materials in an aseptic processing operation.
Replacing sterilizers that operate by one set of principles with sterilizers that operate by another principle (e.g., substituting a gravity displacement steam process with a process using superheated water spray).
Addition to an aseptic processing line of new equipment made of different materials (e.g., stainless steel versus glass, changes between plastics) that will come in contact with sterilized bulk solution or sterile drug components, or deletion of equipment from an aseptic processing line.
Replacing a Class 100 aseptic fill area with a barrier system or isolator for aseptic filling. Once this change has been approved, subsequent process changes for similar product types in the same barrier system or isolator may be submitted as a changes-being-effected-in-30-days supplement.
Replacement or addition of lyophilization equipment of a different size that uses different operating parameters or lengthens the overall process time.
Changes from bioburden-based terminal sterilization to the use of an overkill process, and vice versa.
Changes to aseptic processing methods, including scale, that extend the total processing, including bulk storage time, by more than 50 percent beyond the validated limits in the approved application.
Changes in sterilizer load configurations that are outside the range of previously validated loads.
Changes in materials or pore size rating of filters used in aseptic processing.
3. The following changes for a natural product:12
Changes in the virus or adventitious agent removal or inactivation methods. This applies to any material where such procedures are necessary, including drug substance, drug product, reagents, and excipients.
For drug substance and drug product, changes in the source material (e.g., microorganism, plant) or cell line.
For drug substance and drug product, establishment of a new master cell bank or seed.
4. Any fundamental change in the manufacturing process or technology from that currently used by the applicant. For example:
a. Drug product
Dry to wet granulation or vice versa.
Change from one type of drying process to another (e.g., oven tray, fluid bed, microwave).
b. Drug substance
Filtration to centrifugation or vice versa.
Change in the route of synthesis of a drug substance.
5. The following changes for drug substance
Any process change made after the final intermediate processing step in drug substance manufacture.
Changes in the synthesis or manufacture of the drug substance that may affect its impurity profile and/or the physical, chemical, or biological properties.
6. Establishment of a new procedure for reprocessing a batch of drug that fails to meet the approved specification.
C. Moderate Changes (Supplement-Changes Being Effected)
The following are examples of changes considered to have a moderate potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Supplement-Changes Being Effected in 30 Days
a. For drug products, any change in the process, process parameters and/or equipment, except as otherwise provided for in this guidance.
b. For drug substances, any change in process and/or process parameters, except as otherwise provided for in this guidance.
c. For natural protein drug substances and natural protein drug products:
Any change in the process, process parameters, and/or equipment, except as otherwise provided for in this guidance (e.g., section VII.B.5, VII.D.6).
An increase or decrease in production scale during finishing steps that involves different equipment.
Replacement of equipment with that of a different design that does not affect the process methodology or process operating parameters.
d. For sterile drug products, drug substances, and components, as appropriate:
Changes in dry heat depyrogenation processes for glass container systems for drug substances and drug products that are produced by terminal sterilization processes or aseptic processing.
Changes to filtration parameters for aseptic processing (including flow rate, pressure, time, or volume, but not filter materials or pore size rating) when additional validation studies for the new parameters should be performed.
Filtration process changes that provide for a change from single to dual sterilizing filters in series, or for repeated filtration of a bulk.
Changes from one qualified sterilization chamber to another for in-process or terminal sterilization that results in changes to validated operating parameters (time, temperature, F0, and others).
Changes in scale of manufacturing for terminally sterilized drug products that increase the bulk solution storage time by more than 50 percent beyond the validated limits in the approved application when bioburden limits are unchanged.
e. For synthetic drug substances, redefinition of an intermediate, excluding the final intermediate, as a starting material.
2. Supplement-Changes Being Effected
a. A change in methods or controls that provides increased assurance that the drug will have the characteristics of identity, strength, quality, purity, or potency that it purports or is represented to possess.
b. For sterile drug products, elimination of in-process filtration performed as part of the manufacture of a terminally sterilized drug product.
D. Minor Changes (Annual Report)
The following are examples of changes considered to have a minimal potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. For drugs, changes to equipment of the same design and operating principle and/or changes in scale, except as otherwise provided for in this guidance (e.g., section VII.C.1.c, VII.D.6).
2. A minor change in an existing code imprint for a dosage form drug product. For example, a change from a numeric to alphanumeric code.
3. Addition or deletion of a code imprint by embossing, debossing, or engraving on a solid dosage form drug product other than a modified- release dosage form.
4. A change in the order of addition of ingredients for solution dosage forms or solutions used in unit operations (e.g., granulation solutions).
5. Changes in scale of manufacturing for terminally sterilized drug products that increase the bulk solution storage time by no more than 50 percent beyond the validated limits in the approved application when bioburden limits are unchanged.
6. For natural protein drug products and natural protein drug substances:
An increase or decrease in production scale during finishing steps that does not involve an equipment change.
Replacement of equipment used in manufacturing steps other than finishing steps with that of the same design and operating principle, and capacity with no change in production scale.
VIII. SPECIFICATIONS
A. General Considerations
All changes in specifications from those in the approved application must be submitted in a prior approval supplement unless otherwise exempted by regulation or guidance (21 CFR 514.8(b)(2)(ii)(A)). Specifications are the quality standards (i.e., tests, analytical procedures, and acceptance criteria) provided in an approved application to confirm the quality of drugs including, for example, drug substances, Type A medicated articles, drug products, intermediates, raw materials, reagents, components, in-process materials, container closure systems, and other materials used in the production of a drug. (21 CFR § 514.8(a)(2)(iv)). For the purpose of defining specifications, acceptance criteria are numerical limits, ranges, or other criteria for the tests described. (21 CFR § 514.8(a)(2)(iv)). Examples of a test, an analytical procedure, and an acceptance criterion are, respectively: an assay; a specific, fully described high pressure liquid chromatography (HPLC) procedure; and a range of 98.0-102.0 percent. The recommendations in this section also apply to specifications associated with sterility assurance that are included in NADA and ANADA submissions.13
A regulatory analytical procedure is the procedure in the approved application that is designated for use in evaluating a defined characteristic of the drug substance or drug product. Section 501(b) of the Act recognizes the analytical procedures in the U.S. Pharmacopeia/National Formulary (USP/NF) as the regulatory analytical procedures for compendial items. Tests and associated acceptance criteria and regulatory analytical procedures in addition to those specified in the USP/NF may be required for approving compendial items (section 512(b)(1)(D) of the Act).
The applicant may include in its application alternatives to the approved regulatory analytical procedures for testing the drug. However, for purposes of determining compliance with the Act, regulatory analytical procedures are used.
In sections B through D below, the use of the term analytical procedure without a qualifier such as regulatory or alternative refers to an analytical procedures used to test materials other than the drug.
B. Major Changes (Prior Approval Supplement)
The following are examples of changes in specifications considered to have a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Relaxing an acceptance criterion, except as otherwise provided for in this guidance (e.g., section VIII.C.1.b, VIII.C.1.e).
2. Deleting any part of a specification, except as otherwise provided for in this guidance (e.g., section VIII.D.2).
3. Establishing a new regulatory analytical procedure, including designation of an alternative analytical procedure as a regulatory procedure.
4. A change in a regulatory analytical procedure that does not provide the same or increased assurance of the identity, strength, quality, purity, or potency of the material being tested as the regulatory analytical procedure described in the approved application.
5. A change in an analytical procedure for testing components, packaging components, the final intermediate, in-process materials after the final intermediate, or starting materials introduced after the final intermediate that does not provide the same or increased assurance of the identity, strength, quality, purity, or potency of the material being tested as the analytical procedure described in the approved application, except as otherwise noted. For example, a change from an HPLC procedure that distinguishes impurities to (1) an HPLC procedure that does not, (2) another type of analytical procedure (e.g., titrimetric) that does not, or (3) an HPLC procedure that distinguishes impurities but the limit of detection and/or limit of quantitation is higher.
6. Relating to testing of raw materials for viruses or adventitious agents14: (1) relaxing an acceptance criterion; (2) deleting a test; or (3) a change in the analytical procedure that does not provide the same or increased assurance of the identity, strength, quality, purity, or potency of the material being tested as the analytical procedure described in the approved application.
7. A change in a regulatory analytical method that significantly modifies the extraction and purification procedures.
C. Moderate Changes (Supplement-Changes Being Effected)
The following are examples of changes in specifications considered to have a moderate potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Supplement-Changes Being Effected in 30 Days
a. Any change in a regulatory analytical procedure other than those identified as major changes or editorial changes.
b. Relaxing an acceptance criterion or deleting a test for raw materials used in drug substance manufacturing, in-process materials prior to the final intermediate, starting materials introduced prior to the final drug substance intermediate, or drug substance intermediates (excluding the final intermediate), except as provided for in section VIII.B.6.
c. A change in an analytical procedure used for testing raw materials used in drug substance manufacturing, in-process materials prior to the intermediate, starting materials introduced prior to the final drug substance intermediate, or drug substance intermediates (excluding the final intermediate) that does not provide the same or increased assurance of the identity, strength, quality, purity, or potency of the material being tested as the analytical procedure described in the approved application, except as provided for in section VIII.B.6.
d. Relaxing an in‑process acceptance criterion associated with microbiological monitoring of the production environment, materials, and components that are included in NADA and ANADA submissions. For example, increasing the microbiological alert or action limits for critical processing environments in an aseptic fill facility or increasing the acceptance limit for bioburden in bulk solution intended for filtration and aseptic filling.
e. Relaxing an acceptance criterion or deleting a test to comply with an official compendium that is consistent with FDA statutory and regulatory requirements (21 CFR 514.8(b)(3)(ii)(C)).
2. Supplement-Changes Being Effected
a. An addition to a specification that provides increased assurance that the drug will have the characteristics of identity, strength, quality, purity, or potency that it purports or is represented to possess. For example, the addition of a new test and associated analytical procedure and acceptance criterion.
b. A change in an analytical procedure used for testing components, packaging components, the final intermediate, in-process materials after the final intermediate, or starting materials introduced after the final intermediate that provides the same or increased assurance of the identity, strength, quality, purity, or potency of the material being tested as the analytical procedure described in the approved application.
D. Minor Changes (Annual Report)
The following are examples of changes in specifications considered to have a minimal potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Any change in a specification made to comply with an official compendium, except the changes described in section VIII.C.1.e, that is consistent with FDA statutory and regulatory requirements (21 CFR 514.8(b)(4)(ii)(A)).
2. For a drug, the addition or revision of an alternative analytical procedure that provides the same or increased assurance of the identity, strength, quality, purity, or potency of the drug being tested as the analytical procedure described in the approved application, or deletion of an alternative analytical procedure.
3. Tightening of acceptance criteria.
4. A change in an analytical procedure used for testing raw materials used in drug substance synthesis, starting materials introduced prior to the final drug substance intermediate, in-process materials prior to the final intermediate, or drug substance intermediates (excluding final intermediate) that provides the same or increased assurance of the identity, strength, quality, purity, or potency of the material being tested as the analytical procedure described in the approved application.
IX. CONTAINER CLOSURE SYSTEM
A. General Considerations
The potential for adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug when making a change to or in the container closure system is generally dependent on the route of administration of the drug, performance of the container closure system, and the likelihood of interaction between the packaging component and the dosage form. In some cases, there may be a substantial potential for adverse effect, regardless of direct drug testing for conformance with the approved specification.
A change to or in a packaging component will often result in a new or revised specification for the packaging component. This situation does not have to be considered a multiple related change. Only the reporting category for the packaging change needs to be considered.
B. Major Changes (Prior Approval Supplement)
The following are examples of changes considered to have a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. For liquid (e.g., solution, suspension, elixir) and semisolid (e.g., creams, ointments) dosage forms, a change to or in polymeric materials (e.g., plastic, rubber) of the primary packaging components.
2. For liquid (e.g., solution, suspension, elixir) and semisolid (e.g., creams, ointments) dosage forms in permeable or semi-permeable container closure systems, a change in the ink and/or adhesive used on the permeable or semi-permeable packaging component.
3. A change in the primary packaging components for any drug product when the primary packaging components control the dose delivered to the animal (e.g., the valve or actuator of a metered-dose inhaler or addition of a dosing gun).
4. For sterile drug products, any change that may affect drug product sterility assurance such as:
A change from a glass ampule to a glass vial with an elastomeric closure.
A change to a flexible container system (bag) from another container system.
A change to a prefilled syringe dosage form from another container system.
A change from a single unit dose container to a multiple dose container system.
Changes that add or delete silicone treatments to container closure systems (such as elastomeric closures or syringe barrels).
Changes in the size and/or shape of a container for a sterile drug product.
5. Deletion of a secondary packaging component intended to provide additional protection to the drug (e.g., a carton to protect from light, an overwrap to limit transmission of moisture or gases) or a change in the composition of, or the addition of, a secondary packaging component that may affect the impurity profile of the drug.
6. A change to a new container closure system if the new container closure system does not provide the same or better protective properties than the approved container closure system.
7. For a liquid or semisolid dosage form, an increase in the size of a container that results in an increase of the labeled amount of drug product.
C. Moderate Changes (Supplement-Changes Being Effected)
The following are examples of changes considered to have a moderate potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Supplement-Changes Being Effected in 30 Days
a. A change to or in a container closure system, except as otherwise provided for in this guidance, that does not affect the quality of the drug.
b. Changes in the size or shape of a container for a sterile drug substance.
2. Supplement-Changes Being Effected
a. A change in the size and/or shape of a container for a nonsterile drug product, except for solid dosage forms (see section IX.D.2), without a change from one container closure system to another (21 CFR 514.8(b)(3)(vi)(B)).
b. A change in, or addition or deletion of, a desiccant.
D. Minor Changes (Annual Report)
The following are examples of changes considered to have a minimal potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. A change within the container closure system for a nonsterile drug product, based upon a showing of equivalency to the approved system under a protocol approved in the application or published in an official compendium (21 CFR 514.8(b)(4)(ii)(E)).
2. A change in the size and/or shape of a container containing the same number of dosage units for a nonsterile solid dosage form drug product , without a change from one container closure system to another (21 CFR 514.8(b)(4)(ii)(D)).
3. The following changes in the container closure system of solid oral dosage form drug products as long as the new package provides the same or better protective properties (e.g., light, moisture):
Adding or changing a child-resistant closure, a change from a metal to plastic screw cap, or a change from a plastic to metal screw cap.
Changing from one plastic container to another of the same type of plastic (e.g., high density polyethylene (HDPE) container to another HDPE container).
Changes in packaging materials used to control odor (e.g., charcoal packets).
Changes in bottle filler (e.g., change in weight of cotton or amount used) without changes in the type of filler (e.g., cotton to rayon).
An increase in the wall thickness of the container.
A change in or addition of a cap liner.
A change in or addition of a seal (e.g., heat induction seal).
A change to a new container closure system when the container closure system already is approved in the NADA or ANADA for other strengths of the drug product.
4. The following changes in the container closure system of nonsterile liquid drug products, as long as the new package provides the same or better protective properties:
Adding or changing a child-resistant closure, a change from a metal to plastic screw cap, or a change from a plastic to metal screw cap.
Increasing the wall thickness of the container.
A change in or addition of a cap liner.
A change in or addition of a seal (e.g., heat induction seal).
5. A change in the container closure system of unit dose packaging (e.g., blister packs) for nonsterile solid dosage form drug products, as long as the new package provides the same or better protective properties.
6. The following changes in the container closure system of nonsterile semisolid drug products, as long as the new package provides the same or better protective properties:
Changes in the closure or cap.
Increasing the wall thickness of the container.
A change in or addition of a cap liner.
A change in or addition of a seal.
A change in the crimp sealant.
7. A change in the flip seal cap color, as long as the cap color is consistent with any established color coding system for that class of drug products.
X. MISCELLANEOUS CHANGES
A. Major Changes (Prior Approval Supplement)
The following are examples of changes considered to have a substantial potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Changes requiring completion of appropriate clinical studies to demonstrate equivalence of the drug to the drug as manufactured without the change (21 CFR 514.8(b)(2)(ii)(B)).
2. Addition of a stability protocol or comparability protocol.
3. Changes to an approved stability or comparability protocol unless otherwise provided for in this guidance (e.g., VIII.C, VIII.D, X.C.2).
4. An extension of an expiration dating period based upon (1) data obtained under a new or revised stability testing protocol that has not been approved in the application, (2) full shelf-life data on pilot scale batches using an approved protocol, or (3) limited shelf-life data on production lots.
5. Changes to a drug under an application that is subject to a validity assessment because of significant questions regarding the integrity of the data supporting that application (21 CFR 514.8(b)(2)(ii)(G)).
6. Change in the labeled storage conditions, except as otherwise provided for in this guidance .
B. Moderate Changes (Supplement-Changes Being Effected)
The following are examples of changes considered to have a moderate potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. Supplement-Changes Being Effected in 30 Days
a. Reduction of an expiration dating period to provide increased assurance of the identity, strength, quality, purity, or potency of the drug. Extension of an expiration date that has previously been reduced under this provision should be submitted in a changes-being-effected-in-30-days supplement even if the extension is based on data obtained under a protocol approved in the application.
b. Changes categorized as major changes, other than changes to the components and composition, that have been approved by FDA in the corresponding human drug product.15
2. Supplement-Changes Being Effected
No changes have been identified.
C. Minor Changes (Annual Report)
The following are examples of changes considered to have a minimal potential to have an adverse effect on the identity, strength, quality, purity, or potency of a drug as these factors may relate to the safety or effectiveness of the drug.
1. An extension of an expiration dating period based on full shelf-life data on production batches obtained under a protocol approved in the application (21 CFR 514.8(b)(4)(ii)(F)).
2. Addition of time points to the stability protocol or deletion of time points beyond the approved expiration dating period.
3. A change from previously approved stability storage conditions to storage conditions recommended in Veterinary International Conference on Harmonization (VICH) guidances.
4. Non-USP reference standards:
Replacement of an in-house reference standard according to procedures in an approved application.
Tightening of acceptance criteria for existing reference standards to provide greater assurance of drug product purity and potency.
5. Submitting updated stability data generated on commercial or production batches under an approved stability protocol or commitment (21 CFR 514.8(b)(4)(iii)(I)).
XI. MULTIPLE RELATED CHANGES
Multiple related changes involve various combinations of individual changes. For example, a site change also may involve equipment and manufacturing process changes, or a component and composition change may necessitate a change in a specification. For multiple related changes, where the recommended reporting categories for the individual changes differ, CVM recommends that the submission be in accordance with the most restrictive of the reporting categories recommended for the individual changes. When the multiple related changes all have the same recommended reporting category, CVM recommends that the submission be in accordance with the reporting category for the individual changes.
ATTACHMENT A: TYPE OF OPERATION AND CGMP INSPECTIONS
Section VI states that a change to a different manufacturing site should be submitted in a prior approval supplement when (1) the new manufacturing site has never been inspected by FDA for the type of operation being moved, (2) the move results in a restart of a type of operation that has been discontinued for more than two years at the new manufacturing site, or (3) the new manufacturing site does not have a satisfactory current good manufacturing practice (CGMP) inspection for the type of operation being moved.
A profile class system is used by FDA to assist in (1) managing the CGMP inspection process, (2) evaluating the findings and the compliance follow-up needed, and (3) communicating the results of inspections. A profile class can relate to the manufacture of a particular dosage form (e.g., large volume parenterals, oral liquids), type of drug substance (e.g., sterile bulk by chemical synthesis), or specific function performed at a site (e.g., control testing laboratory). There are profile class codes for major categories of drug substance processes, dosage forms, and manufacturing functions (see table below). However, the system is not comprehensive for all operations performed in the pharmaceutical industry (see "not elsewhere classified" or NEC profile class code).
The term type of operation refers to the specialized or even unique conditions and practices that are employed to manufacture a class or category of drug or to perform a limited segment of the manufacturing process. These conditions and practices exist and are performed within the framework of CGMPs, along with general conditions and practices that contribute to the manufacture of all drug products at a given manufacturing site. The conditions and practices, both general and specific, are inspected to evaluate the CGMP acceptability of a manufacturing site. A wide variety of classes or categories of drugs may be produced at a manufacturing site, or the manufacturing site may produce only a single class of drug or perform a limited segment of a manufacturing process. Each type of operation is represented by a profile class code.
Generally, a satisfactory CGMP status for a profile class code is used to communicate a satisfactory CGMP clearance for all of the products and for all of the operations included within the category that code represents. Thus, the profile class code for a particular dosage form or type of drug substance is used to communicate the CGMP status for all aspects of manufacturing, processing, packing, or holding that are performed at the specific manufacturing site relating to that particular dosage form or type of drug substance, including packaging and labeling operations, testing, and quality control. The profile class code for a particular dosage form or type of drug substance also is used to communicate the CGMP status for manufacturing sites that produce in-process material (e.g., controlled-release beads), package drug products, or label drug products, even if these are stand-alone (e.g., contractor) operations.
A few profile class codes that describe certain types of operations (see items in boldface in table) are provided to report the CGMP status for contractor firms whose only function in the manufacturing process is to perform this operation. If one of these operations (e.g., steam sterilization process) is performed at the manufacturing site involved in producing the drug product/drug substance, the CGMP status for that operation is reported as part of the profile class code for the particular dosage form or type of drug substance. For example, a manufacturing site producing a terminally sterilized small volume parenteral drug product would be reported with the profile class code for the dosage form (SVT), not by the profile code for the sterilization process (SSP).
Certain inspections may be required by program priorities even if the rating for a profile class code indicates an acceptable CGMP status. The current profile codes/classes for drugs are:
ADM |
Aerosol dispensed medication |
NEC |
Not elsewhere classified (when using this class, specific drug products are noted) |
CBI |
Biotechnology crude drug |
OIN |
Ointment, nonsterile (includes cream, jelly, paste) |
CEX |
Plant/animal extraction crude drug |
POW |
Powders (includes oral and topical) |
CFS |
Sterile bulk by fermentation crude drug |
RAD |
Radiopharmaceutical |
CFN |
Nonsterile bulk by fermentation crude drug |
RSP |
Radiation sterilization process |
CHG |
Capsule, prompt release |
SNI |
Sterile noninjectable |
CRU |
Crude bulk drugs-nonsynthesized |
SOP |
Soap |
CSG |
Capsules, soft gelatin |
SSP |
Steam sterilization process |
CSN |
Nonsterile bulk by chemical synthesis |
SUP |
Suppositories |
CSP |
Chemical sterilization process |
SVL |
Small volume parenterals (lyophilized) |
CSS |
Sterile bulk by chemical synthesis |
SVS |
Sterile-filled small volume parenterals |
CTL |
Control testing laboratories |
SVT |
Terminally sterilized small volume parenteral |
CTR |
Capsules, modified-release |
TAM |
Type A Medicated Article |
GAS |
Medical gas (includes liquid oxygen and other) |
TCM |
Tablets, prompt-release |
GSP |
Gas sterilization process |
TCT |
Tablets, delayed-release |
HSP |
Dry heat sterilization process |
TDP |
Transdermal patches |
IMN IMS |
Implant Nonsterile Implant Sterile |
TSP |
Fractional (tyndallization) sterilization process |
LIQ |
Liquis (includes solutions, suspension, elixirs, and tinctures) |
TTR |
Tablets, extended-release |
LVP |
Large volume parenterals |
WSP |
Water sterilization process |
CGMP inspectional status, based on the profile class, is available through FDA's Freedom of Information (FOI) Office. (See Glossary under Satisfactory Current Good Manufacturing Practice (CGMP) Inspection for more information regarding FOI requests.)
Examples of post-approval manufacturing site changes and recommended reporting categories:
An applicant wants to move the manufacture of an immediate-release tablet (TCM) to a different manufacturing site that currently manufactures, and has satisfactory CGMP status for, prompt-release capsules (CHG) and powders for oral solution (POW). This manufacturing site change should be filed in a prior approval supplement because the new manufacturing site does not have a satisfactory CGMP inspection for immediate-release tablets.
An applicant wishes to consolidate product testing to a single analytical laboratory site at a manufacturing site. This manufacturing site produces various solid oral dosage form drug products, has an operational analytical laboratory currently at the site, and satisfactory CGMP inspections for the manufacturing occurring at the facility. Some of the drug products that will be tested at the analytical laboratory when the consolidation occurs are not solid oral dosage form products. Unlike most other production operations, testing laboratories (and other operations in boldface in the table) are not inspected on a dosage form/type of drug substance specific basis. The satisfactory CGMP inspection of the analytical laboratory, which was performed as part of the CGMP inspection for manufacture of the solid oral dosage form drug products, is considered to apply to all dosage forms, including those not actually produced at the site. Therefore the change to consolidate product testing to a single analytical laboratory site with a satisfactory CGMP status may be filed in a supplement-changes-being-effected.
GLOSSARY
Acceptance Criteria: Numerical limits, ranges, or other criteria for the tests described (21 CFR 514.8(a)(2)(iv)).
Assess the Effects of the Change: To evaluate the effects of a manufacturing change on the identity, strength, quality, purity, and potency of a drug as these factors may relate to the safety or effectiveness of the drug (21 CFR 514.8(a)(2)(i)).
Container Closure System: The sum of packaging components that together contain and protect the drug. This includes primary packaging components and secondary packaging components, if the latter are intended to provide additional protection to the drug.
Component: Any ingredient intended for use in the manufacture of a drug, including those that may not appear in such drug.
Drug Product: A finished dosage form, for example, tablet, capsule, or solution, etc., that contains an active ingredient (or drug substance) generally, but not necessarily, in association with inactive ingredients (21 CFR 210.3(b)(4)).
Drug Substance: Any component that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of a disease, or to affect the structure or any function of the human body, but does not include intermediates used in the synthesis of such ingredient. The term includes those components that may undergo chemical change in the manufacture of the drug product and are present in the drug product in a modified form intended to furnish the specified activity or effect (21 CFR 210.3(b)(7)).
Final Intermediate: The last compound synthesized before the reaction that produces the drug substance. The final step forming the drug substance involves covalent bond formation or breakage; ionic bond formation (i.e., making the salt of a compound) does not qualify. Consequently, when the drug substance is a salt, the precursors to the organic acid or base, rather than the acid or base itself, should be considered the final intermediate.
Inactive Ingredients: Any intended component of the drug other than an active ingredient.
In-process Material: Any material fabricated, compounded, blended, or derived by chemical reaction that is produced for, and used in, the preparation of the drug product (21 CFR 210.3(b)(9)). For a drug substance, in-process materials are considered those materials that are undergoing change (e.g., molecular, physical).
Intermediate: A material that is produced during the steps of the synthesis of a drug substance and undergoes further molecular change before it becomes a drug substance.
Package: The container closure system and labeling, associated components (e.g., dosing cups, droppers, spoons), and external packaging (e.g., cartons, shrink wrap).
Packaging Component: Any single part of a container closure system.
Primary Packaging Component: A packaging component that is or may be in direct contact with the dosage form.
Satisfactory Current Good Manufacturing Practice (CGMP) Inspection: A satisfactory CGMP inspection is an FDA inspection during which (1) no objectionable conditions or practices were found (No Action Indicated (NAI)) or (2) objectionable conditions were found, but corrective action is left to the firm to take voluntarily and the objectionable conditions will not be the subject of further administrative or regulatory actions (Voluntary Action Indicated (VAI)). A manufacturing site may have an unsatisfactory CGMP status for a specific profile class or type of operation if, for example, (1) the manufacturing site has not been inspected by FDA and found to be in compliance with CGMPs within two years of the last satisfactory FDA inspection, (2) an Official Action Indicated (OAI) is designated by FDA for the manufacturing site for a different profile class or type of operation, or (3) the manufacturer has not satisfactorily addressed the CGMP inspection observations.
Information about the CGMP status of a firm may be obtained by requesting a copy of the Quality Assurance Profile (QAP) from the FDA's Freedom of Information (FOI) Office. The QAP contains information on the CGMP compliance status of firms that manufacture, package, assemble, repack, re-label, or test human drugs, devices, biologics, and animal drugs. All FOI requests must be in writing (21 CFR 20.40(a)) and should be prepared following the instructions found in the reference entitled A Handbook for Requesting Information and Records from FDA. An electronic version of this reference is available on the Internet at http://www.fda.gov/opacom/backgrounders/foiahand.html.
Secondary Packaging Component: A packaging component that is not and will not be in direct contact with the drug.
Specification: The quality standard (i.e., tests, analytical procedures, and acceptance criteria) provided in an approved application to confirm the quality of drugs, including, for example, drug substances, Type A medicated articles, drug products, intermediates, raw materials, reagents, components, in-process materials, container closure systems, and other materials used in the production of a drug. (21 CFR 514.8(a)(2)(iv)
Type A Medicated Article: Product consisting of new animal drug(s) with or without carrier (e.g., calcium carbonate, rice hull, corn, gluten) and with or without inactive ingredients. A Type A medicated article is intended solely for use in the manufacture of another Type A medicated article or a Type B or C medicated feed (21 CFR 558.3(b)(2)).
1 Public Law 105-115.
2 CVM intends to issue guidance on requesting expedited reviews of NADAs, ANADAs, supplemental NADAs, and supplemental ANADAs.
3 For a change to a product manufactured in a foreign facility, a field copy of the supplement or its amendment is not required to be submitted to an FDA district office. However, FDA recommends that the applicant state that the drug made with the change is manufactured only at a foreign site.
4 Assess the effects of the change means to evaluate the effects of a manufacturing change on the identity, strength, quality, purity and potency of a drug as these factors may relate to the safety or effectiveness of the drug. (21 CFR § 514.8(a)(2)(i)). The term assess or assessment as used in this guidance are not the same as validation. Certain validation information, such as sterilization processes, is considered information that is needed to assess the effect of the change as specified in 21 CFR 514.8(b)(1)(ii) and should be submitted in an NADA or ANADA. Unless otherwise specified by FDA, other validation (e.g., process, equipment) data need not be submitted in the application, but must be retained at the facility and be available for review by FDA (see, e.g., 21 CFR 211.180).
5 If a specification needs to be revised as a result of the change, this would be considered a multiple change (see sections VIII and XI).
6 Recommendations on identifying, qualifying, and reporting impurities can be found in relevant guidances (e.g., VICH GL10(R), Impurities in New Veterinary Drug Substances (Revision), Draft Revised Guidance, (GFI 92) (January 6, 2006)).
7 Manufacturing or processing a drug also includes the preparation (e.g., sterilization, depyrogenation, irradiation, washing) of container closure systems or packaging components. Changes in the site used to fabricate packaging components (e.g., bottles) or manufacture packaging materials (e.g., resins) need not be reported to CVM if there are no other changes (e.g., dimensions, composition, processing aids). If other changes occur, the reporting category should be based on the recommended reporting categories for these changes (i.e., the manufacturing site change does not need to be considered when determining the appropriate reporting category).
8 See Glossary for a definition of satisfactory CGMP inspection.
9 See Attachment A for a discussion of the term type of operation.
10 Certain operations relating to the manufacture, processing, or primary packaging of modified-release solid oral dosage form drug products need not be reported in a prior approval supplement (see sections VI.C.1.c and VI.D.6).
11 For purposes of this guidance document, annual report means a minor changes and stability report according to 21 CFR 514.8(b)(4).
12 For the purposes of this guidance, natural product refers to materials (e.g., drug substance, excipients) that are derived from plants, animals, or microorganisms and that are subject to approval under section 512 of the Act. The specific recommendations for natural products are not applicable to inorganic compounds (e.g., salts, minerals).
13 See FDA Guidance for Industry #48, Submission of Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products (November 1994).
14 In this context, testing for adventitious agents is not considered to include tests that are found in an official compendium (e.g., USP <61>).
15 This change applies only to those approved animal drug products that have identical composition (i.e., formulation), manufacturing processes, and analytical controls to a corresponding approved human drug product. As part of CVM's 30-day assessment to determine the change's eligibility as a change that can be placed into effect according to 21 CFR 514.8(b)(3)(iv), the sponsor should also submit, along with the requirements in 21 CFR 514.8(b)(2)(iii), a copy of FDA's approval letter for the change in the human drug product and a certification that the animal and human drug products are identical with the exception of labeling.
| File Type | application/msword |
| File Title | Guidance for Industry |
| Author | CVM |
| Last Modified By | DPresley |
| File Modified | 2007-02-02 |
| File Created | 2007-02-02 |
© 2026 OMB.report | Privacy Policy