Supporting Statement part A 4-3-2007
Supporting Statement part A 4-3-2007.doc
Risk Perception, Worry, and Use of Ovarian Cancer Screening among Women at High, Elevated, and Average Risk of Ovarian Cancer
OMB: 0920-0744
Supporting Statement for
Risk Perception, Worry, and Use of Ovarian Cancer Screening Among Women at High, Elevated, and Average Risk of Ovarian Cancer
PART A.
April 9, 2007
Technical Monitor:
Lucy A. Peipins, Ph.D.
Division of Cancer Prevention and Control
Phone: 770-488-3034
Fax: 770-488-4639
E-mail: [email protected]
Supported by:
Centers for Disease Control and Prevention
National Center for Chronic Disease Prevention and Health Promotion
Division of Cancer Prevention and Control
4770 Buford Highway., NE, Mailstop K55
Atlanta, GA 30341
TABLE OF CONTENTS
A. Justification 1
A1. Circumstances Making the Collection of Information Necessary 1
A2. Purpose and Use of the Information Collection 2
A3. Use of Improved Information Technology and Burden Reduction 5
A4. Efforts to Identify Duplication and Use of Similar Information 5
A5. Impact on Small Businesses or Other Small Entities 7
A6. Consequences of Collecting the Information Less Frequently 7
A7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 7
A8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency 7
A9. Explanation of Any Payment or Gift to Respondents 10
A10. Assurance of Confidentiality Provided to Respondents 10
A11. Justification for Sensitive Questions 12
A12. Estimates of Annualized Burden Hours and Costs 13
A13. Estimates of Other Total Annual Cost Burden to Respondents or
Recordkeepers 15
A14. Annualized Cost to the Government 15
A15. Explanation for Program Changes or Adjustments 15
A16. Plans for Tabulation and Publication and Project Time Schedule 15
A17. Reason(s) Display of OMB Expiration Date Is Inappropriate 16
A18. Exemptions to Certification for Paperwork Reduction Act Submissions 16
Attachments
A. Legislative Authority
B. 60-Day Federal Register Notice
C. Documentation of CDC IRB Approval
D. Documentation of Henry Ford Health System IRB Approval
E. Data Collection Instrument: Baseline Questionnaire
F. Data Collection Instrument: Follow-Up Questionnaire
G. Introductory Letter
H. Thank-You Letter for Baseline Survey Participants
I. Reminder Letter for Follow-Up Survey
J. Thank-You Letter for Follow-Up Survey Participants
A. JUSTIFICATION
A1. Circumstances Making the Collection of Information Necessary
The Centers for Disease Control and Prevention (CDC), Division of Cancer Prevention and Control, is requesting Office of Management and Budget (OMB) approval to conduct a study of risk perception, worry, and use of ovarian cancer screening among women at high, elevated, and average risk of ovarian cancer. The information collection for which approval is sought is in accordance with CDC’s mission to conduct, support, and promote efforts to prevent cancer and to increase early detection of cancer, authorized by Section 301 of the Public Health Service Act (42 U.S.C. 241). A copy of the legislation is included in Attachment A—Legislative Authority.
Accounting for 19,177 cases and 14,682 deaths in 2002, ovarian cancer is the most frequent cause of death from gynecologic malignancy in the United States (USCS, 2005). Although diagnosis at an early stage affords an excellent five-year survival of over 90%, only 25% of women are diagnosed at this early stage. For the ovarian cancer patients presenting with distant disease, the five-year survival is approximately 28% (Ries et al., 2000). Methods for ovarian cancer screening include transvaginal ultrasound and CA125 serum testing. However, no current screening methods have yet been shown effective from a population perspective. Any routine screening test for ovarian cancer must have high specificity and a strong likelihood that a positive test is a cancer (positive predictive value) because a routine screening that generated a large number of false-positive results would require many women to undergo invasive surgical procedures such as laparoscopy or laparotomy to rule out a positive diagnosis.
Because current screening methods are not appropriate for broad population use, identifying a woman’s risk of ovarian cancer plays a key role in determining whether she is a candidate for screening. A family history of ovarian or breast cancer is an important indicator of risk. A woman’s level of risk—average, elevated, or high—is objectively determined by the number of first and/or second-degree relatives with breast or ovarian cancers and their age at cancer diagnosis (USPSTF, 2005; NIH Consensus Development Panel, 1995). It is only for high-risk women (i.e., women with a strong family history of cancer suggestive of a hereditary genetic mutation such as BRCA1 or BRCA2) that the currently available screening modalities of CA125 testing and transvaginal ultrasound are recommended (NIH Consensus Development Panel, 1995). A recent review by the US Preventive Services Task Force determined that these high-risk women would benefit from genetic counseling that allows informed decision making about testing and prophylactic treatment (USPSTF, 2005).
Although
the literature demonstrates a positive association between screening
behavior and family history (Yoon et al., 2003), a recent study found
that women most likely to report high levels of perceived risk and
high levels of screening for ovarian cancer were not those at the
highest objective risk (Andersen et al., 2002). Additionally, many
women at high risk for ovarian cancer may not be getting recommended
screening (Drescher et al., 2000). These studies suggest that women
tend to overestimate their risk for ovarian cancer, irrespective of
their objective risk as determined by their age and family history.
Furthermore, genetic counseling only
shows a limited effect on improving the accuracy of a woman’s
perceived risk
(Hopwood et al., 2001; Braithwaite et
al., 2004; Lerman et al., 1995; Leventhal et al., 1999; Watson et
al., 1999).
This lack of agreement between objective and perceived risk may be due to an influence of demographic and contextual factors on risk perceptions (Leventhal et al., 1999), limitations in the measurement of perceived risk (Schwartz et al., 1997; Hopwood, 2000; Hopwood 2003; Woloshin et al., 1999), or a failure to take into account the cognitive and emotional components of perceived risk (Miller et al., 1999; Slovic et al., 2004). These demographic and contextual factors can include age, education, marital status, style of coping with potentially threatening information, and knowledge and beliefs about ovarian cancer. Among the cognitive and emotional constructs that have been suggested as an influence on risk perception is the experience of cancer in a family member or friend (Montgomery et al., 2003; Foster et al., 2002; Fiandt et al., 1999). An additional component in both risk perception and screening is anxiety or worry. Research shows that women with any family history of ovarian cancer also report increased worry and high levels of perceived risk (Wardle et al., 1993; Schwartz et al., 1995; Robinson et al., 1997). This suggests that worry or anxiety proneness can interfere with the ability to understand risk and follow through with appropriate screening. Consequently, we see that a number of predictors and/or moderators can be important in making judgments about risk and in making the decision to undergo screening. An investigation of the interrelationships among these factors is needed to shed light on the processes involved in the construction of perceived risk and its influence on screening behavior.
A2. Purpose and Use of the Information Collection
The purpose of this study is to examine the effects of a family history of cancer, knowledge about ovarian cancer, worry or anxiety, and perceived risk of cancer on the likelihood of being screened for ovarian cancer by CA125 testing and/or transvaginal ultrasound testing among women at average, elevated, or high risk of ovarian cancer in a managed care population. We hypothesize a pathway leading from objective risk of ovarian cancer as measured by a woman’s family history of cancer, to the formulation of her perceived risk, to intent to undergo screening and finally, to actual screening behavior. Our primary outcomes of interest are perceived risk and ovarian cancer screening behavior. Factors that may influence perceived risk include experience of a friend or relative with cancer, style of coping with stressful situations or information, and knowledge about ovarian cancer as well as a family history of cancer (see Figure A2-1 below).
Figure A2-1: Hypothesized Relationships Among Variables Related to Risk Perception,
Intent to Screen, and Actual Screening Behavior
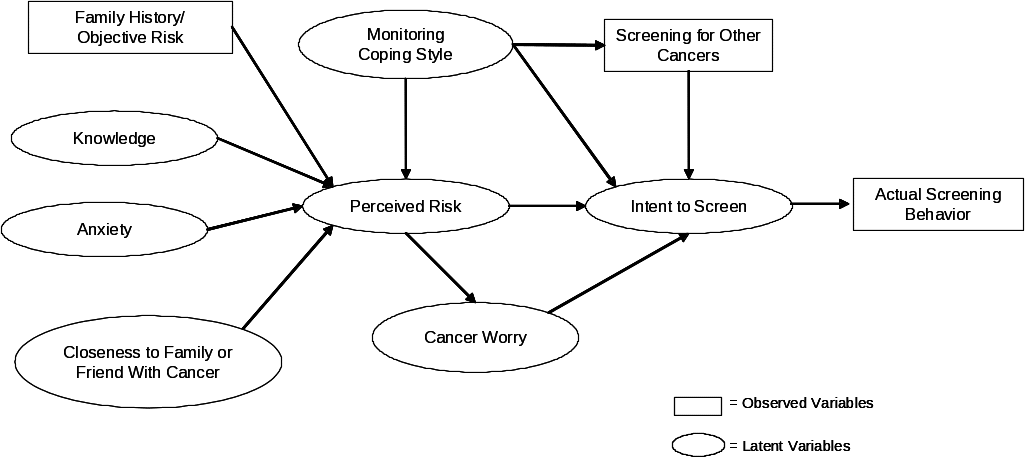
As a federal health agency, it is important that CDC understand the components of perceived risk and its impact on screening behavior in order to help women make appropriate screening decisions. To examine perceived risk and its influence on screening behavior we propose to administer a survey to a random selection of women enrolled in a managed care organization. We will follow-up with these women one year later by administering a second telephone survey to assess whether any changes had occurred in perceived risk or worry and whether intent to undergo screening is followed by actual screening behavior. By working with Henry Ford Health System (HFHS), a managed care organization in the Detroit, Michigan area, we will recruit our sample from a large, racially diverse population. An initial eligibility telephone screening of up to 32,000 women will identify subgroups of average-, elevated- and high-risk women. Approximately 2,000 randomly selected elevated- and average-risk women, and all high-risk women will be surveyed by telephone to collect information on family history of cancer, perceived risk, worry, coping style, anxiety, personal experiences with cancer in family or friends, and intent to undergo screening or actual screening behavior. A one-year follow-up telephone survey will be conducted to assess change in perceived risk, family history and ovarian cancer screening activity.
Through this effort, we will address the following research questions:
1. What are the predictors of a perception of being at high risk for ovarian cancer?
We hypothesize that a strong family history of breast and ovarian cancer, having experienced cancer in one’s family or among friends, cancer knowledge, and an information style of coping with potentially threatening information is positively associated with a perception of high risk of ovarian cancer.
2. What characteristics influence a woman’s likelihood of being screened for ovarian cancer through CA125 or transvaginal ultrasound? What are the strongest predictors of screening behavior?
We hypothesize that a positive family history of cancer, a perception of higher risk, a higher level of cancer worry, prior screening for other cancers, and an information-seeking style of coping with potentially threatening information are associated with a higher likelihood of screening.
3. Is a perception of being at high risk for ovarian cancer also associated with increased cancer worry and anxiety? Does knowledge about ovarian cancer affect that relationship?
We hypothesize that a high level of anxiety and a higher perceived risk will be positively associated with a higher level of cancer worry.
4. What is the relationship between intent to undergo screening and actual screening behavior? Is this relationship moderated by change in family history or change in risk perception or other characteristics?
We hypothesize that the proportion of women reporting intent to undergo screening will be smaller than those who actually undergo screening and that this will be influenced by changes in perceived risk and family history of cancer.
Gaining a comprehensive understanding of the role of risk perception in cancer screening adherence is a priority area for behavioral research in cancer prevention and control (Miller et al., 2004). Although objective risk estimates are typically given by providers or counselors to motivate behavioral change, more needs to be known about the correspondence between objective risk and the extent of under- and overestimation in individuals’ perception of their own risk (Vernon, 1999). Gaining a thorough understanding of the factors that lead to an accurate perception of individual risk is essential in developing strategies to discourage women from undergoing excessive or unwarranted screening and, at the same time, encourage appropriate screening. To minimize psychological distress and overestimation of risk, we need to know more about the constellation of affects, beliefs and expectations that influence screening behavior. The results of this investigation could have important implications for encouraging the appropriate use of screening and for informing educational and communication efforts aiming to maximize screening effectiveness while minimizing worry for average- and elevated-risk women.
Our goal is to advance research efforts, inform counseling practice, and provide information for decision-making strategies and risk communication through an understanding of the process involved in risk perception and its subsequent effect on screening behavior. Thus, the primary audiences for this study are researchers in cancer prevention and control, genetic counselors and physicians. Knowledge about how people perceive their vulnerability to cancer can help us construct interventions that will reduce risk perception biases. For example, we may learn that there are specific subgroups of women who are especially prone to distress regarding cancer risk—for instance, those who have experienced cancer in their family members or among friends. With this knowledge, we may recommend that physicians identify patients with this background for counseling on ovarian cancer risk and screening. Furthermore, these findings can inform communication aimed at family members who are undergoing the stress of cancer in their family or among friends. Counselors could address women’s reactions to a parent’s or friend’s death from cancer and could explore those reactions and their impact on perceived risk and distress (Zakowski et al., 1997).
With rapid growth in genetic and molecular medicine, information on susceptibility to a host of diseases is likely to be available to individuals who want to avoid or reduce potential health problems. Understanding how risk information and perceived risk may motivate behavioral change is an important step in developing ways to communicate information so that it is most useful to individuals.
A3. Use of Improved Information Technology and Burden Reduction
The proposed project involves the use of a CATI (computer-assisted telephone interviewing) system to conduct a baseline questionnaire to women at average, elevated, or high risk of ovarian cancer, identified from the membership records of Henry Ford Health System (HFHS), and a follow-up questionnaire administered to the same respondents one year later. Interviewers will ask survey questions using the CATI system. All survey participants’ responses will be entered directly into a data file, without additional data entry required. A direct benefit to respondents of this type of data collection is the reduced burden experienced by participants due to complicated skip patterns that are managed by the CATI system. Other benefits of administering the questionnaires via a CATI system include: (1) the system permits real-time error checking and correction, (2) the CATI system enables the interviewer to correct problems while respondents are still available, (3) the CATI system requires that, except for the open-ended questions, all interviewers enter the same codes for the same answers provided by respondents to any given question, (4) the many range and logic checks programmed into the survey will produce a clean data set, and (5) the data collection systems include validation of submitted data, real-time summation during numerical data entry, and uploadable and downloadable data files and spreadsheets.
Efforts have been made to design instruments that are user-friendly and understandable. The research team has carefully considered the content, appropriateness, and phrasing of questions. Cognitive interviews were conducted with nine individuals to ensure comprehension of the content and wording of the instrument items. This process has helped refine the content and eliminate extraneous items and response categories. Furthermore, this process has helped ensure that the instruments are the least burdensome for the respondents.
A4. Efforts to Identify Duplication and Use of Similar Information
Based on an extensive review of the literature, a review of the scientific projects described in the CRISP National Institutes of Health database, attendance at conferences, and personal communication with experts in the field, CDC has determined that the planned data collection efforts do not duplicate any other current or previous data collection efforts.
Existing studies of the relationships between risk perception and screening practices have largely focused on breast cancer and have been conducted primarily among patients seen at cancer centers, women from high-risk families identified through registries, and other narrowly selected groups of women (Vernon et al., 1999; McAllister 2003; Absetz et al., 2002; Erblich et al., 2003; Hopwood et al., 2001; Isaacs et al., 2002) We proposed this study to involve average-risk women as well as high-risk women randomly selected from a managed care organization. Two recent studies on ovarian cancer risk and screening found that women most likely to report high levels of perceived risk and high levels of screening for ovarian cancer were not those at the highest objective risk (Andersen et al., 2002) and many women at high risk for ovarian cancer may not be getting recommended screening (Drescher et al., 2000). These studies highlight the need to further examine the processes involved in the development of risk perception among women from a broad range of objective risk categories.
A search of the National Institutes of Health CRISP database reveals a number of studies involving cancer and risk perception. These include studies of the influence of news coverage on risk perception, development of support materials for relatives of persons receiving BRCA results, influence of monitoring style of information processing on risk perception, changes in perceived risk of prostate cancer, family communication about risk in melanoma-prone families, measurement of risk perception, and the development of a decision tool that educates women about breast cancer risk and risk reduction. Our study covers some aspects addressed in these investigations, specifically the measurement of individuals coping style as well as measurement of changes in perceived risk over time. However, our focus is on elucidating the determinants of perceived risk and of the effect of risk perception on screening. To that end, we will include among the factors that influence a woman’s perceived risk her direct experience with cancer illness or death in relatives and friends. We will extend earlier research in this area (Wardle, 1995; Absetz et al., 2002; Zakowski et al., 1997; Montgomery et al., 2003) by elaborating on particular aspects of a woman’s relationships with affected friends or relatives that may lead to a heightened sense of vulnerability. These include strength of the relationship, observation of negative change in family or friend, physical and psychological resemblance to the relative or friend and communication about the cancer experience. To our knowledge, these particular aspects of perceived risk have not been explored. Additionally, we will examine the various factors influencing perceived risk to determine the strongest predictors of screening behavior.
Along with our extensive literature review and search of scientific projects in the CRISP database, we have sought out other possibly similar studies through conference presentations and discussions with experts in the field. Over the last several years, CDC has participated in poster presentations describing the study design to attendees at the International Meeting on the Psychosocial Aspects of Genetic Testing for Hereditary Cancer and CDC’s Public Health Genomics Conference. We obtained no information on data collection efforts comparable to the proposed study through conversations with conference attendees.
A5. Impact on Small Businesses or Other Small Entities
No small businesses or other small entities will be involved in this study.
A6. Consequences of Collecting the Information Less Frequently
In this data collection effort, a baseline telephone survey will be conducted with approximately 2,000 eligible women to collect information on family history of cancer, perceived risk, worry, anxiety, personal experiences with cancer in family or friends, and intent to undergo screening or actual screening behavior. A follow-up telephone survey will be conducted with these same women one year later.
This is a one-time study with two data collection points. While there are no legal obstacles to reducing the burden further, collecting this information less frequently would detract from the purpose of the study. Reducing the respondent burden below the estimated levels (that is, reducing the number of participants) would reduce the power of the study to detect outcome measure differences (perceived risk and screening) among women at high, elevated and average risk and thus diminish the utility of the study. In addition, failing to collect data at two points in time would prevent us from assessing change in perceived risk, family history and ovarian cancer screening activity from baseline to the follow-up survey. Understanding whether perception of risk changes over time and the factors that may influence those changes will inform our use of this construct to communicate information and motivate behavior change.
Because perceived risk plays such a critical role in behavioral change models, the negative consequences of not collecting this information would place a limit on our ability to inform communication and educational efforts that attempt to bring perceived and objective risk into closer alignment.
A7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
This project fully complies with all guidelines of 5 CFR 1320.5.
A8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A notice for public comments on the proposed data collection activities required by 5 CFR 1320.8(d) was published in the Federal Register on March 27, 2006 (Vol. 71, No. 58, pages 15187–15188). CDC has received no comments in response to this notice. A copy of the notice is included in Attachment B—60-Day Federal Register Notice.
The study protocol, data collection plan, identification of a partner managed care organization, data collection instrument and analysis plan are based on discussions with the project team held from August 2003 to February 2006. This project team is composed of CDC staff, ORC Macro staff, Henry Ford Health System staff, and two consultants.
Sharon Alford, PhD, MPH
Henry Ford Health System
Josephine Ford Cancer Center
Henry Ford Health System
1 Ford Place, 5C
Detroit, MI 48202
Phone: (313) 874-9413
Danielle Beauchesne, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Steven Coughlin, PhD
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-4776
Nicola Dawkins, PhD, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
James Dayton, MBA
ORC Macro
126
College Street
Burlington, VT 05401
Phone: (802) 863-9600
Nikki Hawkins, PhD
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-4229
Ronaldo Iachan, PhD, MS
ORC Macro
11785 Beltsville Drive
Calverton, MD 20705
Phone: (301) 572-0200
Rebecca Jackson, MD
San Francisco General Hospital
1001 Potrero Avenue, #6D
San Francisco, CA 94110
Phone: (415) 206-3050
Steven Leadbetter, MS
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-4143
Lucy Peipins, PhD
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-3034
Christine Phelan, BA
ORC Macro
126
College Street
Burlington, VT 05401
Phone: (802) 863-9600
Maren Scheuner, MD, MPH
555 36th Street
Manhattan Beach, CA 90266
Phone: (310) 545-2564
Lawrence Scholl, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Gretchen Simmons, MPH, CHES
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Robert Stephens, PhD, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Paula Yoon, ScD
Office of Genomics and Disease Prevention
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mail Stop K89
Atlanta, GA 30341-3717
Phone: (770) 488-8436
Ye Xu, MA, MS
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Susan Zaro, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Comments on early drafts of the questionnaire and protocol were received from Dr. Sarah Kobrin, Division of Cancer Control and Population Sciences, National Cancer Institute. In addition, Dr. Harland Austin from the Department of Epidemiology at Emory University in Atlanta, Georgia reviewed and provided comments on the protocol.
Funding Sources
Project activities will be supported by CDC Task Order Contract 200-2002-00574, Task Order 15, Risk Perception, Worry, and Use of Ovarian Cancer Screening Among Women at High, Elevated, and Average Risk of Ovarian Cancer.
A9. Explanation of Any Payment or Gift to Respondents
Incorporating modest incentives to aid in recruitment is considered justifiable in order to draw participant’s attention to the study and gain cooperation in completing the survey. In addition, monetary remunerations are associated with higher response rates in questionnaire study designs (Singer 1981; Singer et al., 1997; Whiteman et al., 2003). High response rates are essential to ensure that study findings are representative of the study population. The payment amounts being offered for this study were considered reasonable and appropriate by the CDC IRB Committee and the HFHS IRB committee (Attachment C—Documentation of CDC IRB Approval and Attachment D— Documentation of Henry Ford Health System IRB Approval) and are described below.
Participants in the survey will be provided with a modest incentive for participation in this survey. Participants will be provided a $15 gift card upon completion of the baseline survey, which is estimated to take approximately 35 minutes to complete. Follow-up survey participants will be provided a $10 gift card upon completion of the follow-up survey. The follow-up survey is designed to take only 15 minutes to complete as compared to the baseline survey, thus the reduced amount for the follow-up survey was deemed appropriate.
A10. Assurance of Confidentiality Provided to Respondents
The CDC Privacy Act Officer has reviewed this submission and determined that the Privacy Act applies. The relevant Privacy Act system of records is “Epidemiologic Studies and Surveillance of Disease Problems.”
The Henry Ford Health System, as part of the sub-contractual agreement with ORC Macro, will provide the data collection contractor, ORC Macro, with names, telephone numbers and mailing addresses of prospective respondents. ORC Macro will use respondent names and addresses to mail the introductory letter (Attachment G); the thank you letter and payment for participation in the baseline survey (Attachment H); the follow-up survey reminder letter (Attachment I); and the thank you letter and payment for participation in the follow-up survey (Attachment J). The contractor will also use names and telephone numbers to contact respondents for participation in the telephone surveys (Attachments E and F). Although each respondent will be assigned a unique identification number in the study database, the contractor maintains the linking information and retains the ability to re-link response data to identifying information throughout the data collection period (2-3 years). This linking is required to facilitate follow-up with respondents, including customization of items on the follow-up questionnaire (see question #12 in Appendix F) based on answers to the baseline questionnaire.
The data files delivered to CDC will exclude personal identifiers. The survey data will be analyzed in the aggregate, and no individual respondents will be identified. In both the baseline survey and the follow-up survey, women will be assured that what they say will be kept private. Their answers will not be linked to their name or to other personal information in any report or publication. Only study staff will have access to identifying information. Data from the eligibility screener portion of the questionnaire will be counted as a category of potential responses to the invitation to participate in this survey. The question on month and year of birth on page 2 of the eligibility screener will be used to determine inclusion or exclusion from the study, and to verify data obtained from HFHS. ORC Macro staff, at the end of the study (after data have been analyzed and findings disseminated), will destroy all electronic files and hard copy documents providing the linkages between HFHS patients’ unique identifiers and participants’ assigned study identification numbers and all electronic and hard copy documents containing names and contact information for HFHS patients that were provided by HFHS.
Key safeguards have been put in place to assure respondents that their responses will be treated in a confidential manner. Before asking survey questions in the baseline survey and the follow-up survey, interviewers will obtain verbal informed consent by reading from an informed consent script embedded after the survey introduction (Attachment E—Data Collection Instrument: Baseline Questionnaire and Attachment F—Data Collection Instrument: Follow-Up Questionnaire). Interviewers will review this informed consent statement with participants and respond to any questions prior to beginning the survey. The informed consent script is written in simple language (grades 7.9 and grades 7.5 Flesch-Kinkaid reading levels). It includes a brief description of the study and contains the following key points:
Purpose of the study
Study procedures
Question topics
Estimated time required to participate
Disclosure of incentive
Potential risks and benefits
Statement that participation is voluntary
Telephone numbers of persons they may contact with further questions
Authority for the data collection
Interviewers will ensure that each respondent understands that participation is voluntary, that she can refuse to answer any questions or withdraw at any time, that their answers to questions will be kept separate from their name or any other personal information, and that data will be reported at the aggregate level. By taking the steps of de-identifying datasets and reporting only aggregate information from the survey, we provide additional assurance of the treatment of responses in a confidential manner.
Because we will be asking respondents potentially sensitive information in the form of a detailed family history of cancer, whether they have undergone BRCA1/BRCA2 genetic testing, and the results of those genetic tests, CDC is seeking a 301(d) Certificate of Confidentiality of the Public Health Service Act for this study. CDC handles the review and issuance of certificates for research projects funded by CDC. Upon confirmation of OMB approval for a data collection activity, a six-member CDC Confidentiality Review Group considers applications and determines whether confidentiality authorization will be granted. Once granted, CDC prepares the certificate. This certificate is important to protect sensitive individual information and provides additional assurance that all answers given by participants will be kept private and that no information will be shared with anyone outside the study staff, even under court order. Women may not be willing to share this sensitive information without such an assurance of confidentiality. Advisements to respondents will be modified as needed upon receipt of this executed Certificate of Confidentiality.
Additionally, ORC Macro will provide standard security safeguards for protecting the collected data. All contractor staff working on the project will sign a confidentiality statement that emphasizes the importance of confidentiality, outlines staff obligations, and prohibits the disclosure of confidential information and states specifically that the data will be treated in a confidential manner and that researchers will not use the information for anything other than data analysis consistent with the study purposes as presented to the IRB. The approval letter from the CDC IRB is provided as Attachment C and the approval letter from the HFHS is provided as Attachment D.
HFHS, through its legal department, will set up a data use agreement with ORC Macro specifying the proper handling and use of names and personal information to be used for the recruitment of subjects. The data use agreement specifies that ORC Macro can use the data provided through the end of the study (i.e., through the end of data analysis and dissemination of results). Under the data use agreement with HFHS, ORC Macro will not release respondent identifiers to CDC but will provide CDC and HFHS with a de-identified analysis dataset. In the de-identified dataset provided to HFHS, the added precaution of including only age at time of interview (month and year of birth will not be provided) and race/ethnicity only in the aggregate will be taken. In addition, ORC Macro will be asked to certify by signing the contract that they will abide by current HIPAA regulations in their handling of HFHS data. HFHS maintains HIPAA standards in its clinical and research practices that are also expected of all collaborating institutions. To ensure that the confidentiality of patients is maintained, HFHS will send ORC Macro’s call center office the list of patient names, addresses, and phone numbers as a password protected encrypted file burned to a CD. The password will not accompany the file, but will be sent under separate cover. In addition, HFHS will use a traceable shipping service so that the file can be monitored in transit with the assurance of a correct and safe delivery.
All personnel who will be engaged in screening potential participants and/or interacting with participants during the data collection periods will be thoroughly trained on-site at ORC Macro. Project-specific interviewer training will address confidentiality and security issues particular to the project. Written protocols will be provided that will outline the necessary steps for each portion of the study. Interviewing staff who are selected specifically for this project will attend a half-day training session that will include a review of the study’s background, study protocols, and review of the surveys. Role-play scenarios will be used to illustrate various situations, and specific emphasis will be placed on consent procedures and maintaining confidentiality. Included in the interviewer training will be confidentiality and security issues particular to the project, a discussion of the definitions of the types of cancers, cancer risk factors and other topic-specific questions. Interviewer training will also familiarize interviewers with any resources for assistance, should a respondent indicate a need for such services. ORC Macro project management will develop a project-specific training manual to be used at the training sessions.
CATI interviewers will keep completely confidential the names of respondents, all information or opinions collected in the course of the survey, and any information about respondents learned incidentally during the survey.
To improve data security, response data sets will contain no directly personally identifying information about the respondents. Each respondent’s answers will be identified solely through a unique master identification number that is unrelated to social security number, phone number, or other personal information. ORC Macro’s offices maintain controlled access at all times; confidential hard copy project information is kept in locked files and electronic data are stored on a password protected computer, with access limited to members of the project management team. CDC and HFHS will only have access to de-identified information from the questionnaires. The dataset provided to HFHS will have only age at interview (and not month and year of birth) and race/ethnicity in aggregate form. Thus HFHS will have no means of indirectly identifying individual women from variables provided in the dataset and linking them with responses. The participants’ names and other factors that could identify them as study participants will not appear in study presentations or publications.
A11. Justification for Sensitive Questions
The proposed data collection includes very sensitive information related to the respondent’s personal and family history of cancer. In addition, question concerning educational level and income may be viewed as sensitive by a portion of respondents. Finally we will also be asking women about their race and ethnicity. Although our sample size will not permit stratification by race for all outcomes of interest, we will be able to examine the determinants of perceived risk by race. There may be important differences in the psychological constructs such as cancer worry, anxiety and perceived risk by race that we will want to carefully examine (Consedine et al., 2004). Furthermore, race as well as income and medical care access will be important control variables in multivariate analyses. The sensitivity of the data to be collected necessitates the privacy protection.
Sensitive information is required in order to describe and understand the relationship between objective risk and perceived risk, and we will ask women to provide a detailed history of cancer in their family as part of their objective risk assessment. A strong family history of cancer may be indicative of a genetic mutation such as BRCA1/BRCA2. We will also ask if women had undergone genetic testing for BRCA1/BRCA2 and the results of that test. Both family history of cancer and genetic testing could be considered sensitive information. Knowing if a woman has a strong family history of cancer is imperative for proper classification in the study as a person with high objective risk. In addition to providing an assessment of objective risk, the questions on genetic testing will allow us to examine the behavior of pursuing genetic testing as a demonstration of a woman’s perceived high risk.
Participants may find thinking about and discussing cancer unpleasant or may feel uncomfortable answering some questions about their experiences with cancer. To minimize psychological distress, participants will be told that they may skip over any questions they do not want to answer and stop participating at any time. Interviewers will answer any respondent questions about the study prior to beginning the survey, and participants will be given telephone numbers of the study coordinator at ORC Macro, the Principal Investigator at CDC, or the Associate Director for Science at CDC to answer questions pertaining to the study. Also, at the end of the survey, participants will be provided with the website for CDC’s Division of Cancer Prevention and Control should they desire further general information about cancer or screening for cancer.
A12. Estimates of Annualized Burden Hours and Costs
Burden
The estimate of burden for the instruments is based on cognitive interviews with nine respondents.
Telephone Screener
This portion of Table A12-1 represents the annualized burden for completing the telephone screener. The screener will be administered to approximately 32,000 women. Based on prevalence estimates, 1,200 of these women will be at elevated and high risk, and all 1,200 will be included in the baseline survey. Of the remaining 30,800 women screened—all who, according to prevalence estimates, will be considered at average risk for ovarian cancer—800 will be randomly sampled for inclusion in the baseline survey.
Baseline Survey (Women 30 and Older)
This CATI survey will be conducted with women aged 30 and older who are selected to participate via the telephone screener. We will ask 2,000 women a series of questions that are estimated to take 35 minutes, and we expect that 1,900 will complete the survey. Questions will cover key variables related to ovarian cancer screening including coping, anxiety, perceived risk, worry, personal cancer history, family cancer history, closeness with family or friends who have had cancer, screening behavior, and knowledge of ovarian cancer.
Follow-Up Survey (Women who Completed Baseline Survey)
A follow-up questionnaire will be administered, also using a CATI program, to the women included in the baseline questionnaire. Each of the women will be contacted one year after they complete the baseline survey. The researchers anticipate a 15 percent attrition of the sample between baseline and completion of follow-up leaving approximately 1,600 women in the follow-up sample (85% * 1,900). In the follow-up, women will be asked a series of questions that are estimated to take 15 minutes. The purpose of this data collection effort is to determine if risk perception has changed and to ask about screening for ovarian cancer since the baseline questionnaire was administered.
All data will be collected over a three-year time period. There are no costs to respondents except their time to participate in the survey. The total data collection burden annualized over a three-year time period is 1,411 hours.
Table A12-1: Estimated Annualized Burden Hours
Type of Respondent |
Form Name |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (in Hours) |
Total Burden Hours |
Women aged 30+ |
Telephone screener |
10,667 |
1 |
5/60 |
889 |
Baseline survey (women 30 and older) |
667 |
1 |
35/60 |
389 |
|
Follow-up survey (women who completed baseline survey) |
533 |
1 |
15/60 |
133 |
|
Total 1,411 |
|||||
Respondent Cost
Table A12-2 presents the calculations for cost of burden hours. Average hourly wages were used to calculate the cost of burden for women to participate. Hourly wage information is from the U.S. Department of Labor, Bureau of Labor Statistics web site (http://www.bls.gov/home.htm) and is specific to all workers in the Greater Detroit primary metropolitan statistical area (http://www.bls.gov/oes/2004/november/oes_2160.htm#b00-0000). The total estimated annualized respondent cost (including the telephone screener, baseline survey, and follow-up survey) is $29,574.56.
Table A12-2: Estimated Annualized Burden Costs
Type of Respon- dent |
No. of Respondents |
No. of Responses per Respondent |
Average Burden per Response (in Hours) |
Total Burden Hours |
Hourly Wage Rate |
Total Respondent Costs |
Women aged 30+ |
10,667 |
1 |
5/60 |
889 |
$20.96 |
$18,633.44 |
667 |
1 |
35/60 |
389 |
$20.96 |
$8,153.44 |
|
533 |
1 |
15/60 |
133 |
$20.96 |
$2,787.68 |
|
Total $29,574.56 |
||||||
A13. Estimates of Other Total Annual Cost Burden to Respondents or Recordkeepers
Respondents will incur no capital or maintenance costs to complete this data collection.
A14. Annualized Cost to the Government
Two types of government costs will be incurred: (1) government personnel, and (2) contracted data collection.
The Technical Monitor is assigned for 15% of her time, and the other CDC staff (epidemiologist, statistician and behavioral scientist) will each assign 5% of their time. Assuming an annual salary of $101,478 for the Technical Monitor, $107,447 for the statistician, $116,401 for the epidemiologist, and $75,773 for the behavioral scientist, a total paid to government personnel annually is $30,203.
The data collection is being conducted under a contract with ORC Macro who has a sub-contract with HFHS. The study execution portion of this contract is for a total of $854,857. The collection (including data collection, data management, data analysis, and dissemination of results) will last three years, making the annualized cost of the data collection $284,952.33.
Therefore, total annualized costs to the federal government for this data collection are $315,155.
A15. Explanation for Program Changes or Adjustments
This is a new data collection.
A16. Plans for Tabulation and Publication and Project Time Schedule
Project Time Schedule
Table A16-1 presents the estimated timeline for this study. A three (3)-year clearance is requested.
Table A16-1: Project Time Schedule
Study Activity |
Estimated Date of Completion |
Study logistics |
1-2 months after OMB approval |
Baseline Survey |
|
Recruitment & data collection |
3 -9 months after OMB approval |
Analysis, interpretation, and reporting |
10-15 months after OMB approval |
Follow-Up Survey |
|
Recruitment & data collection |
16-21 months after OMB approval |
Analysis, interpretation, and reporting |
22-25 months after OMB approval |
Publication Plan
Results of the study will be disseminated through presentations at scientific meetings and publications in peer-reviewed journals. We will initially focus on the hypotheses outlined for this study (in Section A2) and anticipate the development of manuscripts on the following topics:
Correlates of perceived risk of ovarian cancer in a managed care population
Characteristics influencing a woman’s likelihood of undergoing screening for ovarian cancer
Determinants of follow-through of the intent to undergo screening for ovarian cancer and adherence to ovarian cancer screening
Cancer worry, knowledge about cancer, and perceived risk of ovarian cancer
Changes in perceived risk of ovarian cancer over time
All abstracts, poster presentations, and manuscripts will undergo CDC clearance review prior to submission to conferences or journals. Women who participate in the survey(s) will be provided with CDC Division of Cancer Prevention and Control’s website, which provides links to published articles.
A17. Reason(s) Display of OMB Expiration Date Is Inappropriate
Exemption to display of OMB expiration date is not being sought.
A18. Exemptions to Certification for Paperwork Reduction Act Submissions
No certification exemption is being sought.
August 22, 2006 Page
| File Type | application/msword |
| File Title | Supporting Statement for |
| Author | sxw2 |
| Last Modified By | arp5 |
| File Modified | 2007-04-09 |
| File Created | 2007-04-03 |
© 2026 OMB.report | Privacy Policy