Supporting Statement part B 4-3-2007
Supporting Statement part B 4-3-2007.doc
Risk Perception, Worry, and Use of Ovarian Cancer Screening among Women at High, Elevated, and Average Risk of Ovarian Cancer
OMB: 0920-0744
Supporting Statement for
Risk Perception, Worry, and Use of Ovarian Cancer Screening Among Women at High, Elevated, and Average Risk of Ovarian Cancer
PART B. Collections of
Information Employing
Statistical Methods
TABLE OF CONTENTS
B. Collections of Information Employing Statistical Methods
B1. Respondent Universe and Sampling Methods
B2. Procedures for the Collection of Information
B3. Methods to Maximize Response Rates and Deal With Non-response
B4. Tests of Procedures or Methods to Be Undertaken
B5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or
Analyzing Data
References
B1. Respondent Universe and Sampling Methods
Study Population and Setting
This study will be conducted within the Henry Ford Health System (HFHS) in the Detroit, Michigan area. HFHS is a large, integrated health system serving the primary and specialty health care needs of residents in southeastern Michigan, including Detroit and its surrounding metropolitan area. Out of the 231,089 enrolled members, 53% or 122,369 are women. The racial distribution of HFHS is: 30% African American, 64% White, and approximately 5% “other” racial designations. At baseline, HFHS will provide a list with names and contact information for approximately 60,000 women age 30 and older enrolled in their system.
Participant Inclusion and Exclusion Criteria
Those eligible to participate in the baseline and follow-up surveys will be women 30 years of age or older who have not been diagnosed with ovarian cancer and who report not having had both of their ovaries removed. Women under the age of 30 will be excluded because ovarian cancer incidence and risk is lower for younger women (Goodman et al., 2003), and women who have had their ovaries removed will be excluded because they are not at risk for ovarian cancer. While none of these groups are explicitly targeted by the study, the sample of patient names provided to ORC Macro by staff of HFHS could include: pregnant women, HIV/AIDS-affected persons, mentally disabled persons, economically disadvantaged persons, and educationally disadvantaged persons. In addition, participants will be able to speak and understand English and respond to survey questions in English. Although managed care organizations do not typically collect information on language or ethnicity, census data show that English is the language predominantly spoken in the geographic area that is the source of our study population. Only 1.8% of the population 5 years of age and over in the Detroit, Michigan primary metropolitan statistical area speaks English “not well” or “not at all” (US Bureau of the Census, 2006). Thus, the proportion excluded because of an inability to speak English would be very small and would not represent a major segment of the population. In addition, several of the instruments used in this study, such as the State-Trait Anxiety Inventory (STAI), have been developed and tested in populations that were primarily English speaking.
Sample Size Estimates and Sampling Procedures
Using prevalence estimates of high, elevated, and average risk women found in the literature (Isaacs et al., 2002; Drescher, et al., 2000, Schwartz et al., 1995; Andersen et al., 2002), we estimate that by screening 32,000 women, we would attain 200 and 1,000 high-risk women and elevated-risk women respectively. These estimates are based on conservative prevalence estimates of approximately 0.6% (Drescher et al., 2000) for the high-risk group and 3.1% for the elevated-risk group. Time and resource considerations also provided practical constraints in determining how many women could be screened initially (i.e., 32,000 women) to obtain the sample sizes for these groups.
Our initial eligibility screening of 60,000 women will account for an estimated 15% incorrect telephone numbers; an estimated 15% unable to reach to determine willingness to participate (due to messages left on answering machines and not returned, busy signals); and an estimated 25% initial refusals to participate in the screening for those women who are reached—this leaves approximately 32,000 women who will be contacted and screened. Of the 32,000 women screened and eligible, approximately 28,500 are expected to fall into the category of average risk for ovarian cancer with no family history of breast or ovarian cancer. We will randomly select and recruit 400 women from this group into the baseline survey. From the eligibility screening, we will be also able to identify an average risk group of approximately 2,300 women with a more limited breast or ovarian cancer family history. From this group we will randomly select 400 women. This will provide us with a total average-risk group of 800 women, 400 of whom have had some limited cancer experience in their family. Women whose family history of cancer indicates high or elevated objective risk levels (n=1,200) will be selected for the sample with certainty. We will interview approximately 2,000 women and expect a 5% failure to complete an interview leaving a baseline sample size of n=1,900 for this study (Figure B1-1). We expect that most of the women who complete the screener will continue through the interview if selected. Furthermore, the CATI system can suspend and resume an interview should a participant need to take a break for whatever reason, which will make completion of interviews more feasible. Figure B1-1 depicts the sampling framework.
Figure B1-1: Sampling Framework
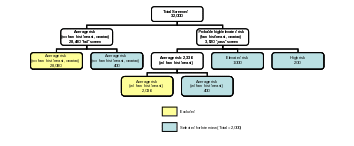
Based on the stable health system enrollments, coupled with updated contact information from the baseline survey, we anticipate successful re-contact and re-interview of an estimated 85% of the baseline sample at 12-month follow-up. Therefore, we expect a sample size of approximately 1,600 for the follow-up sample. For the probable high/elevated risk and average-risk groupings based on family history of cancer, follow-up sample sizes are projected n=960 and n=640, respectively.
Although these sample sizes are based on the overall follow-up response rate of 85%, we may observe differential response rates among subgroups, where those with higher than average perceived risk of developing ovarian cancer or those with a strong family history of cancer may potentially have greater interest in the survey. Consequently, these respondents may be more likely to participate in the follow-up survey than those who perceive themselves to be at average risk. To the degree that perceived risk and objective risk are concordant, the follow-up response rates and sample sizes for the high and elevated objective risk subgroups may exceed the conservative sample size estimates.
Precision of Study Estimates and Power
Most of the estimates of interest in the study will take the form of prevalence rates, percentages or proportions (e.g., percentages of women who fall into objective or perceived risk groupings, or percentages of women who undergo screening, overall or by risk subgroups). Examples include the percentage of women in the elevated or high risk subgroups; percentage of women who undergo screening in each risk subgroup and overall; and the percentage of women who perceive themselves to be at higher than average risk for ovarian cancer.
Table B1-1 provides the standard errors for percentages or proportions as the expected precision of study estimates. The standard errors are based on expected sample sizes for specific risk subgroups and the overall target population at both baseline and follow-up. The table provides standard errors for prevalence rates in the range of 3% to 35%, consistent with the screening rates for average, elevated and high risk as reported in the literature (Isaacs et al., 2002, Drescher et al., 2000, Schwartz et al., 1995; Drescher et al., 2004; Andersen et al., 2002). This considerable variability in the prevalence of screening and higher than average risk perception for ovarian cancer is due not only to differences in family histories of cancer but also to different recruitment methods that were employed to capture the respective study populations (Andersen et al., 2004). Our study most closely approximates a general population and thus, is likely to have lower prevalence estimates relative to clinic populations or populations recruited from physician referrals.
To illustrate the use of Table B1-1, hypothetically assume that our findings at baseline indicate that 20% of women with elevated risk for ovarian cancer have been screened. Then the corresponding standard error of this prevalence estimate from Table 2 is 1.3% and the 95% confidence interval is 20.0% +/- 1.96*1.3% or (17.5%, 22.5%).
Another key outcome measure is the percentage of women who perceive themselves to be at higher risk for ovarian cancer than other women. If we find, for example, that 35% of the average-risk women in our study at follow up perceive themselves at higher than average risk for ovarian cancer, then the corresponding standard error from Table 2 is 2.3%. The 95% confidence interval of higher than average risk perception among average risk women is 35.0% +/- 1.96*2.3% or (30.5%, 39.5%).
We expect to see the smallest prevalence differences (i.e., most similarity in perceived risk and screening) between women at elevated and at high risk. The study will be sufficiently powered (i.e., power=80%, alpha = 0.05) at both baseline and follow up, to detect any cross-sectional outcome measure differences of 7.7% (baseline) and 9.5% (follow-up) or greater that are found between the high and elevated risk groups.
Table B1-1: Standard Errors (%) of Estimated Prevalence Rates Among Objective Risk Level Subgroups and Overall Study Population at Baseline and Follow-Up
Objective Risk Subgroup Samples (n) |
Estimated Prevalence |
||||
3% |
5% |
20% |
25% |
35% |
|
High |
|||||
Baseline (200) |
1.2 |
1.5 |
2.8 |
3.1 |
3.4 |
Follow-up (127) |
1.5 |
1.9 |
3.5 |
3.8 |
4.2 |
Elevated |
|||||
Baseline (1,000) |
0.5 |
0.7 |
1.3 |
1.4 |
1.5 |
Follow-up (637) |
0.7 |
0.9 |
1.6 |
1.7 |
1.9 |
Average |
|||||
Baseline (700) |
0.6 |
0.8 |
1.5 |
1.6 |
1.8 |
Follow-up (446) |
0.8 |
1.0 |
1.9 |
2.1 |
2.3 |
Overall |
|||||
Baseline (1,900) |
0.4 |
0.5 |
1.0 |
1.0 |
1.1 |
Follow-up (1,210) |
0.5 |
0.6 |
1.1 |
1.2 |
1.4 |
It is also useful to examine the relative standard error (RSE) of estimated prevalence rates. Expressed as percentages, the RSE of a percentage is defined as the standard error of the percentage estimate divided by the percentage itself [i.e., RSE(p) = SE(p) / p]. We consider an estimate to be sufficiently precise if the corresponding RSE is 30% or less. For example, if 25.0% of all women at baseline have ever been screened for ovarian cancer, the corresponding standard error from Table 2 is 1.0%, the RSE = 1.0% / 25.0% = 4.0%, and we conclude that the prevalence estimate is precise. The high objective risk subgroup, with the smallest sample sizes, will have the least precise estimates among the risk subgroups. Nevertheless, the high risk subgroup estimates will be sufficiently precise for prevalence rates as low as 8.1%, which are well below the prevalence rates of ovarian cancer screening and higher than average perceived risk among the elevated- and high-risk groups as reported in the previously cited literature.
We will use the adjusted odds ratio from the various multivariate logistic regression models to make inferences about the significance of the effects of numerous model covariates. For dichotomous covariates (e.g., screened vs. not screened for ovarian cancer) the study sample will provide power of 80% to detect odds ratios of 2.0 or greater at a 0.05 significance level for key study outcomes.
We are also proposing to use structural equation modeling (SEM) to examine relationships among the constructs identified in our conceptual model and variables assessed from the baseline and follow-up surveys. There is no minimum sample size required in SEM, though models based on an N < 100 are not recommended and a minimum of 10 cases for every parameter estimated in the SEM has been suggested (Bentler and Chou, 1988; Kline 1998).
Analysis Plan
We plan to conduct a series of analyses employing multivariate techniques to address the research questions outlined in Section A2—Purpose and Use of the Information Collection. Initially we will employ descriptive statistics to summarize the characteristics of the sample and examine the distributions of individual variables. For scales that were drawn from the literature and are being used as they were originally designed (e.g., anxiety, coping, and worry), scale scores will be calculated as described by the instruments’ developers. Descriptive statistics on the scale scores will be compared to those reported in the literature. Similarly, we will calculate reliability coefficients and compare to those reported in the literature. Exploratory factor analysis will be used to validate subscales of importance for the items of the closeness scale.
The first analysis will characterize the predictors of perceived risk. We hypothesize that a family history of cancer, closeness of relative or friend with cancer, and an information seeking style of coping will be associated with a perceived high risk of ovarian cancer. We will examine the bivariate associations between perceived risk and objective risk (family history of breast/ovarian cancer), age, race, education, marital status, coping style, worry, cancer knowledge, and closeness of relative or friend with cancer. These analyses will be conducted using cross tabulation procedures (e.g., chi-square) for categorical variables and between-group procedures (e.g., analysis of variance and independent samples t-test) for continuous variables. In the event that the data do not satisfy assumptions regarding normality, appropriate transformations will be used to adjust the data prior to the analyses. Potential predictors identified from the bivariate analysis will be included in multivariate analyses. Because perceived risk is an ordinal outcome, we can use polytomous logistic regression modeling technique or we can model risk as higher than average vs. average or lower risk. Table B1-2 shows how the results of such analyses will be presented.
Table B1-2: Odds Ratios and 95% Confidence Intervals for Perceived High Risk of Ovarian Cancer
Crude OR (95% CI) Adjusted OR (95% CI)
Age
Race/ethnicity
Objective risk (family history)
High
Elevated
Average
Coping style
Closeness of friend/family
Other demographics
Ovarian cancer knowledge
Another approach in identifying the determinants of perceived risk is to conduct logistic regression modeling of perceived risk as described above, except separately for each level of objective risk (high, elevated, average). This analysis would identify the characteristics of average-risk women that are associated with overestimation of risk and the characteristics of elevated and high risk women that are associated with an accurate perception of their risk. Control variables would include the same variables as above. Odds ratios for the above listed variables would be presented separately for each risk subgroup.
The second analysis will focus on the predictors of cancer worry. We hypothesize that a high level of anxiety and a higher perceived risk will be positively associated with a higher level of cancer worry. This relationship will be mediated by knowledge about cancer and closeness of the family member or friend with cancer. To address this hypothesis, a dichotomous or polytomous (mild, moderate and severe) cancer worry outcome variable will be constructed from the three questions that describe the participants’ level of concern about ovarian cancer. As with earlier analyses, bivariate associations between cancer worry and cancer knowledge, anxiety, closeness of family or friend with cancer, family history of cancer, and demographic variables will be described through cross tabulations or between-group analyses. Logistic regression modeling will be used to characterize the strongest predictors of cancer worry.
The third analysis will investigate the determinants of ovarian cancer screening. We hypothesize that having a close relative or friend with cancer, a high level of cancer worry, anxiety, a family history of cancer, having been screened for other cancers, and a perception of increased risk of cancer will be significant predictors of a higher likelihood of undergoing screening through CA125 or transvaginal ultrasound. We will first consider CA125 and transvaginal ultrasound separately and then combined to create a screened vs. not screened outcome variable. Bivariate analyses and logistic modeling procedures will be used to search for the best combinations of variables that predict the use of these screening tests. Table B1-3 shows how results of this analysis would be presented.
Table B1-3: Odds Ratios and 95% Confidence Intervals for Having Undergone Ovarian Cancer Screening in the Past Year
Crude ORs (95% CI) Adjusted ORs (95% CI)
Age
Race/ethnicity
Other demographics
Objective risk (family history)
High
Elevated
Coping style
Closeness of family/friend
Ovarian cancer knowledge
Prior screening for other cancers
Perceived risk
Anxiety
Worry
A fourth analysis will examine follow-through on screening for women who reported intent to undergo screening. We hypothesize that a smaller proportion of women who report intent to undergo screening will actually do so during the follow-up period. We will use baseline and follow-up data on perceived risk, family history and intent to undergo screening to obtain descriptive measurements of longitudinal outcomes. We are interested in the concordance between reporting the intent to undergo screening versus actual screening behavior at follow-up for each risk group. The proportion of women rescreened and proportion of women following through on intent to undergo screening will be calculated.
In addition to the analyses addressing the primary research questions outlined above, the data may lend themselves to an examination of the relationships among all constructs and variables using structural equation modeling or path analysis. Figure A2-1 presents a conceptual model of the hypothesized relationships between the constructs and variables to be tested. The analysis would be used to test the fit of a correlation matrix of all variables against two or more causal models. A regression is done for each variable in the model as dependent on those variables which the model indicates by the path arrows. The regression weights predicted by the model are compared with the observed correlation matrix and a goodness-of-fit statistic is calculated. This confirmatory type of analysis will be considered if the usual assumptions of regression modeling and appropriate model specification of variables can be met.
B2. Procedures for the Collection of Information
Study Design
In order to investigate the determinants of perceived risk and ovarian cancer screening, we propose to conduct a survey that has both cross-sectional and longitudinal study design elements. We will use a baseline survey administered by telephone to women at high, elevated and average risk of ovarian cancer identified from membership records of HFHS, and a follow-up telephone survey administered to the same respondents one year later. The baseline survey will provide data for numerous cross-sectional analyses related to our outcomes of interest, while the matched baseline and follow-up data will facilitate descriptive longitudinal outcome measurements. Our outcomes of interest include perceived risk of ovarian cancer, intent to undergo ovarian cancer screening and actual screening behavior.
Conducting the study in a managed care setting rather than a clinical setting that would serve primarily high-risk women provides us with a population more representative of the general population of women with access to health care, provides up-to-date contact information for women in the health system, and allows us to examine perceived risk in elevated and average risk women. Furthermore, a managed care population is likely to have fewer barriers to health care which would allow us to elucidate more clearly the relationship between the variables we are examining and actual screening behavior.
Finally, the procedures involved in a computer-assisted telephone interviewing (CATI) such as controlled timing of calls, automatic referral of problem calls, and the ability to assign calls to specific interviewers, are likely to ensure a better response rate than a mailed questionnaire or non-CATI interviewing procedures (Couper et al., 1998).
There are four components of the study design.
HFHS staff will conduct an administrative data review of their enrollees to identify potential participants who are 30 years of age or older. This records review also will exclude from the study any women noted to have a personal history of ovarian cancer. From this review, HFHS will forward a list of names and contact information for approximately 60,000 women to the research team and ORC Macro. ORC Macro call center staff estimate that a list of approximately 60,000 names would be needed in order to be able to reach and screen 32,000 women after taking into account wrong numbers, refusals, answering machines, and other unsuccessful attempts at contact. ORC Macro will enter the contact information for the women into their computer database to be used with the CATI system. If necessary, up to 15 attempts will be made to contact women from this initial list; we anticipate that 32,000 will be successfully contacted and assessed for study eligibility.
Interviewers will conduct a short, five-question eligibility screening survey with women who are successfully contacted by telephone (estimated at approximately 32,000 women). Because the proportion of high-risk women (i.e., women with a family history suggestive of a BRCA1 or BRCA2 genetic mutation) is small, we must conduct an eligibility screening of a large number of potential participants to identify and select all high-risk women and a random sample of average risk women for the cross-sectional survey. These questions will identify women who meet the study’s eligibility criteria (e.g., women who are at least 30 years of age, have not had both their ovaries removed, and have not been diagnosed with ovarian cancer) and who may be classified into one of three risk subgroups based on family history of cancer. In this eligibility screening we will ask about any breast or ovarian cancer among first and second degree relatives. Answers to these questions will be used only for eligibility assessment and not for data analysis.
Based on responses, we will be able to identify a subgroup of women at probable high/elevated risk of developing ovarian cancer and two subgroups of average risk women--a subgroup of women with a more limited family history and a subgroup of women with no family history of breast or ovarian cancer (Table B2-1). We will invite all women at high/elevated risk of ovarian cancer and a random sample of women from the average risk subgroups (an average of every nth person contacted) to participate. The sampling rate for average-risk women (30,800/800) differs from that of average risk with some family history (2,300/400). Selection of women for the baseline survey will happen during the eligibility screening using the CATI system programming capability. Table B2-1 displays the criteria for this initial risk classification.
Table B2-1: Definition of Risk for Ovarian Cancer Based on Family History
Probable High/Elevated Risk |
Personal history of breast cancer |
One or more 1st-degree relative or a 1st- and a 2nd-degree relatives with ovarian cancer |
|
Two or more relatives (1st- or 2nd-degree) with breast cancer |
|
Average Risk With Breast or Ovarian Cancer Family History |
Only one 1st- or 2nd-degree relative with breast cancer |
Only one 2nd-degree relative with ovarian cancer |
|
Average Risk |
No 1st- or 2nd-degree relatives with breast or ovarian cancer |
No personal history of breast cancer |
If a woman is eligible for participation and is selected by the programmed CATI system to be included in the sample, she will be consented and will undergo an interview. Using the CATI system, interviewers will conduct a baseline cross-sectional survey of approximately 2,000 women at average, elevated or high risk for ovarian cancer, age 30 and older, identified in the eligibility screener. The survey instrument includes a total of 133 questions and takes approximately 35 minutes to administer. The questions measure key constructs related to ovarian cancer screening including coping, anxiety, perceived risk, worry, closeness with family or friends who have had cancer, screening behavior, and knowledge about ovarian cancer (see Attachment E—Data Collection Instrument: Baseline Questionnaire).
Interviewers will re-contact women who participated in the baseline survey and who agreed to be contacted again for the follow-up survey approximately one year later to determine if their family history of cancer, risk perception, or screening behavior has changed since the initial survey was completed. The follow-up telephone survey is expected to last no more than 15 minutes (see Attachment F—Data Collection Instrument: Follow-Up Questionnaire).
Concepts, Variables, and Instruments Used in Baseline Survey
Family History of Ovarian/Breast Cancer
Participants will be asked a series of questions about breast and ovarian cancer in first and second-degree female relatives on both the mother’s and father’s side of the family. Participants will be asked whether any grandmother, mother, sister, aunt, daughter, or half-sister had ever been diagnosed with breast or ovarian cancer, and at what age they were diagnosed. We will also seek information on whether any blood relatives had cancer other than breast or ovarian cancer. The information sought will include the type of relative (father, mother, grandparents, aunts, uncles, sisters, brothers or children), type of cancer, and age at diagnosis of the cancer.
Based on the U.S. Preventive Services Task Force guidelines for genetic risk assessment (2005), women will be categorized into one of three risk subgroups: high risk, elevated risk and average risk of ovarian cancer. Women assigned to the high-risk group will have a strong family history of breast/ovarian cancer indicative of a possible inherited genetic mutation such as BRCA1or BRCA2. This group would include women with two or more first or second degree relatives with ovarian cancer; two first degree relatives with breast cancer, one of whom was diagnosed at age 50 years or younger; a combination of three or more first or second degree relatives with breast cancer; a combination of both breast and ovarian cancer among first and second degree relatives; and a first or second degree relative with both breast and ovarian cancer (USPSTF 2005). Women with one first degree relative with ovarian cancer will be assigned to the elevated risk group (Andersen et al., 2002). Women with no relatives with breast or ovarian cancer, or those with only one first degree relative with breast cancer or only a second degree relative with ovarian cancer will be assigned to the average risk group. Self-reports of cancer in family members, particularly first degree relatives, have been found to be accurate and reliable (Murff et al., 2004).
Perceived Risk
Perceived risk of developing ovarian cancer in the next 10 years and during a participant’s lifetime will be assessed with two questions using a 5-point Likert-type response scale. Women will be asked whether their 10-year and lifetime risk of developing ovarian cancer is much higher, higher, about the same, lower or much lower, than risk in most women their age. These perceived risk measures have been used previously in studies and have demonstrated stability and validity (Schwartz et al, 1995; Lloyd et al., 1996).
Cancer Worry
We have adapted the Breast Cancer Worry Scale (Lerman et al., 1991; Lerman and Schwartz, 1993) to assess participants’ levels of concern about ovarian cancer and the extent to which it affects daily functioning. Using the responses of never, rarely, sometimes, a lot, and all the time, participants will report how often they have thought about their chances of developing ovarian cancer, how often thoughts of getting ovarian cancer have affected their mood and how often these thoughts have affected their ability to perform daily activities. This scale is one of two predominant measurement strategies for cancer worry used in cross-sectional and prospective studies involving women of different racial/ethnic groups, the general population and high risk women (Hay et al., 2005). It has also been used in studies of ovarian cancer screening (Drescher et al., 2000; Anderson et al., 2002).
Anxiety
The State-Trait Anxiety inventory (STAI) (Spielberger, 1983) is a well-validated questionnaire that measures underlying (trait) and situational (state) anxiety. Participants are asked how they generally feel and how they currently feel regarding a series of statements about being calm, tense, upset, relaxed or worried using a 4-point Likert-type scale (1=not at all, 2=somewhat, 3=moderately, 4=very much). Scores are summed and higher scores indicate higher anxiety. Extensive reliability and validity data have been obtained for this instrument. Normative data are available in the STAI manual (Spielberger, 1983).
Coping Style
Dispositional monitoring or coping style will be assessed using the Abbreviated Miller Behavioral Style Scale (Miller, 1987). Participants are asked to describe how they would typically react to two vignettes that portray potentially stressful situations—fear of pain during dental work and fear of losing a job. The questions about these scenarios are answered yes or no. Responses to these questions are indicative of a monitoring (active scanning for information) or blunting (avoiding or distracting) style of coping with threatening information. The dimension of coping that this validated scale measures has been shown to have direct relevance to the proposed outcomes of interest for our survey (Schwartz et al., 1995).
Closeness Scale
The experience of cancer in friends or relatives, the closeness of that relationship, and the survival of the family member may have a significant impact on a woman’s perceived vulnerability to cancer (Montgomery et al., 2003; Hopwood et al., 2001; Chalmers and Thomson, 1996; Fiandt et al., 1999). We have developed a new 10-item scale to assess closeness for this study. Participants are asked to identify the relative or friend with cancer to whom they felt the closest; whether they survived their cancer; if they did not, their age at death; and the participant’s age at the time of her friend’s or relative’s death. Participants will be asked about how much time was spent with the friend or relative, how much negative change they observed in that person, how often they spoke with the friend or relative about their cancer, how much they resemble the friend or relative physically or in terms of personality, how often they think about the cancer experience and how much the experience has affected the participant. For each item, response options include three levels: (1) not close or never, (2) somewhat or sometimes, and (3) very or a lot. Factor analyses will be conducted to identify important subscales for further analyses.
Ovarian Cancer Knowledge
Knowledge of factors linked to risk for ovarian cancer will be assessed with 9 questions developed for use in this study. The questions were based on the existing literature on ovarian cancer and include factors that have been linked with the development of ovarian cancer as well as factors that have no known links with cancer. Participants will be asked whether seven factors increase, decrease, or have no effect on a woman’s chances of getting ovarian cancer. These include: being hit in the abdomen; having one or more close relatives with ovarian cancer; giving birth; having breast, colorectal or endometrial cancer; getting older; having many sexual partners; and taking oral contraceptives. Participants will also be asked about the truth or falsity of the statements that women with ovarian cancer never experience symptoms and ovarian cancer causes more deaths than breast cancer. The number of correct responses will be summed to yield a total knowledge score.
Cancer Screening Behaviors
Our primary outcome of interest is ovarian cancer screening using CA125 or transvaginal ultrasound. Participants will be asked if they have ever heard of a CA125 test and if so, whether they have had a CA125 test. Women who respond positively will be asked when they had the test and the reasons for the test. This same series of questions will also be asked about having undergone transvaginal ultrasound testing. Participants will also be asked whether they intend to undergo CA125 testing or transvaginal ultrasound testing in the future. Information also will be collected on up-to-date cervical, breast and colorectal cancer screening using questions developed for the Cancer Control Module of the 2000 National Health Interview Survey (NCHS, 2006).
Ovarian Cancer Seriousness and Controllability
Participants will be asked whether they strongly agree, agree, disagree or strongly disagree with the statements, “getting ovarian cancer would be a very serious problem” and “there is a lot I can do to prevent ovarian cancer.”
Concepts, Variables, and Instruments Used in the Follow-Up Survey
Perceived risk, any additional family members diagnosed with cancer since the baseline survey, cancer worry, and screening for ovarian cancer since the baseline survey will be assessed in the follow-up survey using the same measures as in the baseline survey.
Conduct of Survey
HFHS staff will conduct a review of their administrative data to ensure that potential participants are females 30 years of age or older, and without a history of ovarian cancer. From this activity, a list of names, telephone numbers, and addresses for approximately 60,000 women will be shared with the research team at ORC Macro. An introductory letter about the research study will be sent to all women on the list (see Attachment G—Introductory Letter). From this list, ORC Macro estimates that approximately 32,000 will actually be reached. ORC Macro interviewers will screen these women to determine their interest and eligibility to participate in the baseline survey. Interviewers will review basic information about the study, ask five eligibility questions, and then let women know if they are eligible to participate. Eligible women who are interested in participating in the study will be invited to continue with the baseline survey. Before asking survey questions in the baseline survey, interviewers will obtain verbal informed consent by reading from an informed consent script embedded after the introduction. This section explains the nature and purpose of the research study, why the respondent is being asked to participate, the nature of the questions, and the expected length of time needed to complete the survey. Interviewers will ensure the respondent understands that participation is voluntary, that she can refuse to answer any questions or withdraw at any time, that identifying information will be removed from all datasets, and data will be reported at the aggregate level. Participants will be informed that they will be provided with a $15 gift card as an incentive for their participation. After the interviewer and respondent have discussed these topics, and the respondent has given her verbal consent to participate, the baseline survey will begin. At the end of the baseline survey, participants will be asked if they are willing to be contacted again in about one year to ask them a few additional questions. They will be informed that participation in the follow-up survey would take no more than 15 minutes of their time and, as an incentive, they will receive a $10 gift card. Participants will receive a thank-you letter with contact information should they want further information about the study (Attachment H—Thank-You Letter for Baseline Survey Participants), and a reminder letter for the follow-up (Attachment I—Reminder Letter for Follow-Up Survey).
Finally, interviewers will re-contact women who participated in the baseline survey and who agreed to be re-contacted approximately one year later to determine if their family history of cancer, their risk perception, or their ovarian cancer screening behavior has changed since the baseline survey was completed. Interviewers will remind women about their participation in the baseline study, review the purpose of the study and ask if they are interested in learning more about the follow-up study. Women who are interested in hearing more about the follow-up study will be given basic information about the follow-up survey and asked if they would like to continue their participation in the study by answering a few additional questions. Before asking survey questions, interviewers will obtain verbal informed consent by reading from an approved informed consent script embedded right after the call introduction section. This introduction to the follow-up survey will fully explain the nature and purpose of the follow-up study, why the respondent is being asked to participate in the follow-up study, the nature of the questions to be asked, and the expected length of time needed to complete the follow-up survey. In addition, ORC Macro interviewers will ensure the respondent understands that participation in the follow-up survey is voluntary; she can refuse to answer any question or withdraw from the follow-up survey at any time without penalty or consequence; and any identifying information will be removed from all datasets and data will be reported at the aggregate level. The potential risks and benefits of participation will be described, and the participant will be told that she will receive a $10 gift card as an incentive for participating in the follow-up study. Participants will receive a thank-you letter with contact information should they have any additional questions about the study (Attachment J—Thank-You Letter for Follow-Up Survey Participants).
Interviewers will ask survey questions using the CATI system. All survey participants’ responses will be entered directly into a data file, without additional data entry required. The system permits real-time error checking and correction. The CATI system also enables the interviewer to correct problems while respondents are still available. In addition, the CATI system requires that, except for the open-ended questions, all interviewers enter the same codes for the same answers provided by respondents to any given question. Moreover, the many range and logic checks programmed into the survey will produce a clean data set. The data collection systems include validation of submitted data, real-time summation during numerical data entry, and uploadable and downloadable data files and spreadsheets. Study data will be maintained on a UNIX computer and accessed primarily through SAS statistical analysis software. Final, de-identified datasets will be provided to CDC in a SAS and ASCII format.
Quality Assurance and Quality Control
ORC Macro staff will perform frequency checks of the data on a regular basis from the initial CATI testing phase to the operational pre-test to actual survey administration. Checks occur again every day for the first three days of data collection, and weekly thereafter. Checks of verbatim responses will allow the call center manager to determine whether additional response categories are needed for an item, or whether an item is proving difficult for respondents to answer. Any inconsistencies or problems that appear in the frequency and verbatim checks will be reported to the study team. Open-ended responses will be entered verbatim and later categorized. The ORC Macro data processing staff will compile frequency tables on the survey data for the project team to review.
B3. Methods to Maximize Response Rates and Deal With Non-response
As described in section B.1., our initial eligibility screening of 60,000 women will account for an estimated 15% incorrect telephone numbers; an estimated 15% unable to reach to determine willingness to participate (due to messages left on answering machine and not returned, busy signals); and an estimated 25% initial refusals to participate in the screening for those women who are reached—this leaves approximately 32,000 women who will be contacted and screened. We will make up to 15 attempts to reach and assess eligibility for the 32,000 women. Once screened for eligibility, we will randomly select average-risk women, and select with certainty all elevated and high-risk women to complete the survey. Once having passed the eligibility screening and random selection, we will interview approximately 2,000 women and expect a 5% failure to complete an interview leaving a baseline sample size of n=1,900 for this study. Because we will have already eliminated most refusals in the initial eligibility screening, we expect that most of the women who complete the screener will go on through the interview if selected. Based on the stable health system enrollments, coupled with updated contact information from the baseline survey, we anticipate successful re-contact and re-interview of an estimated 85% of the baseline sample at 12 months follow-up. Therefore we expect a sample size of approximately 1,600 for the follow-up sample. One advantage of a CATI system is its use in achieving high completion rates. The CATI system can suspend and resume an interview should a participant need to take a break for whatever reason, which will make completion of interviews more feasible. Additionally, a modest incentive of a $15 gift card will be offered to those completing the baseline survey and an additional $10 gift card will be offered to those women completing the follow-up. Monetary remuneration has been associated with higher response rates in survey study designs (Singer 1981; Singer et al., 1997; Whiteman et al., 2003).
Because maintaining a low refusal rate is essential to high-quality data collection, ORC Macro staff will thoroughly train its interviewers on techniques to avoid refusals and unnecessary break-offs. Interviewers and supervisors discuss situations in which it may be necessary to call back to complete an interview. The importance of the first few seconds and minutes of contact are emphasized. Interviewers are given strategies for dealing with the various responses they may encounter in attempting to conduct the interview. Common refusal situations and effective replies are reviewed with the interviewers so they are fully informed and prepared with responses that emphasize the importance of the survey, indicate for whom the study is being conducted and convey flexibility in setting appointments to complete the interview at the respondent’s convenience.
B4. Tests of Procedures or Methods to Be Undertaken
The measures were tested for clarity of wording and appropriate response categories through cognitive interviewing methods. Nine individuals, varying by levels of education, race, age, and socioeconomic status participated in cognitive interviews in order to ensure that a diverse range of respondents can complete the measures. Cognitive interviews are semi-structured interviews that allow researchers to determine: the meanings of questions and response choices for respondents; best sequencing of questions; whether response categories are appropriate; best question and response wording; and any questions where respondents have difficulty answering. Based on feedback received in the cognitive interviews, one scale was dropped completely and several other measures (created by the investigators) were revised to account for lack of clarity. In addition, the order of the scales was changed based on participant feedback.
B5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
All questionnaires, study design and statistical methods were developed and reviewed extensively by the study team composed of staff from CDC/NCCDPHP, ORC Macro and HFHS. ORC Macro will conduct the survey from their call center. Staff from CDC/NCCDPHP, ORC Macro and HFHS will participate in the analysis of data and development of scientific manuscripts. Review and comments were also sought from experts in the field of obstetrics/gynecology and genetics (Drs. Jackson and Scheuner). These consultants will participate in data analysis and manuscript development.
Sharon Alford, MPH, PhD
Henry Ford Health System
Josephine Ford Cancer Center
1 Ford Place, 5C
Detroit, MI 48202
Phone: (313) 874-9413
Danielle Beauchesne, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Steven Coughlin, PhD
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway, NE Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-4776
Nicola Dawkins, PhD, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
James Dayton, MBA
ORC Macro
126
College Street
Burlington, VT 05401
Phone: (802) 863-9600
Nikki Hawkins, PhD
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-4229
Ronaldo Iachan, PhD, MS
ORC Macro
11785 Beltsville Drive
Calverton, MD 20705
Phone: (301) 572-0200
Rebecca Jackson, MD
San Francisco General Hospital
1001 Potrero Avenue, #6D
San Francisco, CA 94110
Phone: (415) 206-3050
Steven Leadbetter, MS
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway, NE Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-4143
Lucy Peipins, PhD
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-3034
Christine Phelan, BA
ORC Macro
126
College Street
Burlington, VT 05401
Phone: (802) 863-9600
Maren Scheuner, MD, MPH
555 36th Street
Manhattan Beach, CA 90266
Phone: (310) 545-2564
Lawrence Scholl, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Gretchen Simmons, MPH, CHES
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Robert Stephens, PhD, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Paula Yoon, ScD
Office of Genomics and Disease Prevention
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mail Stop K89
Atlanta, GA 30341-3717
Phone: (770) 488-8436
Ye Xu, MA, MS
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
Susan Zaro, MPH
ORC Macro
3 Corporate Square, Suite 370
Atlanta, GA 30329
Phone: (404) 321-3211
The Technical Monitor for this project is:
Lucy Peipins, PhD
Epidemiologist
Division of Cancer Prevention and Control
Centers for Disease Control and Prevention
4770 Buford Highway NE, Mailstop K55
Atlanta, GA 30311
Phone: (770) 488-3034
Fax: (770) 488-4639
E-mail: l[email protected]
REFERENCES
Absetz P, Aro AR, Sutton SR. Factors associated with breast cancer risk perception and psychological distress in a representative sample of middle-aged Finnish women. Anxiety, Stress and Coping, 2002;15(1):61-73.
Andersen MR, Nelson J, Peacock S, Giedzinska A, Drescher C, Bowen D, Urban N. Worry about ovarian cancer risk and use of screening by high-risk women: how you recruit affects what you find. Am J Med Genetics 2004;129A:130-135.
Andersen MR, Peacock S, Nelson J, Wilson S, McIntosh M, Drescher, Urban N. Worry about ovarian cancer risk and use of ovarian cancer screening by women at risk for ovarian cancer. Gynecologic Oncology, 2002;85:3-8.
Bentler PM, Chou CP. Practical issues in structural modeling. In J.S. Long (editor), Common Problems/Proper solutions: Avoiding Error in Survey Research (pp. 161-192). Newbury Park, CA: Sage, 1988.
BLS (Bureau of Labor Statistics). Metropolitan Area Employment and Unemployment: September 2005. U.S. Department of Labor, Washington, DC, 2005.
Braithwaite D, Emery J, Walter F, Prevost AT, Sutton S. Psychological impact of genetic counseling for familial cancer: a systematic review and meta-analysis. JNCI 2004;96(2):122-133.
Chalmers K, Thomson K, Coming to terms with the risk of breast cancer: perceptions of women with primary relatives with breast cancer. Qualitative Health Research 1996;6(2):256-282.
Consedine N, Magai C, Krivoshekova YS, Ryzewicz L, Neugut AI. Fear, anxiety, worry and breast cancer screening behavior: a critical review. Cancer Epidemiology, Biomarkers and Prevention 2004;13(4):501-510.
Couper MP, Baker R, Bethlehem J, Clark CZF, Martin J, Nicolls II, WL, O’Reilly JM. Computer Assisted Survey Information Collection. New York: Wiley, 1998.
Drescher C, Holt SK, Andersen MR, Anderson G, Urban N. Reported ovarian cancer screening among a population-based sample in Washington state. Obstetrics and Gynecology 2000;96:70-74.
Drescher CW, Nelson J, Peacock S, Andersen MR, McIntosh MW, Urban N. Compliance of average- and intermediate-risk women to semiannual ovarian cancer screening. Cancer Epidemiology, Biomarkers and Prevention 2004;13(4):600-606.
Erblich J, Bovbjerg DH, Norman C, Validmarsdottir HB, Montgomery GH. It won’t happen to me: lower perception of heart disease risk among women with family histories of breast cancer. Prev Med 2000;31:714-721.
Fiandt K, Pullen CH, Walker SN. Actual and perceived risk for chronic illness in rural older women. Clinical Excellence for Nurse Practitioners, 1999;3(2):105-115.
Foster C, Watson M, Moynihan C, Ardern-Jones A, Eeles R. Genetic testing for breast and ovarian cancer predisposition: cancer burden and responsibility. Journal of Health Psychology 2002;7(4):469-484.
Goodman MT, Howe HL, Tung KH, Hotes J, Miller BA, Coughlin SS, Chen VW. Incidence of ovarian cancer by race and ethnicity in the United States, 1992-1997. Cancer 2003;97(10):2676-2677.
Hay JL, Buckley TR. Ostroff JS. The role of cancer worry in cancer screening: a theoretical and empirical review of the literature. Psycho-Oncology 2005, 14(7):517-534.
Hopwood P. Breast cancer risk perception: what do we know and understand? Breast Cancer Res 2000;2:387-391.
Hopwood P, Howell A, Lalloo F, Evans G. Do women understand the odds? Risk perceptions and recall of risk information in women with a family history of breast cancer. Community Genetic 2003;6:214-223.
Hopwood P, Shenton A, Lalloo F, Evans DGR, Howell A. Risk perception and cancer worry: an exploratory study of the impact of genetic risk counseling in women with a family history of breast cancer. J Med Genet 2001;38:139-142.
Isaacs C, Peshkin BN, Schwartz M, DeMarco TA, Main D, Lerman C. Breast and ovarian cancer screening practices in healthy women with a strong family history of breast or ovarian cancer. Breast Cancer Research and Treatment 2002;71:103-112.
Kline RB. Principles and Practice Structural Equation Modeling. New York: The Guilford Press, 1998.
Lerman C, Lustbader E, Rimer B, Daly M, Miller SM, Sands C, Balshem A. Effects of an individualized breast cancer risk counseling: a randomized trial. JNCI, 1995;87:286-292.
Lerman C and Schwartz M. 1993. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat 28:145-155.
Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. 1991. Psychological side effects of breast cancer screening. Health Psychol 10:259-267.
Leventhal H, Kelly K, Leventhal EA. Population risk, actual risk, perceived risk, and cancer control: a discussion. JNCI 1999;25:81-85.
Lloyd S. Watson M, Waites B, Meyer l, Eeles R, Ebbs S, Tylee A. Familial breast cancer: a controlled study of risk perception, psychological morbidity and health beliefs in women attending for genetic counseling. Br J Cancer 1996, 74;482-487.
McAllister M. Personal theories of inheritance, coping strategies, risk perception and engagement in hereditary non-polyposis colon cancer families offered genetic counseling. Clinical Genetics, 2003;64(3):179-189.
Miller SM. 1987. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. Journal of Personality and Social Psychology, 53:345-353.
Miller SM, Bowen DJ, Campbell MC, Diefenbach MA, Gritz ER, Jacobsen PB, Stefanek M, Fang CY, Lazovich D, Sherman KA, Wang C. Current research promises and challenges in behavioral oncology: report from the American Society of Preventive Oncology Annual Meeting, 2002. Cancer Epidemiology, Biomarkers and Prevention 2004;13:171-180.
Miller SM, Fang CY, Manne SL, Engstrom PF, Daly MB. Decision making about prophylactic oophorectomy among at-risk women: psychological influences and implications. Gynecol Oncol 1999;75;406-412.
Montgomery GH, Erblich J, DiLorenzo T, Bovbjerg DH. Family and friends with disease: their impact on perceived health. Preventive Medicine, 2003;37:242-249.
Murff HJ, Spigel DR, Syngal S. 2004. Does this patient have a family history of cancer: an evidence-based analysis of the accuracy of family cancer history. JAMA, 292(12):1480-1489.
NCHS. National Center for Health Statistics. National Health Interview Survey, NHIS 2000, Version 00.3, Cancer Module. Accessed on April 12, 2006 from http://www.cdc.gov/nchs/nhis.htm.
NIH Consensus Development Panel on Ovarian Cancer. Ovarian cancer: screening, treatment and follow-up. JAMA, 1995;273(6):491-497.
Rees G, Fry A, Cull A. A family history of breast cancer: women’s experiences from a theoretical perspective. Social Science and Medicine 2001;52:1433-1440.
Ries LAG, Eisner MP, Kosary CL. Hankey BF, Miller BA, Clegg LX, Edwards BD (eds.). SEER Cancer Statistics Review, 1973-1997, National Cancer Institute. NIH Pub. No. 00-2789. Bethesda, MD, 2000.
Robinson GE, Rosen BP, Bradley L, Rockert WG, Carr ML, Cole DC, Murphy KJ. Psychological impact of screening for familial ovarian cancer: reactions to initial assessment. Gynecol Oncol, 1997;65:197-205.
Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Annals of Internal Medicine, 1997;127(11):966-972.
Schwartz MD, Lerman C, Miller SM, Daly M, Masny A. Coping disposition, perceived risk, and psychological distress among women at increased risk of ovarian cancer. Health Psychology 1995;14(3):232-235.
Singer, E. 1981: Reference Groups and Social Evaluation. In Social Psychology (pp. 66-93) ed. Morris Rosenberg and Ralph H. Turner. New York: Basic Books.
Singer, E. Gebler, N., Van Hoewyk, J., and Brown, J., 1997: Does $10 Equal $10? The Effect of Framing on the Impact of Incentives. Paper presented at the meeting of the American Association for Public Opinion Research, Norfolk, VA.
Slovic P, Finucane ML, Peters E, MacGregor DG. Risk as analysis and risk as feelings: some thoughts about affect, reason, risk, and rationality. Risk Analysis 2004;24(2):311-322.
Spielberger CD 1983. State-Trait Anxiety Inventory for Adults. Consulting Psychologists’ Press: Palo Alto, CA.
US Bureau of the Census. Census 2000 Summary File 3 (SF 3) - Sample Data, QT-P-17 Ability to Speak English: 2000. Accessed http://factfinder.census.gov, on April 11, 2006.
US Preventive Services Task Force (USPSTF). Genetic risk susceptibility and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Annals of Internal Medicine 2005;143:355-61.
USCS. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2002 Incidence and Mortality. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, 2005.
Vernon S. Risk perception and risk communication for cancer screening behaviors: a review. JNCI 1999;25:101-119.
Wardle J. Women at risk of ovarian cancer. JNCI 1995;17:81-85.
Watson M, Lloyd S, Davidson J, Meyer L, Eeles R, Ebbs S, Murday V. The impact of genetic counseling on risk perception and mental health in women with a family history of breast cancer. Br J Cancer 1999;79(5/6):868-874.
Whiteman MK, Langenberg P, Kjerulff K, McCarter R, Flaws JA. A randomized trial of incentives to improve response rates to a mailed women’s health questionnaire. J of Womens Health 2003,12(8):821-828.
Woloshin S, Schwartz LM, Black WC, Welch HG. Women’s perceptions of breast cancer risk: how you ask matters. Med Decis Making 1999;19:221-229.
Yoon PW, Scheuner MT, Khoury MJ. Research priorities for evaluating family history in the prevention of common chronic diseases. Am J Prev Med 2003;24(2):128-135.
Zakowski SG, Valdimarsdottir HB, Bovbjerg DH, Borgen P, Holland J, Kash K, Miller D, Mitnick J, Osborne M, Van Zee K. Predictors of intrusive thoughts and avoidance in women with family histories of breast cancer. Annals of Behavioral Medicine 1997;19(4):362-369.
| File Type | application/msword |
| Author | sxw2 |
| Last Modified By | arp5 |
| File Modified | 2007-04-09 |
| File Created | 2007-04-03 |
© 2026 OMB.report | Privacy Policy