Attachment B - Implementing the Pesticide Registration Improvement Act ? Fiscal Year 2005
Attachment B - PRIA Implementation Report-FY05.doc
Pesticide Registration Fee Waivers
Attachment B - Implementing the Pesticide Registration Improvement Act ? Fiscal Year 2005
OMB: 2070-0167
Implementing the Pesticide Registration Improvement Act -- Fiscal Year 2005
Second annual report. Report release date: March 1, 2006.
Under section 33(k) of PRIA, EPA is required to publish an annual report describing actions taken under this section during the past fiscal year. The report must include several elements, including a review of the progress made in carrying out the Agency’s obligations under the Act, a description of the staffing and resources associated with the review of and decision-making on applications, and a review of its progress in meeting the reregistration and tolerance reassessment timeline requirements. This second annual report covers Fiscal Year 2005 - October 1, 2004, through September 30, 2005.
Early Implementation Efforts and FY 2005 Enhancements
The first annual report released in March 2005, describes steps the Agency undertook to implement PRIA during its first nine months. These included front end processing and screening, waivers, funds management, and communications. In Fiscal Year 2005, the Agency’s Office of Pesticide Programs (OPP) further refined its procedures and processes in these areas as described below.
Front-End Processing and Screening Procedures
To facilitate the implementation of PRIA, the Agency established front-end screening procedures for new pesticide applications in FY 2004. An intra-agency workgroup interpreted the 90 PRIA registration categories to help both applicants and the Agency consistently place each application in the appropriate PRIA category. These PRIA registration categories reflect the types of applications that the Agency may receive and for which Congress has established a fee and a time frame. The time frame, or decision review time, is the amount of time the Agency is expected to take to review the application and reach a regulatory decision. The Agency intends to update these interpretations in FY 2006 based on its experience in FY05.
Teams of EPA experts from the three registering divisions (conventional chemical pesticides, biopesticides, and antimicrobial) pesticides screen all incoming applications to determine whether they are subject to PRIA, and to assign the application to a PRIA category (if appropriate). By adding a chemist and toxicologist to the expert team for conventional pesticides in 2005, the Agency improved the efficiency of its front-end processing screens. The experts do a cursory screen of the submission for completeness, thus saving both the registrant and the Agency valuable time. The Agency has also conducted an analysis of the frequency of incomplete conventional pesticide applications submitted under PRIA and is working on processes that will result in improved applications for all types of pesticides. Typically within 48-72 hours of receipt of an application, the registrant is sent an invoice requesting payment of the appropriate PRIA registration service fee.
The Agency’s internal tracking system, known as the Office of Pesticide Programs Information Network (OPPIN), was modified to track extensions in due dates and withdrawals resulting from an unpaid PRIA fee. Reports enable Agency managers to monitor the workload in their units. Reports enable Agency managers to monitor the workload in their units. The Agency also enhanced OPPIN to notify registrants electronically when the Agency has received their payments.
The Agency enhanced its existing data management contract for the initial data screen in FY 2004 to reduce study processing time to 10 days, thus ensuring that complete data packages are ready to enter the review process at the beginning of the decision review period if the applicant has correctly formatted the data submission. During FY 2005, the average study processing time for the front end screen was 4.6 days.
Funds Management and Utilization
Section 33(c) of PRIA established the Pesticide Registration Fund. Congress established this fund in the Treasury of the United States to carry out the provisions of PRIA. All registration service fees received by EPA are deposited in this fund, and expenditures from the fund can cover the costs associated with the review and decision-making for applications for which registration service fees have been paid. In FY 2004, the Agency worked with the Mellon Bank to establish the fund and create billing procedures and to coordinate communications on fee receipts between the bank and the Agency. Communications were particularly critical as fee receipt triggers the start of the PRIA review period. The Agency was informed of the receipt of a payment within an average of 7.2 days of receipt by the Mellon Bank, and since May 17, 2005, the Agency automatically sends an acknowledgment of payment to those applicants with an e-mail address on file.
In July 2005, EPA began notifying applicants when a payment is 45 days overdue for all PRIA fee categories except Fast Track applications (because of the short time frames for these actions). The notification provides the applicant 75 days to forward payment before the application is administratively withdrawn. The Agency sent 55 such letters, resulting in 24 withdrawn applications, 26 payments or fee waivers, and 5 that are currently being resolved.
Waivers and Fee Reductions
Section 33(b)(7) of PRIA authorizes the Agency to reduce or waive the registration service fee under certain specified situations. The Agency in FY 2004 developed and posted on the internet guidance on how to apply for waivers of the registration service fee. The Agency reviewed 429 applications and reduced the average number of days to grant a fee waiver from 48 to 24 days during FY 2005. The Agency also established formulas for reducing certain registration service fees based on work completed by the Agency prior to the effective date of PRIA. Section 33(b)(8)(C) authorizes EPA to issue discretionary refunds, including instances where the Agency had completed portions of the review of an application before the PRIA effective date. For fees required for pending new active ingredients and for applications where the registrant has offered to pay the registration service fee voluntarily, the Agency applied this refund provision as a credit toward the application registration service fee. During FY 2005, the Agency reduced registration service fees by $1.6 million based on work completed by the Agency on pending applications prior to the PRIA effective date. The amount in FY 2004 was $3.7 million.
Communications and Outreach
As required by the statute, on March 17, 2004 , the Agency published the schedule of covered applications and registration service fees. Under Section 33(b)(6), these fees increased by 5 percent on applications received on or after October 1, 2005 . The new fee schedule was announced in the Federal Register of June 2, 2005. (Pesticides; Revised Fee Schedule for registration Applications (PDF, 9 p., 85 KB, About PDF)). In September 2005, the Agency sent an electronic reminder of this fee increase to over 4,000 individuals and organizations as an “OPP Update”.
In 2005, the Agency conducted meetings and a number of other outreach efforts on PRIA implementation. Agency staff discussed PRIA implementation during the Chemical Producers and Distributors Association Registration Workshop, with State and EPA Regional staff at the Pesticide Regulatory Education Program, and with the Armed Forces Pest Management Board. During the annual meeting of the Consumer Specialty Products Association, EPA and the Natural Resources Defense Council discussed PRIA implementation, compliance with Pesticide Registration Notice 86-5 (standards for registration application submissions), and fee waivers. EPA provided updates on the status of PRIA actions received and summary statistics during meetings of the Agency’s Federal Advisory Committee, the Pesticide Program Dialogue Committee (PPDC). EPA also has quarterly meetings with the Biopesticide Industry Alliance to discuss PRIA and other common issues and with the United State Department of Agriculture IR-4 program. The USDA IR-4 program is working with a number of small companies with new biopesticide active ingredients. During the 2005 Annual Antimicrobial Workshop, EPA provided additional clarification to the antimicrobial industry on which actions were or were not covered by PRIA.
In FY 2004, the Agency established a website dedicated to PRIA implementation. Through this website, the public can submit questions regarding PRIA implementation. Questions are typically answered within 24 hours. Questions are also addressed by registration Ombudsmen. The Ombudsman also help applicants with issues related to the registration process and completing application forms.
During Fiscal Year 2005, the Agency received $10.6 million in new registration service fees (and carried a balance of $9.7 million forward from FY 2004). From this total of $20.3 million, the Agency spent approximately $11.2 million, carrying the remaining balance of $9.1 million forward to FY 2006.
Agency's FY 2004 and FY 2005 Expenditures from the Pesticide Registration Fund |
For |
FY 2004 Expenditures (figures are in thousands) |
FY 2005 Expenditures (000) |
Payroll |
$2,535.3 |
$7,898.2 |
Contracts |
$1,591.3 |
$2,228.8 |
Worker Protection |
$430 |
$750.1 |
Other Expenses |
$455.8 |
$274.3 |
Total |
$5,012.5 |
$11,151.4 |
PRIA became effective March 23, 2004 , and consequently FY 2004 was only a half year while FY 2005 was the first full fiscal year under PRIA. As was the case in FY 2004, the majority of expenditures from the Pesticide Registration Fund in FY 2005 were spent on payroll costs and to expand data review output through contracts. In order to meet PRIA registration review time frames, payroll expenditures increased more than threefold in FY 2005 to $7.9 million (compared with $2.5 million in FY 2004). A major factor in this increase was that PRIA was in effect for only six months of FY 2004. Expenditures on contracts also increased, but less dramatically than payroll (up to approximately $2.2 million in FY 2005 compared with $1.6 million in FY 2004). Included in the payroll and contract expenses are $472.9K ($430.0K in contracts and $42.9K in payroll) to accelerate the review of new inert ingredients. The amount spent on worker protection was $750,106 ($728.8K for contracts/grants and $21.3K on printing). T he Agency continued to invest in upgrading its information management system (the Office of Pesticide Programs Information Network) to track compliance with the PRIA review time frames and to meet reporting requirements. Other funds went primarily to pay for Federal Register printing costs associated with PRIA registrations.
Waivers of Registration Service Fees
In response to requests for fee waivers and fee reductions, as authorized by PRIA, the Agency waived $11.1 million in registration service fees. The Agency reviewed 429 waiver requests, granting 342 and denying 64. The time for the Agency to reach a decision to grant a waiver declined to an average of 24 days at the end of FY 2005, while the time to deny a waiver request was within 50 to 54 days. The time required to deny a waiver reflects the time the Agency took to obtain missing information in an attempt to be able to grant the fee waiver. The table below summarizes the outcome of the 100 percent and 50 percent waiver requests received in FY 2005. In addition to these waivers, the Agency processed a number of fee waivers requested by the USDA IR-4 program.
Small Business Waiver Requests -- FY 2005 |
Waiver |
Submitted |
Granted |
Denied |
Withdrawn |
100 % |
248 |
197 |
45 |
16 |
50 % |
124 |
104 |
14 |
6 |
Total |
372 |
301 |
59 |
22 |
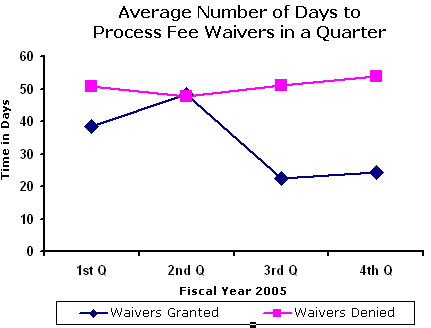
The average number of days EPA took to grant or deny a fee
waiver in FY 2005 is summarized in the table and illustrated in the
graph below. In general, processing times have decreased with a
slight increase in the second quarter of FY 2005 when applicants were
required to submit complete and updated financial information.
Average Number of Days to Process Fee Waivers in a Quarter, 2005 |
Quarter |
To Grant |
To Deny |
1st Q |
38 |
50 |
2nd Q |
48 |
47 |
3rd Q |
22 |
51 |
4th Q |
24 |
54 |
Worker Protection
Section 33(c)(3)(b), EPA is authorized to use 1/17 of the amount of the Fund (but not more than $1 million and not less than $750,000 for any fiscal year) to enhance current scientific and regulatory activities related to worker protection. The Agency worked closely with worker safety stakeholders through the Agency’s Federal Advisory Committee the Pesticide Program Dialogue Committee, to determine which activities to enhance with PRIA funds. Based on the advice of the PPDC, the Agency decided to develop enhancements within focus areas characterized as: Prevention - Safety Training; Response - Poisoning Recognition; Sound Decision Data; and, Inform - Risk Management. Within these areas, PRIA funds were used for the following activities in FY 2005:
National Agricultural Workers Survey - The Department of Labor annually surveys farm workers to collect demographic information. This is the only source of national information on farm worker employment, health and living conditions, and demographic data. PRIA funds have been provided for special focus reports which will be used to enhance program measures, risk management, risk mitigation, communication, outreach, and training.
National Institute of Occupational Safety and Health Sentinel Event Notification System for Occupational Risk (SENSOR) Program - PRIA Funds were used to increase the number of states in the SENSOR program and to expand occupational illness and injury surveillance capacity within state health departments in areas of the country with sizable agricultural worker populations.
The Association of Farmworker Opportunity Programs / AmeriCorps Pesticide Worker Safety Training Program - PRIA Funds were used to increase the number of trainers carrying out education programs to reduce the risks of pesticide use. The program is established at 22 sites in 13 states working with AmeriCorps members to educate farmworkers in rural areas about worker protection measures and safe pesticide practices.
Pesticides and National Strategies for Health Care Providers Initiative - PRIA Funds were used to enhance the national initiative to improve the training of health care providers in the recognition, diagnosis, treatment, and prevention of pesticide poisoning among those who work with pesticides. The University of Washington Pacific-Northwest Agricultural Safety and Health Center will work with decision-makers and faculty at academic institutions and professional associations or organizations to create institutional change in the educational settings for health care providers.
The Migrant Clinicians Network - will work directly with health care providers to change the practice of primary care so that pesticide-related health conditions are recognized, effectively managed, and prevented in practice settings.
The National Pesticide Information Center - PRIA Funds were used to ensure the availability of Spanish speaking staff during the times when Spanish speaking pesticide workers were likely to call for information or reference to clinical services.
Progress in Meeting Decision Times
Number of PRIA Actions Completed in FY 2005
The Agency completed 1098 decisions subject to PRIA during the fiscal year, a substantial increase over FY 2004’s 208. EPA completed 99.8 percent of these decisions met their PRIA due date. The chart below summarizes the number of decisions completed by PRIA category. An application can have more than one decision. The number of decisions depends on the number of product registrations in an application. For instance, in FY 2005, one new antimicrobial active ingredient (A2) was registered that required two decisions. Information on the number of active ingredients and uses registered during a year can be found in the Office of Pesticide Program’s Annual Reports. Generally each application categorized as a Fast Track, Non-Fast Track New Product, and Non-Fast Track Amendment is a single decision.
The average decision time for each PRIA category with completed decisions is shown in days - the number of days it took the Agency to complete a decision once payment was made or a fee waiver was granted. The time frames mandated under PRIA decreased for some categories of decisions in FY 2005 from FY 2004. A decision’s time frame is based on the fiscal year in which the application or decision was received. Even though a fee was paid or a fee waiver was granted in FY 2005, an action received in FY 2004 had a FY 2004 PRIA timeframe with the beginning of the timeframe in FY 2005. The average decision time in the table below does not take into consideration this change in time frame and is an average over the whole Fiscal Year.
While many new active ingredient applications and new use applications appear to have been completed in substantially less time than the decision time frame provided under PRIA, many of these actions were submitted prior to March 23, 2004 , PRIA’s effective date and benefited from work completed before the effective date. Decision times for these actions, such as R1 to R29, are expected to be greater in future years as more recently received decisions are completed.
Among
the FY 2005 completions, the PRIA due dates for 69 (6 percent) were
extended or negotiated by agreement between the Agency and the
applicant. This generally occurred because of missing or deficient
data or information.
Key
to the Table
R – Conventional Pesticides |
EUP – Experimental Use Permit |
A – Antimicrobial Pesticides |
SCLP - Straight Chain Lepidopteran Pheromones |
B – Biopesticides |
PIP - Plant-Incorporated Protectants |
|
SAP – FIFRA Scientific Advisory Panel |
Progress in Meeting Decision Times |
PRIA Category |
Description of Category
|
Number of PRIA “Decision” Completed in FY2004 |
Number of PRIA “Decisions” Completed in FY2005 |
FY05 Average Decision Time in Days |
R1 |
New Active Ingredient, Food Use |
2 |
16 |
365 |
R2 |
New Active Ingredient, Food Use, Reduced Risk |
4 |
8 |
180 |
R7 |
New Active Ingredient, Non-food use, Outdoor, Reduced Risk |
1 |
0 |
|
R9 |
New Active Ingredient, Non-food use, Outdoor, Experimental Use Permit (EUP) submitted before application for registration |
|
1 |
354 |
R14 |
New Use, Additional food use, Indoor Food/Food handling |
|
2 |
360 |
R15 |
New Use, First Food Use |
|
1 |
410 |
R17 |
New Use, Each Additional New Food Use |
1 |
5 |
262 |
R18 |
New Use, Each Additional New Food Use, Reduced Risk |
1 |
11 |
190 |
R19 |
New Use, Additional New Food Use, Bundled, 6 or more |
|
1 |
45 |
R20 |
New Use, Additional New Food Use, Bundled, 6 or more, Reduced Risk |
1 |
5 |
57 |
R23 |
New use, Non-food, Outdoor |
|
9 |
281 |
R24 |
New use, Non-food, Outdoor, Reduced Risk |
2 |
2 |
115 |
R25 |
New use, Non-food, Outdoor with Experimental Use Permit (EUP) (no credit toward new use registration) |
|
2 |
148 |
R26 |
New Use, Non-food, Indoor |
1 |
6 |
200 |
R30 |
New Product, Me-Too, Fast Track |
72 |
222 |
70 |
R31 |
New Product, Non-Fast Track (includes review of product chemistry, acute toxicity, public health pest, efficacy) |
62 |
267 |
232 |
R32 |
New Product, Non Fast Track, new physical form (excludes selective citations) |
1 |
5 |
346 |
R33 |
New manufacturing-use product, Old Active Ingredient, Selective Citation |
3 |
10 |
216 |
R34 |
Non-Fast Track (includes changes to precautionary label statements, source changes to an unregistered source) |
29 |
188 |
130 |
R35 |
Non-Fast track (changes to REI, PPE, PHI, rate and number of applications, add aerial application, modify GW/SW advisory statement) |
4 |
17 |
130 |
R37 |
Cancer reassessment, applicant initiated |
|
1 |
508 |
A42 |
New Active Ingredient Non-food use, Indoor, FIFRA sec. 2(mm) uses |
|
3 |
296 |
A50 |
New use, Non-food, Indoor FIFRA sec. 2(mm) uses |
|
2 |
216 |
A52 |
Experimental Use Permit |
|
1 |
36 |
A53 |
New Product, Me-too, Fast Track |
7 |
79 |
74 |
A54 |
New Product, Non-Fast Track, FIFRA sec. 2 (mm) uses |
2 |
55 |
147 |
A55 |
New Product, Non-Fast Track, Other Uses |
|
5 |
190 |
A57 |
Amendments, Non-Fast Track |
5 |
64 |
121 |
B59 |
New Active Ingredient, Food Use, Microbial/Biochemical, with tolerance |
3 |
6 |
201 |
B60 |
New Active Ingredient, Non-food use, Microbial/Biochemical |
1 |
6 |
293 |
B63 |
New Use, First Food Use, Microbial/Biochemical, with tolerance exemption |
|
2 |
96 |
B65 |
New Use, Non-Food, Microbial/Biochemical |
|
1 |
143 |
B66 |
New Product, Me-Too, Fast Track, Microbial/biochemical |
3 |
4 |
74 |
B67 |
New Product, Non-Fast Track, Microbial/Biochemical |
1 |
40 |
196 |
B68 |
Amendment, Non-Fast Track, Microbial/Biochemical |
1 |
14 |
127 |
B69 |
Straight Chain Lepidopteran Pheromones (SCLP), New Active Ingredient, Food Use or Non-Food Use |
1 |
1 |
179 |
B70 |
SCLP, Experimental Use Permit, (New Active Ingredient, New Use) |
|
3 |
6 |
B71 |
SCLP, New Product, Me-Too, Fast Track |
|
8 |
85 |
B72 |
SCLP, New Product Non-Fast Track |
|
3 |
189 |
B73 |
SCLP, Amendment, Non-Fast Track |
|
11 |
144 |
B75 |
Plant-Incorporated Protectants (PIP), EUP, with Temporary Tolerance or Exemption, No Scientific Advisory Panel (SAP) meeting |
|
2 |
265 |
B80 |
PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, No SAP |
|
1 |
360 |
B81 |
PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, SAP |
|
3 |
330 |
B86 |
PIP, Experimental Use Permit, Amendment, Food Use |
|
3 |
111 |
B88 |
PIP, New Product |
|
2 |
364 |
TOTAL |
|
208 |
1098 |
|
Note:
Appendix A contains a list
of all applications subject to PRIA reviewed during FY2005
(Excel, 192 KB) and includes the decision times for each application.
(Microsoft
Excel Viewer
![]() is
needed to view this file.)
is
needed to view this file.)
Number of PRIA Applications Pending at the End of FY 2005.
The following table summarizes the number of pending registration applications (counted as decisions) in each of the PRIA categories. As of September 30, 2005 , 1178 applications subject to PRIA were pending in the Agency’s registration queue. The number pending at the end of FY 2004 are shown for comparison.
Key to the Table
R – Conventional Pesticides |
EUP – Experimental Use Permit |
A – Antimicrobial Pesticides |
SCLP - Straight Chain Lepidopteran Pheromones |
B – Biopesticides |
PIP - Plant-Incorporated Protectants |
|
SAP – FIFRA Scientific Advisory Panel |
PRIA Category |
Description of Category |
Number of PRIA Decisions Pending at the End of FY2004 |
Number of PRIA Decisions Pending at the End of FY2005 |
R1 |
New Active Ingredient, Food Use |
31 |
27 |
R2 |
New Active Ingredient, Food Use, Reduced Risk |
13 |
10 |
R5 |
New Active Ingredient, Food Use, submitted after an Experimental Use Permit (EUP) |
1 |
0 |
R6 |
New Active Ingredient, Non-food use, outdoor |
7 |
10 |
R7 |
New Active Ingredient, Non-food use, outdoor, reduced risk |
0 |
1 |
R9 |
New Active Ingredient, Non-food use, outdoor, Experimental Use permit submitted before application for registration |
1 |
0 |
R11 |
New Active Ingredient, Non-food use, indoor |
2 |
4 |
R14 |
New Use, Additional food use, Indoor Food/Food handling |
3 |
3 |
R15 |
New Use, First Food Use |
2 |
2 |
R17 |
New Use, Each Additional New Food Use |
81 |
214 |
R18 |
New Use, Each Additional New Food Use, Reduced Risk |
51 |
39 |
R19 |
New Use, Additional New Food Use, Bundled, 6 or more |
18 |
64 |
R20 |
New Use, Additional New Food Use, Bundled, 6 or more, Reduced Risk |
17 |
6 |
R23 |
New use, Non-food, Outdoor |
30 |
44 |
R24 |
New use, Non-food, Outdoor, Reduced Risk |
2 |
1 |
R25 |
New use, Non-food, Outdoor with Experimental Use Permit (no credit toward new use registration) |
0 |
3 |
R26 |
New Use, Non-food, Indoor |
9 |
17 |
R28 |
Import tolerance, New Active Ingredient or first food use |
9 |
12 |
R29 |
Import tolerance, Additional new food use |
6 |
9 |
R30 |
New Product, Me-Too, Fast Track |
81 |
45 |
R31 |
New Product, Non-Fast Track (includes review of product chemistry, acute toxicity, public health pest, efficacy) |
243 |
221 |
R32 |
New Product, Non Fast Track, new physical form (excludes selective citations) |
13 |
17 |
R33 |
New manufacturing-use product, Old Active Ingredient, Selective Citation |
32 |
25 |
R34 |
Non-fast Track (includes changes to precautionary label statements, source changes to an unregistered source) |
110 |
57 |
R35 |
Non-fast track (changes to REI, PPE, PHI, rate and number of applications, add aerial application, modify GW/SW advisory statement) |
49 |
85 |
R36 |
Non-fast track, isomers |
2 |
2 |
R37 |
Cancer reassessment, applicant initiated |
4 |
6 |
A38 |
New Active Ingredient, Food use, with exemption |
1 |
1 |
A41 |
New Active Ingredient, Non-food use, Outdoor, Other uses |
12 |
12 |
A42 |
New Active Ingredient, Non-food use, Indoor, FIFRA sec. 2(mm) uses |
15 |
14 |
A46 |
New Food Use, with exemption |
0 |
6 |
A47 |
New Food use, with tolerance |
0 |
1 |
A48 |
New use, Non-food, Outdoor FIFRA sec. 2(mm) uses |
2 |
1 |
A49 |
New use, Non-Food, Outdoor, Other uses |
5 |
0 |
A50 |
New use, Non-Food, Indoor FIFRA sec. 2(mm) uses |
3 |
5 |
A51 |
New use, Non-Food, Indoor, Other uses |
1 |
3 |
A52 |
Experimental Use Permit |
1 |
1 |
A53 |
New Product, Me-too, Fast Track |
23 |
24 |
A54 |
New Product, Non-Fast Track, /FIFRA sec. 2 (mm) uses |
32 |
28 |
A55 |
New Product, Non-Fast Track, Other Uses |
5 |
10 |
A56 |
New Manufacturing use product, old active ingredient, selective citation |
4 |
7 |
A57 |
Amendments, Non-Fast Track |
36 |
42 |
B59 |
New Active Ingredient, Food Use, Microbial/Biochemical, with tolerance |
15 |
17 |
B60 |
New Active Ingredient, Non-food use, Microbial/Biochemical |
20 |
11 |
B61 |
Experimental Use Permit, Food Use with temporary tolerance exemption, Microbial/Biochemical |
0 |
2 |
B63 |
New Use, First Food Use, Microbial/Biochemical, with exemption |
5 |
10 |
B65 |
New Use, Non-Food, Microbial/Biochemical |
1 |
0 |
B66 |
New Product, Me-Too, Fast Track, Microbial/biochemical |
1 |
0 |
B67 |
New Product, Non-Fast Track, Microbial/Biochemical |
48 |
30 |
B68 |
Amendment, Non-Fast Track, Microbial/Biochemical |
8 |
8 |
B69 |
Straight Chain Lepidopteran Pheromones (SCLP), New Active Ingredient, Food Use or non-Food Use |
1 |
0 |
B70 |
SCLP, Experimental Use Permit, New Active Ingredient, New Use |
1 |
0 |
B71 |
SCLP, New Product, Me-Too, Fast Track |
4 |
0 |
B72 |
SCLP, New Product Non-Fast Track |
3 |
3 |
B73 |
SCLP, Amendment, Non-Fast Track |
1 |
0 |
B75 |
Plant-Incorporated Protectants (PIP), EUP, with Temporary Tolerance or Exemption, No Scientific Advisory Panel (SAP) |
2 |
2 |
B77 |
PIP, Experimental Use Permit, New Active Ingredient, Set. Temporary Tolerance or Exemption, SAP |
0 |
1 |
B80 |
PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, No SAP |
2 |
2 |
B81 |
PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, SAP |
4 |
2 |
B84 |
PIP, Register New Active Ingredient, Set Tolerance/Exemption, SAP |
1 |
1 |
B86 |
PIP, Experimental Use Permit, Food Use , Amendment, |
2 |
2 |
B88 |
PIP, New Product |
5 |
5 |
B90 |
PIP, Amendment, Non-Fast Track |
0 |
3 |
|
|||
Pending Inert Ingredients at the End of FY 2004
PRIA section 33(k)(2)(A)(ii) also requires EPA to provide the number of inert ingredients pending review by the Agency. As of September 30, 2005, the Agency had 32 petitions for new inert ingredients pending review.
Process Improvements in the Registration Program
Section 33(e) of PRIA directs EPA to identify and evaluate reforms to the pesticide registration process with the goal of reducing decision review times for pesticide registration applications. The Agency has made considerable progress during the fiscal year in improving its operations. It has undertaken a number of steps, both internal and external, to explore, develop, and implement improvements in the registration process.
In identifying process improvements, the Agency will not compromise the scientific quality of its assessments as a means toward reducing decision times. The Agency believes that, in terms of receiving recommendations for process improvements, the best means of gathering together suggested improvement areas was through the Federal Advisory Committee Act (FACA) process.
Pesticide Program Dialogue Committee PRIA Process Improvement Workgroup
The PRIA Process Improvement Workgroup was created in FY 2004 under the auspices of the Agency’s Federal Advisory Committee, the Pesticide Program Dialogue Committee, to evaluate process improvements in the registration program. The workgroup is composed of members of registrant companies, pesticide trade associations, public interest groups, and Agency staff. Meetings are open to the public and are held approximately 3 or 4 times a year. Reports of the October 12, 2004, January 25, 2005, and September 14, 2005, PPDC PRIA Process Improvement Workgroup meetings are posted on the internet.
Industry stakeholders identified many areas for improvement in the registration process, including labeling consistency, communication of schedules, and the involvement of the registrants in the decision-making process. Many of the process improvements proposed by the Agency addressed those issues. The Agency continues to work with all stakeholders to evaluate these and other potential improvements to the registration process.
Labeling Committee
Both stakeholders and the Agency recognized that labeling issues should be addressed. The Agency formed a cross-program Labeling Committee to address broad labeling issues and to oversee revisions to the Label Review Manual. A subgroup, the Label Review Manual Team, was formed to revise and continually update the Label Review Manual. The Committee drafted an internal Standard Operating Procedure, prioritized stakeholder issues, obtained concurrence on issues to be addressed, developed a web site for labeling issues, and formulated options on priority issues.
Process Improvements Implemented within the Pesticide Registration Program
The Agency implemented a number of process improvements to monitor workload and assure that PRIA due dates are met. The Agency’s Biopesticide and Pollution Prevention Division analyzed its registration process and divided the work into five phases (screening, publication in the Federal Register where appropriate, preliminary review, secondary review and risk assessment, and document development). For each B fee category (biopesticides B58 to B90), timelines were established for each phase to better manage the workflow and provide a fair and consistent mechanism for calculating the additional time needed if a deficiency had to be addressed. The Agency created a new team to expedite the secondary review of simple actions where no deficiencies were identified in the primary review. This has reduced the time to complete these actions . EPA encourages biopesticide registration applicants to participate in presubmission conferences and provides guidance throughout the application process to increase the number of complete applications.
The Agency’s analysis of its biopesticide workload is one example of numerous activities EPA undertook to reduce the amount of time taken to complete decisions. Workload and compliance with PRIA due dates are monitored in numerous internal Agency meetings. Throughout the pesticide registration program, weekly meetings are held to review the status of pending decisions, due date extensions, and refunds; to identify potential issues and target their resolution; and to coordinate schedules with science support organizations. Justifications for extending or negotiating a PRIA due date and for a PRIA determination not to grant a registration are reviewed and decisions are made at senior management levels. On a bi-monthly basis, progress in meeting PRIA due dates and the pending workload requiring action in the short term are evaluated across all involved organizations and periodically shared with stakeholder groups.
With decreasing time frames, efforts have been increased to monitor the flow of data and documents and to identify missing or deficient data or information. To manage the flow of PRIA applications, the Agency established centralized locations for and electronic documentation of receipt and delivery of risk assessments. It formalized a standard process to screen new chemical data packages for completeness and to identify critical scientific flaws in environmental and ecological data. Incoming data packages are logged in, prepared for contractor review, and shipped to the contractor within a week. Contractors return a draft report within 10 working days. EPA staff complete a final report of deficiencies (if any). The target date for this science screen is 30 days from receipt of the data package by the risk assessor.
Registration Program Workplans
The multi-year workplan for new conventional chemical actions under PRIA was posted in September 2005, and a similar workplan for new uses of conventional pesticides was posted in December 2005. This workplan is updated quarterly to reflect new actions received under PRIA, actions completed, and changes to schedules. For a majority of the new chemical and new use actions listed, the time frame in which the Agency expects to complete its registration decision is shorter than that specified by PRIA. One efficiency improvement came when the Agency identified requests for new uses submitted by USDA’s IR-4 program that were also being requested by registrants. Those requests have been merged into one risk assessment. Additional economies and time-savings were achieved where possible by folding new use assessments into assessments currently being conducted for reregistrations and tolerance reassessment.
The FY 2006 workplan for new biopesticide active ingredients has also been posted. This workplan is updated at least once a quarter to reflect completed actions and changes to the schedule. The workplan for new antimicrobials will be posted by April 3, 2006 .
Formation of Inert Ingredients Assessment Branch
The Agency has created a new Inert Ingredients Assessment Branch in the Office of Pesticide Programs to provide a single unit for all scientific and regulatory activities related to inert ingredients (pesticide ingredients not intended to be pesticidally active, such as surfactants). Inert ingredients in products to be used on food crops must have a tolerance (maximum residue level) or an exemption from tolerance. The new branch is developing processes to reduce decision times for new inert ingredient tolerance petitions. They are also developing a website that will show the status of all the inert ingredient tolerance exemptions that have been reassessed, along with the reassessment reviews.The petition backlog has been reduced significantly during FY 2005 and, by using contractor resources in addition to EPA staff, they anticipate further reductions or elimination of the backlog in FY 2006. The tolerance exemption reassessment effort has been streamlined, resulting in a greater number of completed reassessments in FY 2005 compared to past years. The remaining tolerance exemptions will be reassessed by the August 3, 2006 , deadline.
Scoping Meetings
Scoping is the process of examining and often re-examining an action to determine what specific work is required to complete the action. Another aspect of scoping is determining how the work can be completed in the most efficient manner possible and defining a schedule for the action based on this determination. The Agency’s scientific organizations have also begun scoping exercises, such as problem formulation and risk tiering, to evaluate new registration submissions. The division of the Office of Pesticide Programs that is responsible for benefits assessments participates in chemical team meetings along with the other scientific organizations to further a team approach to review and assessment. This additional coordination permits them to plan more effectively for generating Screening Level Usage Analyses for specific chemicals and ultimately results in more timely decisions under PRIA.
Biopesticide Registrant Assistance
The Biopesticides and Pollution Prevention Division created an email service in FY 2004, known as [email protected], to respond to generic questions about biopesticide registration processes. The Division’s regulatory staff meet on a regular basis to discuss questions raised through this service and provide an answer usually within two to three weeks. The registrant questions and EPA’s responses are posted on the web. To date, the Agency has posted five such questions and responses.
Science Review Improvements
For conducting pesticide environmental risk assessments, the Agency created improvements to its models called, "enter once, use many times". Data, such as environmental chemistry data, are entered into a model once and then used many times while other parameters are modified to view the effects or impacts of alternative exposure scenarios. Level 2 terrestrial and aquatic risk models have been modified to complete a risk assessment within one modeling environment. Other tools are being explored to allow an assessment of risks within the framework of a single model by drawing on outputs of current exposure models and extracting effects endpoints within a single modeling environment. This will result in more efficient use of existing databases. :
In FY 2004, in response to an industry request, the Agency established a waiver decision process for certain studies used for hazard identification. Waivers may be granted if evidence is submitted showing that the additional test is not needed to identify the nature of the hazard. Of 46 repeat dose inhalation toxicity studies originally requested, 35 waivers have been granted, 9 denied, and 2 are still being reviewed.
Progress in Meeting Tolerance Reassessment and Food Use Reregistration Timelines
FY 2005 Accomplishments
During Fiscal Year 2005, the Agency made significant progress in completing risk assessments and risk management decisions for pesticide reregistration. The Agency completed 28 Reregistration Eligibility Decisions (REDs) and issued tolerance reassessment eligibility decisions (TREDs) for 13 active ingredients. These decisions resulted in the completion of 724 tolerance reassessments
Status of Reregistration
Through the end of FY2005, the Agency has completed 271 REDs and must issue 112 more REDs to complete reregistration by the end of FY 2008. EPA’s goal is to complete 46 reregistration eligibility decisions (REDs) and finalize 23 Interim REDs (IREDs) during FY 2006 for pesticides with associated tolerances and to complete a total of 43 REDs in FY 2007 and FY 2008 for pesticides with no food uses or tolerances. This will satisfy PRIA requirements and support the Agency’s tolerance reassessment and reregistration goals. EPA’s schedule for completing these decisions can be found on the Agency’s website.
Status of Tolerance Reassessment
Through the end of FY 2005, the Agency has completed a total of 7,817 tolerance reassessment decisions, addressing over 80 percent of the 9,721 tolerances that require reassessment. EPA is accomplishing tolerance reassessment through both the registration and reregistration programs by revoking tolerances for pesticides that have been canceled, by reevaluating pesticides with pre-FQPA REDs, and through other decisions not directly related to reregistration or registration.
More specifics on the Agency’s progress in meeting its performance measures and goals for pesticide reregistration will be published in the Federal Register, as required by section 4(l) of FIFRA.
Use of Outside Reviewers
During FY 2005, the Agency explored mechanisms for enhancing its work sharing efforts with Canada ’s Pest Management Regulatory Agency (PMRA) and the California Department of Pesticide Regulation (CDPR). In FY 2005, two new active ingredient reviews were completed as part of the Joint Review Program under the North American Free Trade Agreement (NAFTA). Currently, four active ingredients (two conventional chemicals and two biopesticides) are being jointly evaluated by the EPA and PMRA as part of the NAFTA Joint Review Program. Through this process, EPA makes its own registration decision while sharing the risk assessment work. To increase the number of joint reviews, EPA is exploring the barriers to initiating biopesticide joint reviews. In addition, a work-share project on the new active ingredient, metaflumizone, has been initiated with the Pesticide Safety Directorate, United Kingdom .
EPA has also been working with CDPR to expand their capacity to review residue chemistry studies and conduct dietary risk assessments in support of registration decisions. During FY 2005, 25 crop combinations were reviewed through this joint effort.
During FY 2005, the Agency encouraged registrants to submit electronic data evaluation records for studies in support of new active ingredients. This innovation facilitates data interpretation and conserves Agency resources, permitting more rapid decisions, particularly for reduced risk chemicals.
Performance-Based Contracts
Contractors tasked with the review of hazard data were trained in the selection of endpoints and characterization of hazards for human health risk assessment. These contractor services enhanced the production of human health risk assessments.
Appendix A: Decision Review Types for Actions Completed During FY2005
As required by Section 33(k) of PRIA, the following table, as an Excel file, provides the decision times for each decision (application) during FY 2005. Note that decision times indicated in red with an asterisk are decisions completed before the Agency received payment or a waiver was granted. Completion of a registration action before payment is received typically occurs in situations where a voluntary fee payment has been offered for an application that was pending with the Agency prior to March 23, 2004 (the PRIA effective date). Mandatory decision time frames changed for some PRIA action codes and fee categories between FY 2004 and FY 2005. A decision’s time frame is based on the fiscal year in which the application is received. Mandated time frames can be found in the fee schedule published in the (Pesticides; Fees and Decision Times for Registration Applications, March 17, 2004), Federal Register. The Agency’s target due date for completing a decision or action is based on 30 days in a month. The time frames specified in the Consolidated Appropriations Act of 2004 are in months. In the table, if the PRIA due date was met, while the Agency’s target date was not, a date was entered in the column labeled PRIA Due Date. All but four decisions or actions met the Agency’s target date, negotiated due date, or PRIA due date. As EPA improves its reporting capabilities, the Agency may update this table, as necessary.
Table
of completed actions for FY2005
(Excel, 192 KB) (Microsoft
Excel Viewer
![]() is
needed to view this file.)
is
needed to view this file.)
| File Type | application/msword |
| File Title | Implementing the Pesticide Registration Improvement Act -- Fiscal Year 2005 |
| Author | JHOGUE |
| Last Modified By | JHOGUE |
| File Modified | 2007-01-23 |
| File Created | 2007-01-23 |
© 2026 OMB.report | Privacy Policy