Instructions
Instructions CIBMTR Pre_PostTED Manual 4May07.doc
Stem Cell Therapeutic Outcomes Database
Instructions
OMB: 0915-0310
Draft Registration Forms for CIBMTR
e-TED: Pre-TED and Post-TED
(05/07) – last worked on 4May07
Please send any reporting questions or comments about this manual to [email protected]
Welcome to CIBMTR Registration.
If you are new to submitting data to CIBMTR this Manual will assist you in completing the Registration Forms, Pre-TED and Post-TED. If you are a seasoned CIBMTR data collector, these two Forms replace the previous Registration system of Pre-Reg, M-TED, TED and TEDFU, effective July 2007. As of then, all types of HSCT (allo and auto) and all types of CIBMTR Teams (Registering only, CTN and Research Teams) will all complete the same set of Forms on the same schedule:
Pre-TED may be completed up to two weeks prior to the HSCT (if that is helpful to your Center), but must be completed and received by CIBMTR no later than d-0.
Post-TED is completed post-HSCT on approximately d-100, at 6 months, 1 year and annually on the HSCT anniversary as long as the recipient is alive, unless another reportable HSCT occurs, then the “clock” starts over with Pre-TED.
Under new federal legislation, U.S. centers are now required to submit outcomes data on all allogeneic transplants, related and unrelated. To meet the federal requirements, we have modestly expanded the Transplant Essential Data (TED) form to include all necessary data elements. Starting in July 2007, eTED (for expanded TED) Forms will be required for all related and unrelated donor transplants. Additionally, unrelated adult donor and unrelated cord blood transplants that are facilitated by the C.W. Bill Young Transplantation Program will require a Product Form that includes data on HLA typing, graft characteristics and donor infectious disease markers. These data will be used for evaluation of C. W. Bill Young Program operations, including federally required research such as analyses of center-specific outcomes and optimal registry/cord blood bank size.
A Product Form consists of an HLA typing Insert (HLA), IDM Insert (IDM) and Infusion Insert (INF). The HLA and IDM Inserts may be sent with Pre-TED or they can be sent with INF as a “package”, no later than two weeks post-HSCT. This is particularly important for umbilical cord blood (UCB) transplants, as the banks who supplied the product are anxious to ensure the highest quality standards for UCB. CIBMTR sends data back to the banks per the requirements of the SCTOD.
Please note a list of abbreviations on page two of Pre-TED and page one of Post-TED. These are used throughout the Report Forms. Please define for us any abbreviations you use to avoid any misunderstanding.
Some of the sections/questions are designated as “optional for non-U.S. Centers”. These questions are additional to what is collected via the EBMT. We do appreciate non-U.S. centers supplying these data if possible.
Pre-TED
CENTER IDENTIFICATION
CIBMTR Center #: this new number replaces the former CIBMTR Team number and NMDP TC code. There is a look-up tool in FormsNet 2.0 to assist you if you know the old number, but not the new one. EBMT Teams should send the CIC # (Center Identification Code).
Hospital: Refers to the name of your Hospital or Transplant Center. For communication purposes it is important that you only use the official name.
“Unit” is defined in the Abbreviations on Pre-TED pg 2. It represents the type of unit the recipient is transplanted on: Adult, Hematology, Oncology, Pediatric and “other”. There are no definitions – it is for you to decide. If your center has more than one type of unit submitting data and you wish to be able to distinguish between them, make us of this variable to record which side the data is from.
Contact information: The contact person should be the person who completed this Form or who will be able to answer queries about the data submitted. Please keep phone #, fax # and E-mail address accurate and up-to-date. We may need to contact your Center to clarify data submitted before or after we process it. Note the tick box for “changed” to indicate a change to one of these four fields.
Date of this Report: This represents the date the Form is in a “ready to send” state. All questions should be completed unless instructed otherwise. Time saving tip: having another person look over the Form before sending which will eliminate obvious errors.
CIBMTR USE ONLY
This box will not appear of FN2.0 screens. Whether a Report Form is ‘due” or not will be part of your “Forms Due Report”. If submitting this Form via paper (mail or fax), do not write anything in this box. We will note the date received and communicate back to Research Teams whether a Report Form is due “yes” or “no”. BMT-CTN study participants and HSCT utilizing UCB are always Form due “yes”.
RECIPIENT IDENTIFICATION
Universal recipient ID #: Prior to submitting a Pre-TED Form, for the first HSCT for the recipient you will need to obtain a “universal recipient ID #” via the Web. This number replaces the CIBMTR IUBMID # and NMDP Recipient ID #. This number is also known as the CIBMTR Recipient ID# (see top of pages 2-10). There is a look up tool in FN2.0 for use with existing recipients reported prior to July 2007.
Please do NOT use the old numbers in the new system.
If this is not the first HSCT you will need to find out if the recipient already had a number assigned. The easiest thing to do is ask the other center for the number. There will be a tool in FN2.0 to determine if a recipient already has an ID number assigned. This number stays with the recipient no matter where or how many times they are transplanted or followed. Although identifying information is needed to make sure the recipient is ONLY assigned one ID, these data are kept in a secure server that is separate from the database.
A web-based system to assign unique numbers to transplant recipients will be released in July. Centers will use this system to identify patients prior to submission of SCTOD forms. To generate the unique ID, centers will enter the recipient’s name, address, Country of birth, mother’s maiden name, and social security number. This level of identifying data is necessary to ensure that recipients are not already entered in the system and will decrease the chance of duplicate entries/numbers for the same recipient. The identifying data used for generating the unique ID will be stored in a secure database separate from the outcomes database. These identifying data will never be used for any purpose other than generating the unique ID. Once assigned, the unique ID will be used in all data submissions to the SCTOD and CIBMTR.
ID is assigned by: If the ID is assigned by using the CIBMTR secure ID assignment server tick ‘CIBMTR’. If the data submitted is via the EBMT data download, the EBMT ID number is supplied and EBMT box is ticked. We do not anticipate any ‘other’ at this time, but will allow future use of this tick box on an as needed basis.
Study ID#: We are primarily interested in tracking BMT-CTN, RCI-BMT and SCTOD. SCTOD recipients are identified by having a donor, related or unrelated, who is a U.S. citizen. Registration Forms on these recipients must be submitted, in a timely basis, to CIBMTR according to the U.S. government.
Consented for Research: Has the recipient, or legal guardian, signed a consent to use their data for research?
Note: The Office of the General Counsel, US Department of Health and Human Services has determined, with the concurrence of the Office of Civil Rights, that the CIBMTR meets the Privacy Rule’s definition of a public health authority (PHA) and is authorized by law to collect the information necessary to fulfill the legislated mandate to collect data needed to assess outcomes of hematopoietic cell therapy. It is therefore not a “covered entity” under HIPAA. Additionally, transplant centers who fit the definition of covered entities may disclose certain individually identifiable health information to the CIBMTR under 45 CFR 164.512 (Privacy Rule), which allows for the disclosure of an individual’s protected health information without the individual’s written consent or authorization when such a disclosure is made to a PHA that is authorized by law to collect information for the purpose of preventing or controlling disease, injury or disability.
Date of Birth/Gender: Please note order: year, month, day (yyyy/mm/dd).Due to privacy laws these pieces of data are extremely valuable for helping to make sure later Forms are entered to the correct recipient file. DOB likely will appear on later Forms as a means of double checking the Universal/CIBMTR ID # is correct. We apologize if that seems like an inconvenience.
Ethnicity: The OMB has defined ethnicity as culturally or geographically defined, and race as inherited genetic characteristics. The distinction between Hispanic and non-Hispanic is for the purpose of the U.S. Census and access to resources. According to the OMB, “Hispanic” is an ethnic designation based upon where someone (or their ancestors) were raised (e.g. “Latin America”). Hispanic people can be white, black/African American, and native; hence the separate data field for race.
Race: The easiest and most accurate way to collect these data may be to allow the recipient to self designate this answer rather than trying to extract from the patient chart. Some Centers obtain the answer to this question and other socio-economic questions found in the Report Form by grouping them into a separate sheet and giving to the recipient to complete, at their discretion, during the meeting to sign consent Forms. The reason these data are collected is to analyze access to HSCT for all people. Multi-racial people can tick all options that apply. The term Asian is equivalent to the European term Oriental.
DISEASE CLASSIFICATION
Most of the time only one Disease Classification sheet will be submitted. It represents the disease for which the HSCT was performed. Usually that is obvious, but there are a few situations where it is not.
Diagnosis combo (transforms to) Indicate as primary disease |
Send these Disease Classification Sheets |
Date of diagnosis |
MDS to AML |
MDS & AML |
AML |
NHL to another NHL subtype |
Lymphoma (1) |
1st NHL |
CLL to Richter syndrome (DLCL) |
Other Leukemia & Lymphoma |
CLL |
SAA and MDS/AML |
Anemia/hemogl., MDS &/or AML |
MDS, unless AML |
FAN and MDS/AML |
Anemia/hemogl., MDS &/or AML |
MDS, unless AML |
MYE and AMY |
Plasma Cell Disorders (1) |
MYE |
CML & ALL (“blast crisis of CML”) |
CML |
CML |
Date of diagnosis: Date should be the first date of pathologic confirmation as most physicians will not begin treating the disease until at or after that time. The date is important if the interval between diagnosis and HSCT is important to the prognosis. It may be difficult to get the precise date if it is a disease the recipient has had for a long time (e.g. 10 years). The farther away the date is, the less important a precise “day” (or sometimes even month) is. General “date” rule for statistical purposes: if only month and year are know, use the default “day” of “15”, but only if it fits with other known data (e.g. DOB, start of treatment, etc.) If only “year” is known, use “June 15”, again only if it “fits”.
HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT)
Date of this HSCT: If the Form is submitted before d-0, the infusion date may change. When that happens, give the correct date on Post-TED and tick ‘no’ to this question:
![]()
If the infusion begins one day and ends on the next, report the date of the day it was started, not ended (even if one minute before midnight!). Some Centers are experimenting with combinations of cellular therapy and stem cell infusions. Only infusions of stem cells should be considered for the date of this HSCT. Please contact us if there is any question as to what date should be the “Date of this HSCT”.
Chronological number of this HSCT: Include only infusions of stem cells (Note: this is a new instruction, different from prior versions of IBMTR and CIBMTR Report Forms).
If >1, most recent previous HSCT, Date & Type: We hope to receive a Pre-TED for each HSCT that any given recipient received; that is why only the most recent is requested. There may be circumstances where we don’t have the prior data. We appreciate any efforts you can make towards submitting a Pre-TED for prior HSCTs not performed at your Center for which we do not have the prior data. At a minimum, we need the date and type of transplant for HSCT #1 to enter a recipient to our database.
Institution where previous HSCT was performed, if different from current: This information is needed to try to determine if the recipient already exists in our database. Each recipient should exist as only one patient. If two different Teams report the same person each with an HSCT at their center and these HSCTs are not linked, it will create a “duplicate” patient in the database. Please help us avoid this. We use the contact information to 1) search our database 2) contact the other Team for data if we do not have it. The more detailed contact information you provide, the more likely we are to ensure no duplicates exist.
Cell source for this HSCT: The three tissue types listed here all contain stem cells. Some refer to HSCT with peripheral blood as “peripheral blood stem cell transplant” (PBSC), and shorten it further to “stem cell transplant”. The distinction was created as stem cells are not normally found in the peripheral blood. Something must be given to the donor (allo or auto) to cause the stem cells in the bone marrow (BM) to “spill” into the blood for collection. That is the purpose of “priming”.
More than one tissue type may be infused in the same HSCT “procedure”, as well as combinations of cellular therapy (Other, specify). Please contact us regarding the details when more than one cell type is infused in a short period of time (e.g. less than two weeks) if you are not certain how to record the events.
Allo HSCT – donor gender: Auto HSCT skips this question. Indicate the donor as male or female. Multiple donor-HSCT with one at least female and one male donor will check both boxes.
Donor Type: Other terms for the listed options:
Syngeneic: monozygotic twin (one zygote or egg), identical twin (not to be confused with the term HLA-identical sibling), paternal twin.
HLA-identical sibling: may include non-monozygotic twin (a.k.a. dizygotic, fraternal twin). Also may be a regular “garden variety” sibling (birthdates NOT the same), BUT must have identical HLA types.
HLA-matched other relative: includes all other relatives (e.g. parents, aunts, uncles, children, cousins) who have no HLA mismatches. Does NOT include adoptive or step-parents (unless somehow related through DNA).
HLA-mismatched relative: includes siblings that are not HLA-identical, and all other relatives (e.g. parents, aunts, uncles, children, cousins) who have at least one HLA mismatch. Haploidentical (haplo.) means that one half of the HLA type matches the recipient; which is extremely common between parents & children. Does NOT include adoptive or step-parents (unless somehow related through DNA).
Unrelated donor: Shares no known DNA with the recipient. Usually found through an unrelated donor registry.
Registry, specify: A coding system is being developed to avoid submitting the entire name and address of the registry. At this time it is still under development.
Or UCB bank: please supply contact information for the cord blood bank that supplied the umbilical cord blood (UCB).
HLA A->HLA-DPB1: under the given HLA type indicate the number of mismatches for each HLA type (Note: a “match” = “0” mismatches). Please use the specific separate lines for testing by serology (antigenic) and by DNA (allelic). If you are not familiar with HLA typing and the terminology, please find out if you have a resource at your Center to help you understand this very important section. If not, contact us for assistance. If multiple donors are used for this HSCT, leave this section blank, but be sure to answer donor type and the registry from which they are from.
Was there Ex Vivo Graft Manipulation: other than for RBC removal or volume reduction? Ex vivo refers to “outside the body”. Do not record things done to the recipient to affect the graft. If the only purpose of the graft manipulation was to reduce the volume of the collection (plasma removal) or to remove RBC’s (ABO incompatibility, prevent hemolysis), tick “No” even though this is a type of graft manipulation.
T-cell depletion: removes some or all of the T-cells to minimize GVHD. These T-cells may be infused at a later date. Methods of T-cell depletion may include the use of antibodies. For more detail regarding methods of T-cell depletion please refer to the CIBMTR Infusion Insert. This method is only used for allo HSCT.
Tumor purging: removes malignant cells from the collected product. This method is only used for auto HSCT.
Other negative selection: removal of cells by means other than T-depletion or tumor purging; specify what was done.
CD34 selection: also known as ‘positive selection’, collects cells with a CD34+ marker on the surface, as stem cells are known to be CD34+. Commonly done with a CliniMACS/CliniMax or Isolex machine.
Ex vivo expansion: technique to increase the quantity of stem cells.
Other specify:
Performance Score: If the patient is <16 years, please use the Lansky Play-Performance Scale for Children. If performance status is not quantified in the medical record, ask the physician responsible for the recipient’s care, it is NO LONGER acceptable to interpret notes.
CMV-antibodies: Cytomegalovirus: as the virus slowly multiplies the host cell (cyto-)
swells (megalo-), hence the name of the virus. IgM titer <0.91 indicates absence of
previous exposure to CMV. IgG titer <0.91 indicates no acute infection in three months.
Infection in the previous week may give negative results. 50-90% adults test + for CMV
antibody but are asymptomatic. Positive result = ‘reactive’, negative = ‘non-reactive’,
‘not done’ means the test was not performed, and ‘unknown’ indicates you can’t find the
answer (“unknown” may be re-requested in the future). If multiple donors are used for
this HSCT, and at least one is positive, report ‘reactive’ on the donor line, even if the
other/s donor is not-reactive.
PREPARATIVE REGIMEN
Was a preparative regimen given?
Report the total dose to be given as prescribed in the transplant protocol, not the daily dose. If the dose includes a decimal please round down to the nearest whole number if <0.5 or less, round up if >0.5. Indicate which units are appropriate for the dose
Drugs go by many names. Please use the excel file to search for alternate names or ask the transplant unit pharmacist. Only use “other, specify” when the drug really isn’t represented. The therapy recorded here must be part of the patient’s transplant protocol.
When completing Pre-TED for a subsequent HSCT, do not report therapy to treat the recipient’s disease in this section. Only include the treatment if it is considered part of the preparative regimen for the HSCT. If the intended dose does not turn out to be the actual dose, you will NOT have to send corrected information. If your Center is a Research Center you will send the actual dose received in the Harmonized Research Report Form.
Is the intent of the preparative regimen (allo only) myeloablative?
If this is a traditional stem cell transplant, the purpose of the therapy reported here is to
produce pancytopenia for > 1 month, requires a stem cell transplant for marrow
reconstitution and produces initial complete chimerism (a.k.a. ablative therapy). Non
-myeloablative (NST) transplants still utilize therapy; however, the purpose is to prevent
rejection and suppress, but not eliminate the recipient’s hematopoietic/immune system.
Autologous hematopoietic recovery would occur within 1 month without a stem cell
transplant, but with transplant initially produces mixed chimerism. Reduced Intensity
Conditioning (RIC) is anything between myeloablative and non-myeloablative.
Reason/s for NST/RIC:
Common reasons are included: recipient age, co-morbid conditions, Prior-HSCT,
specified in the protocol to do so, and “other” if not one of the prior reasons.
CO-MORBID CONDITIONS
Is there a history of mechanical ventilation? May impact the recipient’s pulmonary
function post-HSCT.
Is there a history of proven invasive fungal infection? Tick ‘yes’ only if the past
fungal infections could be problematic during the HSCT (e.g. a minor nail infection from
many years ago was probably not clinically significant.) When in doubt consult the
transplant physician as to the appropriateness of reporting.
Were there clinically significant co-existing disease or organ impairment at time of patient assessment prior to the preparative regimen?
This refers to serious pre-existing conditions unrelated to the patient’s disease or treatment. Examples of significant coexisting diseases include diabetes mellitus or rheumatoid arthritis (and the transplant is not for rheumatoid arthritis). You must answer each condition ‘yes’, ‘no’, or ‘not done’ (the organ system was not assessed).
Use the most specific category available. ANY history of malignancy, other than the disease for which the patient is being transplanted, should be reported. Many conditions listed in the recipient’s medical history may be completely resolved and unlikely to be of importance during or after the HSCT, e.g. appendectomy from 15 years ago. Please do not report these historic conditions in “other”. If you are unfamiliar with the appropriateness of something listed, please consult with the transplant physician.
If answering this question ‘no’, allo-HSCT skips to Box A: ‘GVHD Prophylaxis’ and auto-HSCT skips to Box B: ‘Post-HSCT Disease Therapy Planned as of Day-0.’
GVHD PROPHYLAXIS (allo only)
Was GVHD prophylaxis planned/given?
The therapy requested in this question refers to something that is done as a preventive measure. It typically is done to all recipients as outlined in their treatment protocol. Recipients of transplants from a syngeneic donor (monozygotic or identical twin) usually do not receive GVHD prophylaxis. If GVHD prophylaxis is used for an identical twin transplant, please provide an explanation. Do not report agents started after the development of Acute GVHD in this section.
POST-HSCT DISEASE THERAPY PLANNED AS OF DAY 0
Is this HSCT part of a planned multiple (sequential) graft/HSCT protocol? If a second HSCT is planned according to the protocol at time of first HSCT answer ‘yes’, even if the recipient does not go on to receive the second HSCT. The use of the word “planned” here does not mean if the recipient relapses we plan to re-transplant them
Is additional post-HSCT therapy planned? If therapy is planned according to the protocol at time of first HSCT answer ‘yes’, even if the recipient does not go on to receive it. The use of the word “planned” here does not mean if the recipient relapses we plan to treat them.
OTHER TOXICITY MODIFYING REGIMEN
Was KGF (palifermin, Kepivance) started or is there a plan to use it?
Was FGF (velafermin) started or is there a plan to use it?
These two newer drugs sound very much alike. If one was used, please confirm which one before ticking the box. If the recipient is part of a study in which you do not know if they received one of these drugs as the study drug or not, tick “masked trial”.
DISEASE CLASSIFICATION SHEET
WHO disease classifications have been adopted with this version Form. If you have any questions about the correct category to tick, please first provide pertinent medical record data and a copy of this Form to the transplant physician for their opinion. For further clarification please do not hesitate to contact us.
General information about Disease Response:
The term 'Primary refractory (less than PR to initial therapy)' should not be taken too literally. It is the equivalent choice for "never CR and less than PR". It doesn't matter if the recipient was in a PR at some point; if they are not in PR at the time of transplant and never achieved CR prior to the transplant, they are primary refractory.
PR: Those who were never CR but are in PR at the time of HSCT are called 1st PR or PR1. There are differing interpretations of what PR2 represents. 1) The numeral one does not literally mean THE FIRST PR, it represents that there never was a CR. PR2 means at some point the recipient was in CR, relapsed and is now in PR.
2) A literal definition where the recipient was in PR, was not in PR and is in PR again.
To avoid confusion please distinguish the type of PR with the following: “without prior CR” and “with prior CR”.
ACUTE LEUKEMIAS
Classification:
AML:
FAB classifications are included in { }.
No “other” category is defined by WHO
ALL
Divide between precursor B-cell and precursor T-cell. If cytogenetic abnormalities present at diagnosis are among the options listed, please also tick the appropriate sub-type.
No “other” category is defined by WHO
Ambiguous lineage
Neither AML or ALL, but still acute leukemia.
Did AML transform from MDS or MPS?
MDS and MPS are on a continuum of disease with AML being a more aggressive phase. The prognosis for someone with prior MDS/MPS is not as promising as de novo AML. That is why it is an important distinction. Also tick ‘yes’ if the MDS/MPS is diagnosed on the same day as AML.
Was AML therapy related?
Agents to treat other diseases can damage the marrow and lead to AML. Please indicate if treatment for a prior condition may have led to AML and indicate the possible source.
Was imatinib mesylate given for pretransplant therapy anytime prior to the start if the preparative regimen?
Also known as Gleevec, Glivec.
Status at Transplantation
Represents the response to all prior therapy. ‘Never treated’ may be appropriate if the recipient had a prior disease, a transplant was in the works, AML was discovered, and a decision was made to proceed to transplant instead of treating the AML. PIF includes as many regimens as were unsuccessfully tried; it is NOT limited to just one regimen. For CR and relapse indicate which number. CR is defined as less than 5% blasts in a cellular marrow with a normal CBC and no other signs or symptoms of (extramedullary) disease, which must be maintained for a minimum of 1 month (or it doesn’t “count”).
For hematologic CR indicate whether it included cytogenetic or molecular remission.
CHRONIC MYELOGENOUS LEUKEMIA (CML)
Classification:
In the new WHO disease classification, the diagnosis CML must include either evidence of the Philadelphia chromosome (a.k.a. Ph+, t(9;22)(q34;q11) or a complex or variant form of t(9;22)) OR demonstrate the bcr/abl gene rearrangement. Tick the combination that best describes the abnormalities identified any time between diagnosis and the start of the preparative regimen. If one of those abnormalities is not found, but CML is still suspected, report in the “Other Leukemias” group under ‘Atypical CML’.
Did recipient receive treatment prior to this HSCT? If yes, tick all the therapy that was used between diagnosis and the start of the preparative regimen.
Status at Transplantation
Represents the response to all prior therapy, indicate type of response and number of that response. For CP and CR only, also tick whether the recipient was in cytogenetic and/or molecular remission.
MYELODYSPLASTIC OR MYELOPROLIFERATIVE DISEASES
Classification:
This disease contains a continuum of disorders and may transform from of subtype to another during the course of the disease; therefore the subtype at first diagnosis and the subtype present just prior to the start of the preparative regimen is requested. If the disease has transformed to AML, the date of diagnosis reported on page one should be that of AML. The MDS/MPS diagnosis date should be reported on p4 and also complete the AML Disease Classification Sheet. No “other” disease classification was indicated by WHO. If you cannot find the appropriate subtype for the recipient, please gather pertinent medical record notes for the recipient and ask the transplant physician to select the subtype.
Other diagnosis: CMML amd JMML are reported here.
Was Janus kinase 2 (jak2) gene mutation positive at any time between diagnosis and the start of the preparative regimen.
Was MDS/MPS therapy related?
Agents to treat other diseases can damage the marrow and lead to MDS/MPS. Please indicate if treatment for a prior condition may have led to MDS/MPS and indicate the possible source.
Status at Transplantation
Represents the response to all prior therapy. Categories are mutually exclusive. Indicate the number for both CR and relapse.
JMML
has a separate response list:

OTHER LEUKEMIAS
Classification:
Includes: atypical CML (see CML for explanation), CLL, PLL, hairy cell leukemia, and other leukemia (specify what the “other” is).
Status at Transplantation
Represents the response to all prior therapy.

LYMPHOMAS
Classification:
Both Hodgkin Lymphoma (HL) and Non-Hodgkin Lymphoma (NHL) subtypes are from WHO disease classification. NHL can transform from one subtype to another, but HL does not “transform” to NHL. When NHL transforms, report the date of diagnosis as the first date of diagnosis, but the subtype is the last transformed type prior to the start of the preparative regimen. If the recipient has HL and develops NHL an important distinction is whether both diseases are considered active. If HL is believed to be in remission, then it is appropriate to report NHL the same as though the recipient had any other prior malignancy in a historical sense. If both are “active” this is one example of an appropriate use of the diagnosis code “900” (see page 6)
![]()
Note: Waldenstrom Macroglobulinemia previously had been collected in the PCD section. It now is listed with Lymphoma. It is the same disorder, just a new location.
Before using the subtype “Other…, specify”, please gather pertinent medical record notes for the recipient and ask the transplant physician to select the subtype.
Status at Transplantation
Represents the response to all prior therapy, through all transformations..

Sensitivity to chemotherapy is defined as:

It is measured based on the last therapy given. If the last therapy was not chemo, it should be reported as PIF/relapse “untreated” (by chemotherapy).
PLASMA CELL DISORDERS
Classification:
Please report the appropriate plasma cell disorder subtype. If the recipient had more than one, please report the most recent:
Diagnosis combination: |
Report as: |
Plasmacytoma first, now has multiple myeloma |
multiple myeloma |
Multiple myeloma + plasma cell leukemia |
plasma cell leukemia |
Multiple myeloma + amyloidosis |
multiple myeloma |
Report the heavy chain sub-type (IgG, IgA, IgD, IgE, or IgM) and the light chain (kappa or lambda). “Light chain only” disease will be kappa or lambda, but not one of the “Ig’s”. “Non-secretory” will not show either an Ig or kappa/lambda. Only use the category “Primary Amyloidosis” if the recipient has NO evidence of multiple myeloma – no matter how much the transplant physician wants you to if they do. In very rare instances a recipient could have two heavy chain types. That is a proper use of “other PCD”. If this is a subsequent HSCT and the two subtypes did not exist prior to the first HSCT, double check with the transplant physician as this could represent oligoclonal reconstitution, not a second sub-type.
Stage at diagnosis:
You may report either Salmon & Durie or the newer I.S.S. (International Staging System). If I.S.S., report either the lab values from diagnosis or the stage based upon those values.
Status at Transplantation
Represents the response to all prior therapy

BREAST CANCER
Classification:
Whether the histology was inflammatory or not is important in disease prognosis. If not readily documented, please ask the transplant physician, who likely knows.

Stage at diagnosis and Metastases: if Stage IV is indicated at diagnosis, skip “stage” and report as metastatic.
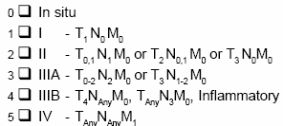
Status at Transplantation
Represents the response to all prior therapy
Adjuvant is appropriate only when diagnosed as Stage II or III, surgery removed all known disease and no relapse occurred prior to the start of the preparative regimen.
Primary refractory means a complete remission was never achieved no matter how many lines of therapy were used and is analogous to ‘no response’ below.
PR1 is reserved for “never in CR”, but a PR was achieved and maintained.

Relapse should only be used after CR is achieved.
Number: indicate for CR, CRU and relapse.
Sensitivity to chemotherapy is defined as:

It is measured based on the last therapy given. If the last therapy was not chemo, it should be reported as “untreated” (by chemotherapy).
“OTHER” DISEASE
Classification:
If the cell infusion is for one of the newer “alternative” uses of cell therapy, please indicate in this box:

Otherwise, this category should be used very infrequently such that we ask you:

[Note – I see the typo – pages 3-10, not 1-8].
OTHER MALIGNANCIES
Classification:
Most of these malignancies are solid tumors. We did not include some of the very rare indications, which can be reported as “other”. The sarcoma group has under gone some updating. If you do not find the sarcoma subtype listed in the recipient’s medical record among this list, please double check with the physician before using “other”; the subtype may just have a new name.
Germ cell tumors that originate in the ovary or testes should be reported as Ovarian or Testicular, respectively.
Status at Transplantation
Represents the response to all prior therapy. Note: some disease categories have changed to using RECIST criteria, which is listed for your convenience. Please tick ‘yes’ if the status you are indicating represents RECIST criteria. If not RECIST, please check the definitions provided in the appropriate Disease Insert found at www.cibmtr.org
Number: indicate for CR, CRU and relapse.
Sensitivity to chemotherapy is defined as:

It is measured based on the last therapy given. If the last therapy was not chemo, it should be reported as “untreated” (by chemotherapy).
NON-MALIGNANT DISORDERS
Classification:
Only the disease subtype is reported. No status of disease pre-HSCT or post-HSCT is collected for non-malignant disease; just a few test results for some of the autoimmune diseases.
Post-TED
There are three time points Post-HSCT for which this single Form is used (see Center Identification box):
Day-100
6 months
HSCT anniversary
At the top of the page is an important instruction:
![]()
As this Form is used for several time points there are some answers that are not appropriate for the first time follow-up is submitted. These answers are designated by a circle instead of a square tick box.
CENTER IDENTIFICATION
See Pre-TED section.
CIBMTR USE ONLY
See Pre-TED section.
RECIPIENT IDENTIFICATION
See Pre-TED section.
Disease:
Please make sure the diagnosis reported here is the same one used on
the Pre-TED Form. The CIBMTR database code for diagnosis is included
after the disease label. e.g.
![]() .
You may use the diagnosis code (e.g. 281) instead of writing out the
diagnosis.
.
You may use the diagnosis code (e.g. 281) instead of writing out the
diagnosis.
HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT)
Chronological number of this HSCT# DCI#:
How to count the number of DCI’s? Look at page two DCI section. From the question “Total # DCI in 10 weeks” add all the DCI’s that would be reported from each DCI reporting box, e.g. DCI infused at 80 and 90 days post-HSCT (from Day-100 Report) and again at 380 and 390 days (from 2nd year Report) = 4 DCI [not 2] on 2nd year Report.
Date HSCT for follow-up: Report the date of the HSCT represented for this follow-up Form. When a subsequent HSCT takes place, the last contact date for the Post-TED prior to the new HSCT should be one day prior to the start of the preparative regimen for the next HSCT or one day prior to the subsequent HSCT when no preparative regimen is used. Follow-up reporting for the prior HSCT is then complete; continue follow-up reporting only on the latest HSCT. Be sure to use the correct HSCT date for the time period you are reporting on.
100 Day Report Only: The time line for reporting a recipient begins with the first dose of the preparative regimen. As centers are able to register recipients prior to the start of the preparative regimen (if it benefits your center to do so), we understand that occasionally the transplant will be delayed or not take place altogether. This is the mechanism to convey when there has been a change.
![]() If
yes, continue to “Initial ANC Recovery” section. If no,
next question.
If
yes, continue to “Initial ANC Recovery” section. If no,
next question.
![]()
If no, this Form should not be completed. If yes, continue with remaining questions:

INITIAL ANC RECOVERY
Initial ANC recovery: ANC refers to the Absolute Neutrophil Count, a subset of the granulocytes, demonstrated in peripheral blood (CBC). Commonly reported units are 500/mm3 = 0.5 x 109/L. ANC recovery is defined as achieving >0.5 x 109/L neutrophils (“segmented neutrophils + band neutrophils”, or “segs & bands”) for three (3) consecutive labs tested on different days*. If >0.5 x 109/L is met by the stated criteria, tick ‘yes’ and report the first date of the three consecutive labs. If not met, tick ‘no’ and report the latest date of assessment.
Calculating the absolute count from a differential:
Differential: the relative number of each type of cell (red, white and platelets) in the
sample typically expressed as a percentage. Do not report the values from a
differential. If the absolute neutrophil count is not given, convert the differential as
follows:
(% neutrophils times total WBC) divided by 100 = absolute neutrophils. If the segs and
bands are reported individually, add them together before doing the calculation:
WBC 5,400/mm3 with 70% segs + 3% bands = 0.73 x 5.4 = 3.942 x 109/L ANC.
*Traditionally, the definition of neutrophil engraftment required selecting the first date of three consecutive days in which the recipient’s ANC was 500/mm3 (0.5x109/L). For various reasons it may not be possible to obtain daily lab values. Under those circumstances you may report neutrophil recovery based upon three consecutive lab values that are more than a day apart as long as the counts show a continual increase, not counts going up and down.
The recipient may receive growth factors or irradiated granulocytes, but NOT unirradiated granulocyte transfusions or “boosts” from the donor. The latter is considered a subsequent HSCT and must be reported as such.
‘Never below’
Once the preparative regimen is started, it takes some time for the recipient’s counts to drop; be sure to locate the lowest count (nadir) before checking the labs for recovery. If the recipient was transplanted for an Immune Deficiency or the preparative regimen regimen was non-myeloablative (NST) or reduced intensity (RIC), the counts may never have been below 0.5 x 109/L. If below for just 1 reading you may not use this option.
‘Previously reported’
Once >0.5 x 109/L has been achieved and reported the tick circle ‘previously reported’ may be used. If the level has not been achieved, continue to tick ‘no’ and report the latest date assessed.
‘Unknown’
As ANC recovery is the heart of HSCT, this answer should rarely be used. It is imperative that every effort be made to track this data for the recipient.
Engraftment: to demonstrate engraftment chimerism tests must be done, which measure the quantity of donor cells compared to host (recipient) cells. While ANC usually represents donor cells, it cannot be proven without chimerism studies.
Did graft failure occur?
Graft failure is defined as a decline in ANC to <500/mm3 (0.5x109/L) for three consecutive days. It may be due to drugs, infection (especially CMV), GVHD and other etiologies. (Note: “failure to engraft” is represented by answering ‘no’ to initial ANC recovery.)
INITIAL PLATELET RECOVERY
Report the initial platelet recovery in this section for achieving levels: 20 x 109/L and/or 50 x 109/L. The date achieved must be at least seven days following the last platelet transfusion and the first date of three consecutive lab values tested on different days that show that level was achieved and maintained.
‘Never below’
Once the preparative regimen is started, it takes some time for the recipient’s counts to drop; be sure to locate the lowest count (nadir) before checking the labs for recovery. If below for just 1 reading you may not use this option.
‘Previously reported’
Once >20x 109/L has been achieved and reported the tick circle ‘previously reported’ may be used. If the level has not been achieved, continue to tick ‘no’ and report the latest date assessed.
‘Unknown’
It is imperative that every effort be made to track this data for the recipient.
Note: this section may change a little – we realize there is a problem of asking two time points and “no”, never below, previously reported and unknown” are not clear as to whether they go with ‘20’ or ‘50’. 4Apr07- “50” has been dropped from Post-TED. DJK
GVHD (allo only)
Graft versus host disease (GVHD) is an immunological phenomenon resulting from the reaction of donor immune cells against major or minor histocompatibility antigens of the recipient. The donor cell primarily responsible is the T-lymphocyte which also explains why GVHD almost never occurs in autologous transplants. The severity of GVHD is determined primarily by the degree of genetic disparity between the donor and the recipient (HLA); in part by the age of the recipient/recipient, and the type of therapy given post-HSCT to prevent GVHD (GVHD prophylaxis).
In the past, GVHD was classified into acute or chronic on the basis of its time to diagnosis following transplant, and other clinical and histological (biopsy or post-mortem) features. Today GVHD should only be classified by clinical and histological (biopsy or post-mortem) features.
Acute GVHD usually begins between 10 and 40 days after HSCT but can appear earlier or later. It occurs in 20-40% of non-T-cell depleted HLA identical sibling transplants. The rate is higher for transplants from mismatched family donors and unrelated donors. The organs usually affected are the skin, gut or liver although other sites (e.g. conjunctiva) may be involved.
Maximum grade of acute GVHD: Please note: this scale is based on clinical evidence
(physician observation), not histology. If there is a difference in the clinical grade
recorded by the physician and a histologic report, use the data from the clinical
documentation noting the difference as a ‘Report Note’ if you wish. Biopsy of affected
organs allows for more precise diagnosis as to the presence or absence of GVHD.
However, overall grading remains clinical and is based on the criteria proposed by
Thomas et al, N Engl J Med 1975.
Maximum extent of chronic GVHD:
Chronic GVHD can occur following acute GVHD or de novo (without prior evidence of aGVHD) and affects 25-50% of long-term survivors of allogeneic transplants. It usually develops after day 100, but has been documented as occurring as early as day 60 and as late as day 400 post-HSCT. The mechanism of tissue damage differs from acute GVHD and a greater variety of organs are affected. There is a simple staging system for grading severity as limited or extensive.
Although according to strict criteria, recipients must have at least skin and/or liver
involvement to be considered “extensive”, involvement of any other target organ has
generally also met the definition. For example, a recipient with only eye involvement or
only mouth involvement would still be considered “extensive.” Note that recipients with limited chronic GVHD can ONLY have skin and/or liver involvement since other manifestations make them “extensive.”
Reporting Stage of Chronic GVHD (Blood 1981; 57:267)
Limited: Localized skin involvement resembling localized scleroderma with or without liver involvement; no other organ involvement.
Extensive: Generalized skin and/or multiple organ involvement
Date of chronic GVHD diagnosis: Report the date of clinical diagnosis recorded in the recipient’s medical record, or if not recorded, you may use the date of histologic confirmation. The date of diagnosis is not necessarily the same as the date of symptoms diagnosis. Between recipient visits the symptoms of GVHD may change from those of acute GVHD to chronic. When the exact date of this progression is not known the “100 day rule” is often applied for the purpose of calculating intervals for statistical analysis. The rule assigns the end date of acute GVHD to 99 days from the date of transplant, and the diagnosis of chronic to day 100. It is only applied when actual dates are not known.
If this is a Follow-Up Report Form and the recipient had chronic GVHD that resolved for at least 30 days, but has reactivated (“flair”); report the new episode and list the new date of diagnosis. If the episode continues from one report to another tick ‘continued from last report.’
DID A NEW MALIGNANCY, LYMPHOPROLIFERATIVE OR MYELOPROLIFERATIVE DISORDER OCCUR?
Date of diagnosis: Should be the date of the pathology report confirming the new malignancy.
New malignancy diagnosis: Please be sure to differentiate between disease that has relapsed and a de novo (“first time”) malignant process diagnosed post-HSCT. Do not report a history of a malignancy diagnosed before the first transplant and now relapsed. Report all new cancers including skin cancers (basal, squamous, melanoma,) new leukemia, myelodysplasia, solid tumor and lymphoproliferative disorders. Cytogenetic abnormalities that appear post-HSCT, but are known to be associated with the pre-HSCT diagnosis should be reported as relapse of the disease and not here. For breast cancer found in the contralateral breast, please report as “relapse of breast cancer” as that is where we will look for these recipients at the time of any studies. Note: PTLD (posttransplant lymphoproliferative disorder) is collected in lymphoma or lymphoproliferative disease, not “other”.
.
SURVIVAL
Survival status at latest follow-up:
Alive:
Date of last actual contact with recipient to determine medical status for this report should be based upon physician contact, which includes the transplant center, referring physician, or other physician currently assuming responsibility for the recipient’s care. We understand this may become difficult the further out from HSCT the recipient becomes.
If only month and year are known, you may estimate the “day” or use “15” (but only if it is compatible with other known dates). If only year is known use “June 15” (but only if it is compatible with other known dates).
If an evaluation was not actually performed on Day 100, 6 months or HSCT anniversary by the transplant center or the physician assuming the recipient’s care, choose the visit as close to Day-100, 6 months or HSCT anniversary as possible.
Questions referring to “current” data should be interpreted as “current for the reporting period represented by the Form.”
Information after the last contact date for this Report Form should be recorded on the next Form.
Dead:
Report the date of death
Lost-to-Follow-Up:
Report the last known date alive.
Recipient’s with this status remain on the “Forms Due – yes” report as we do not want you to forget about them should you become aware of any additional information.
If dead: Main cause of death:
Cause of death (COD): Only one primary cause of death may be specified. If relevant, multiple contributing causes may be listed. If the COD is truly not known, indicate as such. Do not report the final event, “cardiac and/or respiratory arrest”, as the primary COD. What led up to the recipient’s death?
Primary and contributing cause of death
Relapse/Progression/Persistent disease:
Persistence or recurrence of underlying disease for which recipient was transplanted. Be sure the Post-HSCT disease evaluation reflects the presence of disease post-HSCT.
For Aplastic anemia report “rejection/poor graft function”.
HSCT related causes:
GVHD: Provide details aGVHD/cGVHD section as applicable.
Cardiac toxicity: This as primary COD should be fairly rare. Use only if the physician states this as the primary COD and no other causes could be determined.
Infection: can be proven or suspected.
Pulmonary toxicity: Lung failure not from infection, includes bronchiolitis obliterans, radiation pneumonia, etc.
Rejection/poor graft function: Includes failure of marrow to achieve an ANC 0.5 x 109/L (no engraftment or partial engraftment) or loss of graft. May also be recorded as bone marrow failure or aplasia (note: recipient’s transplanted for a disease other than Aplastic Anemia the term “aplasia” does not necessarily refer to the recipient developing SAA.)
(Hepatic) Veno-Occlusive Disease: can be caused by chemo/radiotherapy. Consists of endothelial damage, micro thrombosis of the hepatic venules and sinusoidal fibrosis. It is more common in allogeneic transplants than autologous and typically occurs within 3 weeks of transplant. In the absence of a histological diagnosis, recipients must fulfill the criteria below for a diagnosis of VOD.
CLINICAL CRITERIA FOR VENO-OCCLUSIVE DISEASE OF LIVER
Recipients reported as having veno-occlusive disease of liver based on clinical signs and symptoms only must have two or more of the following with no other identifiable cause for liver disease:
1. Jaundice
(bilirubin
2 mg/dL or > 34 μmol/L)
2. Hepatomegaly with right upper
quadrant pain
3. Ascites and/or weight gain (>5% over
baseline, as generally accepted)
References: McDonald GB, et al. Hepatology 1984; 4:116-122
Jones RJ, et al. Transplantation 1987; 778-783
Other HSCT related COD
New malignancy: Must be diagnosed after the first transplant was performed, if prior, use “other”. Please be sure that the new malignancy differs from the malignant disease for which the transplant was performed (e.g. de novo leukemia, AML diagnosed many years after a transplant for ALL).
Other: Please carefully consider whether the cause of death can be classified into one of the categories provided above. If this is not possible, then indicate and provide details.
Abbreviations: Please become familiar with these abbreviations, which are used throughout these Forms.
MALIGNANT DISEASE EVALUATION FOR THIS HSCT:
Note: non-malignant disease continues to post-HSCT therapy section, unless DCI was performed, then complete DCI section.
WAS A CR EVER ACHIEVED IN RESPONSE TO HSCT(including any therapy planned as of day 0, excluding any change in therapy in response to disease assessment)?
This section collects the data known as “best response to transplant” and is a widely misunderstood data point. The purpose is to capture how well the recipient responded to ONLY the prescribed “transplant package”. It never includes treatment given in response to a disease evaluation. It often is achieved in the first 100 Days, but some diseases like MYE and CLL may take longer, again typically 1 year, but possibly up to 2 years. After that the recipient has likely gotten the most benefit that they will see from the HSCT. Once the recipient relapses or progresses, or receives therapy in response to a disease evaluation, the response to that additional therapy is NOT included in this data field. It will be collected in the ‘current disease status” field. If you have ANY questions about “best response” please contact us.
FIRST RELAPSE OR PROGRESSION AFTER HSCT
(in this period, any type, not persistent disease)
There are three methods to evaluate disease. Not all diseases use all three methods, but every recipient who has an evaluation by a physician has a “clinical” assessment. If the recipient dies, but has not been in for a visit, a physician still must pronounce them dead, hence they have a “clinical” visit. If disease is discovered by autopsy, the date of the assessment should be reported as the date of death, not the actual autopsy date. No data for the recipient can exceed the date of death.
Each method can record relapse or progression, but only the FIRST instance for each method should be reported. Subsequent reporting periods should utilize the “previously reported” option.
ADDITIONAL TREATMENT?
DCI is only appropriate for allogeneic HSC. If yes, complete the DCI section in column on the right side of the page.
Planned: This is generally given within the first year or two post-HSCT as is part of the HSCT “package”. It does NOT mean the “recipient relapsed therefore we planned to treat them”. Please be aware of the distinction regarding planned and not planned therapy.
Not planned: refers to treatment given in response to a disease assessment.
METHOD OF LATEST DISEASE ASSESSMENT
(Record most recent of each)
This section should be completed for every malignant disease. Not all diseases have molecular and/or cytogenetic/FISH (fluorescent In-Situ Hybridization) abnormalities identified with which to monitor disease status. If none exist, tick “not evaluated”. If you don’t know whether the recipient is being monitored by these methods please ask someone at your Center.
Molecular testing: Occasionally a recipient may have a positive test result, but the physician does not believe it represents “disease”. In that instance, tick “yes” to ‘disease detected’ but “no” to “Was this status considered a disease relapse or progression”. E.g. CML HSCT recipient exhibits a low level of BCR-ABL positivity post-HSCT that the physician does not believe represents disease and is not treating it.
All recipients should have some type of medical contact and is reported in the “clinical” category. It does not have to be your transplant Center that did the assessment.
If a previous HSCT was performed for a different disease than this HSCT, give status of original disease.
If this is the recipient’s first HSCT – skip this box. If only applies if a subsequent HSCT is given for a “new malignancy”.
POST-HSCT THERAPY
This box collects data on specific therapies from current CIBMTR studies. It is not intended to collect every type of possible post-HSCT therapy.
Note: KGF and FGF (palifermin and velafermin) sound a lot alike. Please record each of these very carefully.
Masked trial refers to a study in which you do not know if the recipient is receiving the study drug, placebo or something else. Only tick ‘masked trial’ if one of the listed drugs is involved in the study.
DONOR CELLULAR INFUSION (DCI)
Has the recipient received a DCI from the original donor? This section refers to cellular therapy from the original donor, lymphocytes, unstimulated peripheral blood mononuclear cells, dendritic cells, mesenchymal cells, etc. If a bag of cells saved from the HSCT are now infused without a preparative regimen and the reason for the infusion does not pertain to the prior graft (no engraftment, partial/poor engraftment or loss of the graft/late graft failure), please report as Donor Cellular Infusion.
If a different donor was used, report as a subsequent HSCT.
Some recipients have cellular infusions on more than one day. A single DCI section should be completed for all infusions given within a 10-week period, which is a change form the previous 095-Report Forms of 28 days. For example:
Weeks 0 4 8 10 16
^DCI ^DCI ^DCI ^DCI ^DCI ^DCI
These
3 DCI would be reported These 2 DCI’s would be This DCI would
be
in one DCI section. reported together with reported on a
separate
the first DCI section. DCI section.
Please contact us with any specific examples you wish to discuss. List the dates indicated on the timeline prior to contacting us.
Date of the first DCI: In the example above, the first DCI is at approximately the two week mark – report that date. The second DCI “box” date would be the one at the 16 week mark.
Total # DCI in 10 weeks: In the example above the total number is 5.
Types of cell(s) (check all that apply):
The most common type of DCI is the DLI (Donor Lymphocyte Infusion), but as you can see form the list, there are other types of cellular therapy.
Indication: What is the reason the cells are being infused? All of the known indications are listed at the moment. “Other” is to report novel uses; “engraftment” should NEVER be listed as a reason. If the recipient does not have a graft, stem cells are required to attain one and that is reported as a subsequent HSCT.
Maximum Grade of aGVHD: DCI can trigger aGVHD independent of the HSCT. See aGVHD section for details regarding GVHD.
Disease status before next DCI “section”: the response to the DCI includes the grouping (if more than one) within the 10 week period and is only answered if you need to complete data in the next DCI box in this reporting period. “Current status” will collect the response if no additional DCIs were done.
| File Type | application/msword |
| File Title | Draft Registration Forms for CIBMTR |
| Author | dknutson |
| Last Modified By | LWright-Solomon |
| File Modified | 2007-05-11 |
| File Created | 2007-05-04 |
© 2026 OMB.report | Privacy Policy