Supporting Statement Part B_revised 10-22-07
Supporting Statement Part B_revised 10-22-07.doc
Evaluation of a Medication Therapy Management Program to Improve Patient Safety in Medicare Beneficiaries
OMB: 0935-0136
Supporting Statement Part B
“Evaluation of a Medication Therapy Management Program on Patient Safety in Medicare Beneficiaries at High Risk of Adverse Drug Events”
Version 10/22/2007
B. Collection of Information Employing Statistical Methods
1. Potential Respondent Universe and Sample Selection Method
Patient Selection:
Trial inclusion criteria are based on the following parameters:
MTM may be of value in an elderly population with specific risk factors.
Inclusion of a broad, heterogenous population reflective of the ambulatory elderly who would be enrolled in community MTM programs as a component of Medicare Part D.
Maximization of patient accrual in a compressed study timeframe
A total of 600 patients will be enrolled in this study at all 3 sites (see Section A2 for a description of sites) combined. Individual site enrollment goals: 200 patients per site.
Inclusion Criteria:
For inclusion in the study, patients must:
Be at least 65 years old at enrollement
Primarily uses English language for written and oral communication
Have three or more comorbid conditions associated with increased healthcare utilization (see Table B1 below)
Have visited a physician at one or more of the clinics on a regular basis (defined as two or more clinic visits over one year prior to the study start) for these conditions.
Have received 8 or more different chronic prescription medications over the six months prior to the enrollment period
Have a telephone line and agree to maintain it for at least six months
Have one of the following situations placing him/her at risk for a DRP:
Any ER visit in past 30 days or Urgent Care visit in past 30 days leading to a change in medication or change in medication dose
New physician visit in past 30 days
Hospitalization in past 30 days
Invasive procedure (a procedure requiring substantive changes to medication taking practices or which requires informed consent) in past 30 days
Change in medication in past 30 days
Three or more providers seen in the past year
Table B1. Comorbidities Associated with Increased Healthcare Utilization
Comorbidity |
ICD9 and Procedure Codes |
Diabetes Mellitus |
250.0 – 250.9 |
Congestive Heart Failure |
428.0 – 428.9 |
Asthma |
493.0 – 493.9 |
Hypertension |
401.0 – 401.9, 402.0 – 402.9, 403.0-403.9, 404.0 – 404.9, 405.0 – 405.9 |
Dyslipidemia |
272.0 – 272.9 |
COPD |
491.0 – 491.9, 492.0 – 492.8 |
Coronary Artery Disease |
414.0 – 414.9 |
Chronic Renal Failure |
585, 586, 593.9 |
Arthritis |
715.0 – 715.9 |
Depression |
311 |
Dementia |
290.0 – 290.9 |
Chronic Pain |
719.40-714.49, 724.0 – 724.9 |
Conditions requiring anticoagulation with warfarin |
V58.61 (chronic anticoag); 427.3 (Afib), 453.8 (DVT) (note: can’t find MHV) |
Exclusion Criteria:
Patients with the following exclusion criteria will not be enrolled in the study:
Terminal condition, where life expectancy is less than 6 months
Patients already enrolled in an MTM program where medication reconciliation and/or assessment of DRPs has occurred in the previous 12 months
Enrollment Estimates in Tabular Form:
We estimate that approximately 50% of eligible patients approached for participation will choose to enroll in the study. Table B2 below displays the estimates at each step of the process.
Table B2. Enrollment Estimates
Site |
Number of Clinic Patients over 65 |
Estimated Proportion Eligible for Enrollment |
Expected Number Eligible for Enrollment (Require 200 patients per site) |
University of Illinois at Chicago |
6,000 |
UIC estimates show 52.5% receive 6 or more medications. At least 50% of these patients should meet all inclusion criteria. |
Over 788 patients should be eligible for enrollment. We expect 50% of patients meeting criteria to enroll. |
Baylor Health Care System |
Over 2,500 (all from 1 Geriatrics clinic site) |
An estimated 50% of BHCS patients receive 6 or more medications. At least 40% should meet all study inclusion criteria. |
Over 619 patients should be eligible for enrollment. We expect 50% of patients meeting criteria to enroll. |
Duke University Health System |
Up to 14,300 available (if all clinics involved) |
Nationally, 50% of patients receive 5 or more medications. At least 40% of these patients would be expected to meet the other inclusion criteria. |
Approximately 2860 patients should be eligible for enrollment. We expect 50% of patients meeting criteria to enroll. |
2. Information Collection Procedures
Data collected at each study site will be forwarded to the Coordinating Center at UIC. Some data will be collected for the purposes of patient care only and will not be forwarded to the UIC Coordinating Center. Study-related data will include demographic data collected at baseline, the list of medications collected by the MTM clinician (photocopied) (Appendix B medication list), the DRP assessment sheets collected by the MTM clinician (photocopied) (Appendix C), MTM clinician time logs (Appendix N), DRP forms faxed to the study subject’s primary care physicians and those returned to the MTM clinician (photocopied) (Appendix D), and patient telephone interviews at 90 and 180 days by a blinded study investigator (Appendixes G and O). Data collected for patient care that double as study-related data forms will be copied at the site will have the patient name crossed out prior to forwarding to the UIC Coordinating Center. All study-related data forms will be express mailed to UIC Coordinating Center on a monthly basis. Data collected for patient care only and not being forwarded to the UIC Coordinating Center include chart synopses (Appendix B Clinical Documentation Tool) and other clinic notes generated by the MTM clinician but not included in the study-related data.
Screening:
In the initial screening (Patient Telephone Screening and Invitation to Participate Script – Appendix J), there are some questions which patients must answer with more than a yes/no answer. Specifically, potential study subjects must be able to provide us with the medication names off of their prescription bottles. Being able to answer all of the screening questions, without having to involve another family member or care provider (for translation purposes), will be sufficient to invite eligible patients to the baseline study visit. At the baseline study visit, potential study subjects will be asked to read and sign informed consent. By doing so, study subjects are indicating that they agree to participate in the research and are eligible for participation. The very first sentence of the informed consent document is: “You have been asked to participate in the research because you are over 65 years of age, primarily speak English, have 3 or more chronic conditions, are taking eight for more medications, have a situation that places you at risk for a drug related problem, and may be eligible to participate.”
Since the intervention will be conducted in English only, indications that a potential study subject does not speak English will be considered grounds for exclusion from the study.
Sample Size Determination:
Based on Adverse Drug Events (primary study outcome): The pilot study and subsequent validation for the tool we are using identified a total of 7016 “symptoms” (potential reported ADEs) from 837 respondents taking one of the index drugs in the study.8 Approximately 55% (3848) of these “symptoms” were classified as possibly and an additional 16.2% (1134) were classified as probably caused by the drugs studied.
Gandhi et al., using a telephone questionnaire, estimated that 181 ADEs were suffered by 661 patients surveyed (mean of 0.27 ADEs per patient) over a 3-month period.16 The mean age and number of medications per patient in this study were 52 and 1.53 respectively. The number of ADEs suffered were significantly associated with number of medications (OR=1.1; 95% CI 1.06-1.15 per additional medication) but not with age. Assuming a linear relationship and extrapolating these results to our study, where patients are taking 8 or more medications with an estimated mean of 12 medications per patient (and not accounting for other factors which may increase the ADE risks in our inclusion criteria), we would expect to see approximately 1.1012 * 0.27 = 0.84 ADEs per patient over a 3 month period (and likely more over a 6 month period). Chrischilles et al., using a self report method for ADE identification, found that ADEs were associated with increasing age, more medications, and poorer self-perceived health suggesting the potential for a higher ADE rate in our selected population.17
In 178 high risk patients (average age 59, average of 8 medications, being discharged from hospital), detectable differences in serious preventable ADEs were found.18 In this study, preventable ADEs had occurred in 1 patient in the intervention group and 8 in the usual-care group (1% vs 11%; P=.01) and the rate of preventable, medication-related ED visits or hospital readmissions was 1% in the intervention group and 8% in those assigned to usual care (P=.03) at 30 days.
A prior study has not been conducted using patient self report of ADEs in a patient population similar to ours. We have specifically selected our patient population according to their increased risk for ADEs and to maximize the effectiveness of the intervention. From the findings of these and other studies, and using a modified version of the validated tool by Jarernsiripornkul et al., we expect to find between 1 and 9 ADEs per patient.8 Using a normal estimation of the Poisson distribution, accounting for pairwise comparisons between our three groups, and setting alpha at 0.05 and study power at 0.80, we will be able to detect an effect size equivalent to a relative risk reduction of 9-26% with a sample of 200 evaluable patients per group (600 total patients), depending on the number of ADEs reported per patient in the usual care group. Since approximately 25% of ADEs are preventable, this study is sufficiently powered to detect elimination of preventable ADEs in all but the very lowest expected ADE rates. The graph below details the minimum detectable effect size for each of the various possible scenarios.
Figure 3. Required sample size according to usual care ADE risk (lambda0) and intervention ADE risk (lambda1).
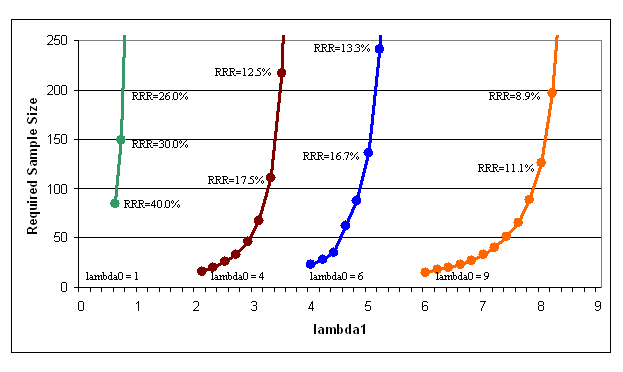
NOTE: The relative risk reduction (RRR) is calculated as (lambda0 – lambda1) / lambda0. Calculations were performed using a normal approximation to the Poisson distribution with adjustment for pairwise comparisons, alpha = 0.05, and power = 0.8.
Based on Medication list discrepancies: Data from the Iowa VAMC indicated that medication lists in their electronic EMR contained 4.4 discrepancies (38% of all medications taken).4 A total of 3.1 omissions (medications the patient was taking that were missing from the EMR) and 1.3 commissions (medications on the EMR that patients were not taking) per patient; 34% of omissions were for prescription medications. A similar study compared congruence of medication lists from three sources (patient, hospital, and GP), identifying between 1 and 3 discrepancies per study subject.3 We were unable to identify any other studies of medication list discrepancies identified in the community setting.
Assuming an average of 1 discrepancy per study subject (compared with the gold standard medication list) in the enhanced medication list and 1.5 discrepancies per patient in the medication list generated by patient interview alone (conservative estimates for this patient population), sample size calculations based on a normal approximation to the Poisson distribution indicated that we needed to recruit 43 patients per arm to have 80% power to show this difference at the 5% significance level.
3. Methods to Maximize Response Rate
Patients found to meet the study inclusion criteria will be sent an advance letter of introduction to the study, followed by a telephone screening call to confirm eligibility and to ask if they will be willing to participate. The advance notice affords the patient time to think about whether he or she wants to participate and not be put on the spot while at a clinic visit. If the patient refuses, the telephone interviewer will also ascertain why they do not want to participate. This refusal data will help us with analysis of the differences between respondents and non-respondents.
The data collection instruments have also been designed to be as brief as possible to minimize the burden on the respondents. The response options are kept to a minimum and patients can respond with a “don’t know” or “refuse” to answer response. The questions have been designed to be as user-friendly as possible.
The use of incentives is expected to increase the response rates and therefore increase the data quality. Participating patients will be reimbursed for their involvement in the study: $10 for the baseline study visit and $10 for each of the completed telephone questionnaires. Each patient will be therefore eligible to receive up to $30 for their participation in the study, regardless of which group they are assigned to.
4. Tests of Procedures
The questionnaires are based on previously administered instruments on earlier studies as described in B3 above. These questionnaires have been previously validated.
5. Statistical Consultation and Independent Review
Data collection and analysis will be completed by the following individuals:
UIC coordinating center and data analysis:
Daniel Touchette, Pharm.D., M.A.
Assistant Professor
UIC College of Pharmacy M/C 886
833 S. Wood Street, Room 164
312 355-3204
Young Ku Choi, Ph.D.
University of Illinois at Chicago
Methodology Research Core
Institute for Health Research and Policy (MC 275)
1747 West Roosevelt
Room 558
Chicago, Illinois 60608
312 996-9486
Baylor Health Care System (BHCS):
Andrew Masica, M.D.
Clinical Scholar/Internal Medicine-Hospitalist
Baylor Institute for Health Care Research and Improvement
8080 North Central Expressway-Suite 500
Lockbox 81
Dallas, TX 75206
214-265-3624
Duke Primary Care Research Consortium (PCRC):
Rowena Dolor
DCRI
2400 Pratt St.
Box 3850 DUMC
Durham, NC 27705
919 668-8596
BIBLIOGRAPHY
Page
| File Type | application/msword |
| File Title | Supporting Statement Part B |
| Author | wcarroll |
| Last Modified By | wcarroll |
| File Modified | 2007-10-22 |
| File Created | 2007-10-22 |
© 2026 OMB.report | Privacy Policy