Appendix A to T_revised 11-5-2007
Appendix A to T_revised 11-5-2007.doc
Evaluation of a Medication Therapy Management Program to Improve Patient Safety in Medicare Beneficiaries
Appendix A to T_revised 11-5-2007
OMB: 0935-0136
Revised 11/5/2007
Appendix A
MTM Clinician Interview Tool
Form Approved
OMB No.
0935-XXXX
Exp. Date XX/XX/20XX
MTM Clinician Interview Tool
Start with a formal form of Introduction and brief description of events to take place
Ask patient to display containers for all prescriptions medications, OTC products, herbal products and nutritional products (if available).
If item(s) not available, ask patient to display a list of medication(s)
If patient cannot provide either have patient verbalize list of medications.
(Prompt the patient to try and remember patches, creams, eye drops, inhalers, sample medications, shots, optic, herbals, vitamins, and minerals).
Do you have any allergies? If so, with what drugs and what was the reaction?
What is your height?
What is your weight?
Does anyone normally help you remember to take your medicines?
If yes, who? (allow that person to assist with answering the questions)
If no, then patient must answer all questions without assistance.
What medicines are you taking at the moment (brand/generic)?
What is the … for each medication listed.
Dosage
Route
Directions for use (sig) as prescribed by the physician
For each medication ask:
How do you take this medication?
What condition does this medication treat?
When did you start the medication or how long have you taken this medication?
When was the last time the dose of this medication was changed?
How many times in the past 2 weeks have you forgotten a dose of this medication?
What time of day do you take this medication?
Do you take anything that you buy without a prescription from a health food store, supermarket, etc? If yes, repeat questions 5, 6, and 7.
Have you recently stopped any medications? Why?
Has the physician changed any medications recently?
Do you have any other conditions for which you are not taking any prescription or non-prescription medications or natural products?
Does the physician periodically check labs, blood pressure, etc to monitor your conditions? If yes, how often, list the last date, any results if known.
Are you suffering any side effects now?
If yes, what side effects?
Which of your medication(s) do you think is (are) causing the problem(s)?
Do you have any questions or concerns about your medications?
Public
reporting burden for this collection of information is estimated to
average 15 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by its
contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c).
Appendix B
MTM Clinical Documentation Tool
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
STUDY ID:________________ DATE:_______________ □ Visit #1 □ Visit #2
Medication Therapy Management Study – Clinical Records for Clinician Pharmacist |
||||||||||||||||||||||||||||
Date: |
/ / |
|
|
|
|
|
|
|
|
|
||||||||||||||||||
Name: |
|
|
|
|
|
|
Site: |
□ Baylor |
□ Duke |
□ UIC |
||||||||||||||||||
|
Last |
First |
|
Middle |
|
|
Primary Care Physician: |
|
|
|||||||||||||||||||
Patient ID: |
|
|
|
|
|
|
PCP Phone Number: |
( ) |
- |
|||||||||||||||||||
DOB: |
/ / |
|
|
|
|
|
PCP Fax Number |
( ) |
- |
|||||||||||||||||||
Height: |
|
□ inch |
Weight: _________ |
□ lbs |
|
Pharmacy |
Name: |
|
|
|||||||||||||||||||
|
|
□ cm |
|
□ kg |
|
Pharmacy |
Phone: |
( ) |
- |
|||||||||||||||||||
Allergies |
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
Drug: |
□ PCN |
□ Sulfa |
Other: |
|
|
Food: |
□ Sulfite |
□ Shell Fish |
Other: |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
Medical History (check where applicable): |
|
|
|
|
|
|
||||||||||||||||||||||
□ |
Anemia |
□ |
Dermatophytosis |
□ |
Hypertension |
|
Other(s): |
|
|
|||||||||||||||||||
□ |
Asthma |
□ |
Diabetes Mellitus |
□ |
Hypokalemia |
|
|
|||||||||||||||||||||
□ |
Atrial Fib/Atrial Flutter |
□ |
DVT/PE |
□ |
Kidney Transplant |
|
|
|
||||||||||||||||||||
□ |
Chronic Renal Failure |
□ |
Gastric Ulcer |
|
□ |
Myocardial Infarction |
|
|
|
|||||||||||||||||||
□ |
Constipation |
□ |
GERD |
□ |
Obesity |
|
|
|
||||||||||||||||||||
□ |
COPD |
□ |
Heart Failure |
□ |
Osteoarthritis |
|
|
|
|
|||||||||||||||||||
□ |
Coronary Artery Disease |
□ |
Hepatitis |
□ |
Osteoporosis |
|
|
|
||||||||||||||||||||
□ |
Depression |
□ |
Hyperlipidemia |
|
□ |
Stroke/CVA |
|
|
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
Most Recent Laboratory Values: |
|
|
|
|
|
|
|
|||||||||||||||||||||
Chemistries |
|
|
Complete Blood Count |
|
Vitals |
|
|
|||||||||||||||||||||
Date Lab Drawn: |
/ / |
|
Date Lab Drawn: |
/ / |
|
BP: _____/____ |
HR ___ |
Date / / |
||||||||||||||||||||
Na (mEq/L) |
|
|
Hemoglobin (g/dL) |
|
|
BP: _____/____ |
HR ___ |
Date / / |
||||||||||||||||||||
K (mEq/L) |
|
|
|
Hematocrit (%) |
|
|
|
|
|
|||||||||||||||||||
Glucose (mg/dL) |
|
|
WBC (/ul) |
|
|
Diabetes |
|
|||||||||||||||||||||
Creatinine (mg/dL) |
|
|
Platelets (/mcl) |
|
|
Date Lab Drawn: |
/ / |
|||||||||||||||||||||
BUN (mg/dL) |
|
|
|
|
|
HbA1C (%) |
|
|||||||||||||||||||||
|
|
|
|
Lipid Panel |
|
|
|
|
||||||||||||||||||||
Liver Function Tests |
|
Date Lab Drawn: |
/ / |
|
Drug Levels: (name) |
|||||||||||||||||||||||
Date Lab Drawn: |
/ / |
|
TC (mg/dL) |
|
|
Date Lab Drawn |
/ / |
|||||||||||||||||||||
AST (U/l) |
|
|
|
LDL (mg/dL) |
|
|
Level: |
|
||||||||||||||||||||
ALT (U/l) |
|
|
|
HDL (mg/dL) |
|
|
Goal: |
|
||||||||||||||||||||
|
|
|
|
TG (mg/dL) |
|
|
|
|
|
|||||||||||||||||||
Coagulation |
|
|
|
|
|
MTM Clinic Only: |
|
|
||||||||||||||||||||
Date Lab Drawn: |
/ / |
|
Thyroid Panel |
|
|
|
|
|
|
|||||||||||||||||||
INR: |
|
|
|
Date Lab Drawn |
/ / |
|
|
CrCl (ml/min) |
|
|
||||||||||||||||||
Goal INR: |
|
|
|
TSH (μIU/ml) |
|
|
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
Specialist Name: |
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
Phone #: |
( ) |
- |
||||||||||||||||||
STUDY ID:________________ DATE:_______________ Page ___ of _____ □ Visit #1 □ Visit #2

|
Medication Name |
Strength |
Frequency |
Indication |
Initiation of Drug |
Last |
Prescriber |
Source |
Is pt. taking the drug? |
How is pt taking the |
|
Generic (trade) |
Dosage Form and # |
(Ex: qday, bid, |
(Ex: DM,HTN, |
≤ 30 days, 1-6 months, |
Titration Date |
Name |
Medical Record (MR), Patient (Pt),Caregiver |
(reported by pt) |
drug? |
|
|
tabs (Ex: 25 mg x2) |
tid, qid, qod) |
etc.) |
> 6 months |
|
|
(Cg), or Other (Oth) |
|
(Ex: am/pm) (reported by pt.) |
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
2 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
3 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
4 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
5 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
6 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
7 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
8 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
9 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
10 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
Note: Use another form if additional medications need to be entered.
STUDY ID:________________ DATE:_______________ Page ___ of _____ □ Visit #1 □ Visit #2
|
Medication Name |
Strength |
Frequency |
Indication |
Initiation of Drug |
Last |
Prescriber |
Source |
Is pt. taking the drug? |
How is pt taking the |
|
Generic (trade) |
Dosage Form and # |
(Ex: qday, bid, |
(Ex: DM,HTN, |
≤ 30 days, 1-6 months, |
Titration Date |
Name |
Medical Record (MR), Patient (Pt),Caregiver |
(reported by pt) |
drug? |
|
|
tabs (Ex: 25 mg x2) |
tid, qid, qod) |
etc.) |
> 6 months |
|
|
(Cg), or Other (Oth) |
|
(Ex: am/pm) (reported by pt.) |
|
|
|
|
|
|
|
|
|
|
|
_1 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_2 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_3 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_4 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_5 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_6 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_7 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_8 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_9 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_0 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_1 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_2 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_3 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_4 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_5 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_6 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_7 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_8 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_9 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
_0 |
|
|
|
|
□ ≤30d □1-6m □ >6m |
|
|
□ MR □ Pt □ Cg □ Other |
□ 0-30% □ 30-80% □>80% |
|
Note: Use another form if additional medications need to be entered.
Appendix C
Modified PCNE Drug Assessment Form
M
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
For every drug the patient is receiving, assess each of the following DRPs. Mark all that apply .
STUDY ID:________________ DATE: _______________ □ Visit #1 □ Visit #2
Patient Name: Date:
General Drug Related Problem: check box if “yes” |
Specific Drug Related Problem (Modified PCNE Problem Code) |
Yes? (circle) |
Cause Code/comments |
||
1. The patient is having an adverse drug event (ADE) as a result of the drug. |
|
A B C
|
|
||
Medication Problem Identified Action/plan/recommendation RPH Date Resolved RPH 1. 2. 3. |
|||||
|
|
|
|
|
|
2. There is a problem with the choice of the drug for the indication in this patient. |
|
A
B
C
D
E
F
|
|
||
Medication Problem Identified Action/plan/recommendation RPh Date Resolved RPH 1. 2. 3. |
|||||
Public
reporting burden for this collection of information is estimated to
average 30 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by its
contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c).
|
|||
3. There is a problem with the drug dose being taken by the patient. |
|
A
B
C D
|
|
Medication Problem Identified Action/plan/recommendation RPh Date Resolved RPH 1. 2. 3. |
|||
|
|||
4. The patient is having difficulties with taking the drug. |
|
A
B
|
|
Medication Problem Identified Action/plan/recommendation RPH Date Resolved RPh 1. 2. 3. |
|||
|
|
|
|
5. The patient is having or at risk for a significant drug interaction. |
|
A
B
|
|
Medication Problem Identified Action/plan/recommendation RPh Date Resolved RPh 1. 2. 3. |
|||
|
||||
6. There are other problems the patient is having with their drug therapy. |
|
A
B
C
D
|
|
|
Medication Problem Identified Action/plan/recommendation RPh Date Resolved RPh 1. 2. 3. |
||||
|
|
|
|
|
7. The patient is at risk for a potential ADE.
|
|
A
B |
|
|
Medication Problem Identified Action/plan/recommendation Rph Date Resolved RPh 1. 2. 3. |
||||
Insert 1 or more Cause Code for every affirmative DRP using the PCNE DRP Causes List (attached)
Pharmacist initials
_________________________ _____
_________________________ _____
_________________________ _____
Appendix D
MTM Clinician/Physician Communication Fax Form
M
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
STUDY ID:________________ DATE: _______________ □ Visit #1 □ Visit #2
Medication Therapy Management Study
Physician Communication Fax Form
TO: Physician name: Fax
Regarding patient: DOB
From: Pharmacist Fax
Phone
Your patient is participating in a multi-center AHRQ study where they have randomly received a Medication Therapy Management intervention from a study pharmacist for the purpose to reconcile their medications and identify any drug related problems.
The following medication related problems have been identified for your patient. Recommendations to address the drug related problem are included.
Please complete the form below by
When completed fax back to
Drug Related problem Recommendation Comments
□ I accept the recommendation □ I do not accept the recommendation
□ I am not managing this medication please contact:
MD Name: Phone #
□ I request the following alternative order
Drug Related problem Recommendation Comments
□ I accept the recommendation □ I do not accept the recommendation
□ I am not managing this medication please contact:
MD Name: Phone #
□ I request the following alternative order
MD Signature: DEA number
Date:
Pharmacist Signature: Date:
For office use only Fax sent (MM/DD/YYYY)
__________________ Fax received (MM/DD/YYYY) _____________________
Public
reporting burden for this collection of information is estimated to
average 5 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by its
contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
Appendix E
Protocol for Patient Safety Guidelines for MTM Visits
Protocol for Patient Safety Guidelines for MTM Visits
During any MTM visit, the site pharmacists or other research personnel may direct patients to seek additional care as felt to be indicated by their clinical judgment.
If the nature of the condition is believed to require timely attention (within 48 hours) and MTM site is proximal to a clinic and same or next day appointments in that clinic are an option, the MTM pharmacist will try to expedite an office visit with the patient’s primary care provider. Administrative, nursing, and physician staff at each of the MTM trial clinic sites will be aware of the potential need to accommodate these visits.
If the nature of the condition is believed to require emergent attention, the MTM pharmacist will refer the patient to the closest emergency room. Examples of such emergent issues include, but are not limited to:
Active chest pain or pattern of accelerating chest pain
Acute neurologic deficit or change in mental status
Active GI bleeding
Acute respiratory distress
Hemodynamic instability
Any condition of major concern to the MTM pharmacist
In instances where MTM is conducted in a clinic location, the MTM pharmacist will notify the clinic’s charge nurse for initiation the transfer process (set of vital signs, call ahead to the closest ER, contact of emergency medical transport for critical patients). In critical cases where the MTM visits are conducted offsite from the clinic and there is no charge nurse available, the MTM pharmacist will contact EMS directly for transport to the emergency room.
Appendix F
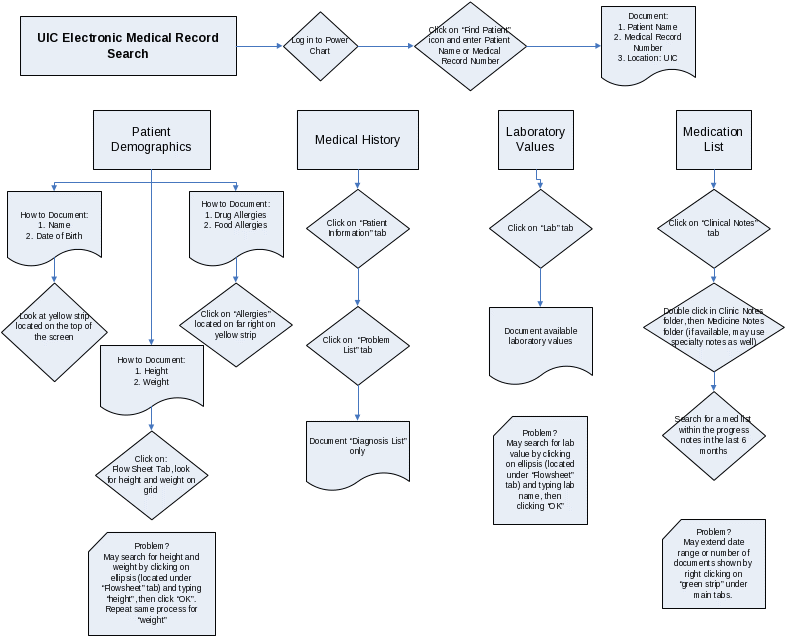
Standardized Protocol for Clinical Synopsis at UIC
Standardized Protocol for Clinical Synopsis at UIC
Appendix G
Telephone Interview Questions
for
Assessing Adverse Drug Events
Telephone Interview Questions for Assessing Adverse Drug Events
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
STUDY ID:________________ DATE: _______________ □ Visit #1 □ Visit #2
Part A
During the last 3 months, have you had any of the following symptoms which you think may be side effects caused by one of your medications?
1. Have you had any of the following symptoms which you think may be due to side effects from a medication related to your skin?
a ❑ bleeding g ❑ pale skin
b ❑ bruising h ❑ puffy skin
c ❑ burning sensation i ❑ pins and needles sensation
d ❑ flushing of skin/ hot flush j ❑ skin rash
e ❑ increased sensitivity k ❑ yellowing of skin
of skin to light l ❑ Other (please indicate)_______________
2. Have you had any of the following symptoms which you think may be due to side effects from a medication related to your hair or nails?
a ❑ change in fingernails c ❑ Other (please indicate) ______________
b ❑ hair loss d ❑ None
3. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your muscles, bones or joints?
a ❑ bone or joint pain e ❑ unsteadiness on feet
b ❑ muscle pain f ❑ unusual or uncontrolled body movement
c ❑ muscle weakness g ❑ Other (please indicate) ______________
d ❑ trembling & shaking of h ❑ None
fingers & hands
4. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your head?
a ❑ headache c ❑ Other (please indicate) ______________
b ❑ migraine headache d ❑ None
5. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your vision?
a ❑ blurred vision c ❑ Other (please indicate) _______________
b ❑ double vision d ❑ None
6. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your eyes?
a ❑ itchy or irritated or inflamed c ❑ unusual movement of the eyes
eyes or eyelids d ❑ Other (please indicate) ______________
b ❑ inability to move eyes e ❑ None
7. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your hearing or ears?
a ❑ change or difficulty in hearing c ❑ ringing, buzzing or noises in ears
b ❑ feeling of fullness in the ears d ❑ Other (please indicate) ______________
e ❑ None
Public
reporting burden for this collection of information is estimated to
average 30 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
8. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your mouth or gums?
a ❑ bleeding from gums c ❑ Other (please indicate) ______________
b ❑ dry mouth or throat d ❑ None
9. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your nose, throat, neck or voice?
a ❑ difficulty talking d ❑ sore throat
b ❑ slurred speech e ❑ Other (please indicate) ______________
c ❑ runny or stuffy nose f ❑ None
10. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your breathing or lungs?
a ❑ cough d ❑ slow breathing
b ❑ difficulty breathing e ❑ Other (please indicate) ______________
c ❑ fast breathing f ❑ None
11. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your heart or circulation?
a ❑ palpitations/ racing heart c ❑ Other (please indicate) ______________
b ❑ missed heart beat d ❑ None
12. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your stomach or digestive system?
a ❑ bloated feeling or gas f ❑ nausea or vomiting
b ❑ decrease in appetite g ❑ vomiting blood or material that looks like
c ❑ indigestion or heartburn coffee grounds
d ❑ increase in appetite h ❑ Other (please indicate) ______________
e ❑ pain or cramps in i ❑ None
lower abdomen
13. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your rectum or bowel movements?
a ❑ black tarry stool d ❑ Other (please indicate) ______________
b ❑ constipation e ❑ None
c ❑ diarrhoea
14. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your kidneys, bladder or urinary system?
a ❑ burning, discomfort or pain e ❑ passing water more often
while passing water f ❑ bloody urine
b ❑ dark brown urine g ❑ Other (please indicate) ______________
c ❑ difficulty in passing water h ❑ None
d ❑ passing water less often
15. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your sexual function (ability)?
a ❑ decrease in sexual desire d ❑ Other (please indicate) ______________
b ❑ decrease in sexual ability e ❑ None
c ❑ increase in sexual desire f ❑ Does not apply
16. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your reproductive (sex) organ?
a ❑ abnormal or change in c ❑ Other (please indicate) _______________
vaginal bleeding d ❑ None
b ❑ burning or irritated penis
17. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your nervous system?
a ❑ confusion or delirium c ❑ dizziness or staggering (vertigo)
b ❑ light-headed when getting up d ❑ increase in convulsions (seizures)
from a lying or sitting position e ❑ Other (please indicate) _____________
or feeling faint f ❑ None
18. Have you had any of the following symptoms which you think may be due to side effects from this medicine related to your mental health?
a ❑ anxiety (nervousness) or f ❑ anger or aggression
agitation g ❑ loss of memory
b ❑ change in mood h ❑ thought of suicide
c ❑ difficulty concentrating or i ❑ reduction in sleeping
learning j ❑ increase sleep or drowsiness
d ❑ hallucinations (seeing, k ❑ Other (please indicate) _______________
hearing or feeling things l ❑ None
that are not there)
e ❑ nightmares
19. Have you had any of the following symptoms which you think may be due to side effects from this medicine?
a ❑ increased sensitivity to cold f ❑ unusual tiredness or weakness
b ❑ excessive thirst g ❑ weight gain
c ❑ fever h ❑ weight loss
d ❑ flu-like symptoms i ❑ Other (please indicate) _______________
e ❑ increase sweating j ❑ None
Total Number of Symptoms Identified in Part A: ___________ (fill in this number of Part B forms).
STUDY ID:________________ DATE: _______________ □ Visit #1 □ Visit #2
Part B
For each symptom reported, ask the following questions (complete one Part B form for each symptom reported in Part A):
1. What is the symptom being reported on this form? ______________________________
2. Where is the symptom located (from form A)?
a ❑ skin l ❑ stomach or digestive system
b ❑ hair, nails m ❑ rectum or bowel movements
c ❑ muscles, bones, joints n ❑ kidneys, bladder, urinary
d ❑ head o ❑ sexual function
e ❑ vision p ❑ reproductive organ
f ❑ eyes q ❑ nervous system
g ❑ hearing, ears r ❑ mental health
h ❑ mouth or gums s ❑ general/constitutional
i ❑ nose, throat or voice
j ❑ breathing or lungs
k ❑ heart or circulation
3. What medication(s) do you believe is causing the problem?
________________________________________
________________________________________
________________________________________
4. How much has this symptom(s) bothered you at its worst ?
a ❑ minimally d ❑ severely
b ❑ mildly e ❑ very severely
c ❑ moderately f ❑ does not apply
5. Have you told your doctor about this symptom?
a ❑ yes (go to question 6) b ❑ no (go to question 7)
c ❑ does not apply
If the patient told the doctor about the symptom:
6. In response to your symptom, did the doctor recommend any of the following actions?
a ❑ The doctor did laboratory tests.
b ❑ The doctor recommended continuing taking medication exactly as before.
c ❑ The doctor recommended stopping the medication.
d ❑ The doctor prescribed another medication.
e ❑ The doctor changed the prescription in some other way.
f ❑ The doctor prescribed another drug to treat the side effect.
g ❑ The doctor told you to do something else to treat the side effect.
7. In response to your symptom, did what action did you take?
a ❑ Continued to take medication as before. (End of survey; go to next symptom)
b ❑ Changed the dosage. (End of survey; go to next symptom)
c ❑ Stopped taking the drug. (Go to question 8)
If patient has stopped taking medication:
8. When did you stop this medication? ( __ __ / __ __ ) month / year
9. Why did you stop?
a ❑ I felt I didn’t need it any longer
b ❑ The doctor said I didn’t need it any longer
c ❑ The doctor told me to stop because I was having problems with it
d ❑ I decided to stop because I was having problems with it
e ❑ I felt it wasn’t helping me
f ❑ Other (please explain)
10. Has the symptom you have described gone away?
a ❑ yes b ❑ no c ❑ does not apply
(End of survey; fill out another Part B for each reported Part A symptom)
Appendix H
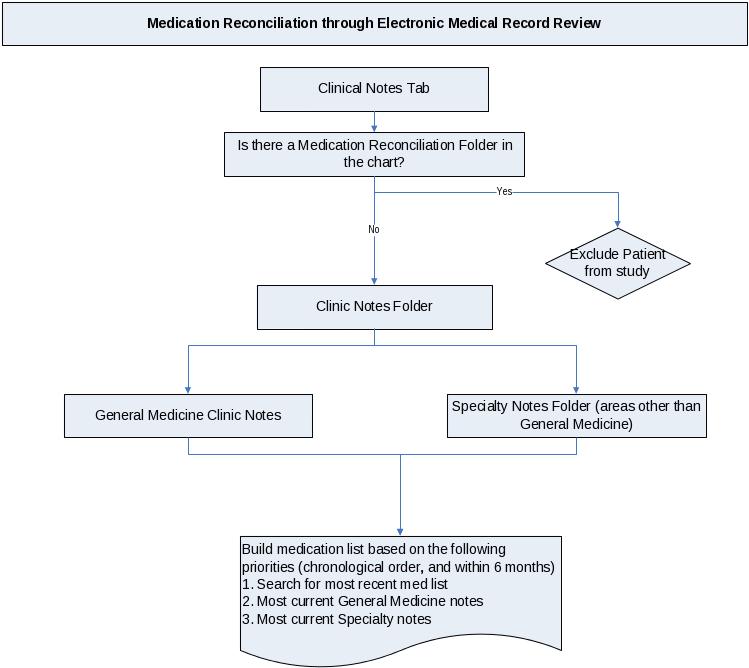
Standardized Protocol for Chart Review
for the
Best Possible Medication History
Standardized Protocol for Chart Review for the Best Possible Medication History at UIC
Appendix I
Initial Patient Contact Letter
Form Approved
OMB No.
0935-XXXX
Exp. Date XX/XX/20XX
Initial Patient Contact Letter
<Date>
Patient Name
Address
City, State Zip
Dear Mr/Ms. LastName:
I am writing to let you know about a New Research Project called the Evaluation of a Medication Therapy Management Program on Patient Safety in Medicare Beneficiaries at High Risk of Adverse Drug Events. This study is being conducted at University of Illinois at Chicago, Baylor Health Care System’s Geriatrics Clinic, and the Duke University Health System. Dr. <site investigator> is the leading the study at <name of site>. The study is testing different ways to review your medication list and identify medication-related problems to improve patient safety.
Your participation in this study is voluntary. Whether you join the study or not, I will continue to be your doctor. Your care with me will not change.
If you would like more information about being in this study, please call <study phone number>, and the study team will call you back as soon as possible.
If you’re sure that you do not want to participate, please call that number in the next ten days and let the study team know that you don’t want to be called.
If you do not call, someone from the study will call you to tell you more about the study.
Please think about this study carefully: understanding how to create an accurate medication list and identify medication-related problems can reduce side effects and prevent hospitalizations. The results of this important research may also help others.
Thank you very much for considering this study.
Sincerely,
<patient’s primary care MD>
Appendix J
Patient Telephone Screening
and
Invitation to Participate Script
Patient Telephone Screening and Invitation to Participate Script
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
PATIENT SCREENING SURVEY
Hello. May I speak with ?
RESPONDENT NOT AVAILABLE |
IF OTHER HOUSEHOLD MEMBER ANSWERS: My name is ____________ and I’m calling from <Name of Institution>. Can you suggest a good time when I could reach him/her?
IF ANSWERING MACHINE/VOICEMAIL: Hello this is ______________ from <Name of Institution> calling for ____________. You may be eligible to participate in a research study here at <Name of Institution> which I recently mailed you a letter about. I was calling to see if you would be interested in participating in this study. Please give me a call back at _________ at your earliest convenience.
[END CONTACT – RECORD CALL-BACK TIME, IF ANY, ON FACE SHEET.]
RESPONDENT AVAILABLE |
Hello, this is ___________ calling from <Name of Institution>. We recently mailed you a letter that said we would be calling you to tell you about a study going on at <name of clinic>. [REMINDER: PATIENT MAY THINK YOU ARE CALLING ABOUT OTHER CORRESPONDENCE FROM THE CLINICS SUCH AS LAB RESULTS]
Did you receive this letter?
IF GOT LETTER:
Great! Just to review, with the letter there was a brochure describing a study we are doing to improve ways to review your medication list and identify medication-related problems to improve patient safety. I’m calling today to see if you would be willing to answer a few questions to see if you may be eligible to participate in the study. Is this a good time?
IF YES, GO TO IRB SECTION.
IF NO: When would be a good time for me to call you back?
Date _____________ Time _________
IF DID NOT GET LETTER:
I’m sorry that you did not get the letter. Let me explain what it was about. In the letter we described a study we are doing to test different ways to review your medication list and identify medication-related problems. Your physician has agreed to let us ask his/her patients if they would be willing to participate. If you want to be in the study, I will ask to meet you at the clinic and have you bring all your medication bottles. At that visit, I will obtain your consent to participate in the study and ask you questions about your medical history and medications. You will be randomly assigned to one of three groups. After that visit, I will contact you by phone again in 3 months and in 6 months to ask if you have had any medication-related problems, urgent/emergency room visits, or hospitalizations. You will receive $30 for being in the study.
Do you have any questions so far?
Public
reporting burden for this collection of information is estimated to
average 5 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
IRB SECTION |
Before we go on, there are a few things you need to know before you decide whether or not you are interested in the study.
Your participation is voluntary. If you choose not to be in the study, it will not affect your health care at <name of Institution>.
If you agree to answer the questions, you may decide to stop at any time or skip questions you do not want to answer. After we’re done, you may decide to withdraw your answers from our study but you must do this in writing. We will provide you with <site investigator’s> mailing address. This address is in the letter we mailed you and will be on the consent form we will ask you to sign when we meet at your visit.
Study records that identify you will be kept strictly confidential to the extent permitted by the Privacy Act. Federal Privacy Regulations provide safeguards for privacy, security, and authorized access.
Except when required by law, you will not be identified by name, social security number, address, telephone number, or any other direct personal identifier in study records disclosed outside of <institution name>.
For records disclosed outside of <institution name>, you will have your own code number. The key to the code will be kept in a locked file in our study office.
Your records may be reviewed to meet federal or state regulations. Reviewers may include representatives from the Agency for Healthcare Research and Quality and the <university> Institutional Review Board.
The study results will be kept on record up to six years after the study is completed. At that time information identifying you will be removed from the study results at <institution>.
I’ve gone through a lot of information. Does this sound like something you’d like to do?
IF YES, GO TO AGREE.
IF REFUSAL READ:
I understand that you don’t want to be in this study. But it would be helpful to us in planning future studies to have a little information about why you are not interested. Could you please let me know why you do not want to be in this study?
DO APPROPIATE REFUSAL CONVERSION USING PAPER CONVERSION SCRIPTS AND RECORD WHY REFUSED
Why refused….
1 = Too busy/No time
2 = Not interested
3 = Too sick/old
4 = Need more information/ more time to think
5 = Have had bad research experience in past
6 = In too many studies already
Other: _________________________________________________________
IF STILL REFUSAL STILL DO REFUSAL QUESTIONS (same set of questions)
AGREE
Now, I need to ask a few questions to see if you would be eligible for the study. It should take no more than 10 minutes.
1. What is your date of birth?
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
IF 65 OR OLDER: ELIGIBLE
IF LESS THAN 65 OR REF OR DNK: INELIGIBLE
2. Has your doctor changed your medication dose or added a new medication within the past month? YES NO
IF YES: Date of medication change
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
► if YES can skip to question 6
3. Have you seen a new doctor in the past month? YES NO
IF YES: Date of new provider visit
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
► if YES can skip to question 6
4. Have you been seen in the Emergency Room in the past month? YES NO
IF YES: Date of ER visit
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
► if YES can skip to question 6
5. Have you been discharged from the hospital in the past month?
YES NO
IF YES: Date of discharge
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
►IF ANSWERED NO TO QUESTIONS 2-5 THEN INELIGIBLE FOR STUDY
6. Have you been told by your doctor that you have any condition that might prevent you from completing this 6-month study?
YES NO
7. Have you had an interview at some time in the past year where you have been asked to bring in all of your medications to a pharmacist, nurse, doctor, or other healthcare professional and been given a list of all your medications?
YES NO
8. Do you have any of the following medical problems?
a. Diabetes YES NO
b. Heart failure YES NO
c. Asthma YES NO
d. High blood pressure YES NO
e. High/abnormal Cholesterol YES NO
f. Emphysema (COPD) YES NO
g. History of heart attack, heart blockage
(e.g. stent placement or bypass surgery) YES NO
h. Poor kidney function YES NO
i. Arthritis YES NO
j. Depression YES NO
k. Memory problems YES NO
l. Chronic pain YES NO
m. Take blood thinner (warfarin/Coumadin)
on a daily basis YES NO
9. Can you tell me the names of your medications, including over the counter medicine, vitamins or supplements, that you take every day? (Write down medication names only. Do not include directions. Use patient prescription bottles to assist in identifying medications)
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
________________________ ________________________
IF INELIGIBLE: Based on your answers, it looks like you are not eligible to participate in the study. The reason could be that you are taking less than 8 medications on a daily basis, have fewer than 3 medical problems, or have not had a change in your medications in the past month. Thank you for taking the time to answer our questions over the phone.
FOR PATIENTS WHO MEET ALL ELIGIBILITY CRITERIA EXCEPT RECENT MED CHANGE:
[ASK IF PATIENT HAS DOCTOR’S APPT COMING UP] If YES: You may be eligible to participate in this study if your doctor makes any changes to your medications. Would you like me to contact you again after this appt to see if you qualify?
Date of MD appt: ____ ____ _______
MM DD YYYY
Please feel free to contact us if you are admitted to the hospital or go to the emergency room as this would also qualify you for this study. Would you like our phone number?
IF ELIGIBLE: Okay, based on these initial questions you may be eligible to be a part of the study. If you agree, I’d like to schedule a time to meet with you at the clinic to review the study in more detail. <Schedule an appointment date/time>.
____ ____ _______ _________ _______________________
MM DD YYYY Time Clinic Location
Please bring your medication bottles to this visit. I will be mailing you a reminder letter with information about your appointment. Thank you for taking the time to answer our questions over the phone. Goodbye.
IF REFUSAL READ ….
I understand that you don’t want to be in this study. But it would be helpful to us in planning future studies to have a little information about why you are not interested. Could you please let me know why you do not want to be in this study?
DO APPROPIATE REFUSAL CONVERSION USING PAPER CONVERSION SCRIPTS AND RECORD WHY REFUSED
Why refused….
1 = Too busy/No time
2 = Not interested
3 = Too sick/old
4 = Need more information/ more time to think
5 = Have had bad research experience in past
6 = In too many studies already
Other: _________________________________________________________
IF STILL REFUSAL DO REFUSAL QUESTIONS (same set of questions)
Appendix K
MTM Patient Visit Log
M
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
MTM Visit Log For Subject No. _______________
DATE |
DRUG RELATED ADVERSE EVENT? |
ANY OF THE FOLLOWING? |
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
___ ___ / ___ ___ / ___ ___ |
______________________________
|
|
Public
reporting burden for this collection of information is estimated to
average 15 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
Appendix L
MTM Study Outpatient Medication Reconciliation Audit Tool
M
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
STUDY ID:________________ DATE: _______________ □ Visit #1 □ Visit #2
INSTRUCTIONS: |
Reviewer: |
|||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
Best Possible Medication History (BPMH) Medication name, dose, route & frequency (including prescription, OTC medications and supplements) |
NO discrepancy |
Intentional Discrepancy |
Undocumented Intentional Discrepancy |
Unintentional Discrepancy |
Resolved |
Discrepancy Comments |
||||
Medication |
Dose |
Route |
Frequency |
0 |
1 |
2 |
3 |
|
Clarification of discrepancies should be noted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BPMH Discrepancy Total |
|
|
|
|
|
|||||
BPMH Discrepancy Type
|
0 |
1 |
2 |
3 |
|
|||||
Type 1= Intentional discrepancy - intentional choice to add, change or discontinue a medication and is clearly documented by physician or patient.
Type 2= Undocumented Intentional Discrepancy - intentional choice to add, change or discontinue a medication by physician or patient but this choice is not clearly documented.
T
Public
reporting burden for this collection of information is estimated to
average 30 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
Appendix M
Personal Medication Form
Personal Medication Form:
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
Bring this form with you and show it to your doctor or pharmacist any time you have a doctor’s appointment, if you have to go to the hospital, and whenever you have a new prescription filled at your pharmacy.
Patient Name: __________________________
Date of Birth: ___/__/_____ Date Form Updated: ___/___/____
mm/dd/yyyy mm/dd/yyyy
Allergies / Reaction: ___________________________________________
___________________________________________
Medications:
-
Start Date /
Stop Date
Name of Medicine
Tablet Strength
How to Use /
When to Use
What is this Medicine for?
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
Public
reporting burden for this collection of information is estimated to
average 15 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
Appendix N
MTM Clinician Time Log
Form Approved
OMB No.
0935-XXXX
Exp. Date XX/XX/20XX
MTM Clinician Time Log:
-
Patient ID
Date
Visit
Time Started
Time Ended
Time Elapsed
(to be filled in by study personnel)
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
❑First Patient Visit
❑Second Patient Visit
❑Physician contact / DRP Resolution
Public
reporting burden for this collection of information is estimated to
average 30 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
MTM Clinical Synopsis Time Log:
-
Patient ID
Date
Visit
Time Started
Time Ended
Time Elapsed
(to be filled in by study personnel)
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
❑First Patient Visit
❑Second Patient Visit
Appendix O
Patient Satisfaction Telephone Survey
Patient Satisfaction Telephone Survey
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
Instructions to interviewer
The following questions are related to study subjects satisfaction with their study or community pharmacist. Please read them to the patient and circle the patient’s level of agreement or disagreement with each item according to the scale.
Satisfaction with MTM services:
SCRIPT: I will read to you ten statements. Each of these statements refers to the care you received during the study. If you visited one of our study clinicians, please rate the care you received from that clinician. If you did not receive care from a study clinician, then rate the care you received from your community pharmacist(s) where you get your prescriptions filled. For each statement, tell me whether you strongly disagree, disagree, are not sure, agree, or strongly agree with the statement. Indicate “not applicable” for the tenth (last) statement if you did not receive care from a study clinician.
-
Item #
Question
Strongly Disagree
Disagree
Not Sure
Agree
Strongly Agree
Not Applicable
1.
Makes me feel secure about taking my medications
2.
Helps me to understand my illness
3.
Does not take time to make sure I understand the importance of my medications
4.
Is impatient and does not listen to my concerns
5.
Gives complete explanation about my medications
6.
Should give more explanations about my medications
7.
Makes me feel like a “number” rather than an individual patient
8.
Makes me feel my care is top priority
9.
Follows up on my questions and concerns
10.
Provides better care than the pharmacist where I get my prescription filled
Overall satisfaction with healthcare received
SCRIPT: Now, I will read to you three statements. Please indicate your satisfaction with the care received by all of your (UIC, Baylor, Duke) healthcare providers during the study by selecting the response that best fits your opinion. The possible responses are “very poor,” “poor,” “fair,” “good,” or “very good.”
-
Item #
Question
Very Poor
Poor
Fair
Good
Very Good
1.
How well staff worked together to provide care
2.
Overall rating of care received during your visit(s)
3.
Likelihood of your recommending our facility to others
Public
reporting burden for this collection of information is estimated to
average 5 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. . All identifiable research data obtained by AHRQ, or by
its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
Appendix P
Baseline Study Visit Questionnaire

B
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
1. What is the date of your birth? Month: _______ Day: _____ Year: _______
2. Are you Latina or Latino? 0 No �1 Yes
3. What is your race? Please select one or more.
[check one]
1 American Indian or Alaska Native
2 Asian
3 Native Hawaiian or other Pacific Islander
4 Black or African American
5 White
4. Do you have any of the following medical problems?
a. Diabetes YES NO
b. Heart failure YES NO
c. Asthma YES NO
d. High blood pressure YES NO
e. High/abnormal Cholesterol YES NO
f. Emphysema (COPD) YES NO
g. History of heart attack, heart blockage (e.g., stent placement or bypass surgery) YES NO
h. Poor kidney function YES NO
i. Arthritis YES NO
j. Depression YES NO
k. Memory problems YES NO
l. Chronic pain YES NO
m. Take blood thinner (warfarin/Coumadin) on a daily basis YES NO
5. Can you tell me the medications, including over the counter vitamins or supplements, that you take every day?
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
6. Has your doctor changed your medication dose or added a new medication within the past month? YES NO
IF YES: Date of medication change
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
7. Have you seen a new doctor in the past month? YES NO
IF YES: Date of new provider visit
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
Public
reporting burden for this collection of information is estimated to
average 30 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. . . All identifiable research data obtained by AHRQ, or
by its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
8. Have you been seen in the Emergency Room or Urgent Care clinic in the past month? YES NO
IF YES: Date of ER/Urgent care visit
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
9. Have you been discharged from the hospital in the past month? YES NO
IF YES: Date of discharge
____ ____ _______ 97 97 9997 98 98 9998
MM DD YYYY REF DNK
10. Have you had an interview at some time in the past year where you have been asked to bring in all of your medications to a pharmacist, nurse, doctor, or other healthcare professional and been given a list of all your medications?
YES NO
11. Have you been told by your doctor or a specialist that you have cancer or any other condition that might prevent you from completing this 6-month study?
YES NO
Appendix Q
Patient Visit Telephone Questionnaire
Patient Visit Telephone Questionnaire
Form
Approved
OMB No. 0935-xxxx
Exp.
Date xx/xx/2010
The purpose of this questionnaire is to collect information about patient visits to the emergency room, hospital, or doctor’s office related to adverse drug events.
SCRIPT:
Have you been keeping a diary or visit log for all of your emergency room visits, hospitalizations, and doctor’s office visits for this study?
IF YES: Great! I will wait on the line while you get the log. I have some questions to ask you and the log will make it easier to answer them.
IF NO: I have some questions to ask you. Please answer them as best you can from memory.
ED VISITS
1. Have you been to the emergency room in the last 3 months?
Yes No
If no, proceed to question 5
2. How many times have you had to go to the emergence room in the last 3 months?
Enter number: _____
If “0” proceed to question 5
3. What was the date (as best as you can remember) that you went for your (first,
second, …) visit to the emergency room? (ENTER DATES IN GRID BELOW)
4. Was this emergency room visit resulting from a side effect to one of your medicines? (ENTER YES / NO IN GRID BELOW)
REPEAT QUESTIONS 3 AND 4 UNTIL ALL ED VISITS (FROM QUESTION 2) ARE REPORTED
PATIENT REPORTED ED VISITS
-
DATE
MEDICATION SIDE EFFECT?
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
Public
reporting burden for this collection of information is estimated to
average 15 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. . . All identifiable research data obtained by AHRQ, or
by its contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c)
HOSPITAL VISITS
5. Have you been admitted to the hospital in the last 3 months?
Yes No
If no, proceed to question 9
6. How many times have you been admitted to the hospital in the last 3 months?
Enter number: _____
If “0” proceed to question 9
7. What was the date (as best as you can remember) that you were admitted for your (first, second, …) hospitalization? (ENTER DATES IN GRID BELOW)
8. Was this hospitalization resulting from a side effect to one of your medicines? (ENTER YES / NO IN GRID BELOW)
REPEAT QUESTIONS 7 AND 8 UNTIL ALL HOSPITALIZATIONS (FROM QUESTION 6) ARE REPORTED
PATIENT REPORTED HOSPITALIZATIONS
-
DATE
MEDICATION SIDE EFFECT?
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
DOCTOR’S OFFICE VISITS
9. Have you visited your doctor in the last 3 months?
Yes No
If no, proceed to next survey
10. How many times have you visited your doctor in the last 3 months?
Enter number: _____
If “0” proceed to next survey
11. What was the date (as best as you can remember) of your (first, second, …) visit to your doctor’s office? (ENTER DATES IN GRID BELOW)
12. Was this doctor’s office visit resulting from a side effect to one of your medicines? (ENTER YES / NO IN GRID BELOW)
REPEAT QUESTIONS 7 AND 8 UNTIL ALL DOCTOR’S OFFICE VISITS (FROM QUESTION 6) ARE REPORTED
PATIENT REPORTED DOCTOR’S OFFICE VISITS
-
DATE
MEDICATION SIDE EFFECT?
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
___ ___ / ___ ___ / ___ ___
□ Yes □ No
END OF SURVEY
Appendix R
Federal Register Notice
and
Response to Comments

Response to Public Comments
Background text from the letters has been removed. Text of actual comment or question is included below for clarity. Responses are underlined.
I. Letter received from Calvin H. Knowlton, Kevin T. Bain, and JoAnne Reifsnyder of “exceleRx,” an Omnicare Company, Philadelphia PA
AHRQ and the investigators thank Calvin H. Knowlton, Kevin T. Bain, and JoAnne Reifsnyder of “exceleRx,” an Omnicare Company, Philadelphia PA, for their comments. These comments have been used to revise and improve the study protocol.
1) Whether the proposed collection of information is necessary for the proper performance of healthcare research and information dissemination functions of AHRQ; including whether the information will have practical utility.
It is difficult to evaluate the clinical utility of the findings produced via the proposed design because of questions surrounding the standardization of the intervention and control of confounding variables. We have the following concerns about the design of the study:
a. Study design favors self selection of patients who are mobile, able to accurately self-report data elements and have sufficient cognitive function and health status to benefit from patient education and participate for the study duration. How then will this study design inform about how to intervene for the oldest and sickest beneficiaries?
Clearly no single study can address all patient populations that might benefit from medication therapy management (MTM). In order for this study to be both practical and generalizable to the broadest range of Medicare beneficiaries, we have targeted those that are mobile, able to accurately self-report data the required elements and have sufficient cognitive function and health status to benefit from the intervention and participate for the study duration. Nevertheless, we recognize that certain patients may be at higher hisk than others. Therefore, the protocol has been revised to focus on those beneficiaries at greater risk of drug related problems (DRPs) and adverse drug events (ADRs) – and thus presumably in the greatest need for medication therapy management (MTM). Entry criteria in the revised protocol include 1). must be at least 65 years old as of August 1, 2007; 2). must have 3 or more comorbid conditions associated with increased healthcare utilization (conditions include diabetes mellitus, heart failure, asthma, hypertension, dyslipidemia, COPD, coronary artery disease, chronic renal failure, arthritis, depression, dementia, chronic pain, and conditions requiring anticoagulation); 3). must have visited a physician at one or more of the clinics on a regular basis (defined as 2 or more clinic visits over 1 year prior to the study start) for these conditions; 4). must have received 8 or more different chronic prescription medications over the 6 months prior to the enrollment period; 5) must have a telephone line and agree to maintain it for at least 6 months; and 6). must have one of the following situations placing him/her at risk for a DRP: a) ER visit in past 30 days, b) new physician in past 30 days, c) hospitalization in past 30 days, d) change in medication in past 30 days, or e) 3 or more providers.
b. Although five specific services are identified, they are not standardized. Lack of a standardized intervention means that it will be impossible to draw valid comparisons between the control and intervention groups.
The intervention has been revised to focus on medication reconciliation and DRP assessment. The intervention will be well-defined, with specific tools or “aids” for implementation, and it will be standardized across the study sites. A “toolkit” will be produced that will allow the intervention to be reproduced once the study is completed.
c. The identified interventions do not address the most critical element of MTM - assessment of the appropriateness of mediations, including identification of untreated indications that would be followed by recommendations to prescribers. In the proposed study, while pharmacists will be addressing continuity of care, the assumption appears to be that the initial prescription choice is always appropriate. We believe this is a major weakness of the study protocol.
Assessment of untreated indications is clearly one type of DRP that will be assessed as part of this study. Follow-up recommendations will be provided to prescribers.
d. Of the five interventions identified in the study protocol, several fall outside the purview of a pharmacist's traditional training such as scheduling follow-up doctor's appointments, obtaining transportation to the clinic or motivating patients to take their medications using motivational interviewing techniques. We are concerned about the feasibility and cost of training pharmacists to undertake these tasks.
The purpose for this study is to assess components of MTM. Providers of MTM may include pharmacists or other healthcare professionals. Thus, a “pharmacist’s traditional training” is not necessarily relevant to the design of the intervention. Nevertheless, as mentioned above, the intervention has been revised to focus on medication reconciliation and DRP assessment - two activities which are likely within the purview of most pharmacists.
e. What is the method of that will be used to achieve an equal or roughly proportional number of enrollees in each group?
Stratified randomization.
Similarly, how is the site effect controlled for, particularly since a disproportionate number of subjects may be enrolled at the UIC site (100 patients)?
There will be an equal number of subjects enrolled at each site.
How will the investigators account for failure to enroll beneficiaries in either group?
Patients will be consented and then randomized to one of the 3 groups.
f. Presumably, participants in the control group will be receiving some level of MTM by virtue of Part D enrollment. How will "usual care", which could contain widely variable applications of MTM, be defined, measured, controlled, and distinguished from the "intervention"?
Patients already enrolled in an MTM program where medication reconciliation and/or assessment of DRPs has occurred in the previous 12 months will be excluded.
g. What methodology will be employed to control for potential confounders residing with the pharmacist; for example, pharmacist tenure/experience with MTM services?
There will be a limited number of pharmacists at each site (1 or 2) and all of the pharmacists will be of similar tenure/experience.
h. How will non-adherence to scheduled monthly MTM program visits and subsequent missing data be accounted for? Will this be a last-observation-carried-forward study? Will beneficiaries who do not keep appointments for some percentage of their scheduled follow-up visits be excluded or treated as controls? Is there a procedure for identifying why patients leave the study?
The intervention has been changed from monthly visits to just 2 visits. With this reduced number of visits we do not anticipate significant non-compliance. Evaluated using intent to treat.
i. Will pharmacists evaluate all of the beneficiary's medications or just those that are Part D covered? Presumably, one would assume the former; however, this should be explicitly stated. And again, how does this differ from "usual care"? Likewise, how will non-Part D medications, particularly samples and OTC medications, be accounted for regarding medication adherence (patient self-report, pharmacy claims, both)?
The program will evaluate all medications. Medication adherence has been removed as an outcome measure for the study.
It seems unlikely that when assessed for 12-month recall of adverse events at the close of the study, participants will be able to relate an accurate history. A participant log or diary might support recall of events.
We will assess this at day 90 and 180 for only the preceding 3 months. Recall over 3 months should not be a problem for most patients. We will use a participant log to assist in recall.
2) The accuracy of AHRQ's estimation of burden (including hours and costs) of the proposed collection(s) of information;
We believe that the investigators may have underestimated the time required to collect information from study participants and to abstract data from clinical records, particularly given the number of tools and measurements that will be employed. Additionally, there appears to be no formal training of pharmacists in the utilization and application of these instruments, which may further underestimate burden.
The revised protocol may contain more data upon which to assess the time requirement. Significant changes have been made to reduce the patient and investigator time. A formal training session will be held for pharmacist who will provide the intervention and tools have been developed to standardize the intervention as mentioned above.
3) Ways to enhance the quality, utility and clarity of the information to be collected;
As we have communicated in the list of questions/concerns about design (above), we believe that the study could be strengthened by:
a. Clearer definitions of the intervention "MTM program" and the "dose" (that is, the specific type and amount of services that the treating pharmacist elects to provide a given beneficiary). If doses are not standard across beneficiaries, as one would expect, what characteristics/criteria will be used to determine dose?
The revised protocol may contain more data upon which to assess these issues. The intervention has been more clearly defined and narrowed in scope, and the number of visits has been reduced.
Explication of techniques for accrual, randomization, and follow up on missed appointments and handling of missing data.
All of these items are included in the revised protocol.
4) Ways to minimize the burden of the collection of information upon the respondents, including the use of automated collection techniques or other forms of information technology.
While we strongly favor and actively use electronic clinical records for MTM, we have found that automated data collection techniques (such as interactive voice response, or IVR) are frequently impractical for collecting data from older adults who may have difficulty hearing, need more time to respond than is typically programmed, and need to have items repeated. However, data collection via telephone with a "live" data collector and web-enabled medication management devices that also track adherence and present questions/collect information from patients are potentially very useful in terms of data accuracy and completeness. One caution exists in terms of interpretation and generalizability of the findings: automated data collection techniques might "cue" the beneficiary in a way that inflates the effect; as such, cues would presumably not be present in standard (non-experimental) application of MTM programs. This could lead to inflation of effect and potential Type 1 error. Studies are needed that explore the feasibility and utility of automated data collection with older adults who are at risk for medication-related problems and poor outcomes.
This study uses no automated collection techniques.
II. Letter received from Michael S. Maddux and C. Edwin Webb of the American College of Clinical Pharmacy, Lenexa, KS
AHRQ and the investigators thank Michael S. Maddux and C. Edwin Webb of the American College of Clinical Pharmacy, Lenexa, KS, for their support and comments. These comments have been used to revise and improve the study protocol.
ACCP recommends the inclusion of additional survey questions that would investigate the process by which beneficiaries are being informed and educated about the availability of MTMPs for eligible enrollees, and how the plans are promoting MTMPs among their enrollees.
The purpose of the proposed study is to evaluated a specific MTM intervention. While important questions, a survey of MTM providers about the process by which beneficiaries are being informed and educated about the availability of MTMPs for eligible enrollees, and how the plans are promoting MTMPs among their enrollees is not within the scope for the study.
III. Letter received from Ronna B. Hauser of the National Association of Chain Drug Stores, Alexandria, VA.
AHRQ and the investigators thank Ronna B. Hauser of the National Association of Chain Drug Stores, Alexandria, VA., for their support and comments. These comments have been used to revise and improve the study protocol.
We strongly believe that any research in the area of MTMP will be very helpful in determining the effect of these programs, but it appears in the proposed project that community pharmacy sites are being excluded from the study. Community pharmacy must be represented in any study evaluating the effectiveness of MTMP, so as to determine potential strengths and/or barriers to providing these programs in the community pharmacy setting. NACDS questions the broad applicability of this research project based on the sites from which study subjects will be recruited and we strongly encourage the involvement of community pharmacy practice sites in this project.
AHRQ recognizes the importance of community pharmacy as one site in which MTM may be provided. No study can be designed to include all aspects of diversity in the site of provision of MTM. For this study we attempted obtain a balance between availability of data needed to assess the impact of the intervention, and the generalizability of the setting of care. In revisions made to the protocol we have focused on developing an intervention that could be conducted in a community pharmacy, and as such may be generalizable to community pharmacies.
Appendix S
Consent Form
Form
Approved
OMB No. 0935-XXXX
Exp. Date XX/XX/20XX
University of Illinois at Chicago
Consent for Participation in Research
“Evaluation of Medication Therapy Management Programs to Improve Patient Safety
in Medicare Beneficiaries at High Risk of Adverse Drug Events”
Why am I being asked?
You have been asked to participate in the research because you are over 65 years of age, primarily speak English, have 3 or more chronic conditions, are taking eight for more medications, have a situation that places you at risk for a drug related problem, and may be eligible to participate. We ask that you read this form and ask any questions you may have before agreeing to be in the research.
This research is being conducted by Daniel Touchette of the College of Pharmacy at the University of Illinois at Chicago (UIC) and at two other study sites, Baylor Health Care System in Texas and the Duke Primary Care Research Consortium in North Carolina. The study is funded by the Agency for Healthcare Quality and Research.
Your participation in this research is voluntary. Your decision whether or not to participate will not affect your current or future relations with the University of Illinois at Chicago. If you decide to participate, you are free to withdraw at any time without affecting that relationship.
Why is this research being done?
This study is being done to find better ways of helping Medicare beneficiaries taking eight or more long-term medications and with an increased risk of having adverse drug events create a complete list of their medications and resolve any drug related problems.
What is the purpose of this research?
The purpose of this research is to find out if a higher level of patient care can prevent adverse drug events. Specifically, we are interested in seeing if interviewing a patient, developing a medication list, and reviewing the list for possible errors, drug interactions, and drug appropriateness can reduce side effects from medications, improve health, and raise satisfaction with healthcare.
What procedures are involved?
If you agree to be in this research, we would ask you to do the following things:
Random Assignment and Clinic Visits
You will be randomly assigned to one of three study groups. Once you provide your written consent to participate in this research, a study investigator will choose a numbered envelope. Your study group assignment will be inside this envelope. The study investigator will not know before opening the envelope which group you will be assigned to.
All study participants will be asked to complete one entry visit and answer two telephone surveys. Depending on the group to which you are assigned, some participants may be asked to come in for two 10-30 minute meetings with a clinician, while others may not have to do so. The first (baseline) visit will be done on the same day as providing this informed consent, right after if possible. The telephone surveys will be done about three and six months later. Some study participants will also be asked to come to UIC for up to 2 more visits between the first (baseline) visit and last telephone survey. As a result of this study, we may request that your physician make changes to your medications. However, no changes will be made to your medications without both your and your physicians consent.
Baseline Study Visit
If you agree to participate in this study, you will be asked to come to a baseline study visit and must bring all of the medications that you are on to this visit. At this visit you will be asked to fill out a short survey with questions about you, your medical history, what medications you are on, and how you take these medications. Answering these questionnaires and completing the visit should take between 5 and 20 minutes.
Patient Diary and Telephone Surveys
If you agree to participate in this study, you will be asked to maintain a diary of all symptoms that may be side effects to medications, as well as doctor, emergency room, and hospital visits you have during the study. You will also be asked to complete two telephone surveys. In these surveys, you will be asked about possible side effects to medications you are taking and about doctor, emergency room, and hospital visits. Some subjects may be asked about their satisfaction with their healthcare. Answering these questionnaires and completing the office visit should take between 20 and 60 minutes.
Information from Medical Records
We may also need to record your name and medical record number in order to get information from your medical record about recent medications, illnesses, allergies, and laboratory and other tests conducted by your doctors. Because we may need access to your health information for this research, if you choose not to allow us to access your medical records we will not be able to include you in the study.
Approximately 200 participants may be involved in this research at the UIC. Another 400 patients from Baylor Health Care System in Texas and the Duke Primary Care Research Consortium in North Carolina may be involved in this research as well.
What are the potential risks and discomforts?
The research has several risks: The main risk of this study is breach of confidentiality. Although the study's investigators will do everything they can to protect confidential information collected from study subjects, there is a small chance that information could be lost, misplaced, or stolen by an individual not involved in the study. This risk is likely less than the risk of similar information being taken from a patient's medical records. Another risk is that some of your medications may be changed, with the possibility that the medication may result in side effects or may not work as well for you. No changes will be made to your medications without both your and your physicians consent.
Are there benefits to taking part in the research?
You may or may not personally benefit from being in this study. A benefit from this study is that medication therapy may be improved upon, leading to the possibility of fewer side effects and better health. You may help us learn how to benefit patients in the future by serving as a subject in this study.
Will I be told about new information that may affect my decision to participate?
During the course of the study, you will be informed of any significant new findings (either good or bad), such as changes in the risks or benefits resulting from participation in the research or new alternatives to participation, that might cause you to change your mind about continuing in the study. If new information is provided to you, your consent to continue participating in this study will be re-obtained.
What about privacy and confidentiality?
The only people who will know that you are a research subject are members of the research team. No information about you, or provided by you during the research, will be disclosed to others without your written permission, except:
if necessary to protect your rights or welfare (for example, if you are injured and need emergency care or when the UIC Institutional Review Board monitors the research or consent process); or
if required by law.
Because we may need access to your health information for this research, if you choose not to allow us to access your medical records we will not be able to include you in the study. You will need to sign a separate authorization form to allow us access your medical records, in addition to this consent form.
By signing this separate authorization form, you are also allowing access of your medical record, and the disclosure of your health information to Dr. Daniel Touchette, his research team, and the Agency for Healthcare Research and Quality (the study’s sponsor). This health information includes name, medical record number, the results of physical exams, blood tests, x-rays and other diagnostic and medical procedures, as well as medical history and medications recommended and/or prescribed. Medical history may include all known hospitalizations, emergency room visits, and clinic visits for 12 months before the study enrollment and throughout the 6 month study period.
You should know that research publications and scientific meetings will never include information about you that would identify you individually. There are University, state and federal agencies and officials who ensure the protection of human subjects in research, and they might access your records in line with their official duties or as required by law.
All of your personal information, research data, and related records will be stored in a locked filing cabinet (if on paper) or in a computer file protected by a password (if electronic). Any information that could be used to identify you will be kept separate from all other health record information and linked using a code available only to the investigators. The surveys, research data, and other research records will be stored for a minimum of six years according to the law. After six years the data will be destroyed using a cross-cut shredder (if paper) or will be deleted using a program that overwrites the data (if electronic).
Authorized representatives of the Agency for Healthcare Research and Quality may need to review records of individual subjects. As a result, they may see your name; but they are bound by rules of confidentiality not to reveal your identity to others.
What if I am injured as a result of my participation?
In the event of injury related to this research, treatment will be available through the UIC
Medical Center. However, you or your third party payer, if any, will be responsible for payment
of this treatment. There is no compensation and/or payment for such medical treatment from the
UIC Medical Center for such injury except as may be required of the University by law.
If you feel you have been injured, you may contact the researcher, Daniel Touchette at 312-355-3204.
What are the costs for participating in this research?
Neither you nor your insurance company will be billed for your participation in this research.
Will I be reimbursed for any of my expenses or paid for my participation in this research?
For participating in this research, you will be paid $10 for the first (baseline) visit and $10 for each of the telephone surveys, for a total of $30. If you withdraw from the study after completing only the first (baseline) study visit, you will be paid $10.
Can I withdraw or be removed from the study?
You can choose whether to be in this study or not. If you volunteer to be in this study, you may withdraw at any time without consequences of any kind. You may also refuse to answer any questions you don’t want to answer and still remain in the study. The investigator may withdraw you from this research if circumstances arise which warrant doing so. If you withdraw or the investigator withdraws you from the study, you will be paid $10 if you have completed the first (baseline) study visit. If you have not completed the first (baseline) study visit, you will not be paid for participating in the study.
Who should I contact if I have questions?
The researcher conducting this study is Daniel Touchette. You may ask any questions you have now. If you have questions later, you may contact the researcher at: Phone: (312) 355-3204
What are my rights as a research subject?
If you have any questions about your rights as a research subject, you may call the Office for Protection of Research Subjects at 312-996-1711.
What if I am a UIC employee?
Your participation in this research is in no way a part of your university duties, and your refusal to participate will not in any way affect your employment with the university, or the benefits, privileges, or opportunities associated with your employment at UIC. You will not be offered or receive any special consideration if you participate in this research.
Remember: Your participation in this research is voluntary. Your decision whether or not to participate will not affect your current or future relations with the University of Illinois at Chicago. If you decide to participate, you are free to withdraw at any time without affecting that relationship.
You will be given a copy of this form for your information and to keep for your records.
Signature of Subject
You have read (or someone has read to you) the above information. You have discussed the procedures, risks and benefits of the study with the researchers. You have been given an opportunity to ask questions and your questions have been answered to your satisfaction. You agree to participate in this research. You will be given a copy of this signed form for your information and to keep for your records. The original copy will be stored in the research file and a copy may be placed in the University of Illinois Medical Center medical record.
Signature Date
Printed Name
Signature of Researcher Date (must be same as subject’s)
Printed name of Researcher
Public
reporting burden for this collection of information is estimated to
average 5 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by its
contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c).
Appendix T
Authorization to Use and Disclose Health Information
Form
Approved
OMB No. 0935-XXXX
University of Illinois at Chicago
Authorization To Use And Disclose Health Information For Research
“Evaluation of Medication Therapy Management Programs to Improve Patient Safety in Medicare Beneficiaries at High Risk of Adverse Drug Events”
You are being asked to permit Daniel Touchette and staff members of the College of Pharmacy to use and disclose protected health information (PHI) that identifies you for the research purposes described below. You are also being asked to permit your doctors and other health care providers to disclose PHI to these Researchers for the purposes described below. The privacy law [45 CFR Parts 160 and 164], Health Insurance Portability and Accountability Act (HIPAA) provides additional protections for PHI. You must sign this authorization if you wish to allow your PHI to be used or disclosed for this research.
Description of protected health information to be used and disclosed
The protected health information that may be used and disclosed includes all information collected during the research described in the Consent for Participation in Research entitled: “Evaluation of Medication Therapy Management Programs to Improve Patient Safety in Medicare Beneficiaries at High Risk of Adverse Drug Events”
The protected health information that may be used and disclosed includes all protected health information in your medical records that is related to the research including illnesses and hospitalizations that occur while you are participating in the research, as described in the Consent for Participation in Research entitled “Evaluation of Medication Therapy Management Programs to Improve Patient Safety in Medicare Beneficiaries at High Risk of Adverse Drug Events.” The health information includes demographic information, the results of physical exams, blood tests, x-rays and other diagnostic and medical procedures, as well as medical history.
The protected health information that may be used and disclosed includes the information as described above, which is collected and maintained by your physicians and other healthcare providers which are identified below:
Alexandra Perez, PharmD, Daniel Touchette, MA, PharmD, and Mary Ann Kliethermes, PharmD.
Research use of your protected health information
The Researchers can use and share your protected health information with each other and with other agencies described below, as required to conduct the research and as described in the informed consent form;
The Researchers can disclose your protected health information to the sponsor of the research, the Agency for Healthcare Research and Quality, as required for the research and if further information is needed to confirm the research;
The Researchers can disclose your protected health information to other collaborators of the research study Baylor Health Care System in Texas and the Duke Primary Care Research Consortium in North Carolina ;
The Researchers can disclose your protected health information to representatives of government agencies (i.e., Food and Drug Administration) where required by law; and
The Researchers can disclose your protected health information to the University of Illinois Medical Center at Chicago and University of Illinois at Chicago representatives including the Institutional Review Board.
Once the Researchers disclose your information to anyone outside of the study, it may be re-disclosed and may no longer be protected by this Authorization and the federal privacy regulations.
Protection of your health information
The Researchers and the Agency for Healthcare Research and Quality agree to protect your health information by using and disclosing it only as permitted by you in this Authorization or as is directed by state and federal law. Further, no publication about the research will reveal your identity without your express written permission. These limitations continue even if you decide to revoke (take back) this Authorization.
Removal of your identifying information (De-Identification)
Once the information that identifies you is removed, the information that remains is no longer subject to this Authorization or to HIPAA. The remaining information may be used and disclosed by the Researchers as permitted by law and may be used and disclosed for other research purposes.
Your options
You do not have to sign this Authorization, but if you do not, you will not be allowed to participate in this research study. However, if you decide not to sign this authorization it will not affect your treatment, payment or enrollment in any health plans or affect your eligibility for benefits.
Expiration of Authorization
This Authorization does not have an expiration date, but can be terminated if you decide to withdraw your permission.
Withdrawal or removal from the study
You may change your mind and revoke this Authorization at any time. To revoke this Authorization, you must write to: Daniel Touchette, 833 S. Wood Street, Chicago, IL 60612. However, if you revoke this Authorization, you may no longer be allowed to continue participation in the research study. Furthermore, even if you revoke this Authorization, the Researchers may still use and disclose health information they already have obtained as necessary to maintain the reliability of the research and to report any adverse effects (bad events) that may have happened to you.
Contact information for questions about my rights under HIPAA
If you have questions or concerns regarding your privacy rights under HIPAA, you should contact the University of Illinois at Chicago Privacy Officer at Ph: (312) 996-2271.
If you have not already received a copy of the Notice of Privacy Practices, you should request one. You will be given a copy of this Authorization after it has been signed to keep for your records.
Signature of Subject
I have read (or someone has read to me) the above information. I have been given an opportunity to ask questions and my questions have been answered to my satisfaction. I authorize the use and disclosure of my protected health information for this research.
Signature of Subject Date
Printed Name of Subject
Public
reporting burden for this collection of information is estimated to
average 5 minutes per response, the estimated time required to
complete the survey. Send comments regarding this burden estimate or
any other aspect of this collection of information, including
suggestions for reducing this burden, to: Doris Lefkowitz, Reports
Clearance Officer, AHRQ, 540 Gaither Road, Room # 5036, Rockville,
MD 20850. All identifiable research data obtained by AHRQ, or by its
contractors and grantees, is protected by the statutory
confidentiality provision found at 42 U.S.C. § 299c-3(c).
Page
| File Type | application/msword |
| File Title | Supporting Statement for “Evaluation of a Medication Therapy Management Program on Patient Safety in Medicare Beneficiaries at H |
| Author | CMS |
| Last Modified By | wcarroll |
| File Modified | 2007-11-05 |
| File Created | 2007-11-05 |
© 2026 OMB.report | Privacy Policy