10-02-07 memo
OMB_Review_Responses_2_070910.doc
Evaluation of a Medication Therapy Management Program to Improve Patient Safety in Medicare Beneficiaries
10-02-07 memo
OMB: 0935-0136
1. Please provide responses to the Medco comment
See attached.
2. confidentiality: please confirm whether the Privacy Act will stand up to a FOIA request. If AHRQ were FOIA’d in regards to this study, would AHRQ still be able to keep all information confidential?
In regard to a FOIA request, the Privacy Act protects confidential and proprietary information. A person’s identity and information would be considered confidential information and therefore be protected by the Privacy Act. It is our understanding that if a court order was issued for the data, all personal identifying information about the respondents would be removed. We have also added the AHRQ Confidentiality Statute on all forms: “All identifiable research data obtained by AHRQ, or by its contractors and grantees, is protected by the statutory confidentiality provision found at 42 U.S.C. § 299c-3(c).”
3. baseline study visit
a. randomization occurs here? Or at the conclusion of the telephone screening? (appendix J)
Randomization occurs at the baseline study visit, after informed consent is signed.
b. Baseline questionnaire (appendix P) given to all 3 groups? Or just to control group? If given to all 3 groups, isn’t this duplicative of the information you will get through appendix A and B?
The baseline questionnaire (appendix P) is asked of all 3 groups by the research assistant. We want to treat all 3 groups the same in the study to minimize any bias that might occur. Also, the research assistant’s skill at (and the process for) soliciting information from the patients is different than that of the MTM clinician and we want comparable information on what the patient believes they are taking from all 3 groups for our baseline demographic information. This is the only point in time where we can get comparable information from all 3 groups, even if it is somewhat duplicative.
c. All patients are told to bring in medications, even those who will ultimately be assigned to control group? (see appendix J)
Yes, all 3 groups will be asked to bring in their medications.
4. where are the burdens associated with appendix P, Q, and S accounted for in table A1 and in ROCIS/ICRAS?
5. will appendix G and O (outcomes survey and patient satisfaction survey) be conducted on the same phone call or will the participant be called on two separate occasions?
Appendixes G and Q will be used for all 3 study groups. Appendix O will be used only for the 2 intervention groups. In the intervention groups, Appendixes G, Q, and O will all be conducted on the same phone call.
6. How will differences between patient self-report and the clinical synopsis be documented? Is there a form for this?
In the medical history, allergies, and laboratory worksheet (page 1 of Appendix B MTM Clinical Documentation Tool), the source of the information will not necessarily be documented. There is white space on the form for comments, if necessary. For the medication list (page 2 of the Appendix B), there is a checkbox in the medication list to identify the source of the information, in order to assist with identifying discrepant information and DRPs.
7. appendix I: please make it clear that participation is voluntary (probably right before the paragraph that says “whether you join the study or not…”)
Done.
8. appendix Q: at what point in the study is appendix Q used?
Appendix Q will be conducted for all study groups at each of the telephone interviews.
9. appendix S: doesn’t the language about access to medical records belong in the HIPAA release? And to the extent that a patient may authorize release of some of the medical data listed in the consent form but not all, how will these discrepancies be handled? Will HIPAA override the consent?
This information is included in the informed consent as part of the required section “What procedures are involved?” In this section of the informed consent, we are required to describe any and all procedures to the patient in lay language. The language in the HIPAA release is much more descriptive and includes specifically what protected health information will be used and disclosed and by whom it will be collected and maintained.
Specific (embedded) comments:
KM1. So essentially, a discrepancy is a type of DRP?
A discrepancy is a specific type of DRP; a difference between the prescriber’s documented intent and the patient’s actual medication taking behavior (but different from adherence)?
KM2. The differences between DRP and ADE are still a bit unclear. According to the supporting statement, it seems like ADEs are the outcomes of some DRPs. However, the definition for DRP above implies that ADEs are a type of DRP.
The following diagram from Aronson et al. (Drug Safety 2005: 28: 851-870) may assist with the interpretation of this terminology:
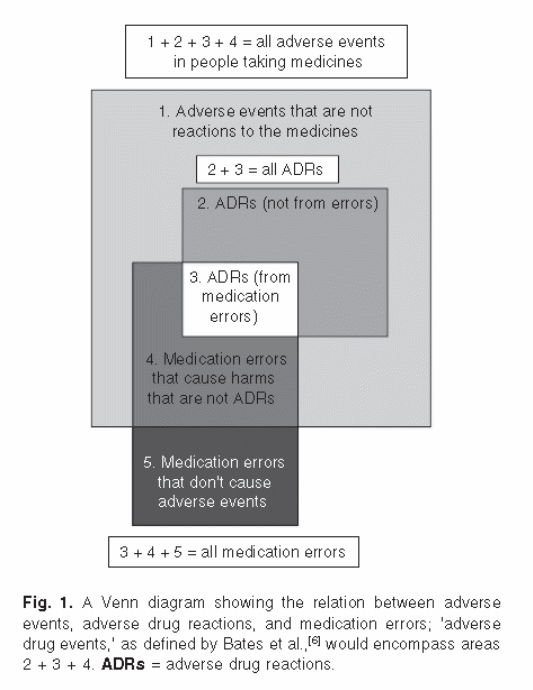
DRPs and ADEs are distinct and different concepts, occurring at different periods of time. A DRP is a potential error or other problem that may or may not already be causing harm to the patient. Identifying DRPs is a preventive measure that is expected to reduce the number of ADEs experienced by the patient. DRPs may or may not be medication errors. They are medication errors if the prescriber was not aware of the potential problem at the time of prescribing the medication. They are not medication errors if the prescriber was award of the potential problem, assessed the risk-benefit, and decided to prescribe the medication anyways (benefit outweighed risk). With DRPs the prescriber’s intent is not yet known, differentiating them from medication errors.
ADEs are harm that has been caused by a drug or inappropriate use of a drug. ADEs can occur even with appropriate use of medications (correct dose and no identifiable DRP), as a side effect of the medication. This type of ADE is frequently termed non-preventable ADE. ADE’s can also be caused by medication errors, often termed an preventable or ameliorable ADE.
KM3. How will this study address the cultural/ethnic/linguistic barriers referenced in the response to A1 if all the respondents are English speakers?
We will know from the initial screening if there are any cultural/ethnic/linguistic barriers, however, the budget constraints for the trial preclude us from examining these barriers in depth, so we are conducting the program with English speakers only.
KM4. This is rather surprising as MR would seem to be the fundamental feature of MTMs. If MTMs are not currently doing MRs routinely, what are they doing?
Also, it is fairly common practice—especially at university medical centers—for doctors to do MRs. Even if patients are not enrolled in an MTM, they may already have MRs. What percentage of patients at these hospitals are expected to NOT have MRs, either through their MTMs or their physicians?
Medication reconciliation (MR) is a generic term that encompasses varying levels of care. For our study, we have defined MR as a process of building a complete medication list based on the most current information taken from prescription bottle directions, patient interview, and medical record information with the goal of reducing medication errors. MR is a time consuming process, without evidence of its impact on patient care and so is not widely utilized in the outpatient setting. As evidenced by the letter from Medco, MR does make up a part of their MTM program, but they are concerned that our proposed process should not become definitive of MTM requirements in the future. Our study, conducted jointly with the American Pharmacists Association, showed that the majority of MTM programs in their first year relied heavily on mass mailings as a form of patient education. Although 60% of MTM programs offered medication review as part of their services, the vast majority utilized a tiered system for triaging patients to various levels of care. Our advisory groups have indicated that a very small proportion of patients are actually receiving medication reviews and that even when used, they are not being conducted on an ongoing basis. From discussions with our clinicians, community providers, and insurers, we have determined that it is unlikely that many patients are receiving MR that includes listings from prescription bottles and a thorough medication history.
KM5 At what point in the enrollment process is this HIPAA waiver obtained? Please provide a copy of it.
A HIPAA waiver is granted by the IRB as part of the study’s approval and is required for collecting patient identifiable information prior to informed consent. This is needed to identify eligible study subjects and obtain their contact information.
I assume that the question here is actually referring to the HIPAA “Authorization to Use and Disclose Health Information for Research.” This document will be presented to the patient for signing at the same time as informed consent. A copy of this document is enclosed.
KM6. So every participant will need to provide this information twice? Once at this screening interview and again at the baseline interview? Can’t they be consolidated so that the participants only have to do it once?
The baseline questionnaire (appendix P) is asked of all 3 groups by the research assistant. We want to treat all 3 groups the same in the study to minimize any bias that might occur. Also, the research assistant’s skill at (and the process for) soliciting information from the patients is different than that of the MTM clinician and we want comparable information on what the patient believes they are taking from all 3 groups for our baseline demographic information. This is the only point in time where we can get comparable information from all 3 groups, even if it is somewhat duplicative.
KM7. Don’t you need a HIPAA authorization to do this? And if you can collect this information from electronic health records, why do you need to rely on patient self-report? On the basis of the chart and billing review, couldn’t you exclude all the non-eligible patients?
HIPAA authorization is required to do this. This statement has been removed from this questionnaire.
KM8. re: our response: This questionnaire may be revised to include 2 additional questions about whether the patient has a terminal condition (life expectancy less than 6 months) and whether they have had a medication reconciliation (explained in lay language as a time when they have been asked to bring all of their medications to a meeting with a pharmacist, nurse, or physician and provided a list of the medications they should be taking) done in the previous 12 months.
Doesn’t it HAVE to be revised to ensure that patients, particularly in the control group, are really not privy to the intervention you are testing?
Both the screening telephone script and the initial office visit have been revised to address concerns regarding enrolling ineligible study subjects.
KM9. Please specify the timeline for when all of the things listed here will happen. Does the baseline visit happen separately from the MTM visits? When does randomization occur? At the baseline visit or before? When does the letter to patients from treating doctor go out? (appendix I) When is the HIPAA waiver for chart review obtained? Does this happen prior to the schedule and billing screening? When does the telephone screening to gauge interest in participating and eligibility screen happen? (appendix J) What is used at the baseline visit (which IC)? What does the control group do at the baseline visit?
We have developed the following graphic to better describe the study’s flow:
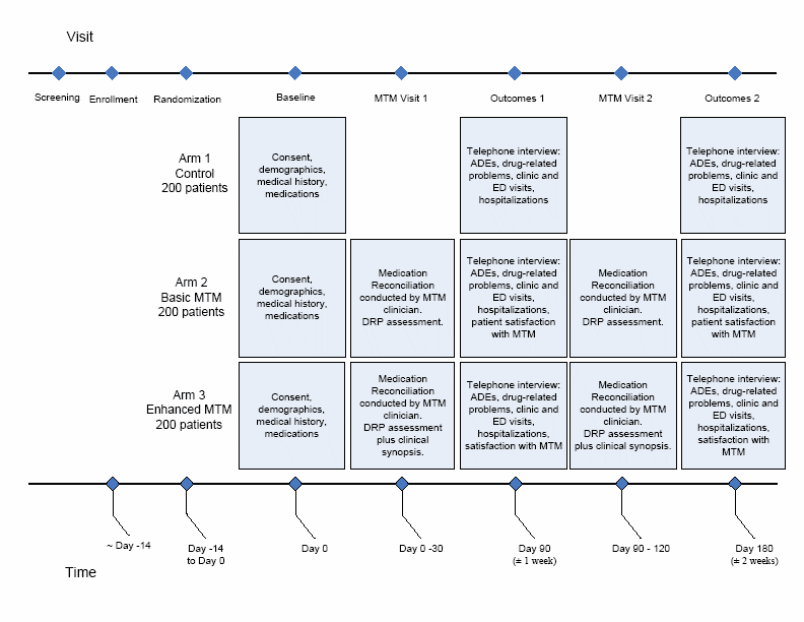
The Appendixes that will be administered at each time are as follows:
|
Screening |
Randomization |
Baseline |
MTM Visit 1 |
Telephone Outcome 1 |
MTM Visit 2 |
Telephone Outcome 2 |
Control
|
J |
Consent HIPAA auth. |
P |
- |
G |
- |
G |
Basic MTM |
J |
Consent HIPAA auth. |
P |
A B (from interview) C D M |
G O Q |
A B C D M |
G O Q |
Enhanced MTM |
J |
Consent HIPAA auth. |
P |
A B (from chart and interview) C D M |
G O Q |
A B C D M |
G O Q |
Notes:
Enrollment, randomization, baseline visit, and MTM visit 1 will all occur on the same day, whenever possible.
Appendix I (Initial Patient Contact Letter) will be sent to the patient prior to the telephone screening visit.
Appendix K (Patient Visit Log) will be given to the patient at enrollment for use throughout the study period.
Appendix L (Outpatient Medication Reconciliation Audit Tool) will be used by blinded investigators in a subset of intervention patients after their first MTM visit.
Appendix N (MTM Clinician Time Log) will be used by the MTM clinicians throughout the study, whenever they are interviewing or addressing patient-related issues.
KM10. What is AHRQ’s plan if the IRB recommends changes to any forms approved by OMB?
We will inform OMB if any major revisions are required, though we are not anticipating any at this point in the process. Once finalized, all forms will be submitted to OMB for their permanent records.
KM11. Re: why aren’t you collecting the burden estimates associated with extracting data from patients’ records, analyzing data from records, and telephone interviews?
OMB would recommend collecting this data.
We have modified Appendix N to include a time log for collecting this data (MTM Clinical Synopsis Time Log)
KM12: Re: collection of time burden estimates for these items would not likely be helpful to the public and would increase the documentation burden of the investigators. Again, we can collect this information if we are required.
This would not be required. However, any time burdens associated with implementing the MTM should be recorded. Along with the time burden for the clinical synopsis, what other time burdens will Appendix N record?
Appendix N will be used to document the time burden for the MTM Clinicians’ intervention, including patient interview, development of the medication list, identification of drug related problems, resolution of drug related problems (preparing and faxing Appendix D to physicians, dealing with any physician requests or questions, and communicating new medication orders to the patient and their dispensing pharmacy as required) and documentation of these activities.
KM13. Re: Note that the Beers’ list is not a list of contraindicated medications in the elderly.
There were a few drugs that were classified as “always inappropriate.” These would presumably constitute age-inappropriate drugs.
The most recent iteration of the Beers Criteria, derived by a consensus panel of experts and considering the limitations in earlier iterations of the Beers list, takes a different approach to classifying the medications. They are listed as “Potentially Inappropriate Medication Use in Older Adults: Independent of Diagnoses” and “Potentially Inappropriate Medication Use in Older Adults: Considering Diagnoses.” Furthermore, the concern is listed and the severity rating. Since we are using well-trained and licensed drug expert clinicians for this study, and not a computer-assisted identification of these medications, we believe that allowing the pharmacist to assess and discuss the risks and benefits of therapy with the patient’s primary care physician is a better approach to patient care. In the training session, we will be including a section on inappropriate medication selection for elderly patients and providing the following paper as required reading by the MTM Clinicians: Fick DM, et al. Updating the beers criteria for potentially inappropriate medication use in older adults. Arch Int Med 2003; 163: 2716-2724.
KM14. Is there a way to provide some validation of the Beers list through this study? (i.e. to track the number of times a potentially inappropriate Beers list drug led to an actual ADE?) That would increase the utility of this ICR a lot.
We are already collecting all of the needed information to do an analysis such as this. However, given the relatively small sample size (600 subjects) we are unlikely to find important results related to this limited list of medications (total of 114 individual medications or classes). The type of analysis proposed would best be conducted using a large dataset. Previous validation studies have already been reported using previous iterations of the Beers list and involving thousands of patients.1-4 As such, we could conduct a post-hoc study, but do not recommend making this hypothesis part of the existing study.
1. Fillenbaum et al. Impact of inappropriate drug use on health services utilization among representative older community-dwelling residents. Am J Geriatric Pharmacotherap 2004; 2: 92-101.
2. Doucet J, Chassagne P, Trivalle C, et al. Drug-drug interactions related to hospital admissions in older adults: A prospective study of 1000 patients. Am Geriatr Soc.
1996;44:944-948.
3. Hanlon JT, Fillenbaum GG, Kuchibhatla M, et al. Impact of inappropriate drug use on mortality and functional stares in representative community dwelling elders. Med Care. 2002;40:166-176.
4. Schmader KE, Hanlon JT, Landsman PM, et al. Inappropriate prescribing and health outcomes in elderly outpatients. Ann Pharmacother. 1997;31:529-533.
KM15. re: All 3 study sites are comprehensive academic medical centers with very few out-of-system office visits.
How do you know this? Hospitals will not have data on other hospitals or offices patients go to. To the extent that some patients in these hospitals do go to other hospitals or private offices, the clinical synopses and the BPMH will not be complete. Insofar as that is true, the BPMH would not really the “best possible” MH, would it? And if this is true, isn’t this a limitation of the study insofar as the most fragmented care—and where an MTM program would be most useful—occurs when patients see different health care providers who do not belong to the same system, who do not have access to an electronic health record, and who do not otherwise communicate with each other or share information on mutual patients?
We believe that, given the comprehensive medical services provided by all three institutions and based on the reports of clinicians practicing at these institutions, there are very few out-of-system specialist visits. It is possible that out-of-system ED visits and hospitalizations may occur. Because of this, we are relying on patient self reports for these particular outcomes. The inability to determine if out-of-system visits occurred is a limitation of this study.
With respect to developing the BPMH, we are building the medication history based on chart records and on patient self report of medications (obtained from their baseline interviews with research assistants). From this, we expect to have the best available medication history. It is possible, but unlikely, that a patient will regularly visit a prescribing specialist outside of our medical centers. In this case, our BPMH may not be the “best possible” and this is a limitation of our methods. Since we are randomizing patients, this type of estimation bias should be equally distributed between the two intervention arms and should not bias our ability to compare discrepancies between the two arms.
We do agree that this intervention might be even more effective in a situation where care is more fragmented and where electronic medical records are not utilized. Unfortunately, conducting the proposed research in such a setting would be far more cumbersome and require additional resources not budgeted for in our study.
KM17. Re: Patient satisfaction questions.
This is still rather confusing. Are these questions (“satisfaction with MTM service”) asking about satisfaction with the MTM clinician or with the pharmacist where they usually get their medications?
If this survey is given to all participants, even control group, how are the control group respondents supposed to respond to question #10?
Won’t intervention groups (arm 2 and 3) be interacting with 2 different pharmacists? The MTM clinician (who is a pharmacist) as well as the pharmacist where they usually get their medications?
Thank you for clarifying your concern. We agree that the PCQ and directions continued to be confusing when applied to our study and have revised the directions and provided a script for the research assistant to follow that addresses these concerns. Unfortunately, there are a limited number of validated patient satisfaction tools that assess pharmacist services. Of the available tools, only this one truly focused on the non-distributive side of pharmacists’ responsibilities. We believe that with these modifications that we have made to the directions, that the concerns above have been addressed.
KM17. The supporting statement says the first MTM visit will happen between days 0-30. But this response basically says that the baseline visit and the first MTM visit will essentially happen both on day 0 (unless the respondent would rather wait to have the first MTM visit)?
Both statements are correct. We are trying to minimize the travel burden on patients and will do our best to have both visits happen on day 0.
KM18. Re: Authorization to Use and Disclose Health Information Document
OMB needs to see this document.
The HIPAA authorization form is attached as Appendix T.
| File Type | application/msword |
| Author | Daniel Touchette |
| Last Modified By | Daniel Touchette |
| File Modified | 2007-09-26 |
| File Created | 2007-09-10 |
© 2026 OMB.report | Privacy Policy