Attachment 4 Follow-up Study Protocol
Attachment 4 GA protocol 4121.doc
Follow-up Study of Chronic Fatigue Syndrome in Georgia (Survey of Chronic Fatigue Syndrome & Chronic Unwellness in Georgia-Baseline Survey)
Attachment 4 Follow-up Study Protocol
OMB: 0920-0638
Survey of Chronic Fatigue Syndrome and Chronic Unwellness in Georgia
National Center for Infectious Diseases, Centers for Disease Control & Prevention, Atlanta, GA
Departments of Medicine and Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta, GA
Principal Investigator: William C. Reeves, MD, MSc
March 15, 2007
Phase 1 (February 2, 2004 to February 14, 2006)
Summary
Chronic fatigue syndrome (CFS) is a complex medical and public health problem. It is estimated that approximately 700,000 adults in the U.S. suffer from CFS. They have been ill for 5-7 years, a quarter are unemployed or receive disability, yet fewer than 20 percent have received medical care for CFS. CFS is a Congressional and DHHS priority and CDC is responsible for its prevention and control. This study of CFS in metropolitan, urban, and rural populations of Georgia addresses major gaps in current knowledge that must be clarified in order to understand CFS and devise optimal control and prevention strategies. Minority racial/ethnic groups appear to disproportionately suffer from CFS and there is evidence of markedly different risks in metropolitan, urban, and rural populations. CFS is defined by self-reported symptoms, because as yet there are no defining physical symptoms or diagnostic laboratory abnormalities. Reasons for the discrepancies between studies attempting to identify risk factors and markers include the heterogeneity of the CFS population and varying rigor applying a standard case definition, which was derived by clinical consensus rather than empirically. This study of CFS in metropolitan, urban, and rural Georgia will estimate the prevalence of CFS in these distinct populations and measure associated risk factors that are important to understand and empirically define the illness. We will use random digit dialing methods to screen residents of metropolitan, urban, and rural communities of Georgia, and interview in detail those reporting fatigue or other unwellness of at least a month’s duration, as well as a random sample of healthy controls. We will assess subjects identified in the telephone survey who appear to meet the case definition of CFS, a random sample of persons reporting other chronic unwellness, and a matched set of healthy controls. Assessments will include clinical evaluation of each subject’s medical and psychiatric status, psychosocial factors, and cognitive functioning. We will also obtain specimens for endocrine and immune measures and future genotype studies. Findings from our study will be used to estimate the prevalence of CFS in defined populations, evaluate demographic and psychosocial factors associated with CFS, and help to develop an empiric case definition for CFS. Our findings will also generate specific hypotheses that will help to further our understanding of the underlying pathophysiology of CFS.
Performance Sites
Centers for Disease Control & Prevention (CDC), Atlanta, GA (OHRP # FW A 00001413)
Departments of Medicine and Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta, GA (OHRP # MI426)
Abt Associates, Inc. 640 North LaSalle Street, Suite 400, Chicago, IL 60610 (OHRP # IRB00001281)
Key Personnel
William C. Reeves, MD, MSc CDC Principal Investigator (PI)
Joann House CDC Project Officer
James F. Jones, MD CDC CDC Deputy PI
Christine Heim, PhD Emory University Emory Deputy PI
Rosane Nisenbaum, PhD CDC Coordinator Biostatistics
Dimitris Papanicolaou, MD Emory University Coordinator Endocrinology
Suzanne D. Vernon, PhD CDC Coordinator Molecular Epidemiology
Abt Associates Inc. CDC has contracted with Abt Associates to implement those tasks necessary to complete the study. This includes collaboration in study design and sampling strategies, random-digit-dialing screening surveys, detailed telephone surveys, and clinical evaluation.
Key CDC/Emory University Personnel Qualifications
Dr. Reeves is Chief of the Viral Exanthems & Herpesvirus Branch (VEHB), Division of Viral and Rickettsial Diseases, NCID and has served as Principal Investigator for CDC’s chronic fatigue syndrome (CFS) research program since 1992. He is a medical epidemiologist with expertise in infectious and chronic diseases. He is an elected member of the American Epidemiologic Society, an elected Fellow American College of Epidemiology, and elected Fellow in the Infectious Diseases Society of America. He is a recipient of the Amador Guerrero Medal (the highest honor awarded by Panama in the field of science and equivalent to the U.S. Medal of Science) awarded by the President of Panama in recognition of his research and service to public health. Dr. Reeves has published 170 peer-reviewed articles of which 30 deal with CFS (encompassing the case definition, prevalence, incidence, outbreaks, risk factors, clinical aspects, laboratory measures, and stress).
Ms House has been Administrative Officer and Deputy Director for VEHB since 1996. Prior to this (1990 to 1996) she was Program Specialist responsible for DVRD program management. She is currently responsible for administrative management of the VEHB CFS and human papillomavirus research programs and serves as Project Officer for all CDC funded studies of CFS. Because optimal administrative management requires ongoing integrated analysis and interpretation of management information related to large epidemiology, clinical, and molecular biology laboratory studies, she participates actively in the CDC CFS Research Group to maintain a level of scientific competence sufficient to place management into an appropriate context.
Dr. Jones is a senior Medical Research Officer in VEHB and will serve as Deputy PI to oversee clinical aspects of this study. He is board certified in Pediatrics and Allergy/Immunology. Prior to accepting a position at CDC last year he was Professor of Pediatrics at the National Jewish Medical Center and University of Colorado School of Medicine. He was responsible for an active CFS research program involving clinical studies, studies of behavior and CFS, studies of immunology and CFS, studies of infectious agents and CFS, and studies of endocrinology and CFS. Between 1986 and 2002 he was been PI on 4 NIH RO-1 grants investigating CFS. He has written 20 book chapters and 75 peer-reviewed publications.
Dr. Heim is an Assistant Professor in the Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine and will serve as Deputy PI to oversee psychiatric and behavioral medicine aspects of this study. She is a research clinical psychologist who is internationally recognized in the field of early-life stress and chronic diseases. In the 3 years prior to relocating to Atlanta from Germany, she was PI on 2 research projects investigating stress and chronic illness. Since 1997 she has written a book on stress and chronic disease, 14 book chapters, and 30 peer-reviewed publications on aspects of this topic. She is an active member of international neuroscience, endocrinology, and psychiatry, socialites, key investigator on two active RO-1 grants, and recipient of a Young Investigator Award. Since relocating to Emory University (partly supported by a CDC IPA) she has been an active collaborator in the CDC CFS research group and has served as co-PI on the Pilot National Survey for CFS (IRB 2936) and on the Clinical Research Center study of CFS in Wichita (IRB 3504).
Dr. Nisenbaum is a senior Biostatistician in VEHB. She has worked on CFS at CDC since 1994. Dr. Nisenbaum has been lead investigator internationally in deriving empiric definitions for CFS and other medically unexplained illnesses. She is also actively involved in studies of other chronic diseases related to infectious agents (cervical cancer and papillomavirus, recurrent respiratory papillomatosis and HPV, and genital herpes). She is author of 18 peer-reviewed publications involving the epidemiology or CFS, behavioral/infectious/immunologic risk factors for CFS, and definitions of CFS. Most recently she has helped to develop mathematical methods for integrating epidemiologic, clinical, gene expression and proteomics data to the study of CFS.
Dr. Papanicolaou is an Assistant Professor of Medicine in the Division of Endocrinology at the Emory University School of Medicine. He is board certified in Internal Medicine and Endocrinology, Diabetes, Metabolism. Since 1996 (when he finished his residency), he has authored 20 peer-reviewed publications on aspects of endocrinology and immunology in areas related to CFS and has written 5 book chapters in this area. In 1998, he relocated from NIH (where he was a senior investigator responsible for clinical studies of endocrinology/immunology and CFS) to Emory and has been an active collaborator in the CDC CFS research group (supported by an IPA). Most recently, he was co-PI on the Clinical Research Center study of CFS in Wichita (IRB 3504) and PI on a CDC funded clinical study of adipose tissue, cytokines and CFS (IRB 3475).
Dr. Vernon is microbiologist and Chief of the VEHB Molecular Epidemiology Group. The Molecular Epidemiology Program combines epidemiology with powerful molecular and genomic technologies to identify markers and risk factors for CFS and to acquire an understanding of the underlying biologic correlated. She has worked on CFS at CDC since 1996 as co investigator in the Longitudinal Study of CFS in Wichita (IRB 1698), the National Pilot Study of CFS (IRB 2936), and on the Clinical Research Center study of CFS in Wichita (IRB 3504). She has served as CDC PI on contracts searching for novel pathogens associated with CFS, protein markers of CFS, isoelectric focusing to identify markers for CFS, endogenous retroviruses associated with CFS, and a contract investigating the economic impact of CFS. She is responsible for a CDC grant to the University of New South Wales to study post-infectious fatigue as a model for CFS, for a contract investigating the economic impact of CFS. Since 1996, she has authored 19 peer-reviewed publications on various aspects of CFS, including the first studies clearly showing a correlation between gene expression profiles and CFS. She also has two pending patents involving laboratory studies of CFS.
Key Abt Associates Personnel Qualifications
As noted above, CDC has contracted with Abt Associates Inc. to collect and deliver data for the Survey of Chronic Fatigue Syndrome and Chronic Unwellness in Georgia. Abt Associates staff are experienced with CDC telephone surveys and clinical studies involving CFS research. Below, we briefly describe the qualifications and responsibilities of Abt Associates’ project team. Upon request, CDC will furnish resumes to the IRB.
Bonnie Randall, a vice president at Abt Associates, is has been Abt Associates project director of all CDC CFS field studies since 1992. Ms. Randall has responsibility for and authority over the scientific, operational, and financial aspects of the task orders conducted under this master contract with CDC. Ms. Randall has two decades of experience in analytic and survey research. She has been associated with CFS studies since 1988.
Senior Survey Director, Marjorie Morrissey, has oversight of the telephone interview component and has day-to-day responsibility (budget, schedule, and staff) for the clinical evaluation component. She, too, has over twenty years of survey management experience and has extensive knowledge of CFS. She was Abt Associates study director for the Longitudinal Study of CFS in Wichita (IRB 1698), the National Pilot Study of CFS (IRB 2936), and on the Clinical Research Center study of CFS in Wichita (IRB 3504).
Senior statistician David C. Hoaglin, Ph.D. collaborated in study design and sampling strategies and will compute sample weights and calculate prevalence estimates and provide technical advice on project design and on analytic protocols. Dr. Hoaglin has provided statistical support for CDC projects, since 1996.
Chief economist, Larry Orr, Ph.D., is assisting CDC with the design the economic impact analysis plan and questionnaire. He will also provide analytic support. Dr. Orr has over thirty years experience in the design and analysis of large-scale research and evaluation projects, including major social experiments in welfare reform, health insurance, home health care, employment and training, and housing.
As study director for the telephone interview component, Survey Director Rebecca Devlin, has day-to-day responsibility (including schedule, budget, and staff). She will work closely with the programmer to implement the CATI versions of the instruments and will create the training materials for supervisors and interviewers. She has also assisted CDC with screener and detailed interview revisions. For the clinical evaluation component, Ms. Devlin will coordinate the trainings for neurocognitive testing and the interviewer-administered psychiatric interview. She has assisted CDC with consent form revisions. Previously, Ms. Devlin implemented the random-digit-dialing telephone survey for the National Pilot Study of CFS (IRB 2936). She also implemented the telephone component of the Clinical Research Center study of CFS in Wichita (IRB 3504).
Senior Analyst Maria Amoruso, task leader for clinical evaluation data collection, has lead responsibilities for implementing the clinic protocol. She has structured the clinic day and is currently working on staffing, space, laboratory, and training issues. She trained clinic staff for the final portion of the Longitudinal Study of CFS in Wichita (IRB 1698), developed data collection instruments for the clinic component of the National Pilot Study of CFS (IRB 2936), and developed and implemented the data collection protocol and quality assurance plan for the Clinical Research Center study of CFS in Wichita (IRB 3504).
Associate Keith Smith will manage data preparation and delivery for the telephone and clinical evaluation components. His specific responsibilities include developing the data entry specifications for paper questionnaires, cleaning questionnaire data, and delivering interim and final data files and documentation. Mr. Smith produced the final data deliverables for the final portion of the Longitudinal Study of CFS in Wichita (IRB 1698) and the telephone and clinical assessment data sets for the National Pilot Study of CFS (IRB 2936). He recently completed and submitted to CDC interim and final data files for of the Clinical Research Center study of CFS in Wichita (IRB 3504).
Field Manager, Sandra Henion, will manage clinic operations in Atlanta and Macon. Ms. Henion has set up and managed four clinics for CDC during the Longitudinal Study of CFS in Wichita (IRB 1698) (1997-2001) and managed clinic operations for the Clinical Research Center study of CFS in Wichita (IRB 3504). She will supervise the local clinic managers.
Study Consultants
Dr. Martin Meltzer, Ph.D., is an NCID senior health economist. He will collaborate with the Principal Investigator in the analysis of the economic data.
Acknowledgment of all funding sources and substantial contributions:
As part of a coordinated Department of Health and Human Services (DHHS) program of chronic fatigue syndrome (CFS) research, CDC has been charged by the Congress to address CFS control and prevention. Congress has specified that CDC conduct population surveys of CFS. Monies appropriated by Congress for this purpose fund this study.
Statement of any conflict of interest:
None of the investigators have any conflict of interest in this project.
Face Page 1 Phase 1Summary 2 Description, Performance Site, and Key Personnel 3-7 Table of Contents 8
Phase 1 Research Plan 9A. Objectives and Specific Aims 9-10 B. Background and Significance 11-22 C. Research Design and Methods 23-57 D. Human Subjects 58-63 E. Literature Cited 64-70 F. Appendix Summary 71-72 Note—these approved Phase 1 appendices are not included in this submission
|
Phase 2Summary 79-74 Description, Performance Site, and Key Personnel 75-78 Phase 2 Research Plan 79A. Objectives and Specific Aims 79-80 B. Background and Significance 81-88 C. Research Design and Methods 89-116 D. Human Subjects 117-122 E. Literature Cited 123-128 F. Appendix Summary 129 F.1. Appendix 1: Press Release and Web Site F.2. Appendix 2: Telephone Interview Advance Letter F.3. Appendix 3: Telephone Non-Contact Letters F.4. Appendix 4: Detailed Telephone Interview F.5. Appendix 5: Descriptive materials sent prior to clinical evaluation F.6. Appendix 6: Physical Examination F.7. Appendix 7: Summary of Specimen Collection F.8. Appendix 8: Questionnaires Concerning Symptomatology F.9. Appendix 9: Assessment of Stress and Coping F.10. Appendix 10: Economic Impact F.11. Appendix 11: Health Services Utilization
|
A. Objectives and Specific Aims
A.1 Objective: Describe epidemiologic and clinical aspects of chronic fatigue syndrome (CFS) in defined Georgia populations.
A.2 Specific aims:
Specific Aim 1 - Determine the prevalence of CFS in different racial/ethnic groups representative of metropolitan, urban, and rural Georgia populations.
Hypothesis – the prevalence of CFS will differ by at least 2-fold between white and black populations.
Hypothesis – the prevalence of CFS will differ by at least 2-fold between metropolitan/urban and rural populations.
Specific Aim 2 - Determine factors associated with CFS in the metropolitan, urban, and rural populations of Georgia. Factors to be studied include: symptoms, demographics (sex, age, race/ethnicity), occupation, quality of life, health services utilization, life experiences, psychiatric comorbidity and endocrine-immune measures.
Hypothesis – symptoms and demographics of CFS will differ in metropolitan, urban, and rural populations.
Hypothesis – occupation, illness characteristics (e.g., type of onset, duration of illness, impairment/disability, physical and psychiatric symptom patterns), and utilization of health services will differ between metropolitan/urban and in rural populations.
Hypothesis – psychosocial factors (e.g., adverse life experiences, personality, coping mechanisms, etc.) will be associated with CFS.
Hypothesis – psychiatric comorbidity (e.g., mood and anxiety disorders) will be associated with CFS.
Hypothesis – endocrine-immune changes will be associated with CFS.
Hypothesis – polymorphisms of genes involved in CNS, neuroendocrine, and immune pathways will be associated with CFS.
Specific Aim 3 - Describe the economic impact of CFS on individuals, families, and the economies of metropolitan, urban, and rural Georgia.
Hypothesis – CFS will be associated with a substantial economic burden on affected individuals, their families, and society throughout Georgia.
Specific Aim 4 - Develop an empiric case definition for CFS based on symptoms and other illness attributes, and evaluate overlap with the 1994 CFS research case definition.
Hypothesis – the illness defined by symptoms and illness attributes (e.g., type of onset, duration of illness, impairment/disability, stress history, physical and psychiatric symptom patterns), and biomarkers will more accurately identify patients with CFS than the consensus 1994 research case definition and will differentiate subcategories of CFS.
Hypothesis –endocrine and immune changes, psychiatric symptoms and type/severity of life experiences, psychological traits, and specific polymorphisms, will vary with fatigue states, symptoms and illness characteristics, and will help to differentiate subgroups of CFS.
Specific Aim 5 - Identify persons representative of the Georgia population with CFS, unwellness and a healthy comparison group to invite for future enrollment in General Clinical Research Center (GCRC) studies at Emory University. Select persons with risk factors for CFS identified in Aim 2 for enrollment in GCRC mechanistic studies. Similarly, select patients with CFS or unwellness based on biological factors identified in Aim 4 for GCRC mechanistic studies.
GCRC proposals based on results of these studies and those of others will be developed under separate protocols and address: 1) specific hypotheses concerning mechanisms and pathophysiology of CFS, 2) specific treatments and interventions, and 3) polymorphisms of genes involved in CNS, neuroendocrine, and immune pathways, as possible examples.
B. Background and Significance
CFS is a complex medical and public health problem. It is estimated that approximately 700,000 adults in the U.S. suffer from CFS. Their median duration of illness is 7 years, a quarter of them are unemployed or receiving disability, yet fewer than 20 percent have received medical care for CFS (Reyes, 2003). Despite more than 3,000 articles in the peer-reviewed medical literature, the pathophysiology of CFS is not well understood. There are no diagnostic laboratory abnormalities or clinical tests. There is no specific treatment or prevention strategy for CFS. CFS is a DHHS priority and CDC is the lead agency for research concerning prevention and control. CFS is also a Congressional priority. Beginning in 1992, Congressional language has stressed legislators’ desire for CDC to conduct community-based CFS surveillance. Recently Congressional language has directed that CDC should target race/ethnicity-specific differences in the occurrence of CFS and should accelerate its CFS research plan to identify the causes, risk factors, diagnostic markers, and economic impact of CFS. This protocol was designed to conduct surveillance of CFS in the metropolitan, urban, and rural populations of Georgia and to address these Congressional mandates. Recently Congress has also directed CDC to accelerate its educational activities for health care providers and this study will collect information concerning health care utilization that is necessary to optimally develop our educational program. Finally, Congress has directed CDC to create a CFS patient registry and information from the Georgia surveillance study (especially as related to rural populations) will be pivotal for developing registry strategies. Each of the Specific Aims discussed below addresses our program objectives and congressional concerns in the context of major unanswered questions concerning the characteristics of CFS in the general population.
B.1 Aim 1 – Prevalence of CFS in Metropolitan, Urban, and Rural Georgia Populations
B.1.a Hypothesis – the prevalence of CFS will differ by at least 2-fold between white and black populations.
B.1.b Hypothesis – the prevalence of CFS will differ by at least 2-fold between metropolitan/urban and rural populations.
An understanding of the prevalence and distribution of CFS is fundamental to focusing etiologic research, targeting health-care and educational programs, and estimating the effects of debilitating fatiguing illnesses on quality of life and productivity. By ranking the burden of CFS and fatiguing illnesses among other national health concerns, a prevalence estimate can help frame appropriate health policy and raise political and public awareness for the concerns that emanate from this misunderstood and stigmatized syndrome.
Previous studies have estimated a wide range of CFS prevalence, from 2.3 to 600 per 100,000 persons [Bates et al., 1993; Jason et al., 1999; Lloyd et al., 1990; Reyes et al., 1997; Steele et al., 1998; Wesley et al., 1997]. Prevalence estimates from those studies cannot be directly compared because of the lack of and inconsistent use of a standard CFS case definition [Reeves et al., submitted], varying degrees of rigor in examining subjects [Holmes et al., 1988] differences in study populations [Reyes et al., 1997; Buchwald et al., 1995; Lloyd et al., 1990; Jason et al., 1999; Minowa et al., 1996], sampling strategies, and methods for estimating prevalence [Fukuda et al., 1994; Lloyd et al., 1990; Sharpe et al., 1991; Kitani et al., 1992]. Physician-based surveillance studies, although they reflect the burden of CFS on the health care system, underestimate the true burden CFS imposes on both individuals and the population.
Only two studies (by CDC and DePaul University) have used population-based random samples to estimate the prevalence of CFS and describe its demographic and socioeconomic characteristics. Both studies used the 1994 CFS Research Case Definition [Fukuda et al., 1994]. The DePaul study estimated a prevalence of 422 per 100,000 in Chicago [Jason et al., 1999] and the CDC study found 235 per 100,000 adults in Wichita to suffer from CFS [Reyes et al., 2003]. These rates were not statistically different. In both studies, CFS primarily affected women, and suggested an increased prevalence, although non-significant, among non whites.
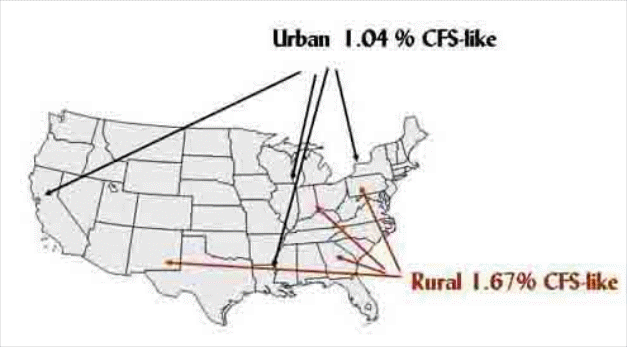
The two studies used different sampling and analysis strategies, confidence intervals for their various estimates overlapped, and there are limitations to their generalizability. Thus, in July 2001, CDC conducted a pilot survey to determine feasibility and test procedures for a National Survey of CFS [Bierl et al., submitted]. To examine regional and metropolitan differences, the pilot survey sampled strata from statistical areas in each of the four US census regions (Northeast, Midwest, South, and West) and was further stratified into urban and rural statistical areas. Urban areas included; Buffalo-Niagara Falls, New York; Chicago, Illinois; Baton Rouge, Louisiana; and Oakland, California. Rural areas included; Franklin County, Pennsylvania, Ripley County, Indiana, Monroe County, Georgia, and Chaves County, New Mexico. We sampled 2,728 households and surveyed 7,317 residents (the screening interview CASRO response rate was 70%1 and 77% of the detailed interviews were completed)[Bierl et al., submitted]. The pilot survey was not designed to rigorously estimate CFS, but CFS-like illness (meets criteria of 1994 CFS Research Case Definition, pending physical examination) was reported by 1.2 percent of the population (similar to both the Wichita and Chicago studies). CFS-like illness was more common in persons with annual incomes < $40,000 and in those with less than a high school education. The survey found no evidence for differences in the prevalence of CFS-like illness by geographic region, but rural rates were 60% higher than those of urban residents.
The pilot study also showed that nation-wide epidemiologic studies of CFS posed significant technical problems because clinical evaluation, which is necessary to confirm classification of CFS, was not practical on a national level. This finding, in conjunction with the tragic events of September 11, 2001 [Heim et al., submitted], precluded a subsequent CFS study on a national level.
We have modified the concept of the National Survey of CFS by limiting data collection to one Southern U.S. state (Georgia). This more focused research is better able to serve the objectives of the National Survey of CFS and additional CDC objectives. Reasons supporting this statement are the following: 1) Logistics - A difficulty in the pilot survey was matching subjects and physicians for clinical evaluations because subjects were, of course, scattered across the continent. Focusing on a single state, Georgia allows operation of regional clinics and greater opportunities for collaboration between and among CDC, Emory University and Abt Associates. 2) Metropolitan, urban and rural differences - The pilot survey results suggest no regional differences in the occurrence of CFS-like illnesses between and among the Midwest, south, west, and northeast, so concentrating on one state (Georgia) should provide generalizable information. The pilot survey findings suggested the importance of further evaluating urban and rural differences in the occurrence of CFS. Again, Georgia supports such a study with a major metropolitan center (Atlanta), well-defined urban areas (Macon and Warner Robins), and rural populations (in counties surrounding Macon/Warner Robbins) with well-defined regional differences. 3) Racial/ethnic differences - As noted earlier, the prevalence of CFS in minority populations must be appropriately evaluated. Population-based studies have indicated that risk for CFS may be elevated the black and Hispanic populations [Steele et al., 1988; Jason et al., 1999; Reyes et al., 2003]. Georgia has well-characterized urban and rural white, black, and Hispanic populations of varying socioeconomic status living in the regions to be studied. The presence of these populations, geographically contiguous to CDC/Emory University, is ideal for public health surveys. Taken together, the proposed GA survey will produce estimates of the prevalence of CFS in metropolitan, urban and rural populations and will elucidate racial/ethnic differences in CFS in these populations.
B.2 Aim 2 - Factors Associated with CFS in Metropolitan, Urban, and Rural Georgia Populations
B.2.a Hypothesis – symptomatology and demographics of CFS will differ in metropolitan, urban, and rural populations. Results from the Pilot National Survey suggest possible differences in risk of CFS between urban and rural communities (see above). Such differences must be evaluated in order to devise and implement appropriate prevention and control activities. Metropolitan, urban and rural populations reflect different demographic profiles, as well as differences in illness attributes and health-seeking behaviors. Data from the Medical Expenditure Panel Survey [Larson, 2003] suggest that there are not only demographic differences between urban and rural communities, but that there are also differences in perceived health, activity, access to health care, and utilization of health services. The sense of community in a population influences several of these parameters [Ahern, 1996]; as does the patient’s perception of their medical care [Ax, 1997].
B.2.b Hypothesis – occupation, illness characteristics (e.g., type of onset, duration of illness, impairment/disability, physical and psychiatric symptom patterns), and utilization of health services will differ between metropolitan/urban and in rural populations. The median duration of illness among persons with CFS identified in community studies varied between 2.5 and 7.3 years (Reyes et al., 2003; Jason et al., 1999]. In spite of this, only half of those with CFS in Chicago were under medical care [Jason et al., 2000] and only 16% of persons identified with CFS in Wichita had received medical care for CFS [Solomon et al., submitted]. Similarly, a clinic-based study in Seattle found that individuals with chronic fatigue, CFS, and fibromyalgia each saw multiple medical physicians, but that those with CFS or fibromyalgia saw alternative providers, such as chiropractors, more frequently than did the other subjects [Bombardier and Buchwald, 1996]. These observations, in the context of the preceding discussion, document the need to determine if occupation, illness characteristics, and utilization of health services by persons with CFS and appropriate comparison groups differ between defined metropolitan, urban, and rural populations.
B.2.c. Hypothesis – psychosocial factors (e.g., adverse life experiences, personality, coping styles) will be associated with CFS. Mainly based on clinical observations, stress (including physical insults such as childhood disease) or emotional traumas have long been considered as major precipitating factors in the development of CFS. Few epidemiological or clinical studies have evaluated the association between severe stress or trauma and CFS or other fatiguing illnesses. One study reported that acute exposure to Hurricane Andrew induced relapses of CFS and symptom exacerbations in a sample of 49 CFS patients living in South Florida [Lutgendorf et al., 1995]. The extent of individual emotional and behavioral stress responses was the single and strongest predictor of the likelihood and severity of the relapse and functional impairment within 4 months after the hurricane. Our group reported that the self-reported chemical, emotional, and physical exposures in Gulf war veterans was related to the prevalence of a multi-symptom fatigue-like illness [Nisenbaum et al., 2000]. Several other studies report elevated rates of CFS in Gulf War veterans, and CFS is associated with combat-related post traumatic stress disorder (PTSD) in these veterans [McCauley et al., 2002; Kang et al., 2003]. It thus appears that severe stress in adulthood might be a precipitating factor of CFS, or at least in a subgroup of CFS patients.
In recent years, much attention has been directed towards understanding the long-term effects of stress during development on long-term health and functioning. Epidemiological studies provide compelling evidence for a strong association between adverse childhood experiences (e.g., abuse or neglect, parental loss and household dysfunction) and unwellness later in life [McCauley et al., 1997; Felitti et al., 1998]. One study involving almost 2000 women reported that those who had been abused as children exhibited increased levels of fatigue and pain in adulthood, and additional symptoms of depression, anxiety, substance abuse and interpersonal sensitivity when compared to women not abused as children [McCauley et al., 1997]. A population-based study conducted in New Zealand reported elevated rates of chronic fatigue in women with childhood adversity [Romans et al., 2002]. One population-based study reported an association between childhood abuse and fatiguing illnesses [Taylor & Jason, 2001]. Childhood abuse also accounted for comorbidity with anxiety disorders (e.g., PTSD). A study in tertiary care patients with CFS found that those with CFS more frequently reported various types of abusive victimization starting in childhood and persisting throughout adulthood, as compared to controls [van Houdenhove et al., 2001]. Another recent clinical study found that an overprotective maternal parenting style and maternal depression, which is often associated with lack of care giving, were associated with a diagnosis of CFS [Fisher & Chalder, 2003].
Since stress exacerbates CFS in adults, these findings support a stress-diathesis model, which postulates that genetic liabilities interact with stressful experiences in determining individual vulnerability to disease, including CFS. This vulnerability likely reflects the combined effects of early experience and genes on the developing brain, resulting in a stable phenotype with different neurobiological expressions, which may determine perceptual thresholds and the "set-point" of neuroendocrine, immune and behavioral reactivity to the environment [Heim & Nemeroff, 2001], thereby contributing to the development of CFS upon further challenge (see Figure 1 below in section on endocrine and immune function).
In addition, certain psychological factors might influence individual adaptation to CFS. One important factor comprises illness attributions. It has been reported that CFS patients tend to attribute their illness to physical causes (like viruses or pollution) and minimize or neglect psychological or personal circumstances. Importantly, these attributions seem to be a risk factor for the exacerbation and perpetuating of CFS and are also accompanied by greater disability [Butler et al., 2001]. Negative coping styles, such as denial or disengagement, have also been shown to be important in the development and perpetuation of CFS [Afari & Buchwald, 2003]. Several other authors suggest an association between CFS and certain personality traits [Fiedler et al., 2000; White & Schweitzer, 2000]. Taken together, it appears that attribution styles, coping strategies and personality traits might influence the development and course of CFS. The proposed study will assess these associations.
B.2.d. Hypothesis – psychiatric comorbidity (e.g., mood and anxiety disorders) will be associated with CFS: Psychiatric disorders that may cause chronic fatigue are exclusionary for a diagnosis of CFS [Fukuda et al., 1994]. Exclusionary psychiatric disorders include major depression with melancholic features, bipolar disorder, psychotic disorders, and eating disorders and substance abuse disorders within 2 years of the onset of the fatigue. However, other psychiatric disorders that are not exclusionary for CFS may be associated with the development and persistence of CFS. Indeed, there are high rates of comorbidity between CFS and psychiatric disorders, mainly with respect to mood, anxiety and somatoform disorders, when compared to individuals with other chronic illnesses or healthy subjects. Rates of comorbidity between CFS and these disorders, determined in community and clinical samples, range between 45-82% for any psychiatric diagnosis, 23-67% for depression, 2-30% for anxiety disorders and 6-28% for somatoform disorders [reviewed in Ax et al., 2001; Afari & Buchwald 2003]. There is also evidence for increased rates of personality disorders among CFS patients [Ciccone et al., 2003]. Because these disorders have symptoms similar to CFS, it is likely that most studies over-estimate rates of comorbidity. The apparent variability in the estimates likely reflects differences in the sampling of CFS cases (community versus clinical samples) and differences in the method of psychiatric assessment (self-report versus diagnostic interview).
The nature of the relationship between CFS and these psychiatric disorders remains obscure but has important implications regarding therapy [Reid et al., 2000}. It is possible that 1) CFS causes psychiatric disorders, 2) psychiatric disorders cause CFS, 3) certain psychiatric disorders are incorrectly diagnosed in CFS patients because of overlap in symptomatology, 4) CFS and certain psychiatric disorders are independent disorders with high rates of comorbidity, and 5) CFS and certain psychiatric disorders share pathophysiologic mechanisms and represent covariates of one underlying process [Nisenbaum et al., 2000; Reyes et al., 1996; Suraway et al., 1995].
B.2.e Hypothesis –endocrine and immune biomarkers will be associated with CFS
Numerous studies have searched for a pathophysiological basis of CFS and other fatiguing illnesses. They have identified multiple neuroendocrine and immune alterations and alterations in sleep and neural circuitry in patients with CFS. One particular area of focus has been hypothalamic-pituitary-adrenal (HPA) axis function in persons with CFS. The HPA axis constitutes one of the major peripheral outflow systems of the brain, serving to adapt the organism to changes in the internal and external milieu. Alterations of the HPA axis may thus represent a common pathway linking antecedent factors, such as infection and stress, with immune disturbances, altered behavior and symptoms of CFS [see, e.g., Heim et al., 2000].

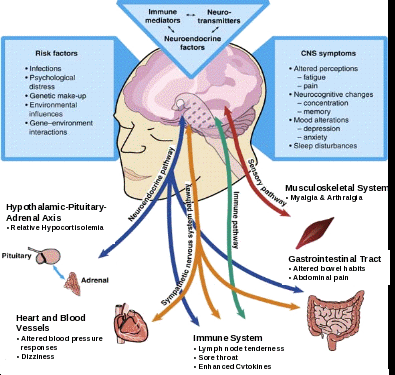 Several
studies have provided evidence for impairment of the HPA axis and
increased immune function in CFS. Patients with CFS exhibit low
cortisol levels compared to healthy controls [Demitrack et al., 1991;
Scott & Dinan 1998; Parker et al., 2001]. Low cortisol
availability might play a causal role in CFS. For example, adrenal
insufficiency, a condition characterized by hypocortisolism, shares
several symptoms with CFS (such as flu-like symptoms, fatigue,
malaise, arthralgia, myalgia, sleep abnormalities, headaches,
dizziness, and decreased memory). Glucocorticoids are important
regulators of immune function. For example, during prolonged stress,
glucocorticoids have a suppressive effect on the production of
inflammatory cytokines, such as IL-6, which might prevent toxic
effects of immune reactions [Munck et al., 1994]. Conversely, a lack
of cortisol effects might be associated with overproduction of
inflammatory cytokines [Heim et al., 2000; Raison & Miller,
2003]. The increased production of inflammatory cytokines may be at
least partially, responsible for the symptom complex of cortisol
deficiency, which greatly overlaps with the symptom complex of CFS
[Papanicolaou et al., 1996]. Thus, chronic cortisol deficiency, as
seen in CFS, could result in chronic overproduction of IL-6 and other
immune mediators. Indeed, overproduction of inflammatory cytokines,
such as IL-6 has been reported in patients with CFS [Gupta, 1999;
Cannon, 1995]. Administration of IL-6 to normal volunteers causes
symptoms of CFS [Papanicolaou et al., 1996]. However, studies of CFS
patients have not uniformly identified elevated serum inflammatory
cytokine levels, nor have they uniformly found altered levels of
serum cortisol. A possible explanation for such disagreements might
be that physiological measures vary with the clinical course of CFS,
including frequent remissions and relapses, which in turn might be
influenced by other factors, such as stress and behavioral styles.
To address the above possibilities in a coordinated manner, the
current study will measure of endocrine-immune markers in saliva and
plasma and will relate these measures to CFS status and illness
attributes (also see Aim 4).
Several
studies have provided evidence for impairment of the HPA axis and
increased immune function in CFS. Patients with CFS exhibit low
cortisol levels compared to healthy controls [Demitrack et al., 1991;
Scott & Dinan 1998; Parker et al., 2001]. Low cortisol
availability might play a causal role in CFS. For example, adrenal
insufficiency, a condition characterized by hypocortisolism, shares
several symptoms with CFS (such as flu-like symptoms, fatigue,
malaise, arthralgia, myalgia, sleep abnormalities, headaches,
dizziness, and decreased memory). Glucocorticoids are important
regulators of immune function. For example, during prolonged stress,
glucocorticoids have a suppressive effect on the production of
inflammatory cytokines, such as IL-6, which might prevent toxic
effects of immune reactions [Munck et al., 1994]. Conversely, a lack
of cortisol effects might be associated with overproduction of
inflammatory cytokines [Heim et al., 2000; Raison & Miller,
2003]. The increased production of inflammatory cytokines may be at
least partially, responsible for the symptom complex of cortisol
deficiency, which greatly overlaps with the symptom complex of CFS
[Papanicolaou et al., 1996]. Thus, chronic cortisol deficiency, as
seen in CFS, could result in chronic overproduction of IL-6 and other
immune mediators. Indeed, overproduction of inflammatory cytokines,
such as IL-6 has been reported in patients with CFS [Gupta, 1999;
Cannon, 1995]. Administration of IL-6 to normal volunteers causes
symptoms of CFS [Papanicolaou et al., 1996]. However, studies of CFS
patients have not uniformly identified elevated serum inflammatory
cytokine levels, nor have they uniformly found altered levels of
serum cortisol. A possible explanation for such disagreements might
be that physiological measures vary with the clinical course of CFS,
including frequent remissions and relapses, which in turn might be
influenced by other factors, such as stress and behavioral styles.
To address the above possibilities in a coordinated manner, the
current study will measure of endocrine-immune markers in saliva and
plasma and will relate these measures to CFS status and illness
attributes (also see Aim 4).
B.3 Aim 3 - Economic Impact of CFS.
The ability of CFS patients to carry out productive lives can be severely limited and CFS likely imposes a large burden on society. Economic cost of illness is an important element in understanding its overall impact and in deciding the allocation of health recourses. Several, clinic-based, studies have found CFS patients to have substantial functional impairment compared with both healthy controls and other chronically ill patient groups [Buchwald et al., 1996; Hardt et al., 2001; Komaroff et al., 1996]. Other clinical studies have reported patients with CFS to be more severely impaired than persons with end-stage renal disease [Hart et al., 1987], heart disease [Bergner et al., 1984], and multiple sclerosis [Komaroff et al., 1996].
However, scant empirical scientific work exists to quantify the economic impact of CFS. Previous studies have addressed consequences of CFS in terms of disability (16%) and unemployment (21%) [Jason et al., 1999; Nisenbaum et al., 2003; Solomon et al., 2003]. CFS imposes a significant burden on both society and those living with the syndrome. Only three studies, all of which were clinic based, have attempted to quantify the impact of CFS, and each showed that people with the syndrome were likely to have lost their job or to be unemployed [Lloyd & Pender, 1992; Bombardier & Buchwald, 1996; McCrone et al., 2003]. Persons with CFS also pose a disproportionate burden on the health care system and their families since they are sick for long periods of time and since there is no known cure for the illness [McCrone et al., 2003].
Persons with CFS incur direct costs associated with healthcare services and products used for the diagnosis, assessment, and management of an illness. Perhaps more important, persons with CFS suffer indirect costs irrespective of their health care. Indirect costs are affiliated with the loss in productivity attributed to a particular illness – that is, forgone income due to a decrease in hours worked or required job change. Medical and nonmedical costs are usually described as resources expended, and productivity losses are described as resources foregone. The individual will experience a lower standard of living due to foregone resources stemming from increased morbidity and mortality. Additionally, the government foregoes tax revenue as well due to lost (reduced) earnings.
Our preliminary estimates [Reynolds et al., submitted] indicate that CFS accounts for $9.1 billion annually in lost productivity. The loss in earnings and wages disproportionately affects women, who carried almost 90% of this burden [Reynolds et al., submitted]. We will use data from the Georgia Survey to precisely measure lost productivity associated with CFS. These costs to society as a whole (i.e., society’s costs related to reduced levels of output, time spent to obtain health care, and lost productivity resulting from change in employment status as a result of morbidity or mortality) emphasize the importance of on-going research to determine causes and develop treatments for this devastating illness.
B.4 Aim 4 Case Definition
B.4.a Hypothesis – the illness defined by symptoms and illness attributes (e.g., type of onset, duration of illness, impairment/disability, stress history, physical and psychiatric symptom patterns), and biomarkers (such as endocrine and immune parameters) will more accurately identify patients with CFS than the consensus 1994 research case definition and will differentiate subcategories of CFS.
The International CFS Research Case Definition [Fukuda et al., 1994] stipulates that patients have the following: 1) clinically evaluated, unexplained, persistent or relapsing chronic fatigue (of least 6 months duration) that is of new or definite onset (i.e., has not been lifelong); 2) is not the result of ongoing exertion; 3) is not substantially alleviated by rest; and 4) results in substantial reduction in previous levels of occupational, educational, social, or personal activities. These descriptors of fatigue are difficult to apply in practice. In addition, the case definition represents a clinical-consensus document that is not based on experimental evidence.
The etiology and pathophysiology of CFS remain unknown, and there is a lack of consensus in the findings of many well-conducted studies both within and between centers [Wilson et al., 2001]. Difficulties with accurate case ascertainment are a major contributor to this problem. Much of the difficulty reflects conceptual and operational problems inherent in classifying an illness defined by symptoms and reported disability [Taylor et al., 2001].
CDC has convened an International Chronic Fatigue Syndrome Study Group, which has focused on aspects of a case definition [Reeves et al., submitted]. The Study Group has recommended a standardized approach to persons with CFS, which we have adopted in this protocol. The Group has also recommended standardized and validated instruments to be used in assessment of fatigue, disability, and other CFS case-defining symptoms, which we will use in this protocol. Individual instruments are discussed below (Section C.2.e Clinical Evaluation). Finally, the Group has recommended studies of patients with chronic unexplained fatigue from which a definition of CFS can be empirically derived, the subject of specific aim 4.
Defining CFS empirically is not trivial. We think that CFS may be categorized as a functional somatic syndrome because its symptoms, suffering and disability do not correlate with objective findings in patients [Barsky & Borus, 1999]. Other functional syndromes include irritable bowel syndrome, chronic pelvic pain, fibromyalgia, atypical or non-cardiac chest pain, tension headache, temporomandibular joint dysfunction, and multiple chemical sensitivities. Wessely et al. [1999] reviewed several characteristics of functional somatic syndromes, including chronic fatigue syndrome, and found substantial overlap of diagnostic features (e.g., symptoms) and in associations with sex, psychological distress, physiology, history of childhood maltreatment and abuse, difficulties in doctor-patient relationship, and response to treatment. Because of this overlap, they suggest a scheme of classification that would consider not only types of symptoms, but also duration of symptoms, associated mood disturbance, patients’ attributions for the symptoms, and identifiable physiological processes. With Specific Aim 4, we will attempt to derive such a classification scheme using data collected during the clinical evaluation and statistical techniques such as structural equation modeling.
B.5 Aim 5 - General Clinical Research Center Studies
A continuing need in the study of CFS is identification of biomarkers that would contribute to patient identification and to determination of pathogenesis. Two approaches are proposed here. The first is the collection of samples from a large number of individuals as is only possible in a population based study, with performance of assays following the completion of subject accrual. Testing for genetic polymorphisms is a good example of this approach.
Genetic studies (including family, adoption, twin, and molecular genetic studies) have now provided compelling evidence that many diseases, including functional somatic syndromes and the major psychiatric disorders, have one or more genetic components. Studies on the genetics of complex functional disorders have been hampered by a variety of factors, such as genetic heterogeneity (a similar phenotype develops from different genotypes), pleiotropy (the same gene contributes to the manifestation of several different disorders, leading to comorbidity) and incomplete penetrance of the phenotype (genetic risk might be uncovered by an environmental trigger) [see Radant et al., 2001; Heim, Bremner & Nemeroff, in press]. Little is known about genetic variants which may confer susceptibility to CFS, but there is reason to believe that CFS does have a genetic component. Twin studies have shown varying degrees of heritability of CFS, and familial clustering of CFS has been observed in several studies [Buchwald et al., 2001; Hickie et al., 2001; Walsh et al., 2001; Endicott 1999; Levine et al., 1998; Torpy et al., 2001]. It is conceivable that polymorphisms in genes involved in neuroendocrine-immune pathways and neurotransmission might confer vulnerability to CFS in interaction with other factors. We will therefore assess single nucleotide polymorphisms in our population-based sample. We will specifically assess mutations or polymorphisms in immune- and neurotransmitter-related genes, including genes of cytokine receptors, adrenergic receptors, selected complement components, serotonin, and transporters as well as dopamine receptors and transporters. Since allergy also appears to be common in CFS, we will also examine genes suspected to be associated with the allergic diathesis.
For example, only recently, a common polymorphism of the serotonin transporter (SERT) gene has been demonstrated to determine the risk to develop psychopathology related to stress, including early adverse experience [Caspi et al., 2003]. Such polymorphisms in several candidate genes will be the initial focus of the study, but DNA samples from each patient will be stored and may be subjected to additional analyses as new candidate loci are identified.
One of the strengths of this study is the ability to evaluate the prevalence of mutations or polymorphisms in candidate genes in the CFS population as compared not only to unaffected populations but also to populations with other fatiguing conditions. One of the recurrent themes in CFS research and clinical management has been the challenge of distinguishing it from the myriad of other conditions which can cause similar symptoms. If particular genetic variations appear preferentially in individuals with CFS, they could be tested in prospective studies as potential biological markers and rapidly incorporated into clinical practice. Additionally, insights into the fatigue process on a molecular level would have wide applicability to other medical conditions, and potentially help elucidate drug efficacy variability and differing host-responses to a variety of pathogens and antigens.
The second can only take place when persons fulfilling strict definitional criteria are identified and entered into appropriate study protocols. The Georgia survey will identify subjects who meet these criteria. They will be asked to grant permission to be contacted for possible enrollment into studies to be performed at Emory University.
C. Research Design and Methods
C.1 Design
C.1.a Study population. This cross-sectional study will use a random-digit-dialing survey to identify, and enroll subjects representative of metropolitan, urban, and rural Georgia populations. To identify persons with CFS and comparison subjects who are unwell and have similar symptoms of CFS, we will enroll Unwell subjects. Unwell subjects are defined as reporting one month or more duration of any of the following CFS defining symptoms (severe fatigue, unrefreshing sleep, impaired memory or concentration, muscle or joint pain). Unwell subjects will be further evaluated to identify the subset with CFS. Well subjects who do not report any of these CFS defining symptoms for one month or longer will comprise the control group.
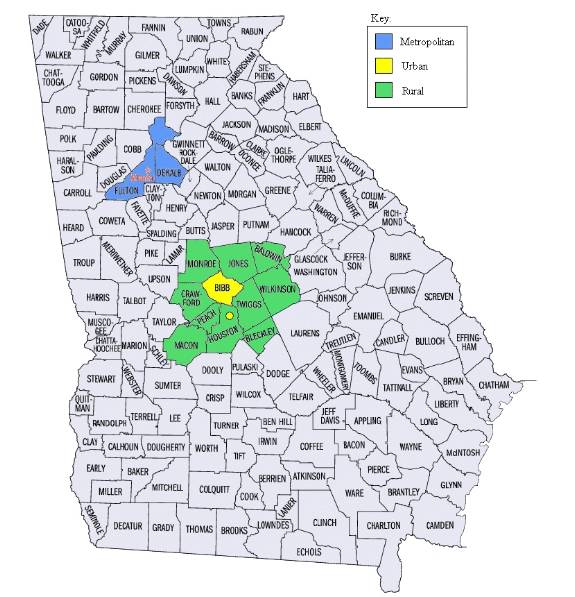 The
metropolitan (Atlanta) population will consist of the residents of
DeKalb and Fulton counties. The urban population will consist of
residents of Macon (Bibb county) and the adjoining city of Warner
Robins (in Houston County). The rural population will include
residents of counties surrounding Bibb, specifically Houston
(excluding Warner Robins), Monroe, Jones, Baldwin, Wilkinson, Twiggs,
Peach, Crawford, Bleckley, and Macon counties. We limited the study
to the Atlanta and Macon areas for logistic reasons (conducting
clinical evaluations). Atlanta is the major metropolitan center of
Georgia and we chose the two counties that encompass its core. Macon
and Warner Robins represent typical urban Georgia, and the areas
surrounding Macon/Warner Robins will represent rural Georgia.
The
metropolitan (Atlanta) population will consist of the residents of
DeKalb and Fulton counties. The urban population will consist of
residents of Macon (Bibb county) and the adjoining city of Warner
Robins (in Houston County). The rural population will include
residents of counties surrounding Bibb, specifically Houston
(excluding Warner Robins), Monroe, Jones, Baldwin, Wilkinson, Twiggs,
Peach, Crawford, Bleckley, and Macon counties. We limited the study
to the Atlanta and Macon areas for logistic reasons (conducting
clinical evaluations). Atlanta is the major metropolitan center of
Georgia and we chose the two counties that encompass its core. Macon
and Warner Robins represent typical urban Georgia, and the areas
surrounding Macon/Warner Robins will represent rural Georgia.
The process of sampling the study population using an RDD sample, involves a number of steps. Please refer to the flowchart below. First, a random sample of telephone numbers from the selected Georgia counties is drawn. These numbers are then screened for business and nonworking numbers using the GENESYS ID Plus sampling system. Nonworking and business numbers are removed from the sample.
Telephone
numbers randomly drawn for selected counties in Georgia.
Addresses appended to telephone numbers through reverse address
matching.

Advance letters sent for telephone numbers where matched addresses
are found.

Sampled telephone numbers dialed to attempt screening interviews.


Respondent(s)
sampled for detailed interview.
No respondents sampled for detailed interviews.
Attempt detailed interview(s) for sampled household member(s).
Then, using a reverse address matching process, addresses are appended to telephone numbers where matched addresses are found. Advance letters are sent to telephone numbers with matched addresses prior to initial contact. When addresses are available, noncontact letters are sent to households where we are having difficulty reaching household members to complete screening interviews.
Following the screening interviews, a detailed interview is attempted with each selected respondent. When addresses are available, noncontact letters are sent to respondents with whom we are having difficulty reaching to conduct detailed interviews. The following sections describe the interviewing process in more detail.
C.1.b Population Survey. The survey will include four components:
A Screening Telephone Interview of a single household informant to provide a household census (for deriving weights to be applied during analysis) and to identify Unwell household members who have at least one of the core CFS-defining symptoms for a month or longer (fatigue, cognitive impairment, unrefreshing sleep or muscle/joint pain) and Well residents (subjects that have none of these problems for at least a month).
A Detailed Telephone Interview of respondents between 18 and 59 years of age including all Unwell identified as fatigued, a random sample of selected Unwell subjects who are not fatigued (i.e., identified with cognitive impairment, pain, or sleep problems) and a random sample of Well to:
determine classification as CFS-like (i.e., those who report fatigue characteristics and symptoms of CFS and report no exclusionary medical or psychiatric conditions) and thus eligibility for clinical evaluation (Aims 1-5).
obtain information on residence, symptom characteristics, demographics, medical/psychiatric comorbidity, and economics (Aims 1-3).
screen for psychiatric comorbidity and life experiences (Aims 1, 2, 4, 5).
Clinical Evaluation (one day) of:
all subjects classified, based on information from the Detailed Telephone Interview, as having CFS-like illness. Clinical evaluation is necessary to confirm classification as CFS (Aims 1-5).
comparison subjects for the purposes of identifying characteristics specific to CFS (Aim 2), to assess impairment and utilization of health services, and for derivation of an empiric case definition of CFS (Aim 4). These will include a random sample of subjects with Chronic Unwellness (not CFS-like), and a random sample of Well subjects. Persons with Chronic Unwellness are defined as being Unwell (with or without fatigue) reporting symptoms lasting for at least 6 months. The Well comparison group will be matched to CFS-like subjects by geography (metropolitan/urban/rural), sex, age and race/ethnicity. The Chronic Unwellness comparison group will be randomly selected (1:1 for CFS-like) from the same geographic area as CFS-like.
GCRC Studies. Finally, selected subjects participating in the Detailed Telephone Interview and those undergoing the Clinical Evaluations will be offered the opportunity to participate in future follow-up studies and in clinical research studies of fatiguing illness, which will be conducted at Emory University (Aim 5).
C.2 Methods
In this section we describe the data collection process from initial contact through GCRC studies. To facilitate communication throughout the study, we will host an Internet website that subjects can visit to learn more about the study, get answers to frequently asked questions, and ensure themselves of the study’s legitimacy.
C.2.a Advance and follow up letters. Prior to calling selected telephone numbers, advance letters will be sent to all households where the randomly generated telephone number could be matched to an address. These letters will notify respondents of the survey, explain its purpose and sponsor, and alert respondents to expect a call from a telephone interviewer.
Potential respondents who are initially reluctant to cooperate may be sent follow-up letters, emphasizing the importance of their participation in the study and giving them a toll-free telephone number to call to schedule or complete an interview. Four “conversion” letters were designed for this study. The first letter will be sent to households we have difficulty contacting by telephone for the Screening Telephone Interview. The second letter will be sent to households reluctant to complete the Screening Telephone Interview. The third letter will be sent to adult subjects reluctant to complete the Detailed Telephone Interview. The fourth letter will be sent to households where we are having difficulty reaching a subject selected for a Detailed Telephone Interview. These letters are appended.
C.2.b Telephone interview overview. Professional interviewers trained to administer the Screening and Detailed questionnaires will conduct all telephone interviews. All telephone interviews will be administered using computer-assisted telephone interviewing (CATI). The scripts that the telephone interviewers will use to contact respondents and conduct the interviews are included in the questionnaires. Informed consent statements are included in both the screening and detailed telephone interview questionnaires. The consent statements for both of these interviews will be administered as soon as the respondent is on the telephone. Telephone interviewers will be prepared to discuss any aspect of the respondent’s participation at greater length, including respondent rights and the telephone numbers of staff who can be contacted for more or different information. The interview will not proceed until the informed consent statement has been read, exactly as written, to each respondent, and all of the respondent’s questions have been answered.
We request a waiver of documentation for the verbal consent for the screening and detailed telephone interview questionnaires. 1) These questionnaires involve no more than minimal risk to the participants. The interview provides participants with a number to call (Dr. James F. Jones) if they think that they have been injured in this study. Dr. Jones (CDC Co-PI) has considerable clinical experience managing persons with CFS and other illnesses and is available at all times. He is backed up by Dr. Dimitris Papanicolaou (Emory Co-investigator). Previous versions have been administered with verbal consent in previous CDC studies of CFS (the Longitudinal Study of CFS in Wichita - IRB 1698) and the Pilot National Survey for CFS - IRB 2936) and there have been no adverse events. 2) The waiver or alteration will not adversely affect the rights and welfare of the participants. The verbal consent explains the study, the reasons it is being conducted, the nature of the questions, discusses possible risk, and informs the participant that she/he can choose not to answer any question or terminate the interview at any time. The interview also informs participants that they can call the CDC Deputy Director for Science at a toll free number if they have any questions about their rights in this study. Finally, individual potentially sensitive sections (e.g., exclusionary medical conditions and the life experiences screener) have introductory text informing the participant as to why the questions are being asked, their potentially sensitive nature, and that responses are completely voluntary and that he/she is free to not answer any question. 3. Because of the nature of random-digit-dialing telephone surveys, it is not practical to obtain written informed consent. 4) Whenever appropriate, participants will be provided with additional pertinent information after participation. The interview closes informing participants that if they have any questions about this research study they can contact Dr. Jones.
C.2.c Screening Telephone Interview. The objective of the Screening Telephone Interview is to identify subjects to interview in detail. A copy of the Screening Telephone Interview is appended. Screening will be similar to that in previous CDC population surveys for CFS.
The Screening Telephone Interview will contact a responsible adult informant for the selected households by asking for a respondent “who knows about the general health of the people in the household.” She/he will be asked to provide demographic information for all household residents. Household residents include: a] related family members residing in the living unit, b] unrelated people who share the housing unit (e.g., lodgers, foster children, wards, or employees), c] a person living alone in a housing unit, d] a group of unrelated people sharing a housing unit (e.g., partners or roomers), e] persons who are temporarily away (e.g., business, vacation, at boarding school, in the hospital). Persons not considered household members and who will not be enumerated include: a] students living away at college, b] persons who usually live elsewhere (e.g., “snowbirds” who live in a warmer climate during winter months but live elsewhere during the remainder of the year), c] persons living in group quarters (e.g., dormitories, nursing homes, military barracks).
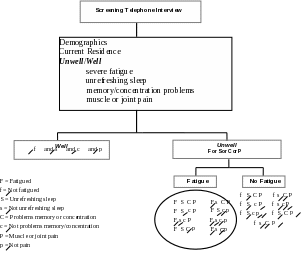
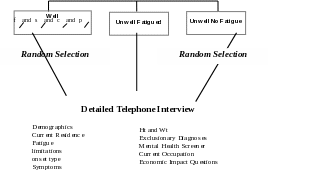
We will also ask the respondent to identify Unwell household residents who have at least one core symptom of CFS for at least one-month duration (e.g., fatigue, unrefreshing sleep, problems with memory or concentration, and muscle or joint pain). Subjects selected for the Detailed Telephone Interview will be adults between 18 and 59 years of age and include all fatigued Unwell, a random sample of the Unwell not fatigued, and a random sample of the Well Subjects.
C.2.d Detailed Telephone Interview. The primary objective of the Detailed Telephone Interview is to identify Unwell subjects who fulfill criteria of the 1994 CFS Research Case Definition (i.e., have CFS-Like illness) and recruit them for clinical evaluation to confirm whether they have CFS and to obtain additional information needed for weighting during data analysis. The Detailed Telephone Interview will also 1) obtain data concerning characteristics of Well and Unwell respondents from metropolitan, urban, and rural populations; 2) obtain data from Unwell respondents for use in developing an empiric case definition (this includes information on mental health problems that may be related to the pathophysiology of CFS but are not exclusionary, such as anxiety disorders); 3) obtain data from Well and Unwell respondents for use in determining associations between life experiences and unwellness.
A copy of the Detailed Telephone Interview is appended. As with the Screening Telephone Interview, Detailed Interviews will be administered as CATI. We will attempt to complete the detailed interview immediately following the initial screening contact. If this is impossible, we will schedule an appropriate time.
We will conduct Detailed Telephone Interviews with all fatigued Unwell subjects between 18 and 59 years of age (i.e., those who could be CFS), randomly selected not fatigued Unwell, and randomly selected Well subjects between 18 and 59 years of age from within the specific geographic area. Sample size considerations are discussed below.
The detailed interview will:
Confirm the respondent is the individual who was identified by the household representative during the Screening Telephone Interview.
Document current residence.
Document demographic characteristics (age, sex, race, and ethnicity).
Document status of fatigue and other symptoms by a modified version of the CDC Symptom Inventory used in previous population surveys of CFS conducted by CDC. This will record presence or absence of specific symptoms, and if they have been present for at least 6 months.
Screen for medical conditions that exclude classification as CFS, by a modified version of the CDC screener used in the previous population surveys.
Screen for psychiatric conditions that exclude classification as CFS and comorbid conditions and experiences thought to be associated with CFS.
We will screen for exclusionary psychiatric disorders by an instrument we have compiled from the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002). Exclusionary psychiatric conditions identified by this instrument include: bipolar disorder, psychotic disorders, eating disorders, and alcohol and substance abuse. During the clinic visit, melancholic (and other) subtypes of depression will be determined in a detailed psychiatric interview.
Screen for psychiatric comorbidity and lifetime experiences.
Because psychopathology is highly prevalent in patients with CFS and may be related to the clinical course of CFS, we will assess lifetime and current psychiatric disorders that are not exclusionary for CFS, such as depression. We will include a screening for mood, anxiety (e.g., PTSD), and somatoform disorders to determine associations between psychopathology and CFS and to identify persons to recontact for future clinical studies at Emory University. The screening instrument was compiled from the SCID (First et al., 2002).
Because of the increasing evidence for a role of stress during development as a risk factor for CFS, and because of the evidence that symptoms of CFS are often exacerbated by acute life stress, we will include a detailed assessment of stress history in the present study. We will document lifetime experiences with the Childhood Traumatic Events Scale (CTES), the Recent Traumatic Events Scale (RTES) (Pennebaker and Susman, 1988), and four items of the Parental Bonding Instrument (PBI) (Parker, 1979). The RTES measures major life events in the preceding 3 years.
Document occupation.
Gather information to estimate direct and indirect economic costs associated with CFS and chronic unwellness.
B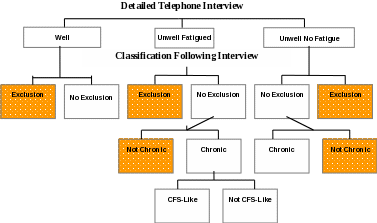 ased
on information from the Detailed Telephone Interview, Well
and Unwell
subjects will be classified as having or not having an exclusionary
medical or psychiatric condition. Unwell
participants
will be classified as Chronically
Unwell if
symptoms have persisted 6 months or longer. Chronically
Unwell patients
with fatigue who do not have an identified exclusionary condition
will be classified as CFS-Like
if they fulfill criteria of the 1994 CFS research case definition.
Chronically
Unwell
participants (with or without fatigue) who do not meet criteria for
CFS
will be classified according to the nature of their symptoms and the
presence or absence of comorbid psychiatric conditions identified in
the interview.
ased
on information from the Detailed Telephone Interview, Well
and Unwell
subjects will be classified as having or not having an exclusionary
medical or psychiatric condition. Unwell
participants
will be classified as Chronically
Unwell if
symptoms have persisted 6 months or longer. Chronically
Unwell patients
with fatigue who do not have an identified exclusionary condition
will be classified as CFS-Like
if they fulfill criteria of the 1994 CFS research case definition.
Chronically
Unwell
participants (with or without fatigue) who do not meet criteria for
CFS
will be classified according to the nature of their symptoms and the
presence or absence of comorbid psychiatric conditions identified in
the interview.
At the conclusion of the Detailed Telephone Interview, the CATI program will determine subject eligibility for the clinical evaluation component. If subjects are eligible, the CATI program will display a script for the telephone interviewer to read to subjects informing them that they have been selected for the clinical evaluation component. Next, the CATI interviewer will be prompted to collect information to facilitate follow-up by clinics for scheduling purposes. Only respondents who did not have an exclusionary medical or psychiatric condition identified during the Detailed Telephone Interview will be eligible for clinic evaluation. We will attempt to clinically evaluate all respondents with CFS-like illness, an equal number of randomly selected Chronically Unwell not CFS-like (fatigued or not fatigued) subjects, and randomly selected Well subjects matched 1:1 to CFS-Like subjects by geography, sex, age, race/ethnicity.
C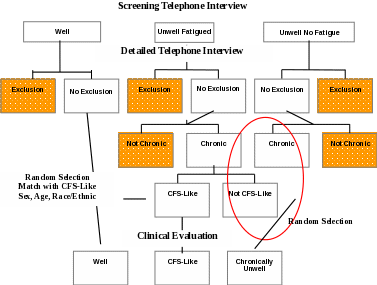 .2.e
Clinical Evaluation.
The objectives of the Clinical Evaluation are: 1) to classify
subjects as CFS,
Chronically
Unwell not CFS,
or Well
[Specific Aims 1-5]; 2) obtain detailed information pertinent to the
symptom domains of CFS in order to determine its characteristics
[Specific Aim 2] for use in deriving an empiric definition (Specific
Aim 4), as stratification variables [Specific Aim 2], and to identify
subgroups for participation in GCRC studies [Specific Aim 5]; 3)
obtain information to describe the economic impact of CFS (Specific
Aim 3), and; 4) obtain information concerning stress history, coping
mechanisms, and economic impact [Specific Aims 2 – 5].
.2.e
Clinical Evaluation.
The objectives of the Clinical Evaluation are: 1) to classify
subjects as CFS,
Chronically
Unwell not CFS,
or Well
[Specific Aims 1-5]; 2) obtain detailed information pertinent to the
symptom domains of CFS in order to determine its characteristics
[Specific Aim 2] for use in deriving an empiric definition (Specific
Aim 4), as stratification variables [Specific Aim 2], and to identify
subgroups for participation in GCRC studies [Specific Aim 5]; 3)
obtain information to describe the economic impact of CFS (Specific
Aim 3), and; 4) obtain information concerning stress history, coping
mechanisms, and economic impact [Specific Aims 2 – 5].
In summary, the following will be assessed during clinical evaluation: 1) physical and laboratory examinations for classification purposes; 2) saliva and blood collection for endocrine/immune function and future genetic studies; 3) questionnaires concerning symptomatology; 4) neurocognitive assessment; 5) psychiatric evaluation; 6) assessment of early and adult life experiences; 7) assessment of stress, coping and personality traits; 8) assessment of economic impact; and 9) assessment of health care utilization.
Each subject scheduled for Clinical Evaluation will receive a packet of materials prior to the appointment. This will include a letter describing the study, an informed consent document for review (8TH grade reading level), and a questionnaire to complete before arriving at clinic. The packet will also include 4 salivette devices and written instructions for the collection of saliva samples at home (see below). Upon arrival at the clinic, the coordinator will explain the study, answer any questions, and confirm eligibility for clinical evaluation. A subject will be ineligible for clinical evaluation if his/her body mass index (BMI) is 40 or greater, if the subject is not between 18 and 59 years of age, or if the subject has been pregnant within the past 12 months. Also, if the registered nurse determines the subject is suffering an acute illness (based on basal temperature and observation), the subject will be temporarily deferred until he/she is no longer contagious. Finally, a matched Well subject may be ineligible if his/her matching criteria (such as age, race, or ethnicity) no longer match the CFS-like subject with whom he/she has been paired.
Clinic coordinators will administer the informed consent to eligible subjects. Descriptive materials sent to subjects prior to Clinical Evaluation are appended (research instruments are appended separately).
The following table is an example of a work plan at clinic. All instruments are appended.
Exhibit 2.5
Clinic Flow for a Typical Clinic Day
|
||||
|
Subject 1 |
Subject 2 |
Subject 3 |
Subject 4 |
7:30 am |
Informed Consent |
Informed Consent |
|
|
8:00 am |
Blood Draw |
Blood Draw |
Informed Consent |
Informed Consent |
8:30 am |
BREAKFAST |
BREAKFAST |
Blood Draw |
Blood Draw |
9:00 am |
Medical & Meds Hx |
WRAT/CANTAB |
BREAKFAST |
BREAKFAST |
9:30 am |
Buffer |
|
WRAT/CANTAB |
Questionnaires |
10:00 am |
WRAT/CANTAB |
Medical & Meds Hx |
|
|
10:30 am |
|
Buffer |
Questionnaires |
WRAT/CANTAB |
11:00am |
Questionnaires |
Questionnaires |
Medical & Meds Hx |
|
11:30 am |
|
|
Buffer |
Questionnaires |
12:00pm |
LUNCH |
LUNCH |
Questionnaires |
Medical & Meds Hx |
12:30 pm |
SCID & PDQ4 |
Physical Exam |
LUNCH |
LUNCH |
1:00 pm |
|
|
SCID & PDQ4 |
Buffer |
1:30 pm |
|
Questionnaires |
|
Physical Exam |
2:00pm |
|
|
|
|
2:30 pm |
Physical Exam |
SCID & PDQ4 |
|
Questionnaires |
3:00pm |
|
|
Questionnaires |
SCID & PDQ4 |
3:30pm |
Questionnaires |
|
|
|
4:00pm |
|
|
Physical Exam |
|
4:30 pm |
|
|
|
|
5:00pm |
|
|
|
|
1. History, Physical Examination, Review of Medications and Laboratory Examinations
A past medical history, review of medications, and physical examination are specified by the CFS research case definition as necessary to rule-out other medical causes of chronic fatigue and to classify a subject as CFS. The history, physical examination, and review of medications have been modified from past CDC studies of CFS. The case definition also specifies screening laboratory tests (a complete blood count [CBC] with differential, c-reactive protein, alanine aminotransferase [ALT] [SGPT], albumin, alkaline phosphatase, asparatate aminotransferase [AST] [SGOT], total bilirubin, calcium, carbon dioxide, chloride, creatinine, glucose, potassium, total protein, sodium, urea nitrogen BUN, antinuclear antibodies, rheumatoid factor, TSH, free T4, and urinalysis).
An interviewer will collect information from each patient regarding past medical history and use of medications. Each patient will undergo a complete physical examination by the local study physician.
A fasting blood sample and a urine specimen will be collected after the patient has given informed consent. The blood (30 ml) will be collected by venipuncture. The patient will be given breakfast after the blood draw.
2. Assessment of Endocrine and Immune Markers, and Genetic Polymorphisms
As described in the Background section, alterations of the HPA axis have been implicated in the pathophysiology of CFS. Specifically, decreased cortisol levels (relative to controls) have been postulated to play a causal role in CFS due to a potential lack of glucocorticoid control of immune function (see above). Because a) the HPA axis is a self-regulated dynamic feedback system and b) ACTH and cortisol are secreted in a pulsatile fashion, single time-point measures of cortisol cannot be interpreted in terms of HPA axis functioning. An adequate assessment of HPA axis function requires multiple serial sampling (to test basal activity) or dynamic testing using pharmacological or psychological challenges (to test reactivity/feedback sensitivity). Because both approaches are impossible during the 1-day clinic visit of this study, we decided to use serial collection of saliva samples, which the subjects will perform at home, in order to obtain information on HPA axis functioning in this study.
The assessment of cortisol concentrations in saliva is a widely used method in psychobiology [see Kirschbaum & Hellhammer 2000]. Among the advantages of salivary cortisol measures, as compared to serum cortisol measures, is the fact that the sample collection method is non-invasive and will not lead to HPA axis activation. In addition, salivary cortisol concentrations represent the free fraction of circulating, which is not bound to CBG, and therefore reflect the biologically active fraction of cortisol. A number of recent clinical studies have provided evidence that there is a pronounced surge of cortisol secretion immediately after awakening. Measures of the cortisol response curve to awakening have been validated as a useful marker for HPA axis function in a variety of studies. This marker has also been shown to vary with clinical measures, such as chronic stress, pain and chronic health problems and CFS [Schulz et al., 1998; Pruessner et al., 1999; Kudielka & Kirschbaum 2003; Gaab et al., 2002]. It has also been shown that the cortisol response to awakening is highly correlated with cortisol responses to dynamic challenge of cortisol secretion with a low dose of ACTH1-24 [Schmidt-Reinwald et al., 1999].
We will use this measure in the proposed study. Subjects will be instructed to collect saliva using Salivette devices (Sarstedt, Germany) at home on a regular workday within 3 days of their clinic visit. Saliva will be collected immediately after awakening (0 minutes) as well as 30, 45 and 60 minutes after awakening. Subjects will not be allowed to brush their teeth or consume coffee, food or cigarettes throughout the sampling period. Subjects will store salivettes in the refrigerator and will bring the salivettes to clinic, where they will be stored at -20 C until assayed. Instructions that patients will receive for saliva collection are appended with materials to be sent to them prior to the clinic appointment.
Blood will be collected from subjects when they arrive at clinic. This blood will be used for standard laboratory exam to exclude underlying abnormalities (see above). We will also determine cytokine concentrations in this sample. Blood for these measures will be collected between 8 and 9 am through direct venipuncture. All subjects will be requested to be fasting for 10 hours before coming to the clinic. We will measure a variety of cytokines and serum C-reactive protein, an end-product of IL-6 action.
We will collect cells for genotyping studies from the tubes with anticoagulants used to obtain plasma specimens and the clots from tubes used to obtain serum.
For a summary of saliva and blood collection and handling, see Appendix 7.
3. Questionnaires Concerning Symptomatology (Appendix 8)
Symptom Inventory. We will use the CDC Symptom Inventory to collect information on occurrence, frequency, and severity of symptoms common in CFS and other fatiguing illnesses. This symptom checklist has evolved through several population-based studies. This instrument takes 5 to 10 minutes to complete.
Medical Outcomes Study 36-item short-form health survey (SF-36v2) (Ware & Sherborne, 1992): a general indicator of health status (function and well-being) and includes primarily non-psychiatric health status questions. The SF-36v2 is a well-researched, reliable and valid broad-based, sophisticated instrument with population norms and normative data for a wide variety of medical conditions. The SF-36v2 assesses health-related quality of life in 8 areas: 1) limitations in physical activities because of health problems; 2) limitations in social activities because of physical or emotional problems; 3) limitations in usual role activities because of physical health problems; 4) bodily pain; 5) general mental health; 6) limitations in usual role activities because of emotional problems; 7) vitality (energy and fatigue); and 8) general health perceptions. This instrument takes 10 to 15 minutes to complete.
Multidimensional Fatigue Inventory (MFI) (Smets et al., 1995): a 20-item self-report instrument designed to measure fatigue, covering the dimensions General Fatigue, Physical Fatigue, Mental Fatigue, Reduced Motivation and Reduced Activity. This instrument was tested for its psychometric properties and found to have reasonable internal consistency (Cronbach’s alpha between 0.53 and 0.93) and construct validity in samples with CFS. This instrument takes 5 to 10 minutes to complete.
Sleep Assessment. We will screen for sleep abnormalities using the Emory list, which includes the Epworth sleepiness scale, questions regarding circadian rhythm, specific questions about movement problems, as well as general sleep problems. This instrument takes 10 to 15 minutes to complete.
Brief Pain Inventory. (BPI) (Cleeland, 1991): In its original form, this instrument was developed to measure the severity of cancer pain and its impact on the patient’s functioning, but it can also be used for other chronic illnesses. The BPI includes 20 items regarding to the intensity of pain at different stages, and the extent of interferences with other activities. It also records the location of the pain and subjects are asked to select words from an item list which best describe their pains. The questionnaire takes 10 to 15 minutes to complete. Cronbach’s alpha varies between 0.87 for the intensity items and 0.91 for the interference scale. Advantages of Brief Pain Inventory (BPI) include: self-report instrument; Good reliability; shorter than MPQ; includes pain history and interference with activities; pain intensity is rated, and it has been use in a multicenter study in the US. Its disadvantages include: moderate validity; and assessment of only sensory dimension pain. This instrument takes 10 to 15 minutes to complete.
4. Neurocognitive Assessment (Appendix-9)
Cambridge Neuropsychological Test Automated Battery (CANTAB)
The CANTAB has been used to assess neurocognitive performance in modeling studies of CFS (Robbins & Sahakian, 1994; Capuron et al., 2001). The CANTAB has modules for several neurocognitive functions and processes including psychomotor and motor speed, reasoning and planning abilities, memory and attention, and frontal, temporal and hippocampal dysfunctions. Thus, it allows assessment of neurocognitive dysfunctions associated with neurologic disorders, pharmacologic manipulations, and neurocognitive syndromes. The mean time for the battery we will administer is one hour. The tests are independent of language and non-sensitive to gender. Parallel versions of these tests can be used for subsequent evaluations. All tests of the CANTAB are computerized, presented on a touch screen and, thus, testing is standardized and data is instantly recorded. The CANTAB includes the following modules:
Psychomotor Coordination and Motor Speed: The Reaction Time Test measures subject’s speed of response to a visual target where the stimulus is either predictable (simple reaction time) or unpredictable (choice reaction time). This test requires approximately 5 minutes.
Reasoning and Planning Abilities: The Stockings of Cambridge task assesses subject’s ability to engage in spatial problem solving. It makes substantial demands on executive function and is sensitive to frontal-lobe deficits. It requires 7-10 minutes.
Memory: There are three modules: (a) The Spatial Working Memory module requires that subjects find a blue token in a series of displayed boxes and use these to fill up an empty column, while not returning to boxes where a blue token has been previously found. Some studies have shown this to be impaired in CFS patients (Joyce et al., 1996). It requires 5-10 minutes. (b) The Pattern Recognition Memory test screens visual recognition memory in a 2-choice forced discrimination paradigm and is sensitive to temporal or hippocampal dysfunctions. It requires about 3 minutes. (c) The Spatial Recognition Memory test is a 2-choice forced discrimination paradigm that requires about 2-3 minutes.
Attention: There are two modules: The Attentional Shift: Intra/Extra Dimensional Shift task is a test of rule acquisition and reversal, featuring visual discrimination and attentional set shifting. It is sensitive to cognitive dysfunction in Parkinson disease and frontal-lobe deficits and requires approximately 5 minutes. The Sustained Attention: Rapid Visual Information Processing module is a visual continuous performance task (vigilance) with a small working memory component. It is impaired in patients with frontal lobe pathology. Its duration is 4 minutes.
Reading Subtest of the Wide-Range Achievement Test (WRAT3) (Wilkinson, 1993). General intelligence must be estimated to interpret each subject’s CANTAB performance relative to normative ranges. Because a full intelligence test is too time-consuming, and because there is no specific hypothesis to be tested related to associations between intelligence and CFS, we will use a brief reading test that was previously shown to highly correlate with general intelligence and widely used to approximate general intelligence levels. The test employed will be the Wide-Range Achievement Test (WRAT3) Reading Subtest. The Reading Subtest focuses on recognizing and naming letters and pronouncing printed words with increasing complexity and unfamiliarity. This test was developed to measure the development of skills of reading. It was designed to eliminate as much as possible the effects of comprehension. Norms and reliability data are provided. Absolute scores, standard scores and grade scores are provided for the subtest. Two equivalent alternate test forms are provided. Each can be used for persons aged 5 through 75 years of age. Test administration time is 5 minutes.
5. Psychiatric Evaluation (Appendix 10)
All subjects will undergo a general psychiatric evaluation administered by experienced licensed psychiatric social workers, clinical psychologists, psychiatric nurse practitioners, or certified research nurses with experience in psychiatric assessments. The objective of the psychiatric evaluation is to identify psychiatric conditions that would exclude diagnosis, identify psychiatric comorbidity, and measure psychologic characteristics postulated as associated with CFS. Psychiatric evaluation will include:
Structured Clinical Interview for DSM-IV (SCID) [First et al., 2002]: The SCID is a semistructured interview for making the major Axis I DSM-IV diagnoses. It is administered by a clinician and includes an introductory overview followed by nine modules, seven of which represent the major axis I diagnostic classes: Screening module, mood episodes, psychotic symptoms, psychotic disorders, mood disorders, substance use disorders, anxiety disorders, somatoform disorders, eating disorders, and adjustment disorders. Because of its modular construction, it can be adapted for use in studies in which particular diagnoses are not of interest. Using a decision tree approach, the SCID guides the clinician in testing diagnostic hypotheses as the interview is conducted. We will use the Research Version of the SCID for this study. The output of the SCID is a record of the presence or absence of each of the disorders being considered, for current episode (past month) and for lifetime occurrence. It requires about 30 – 60 minutes to obtain the psychiatric status of subjects.
Personality Diagnostic Questionnaire-Revised (PDQ-4) (Hyler et al., 1990): The former version (PDQ-R) has shown good internal consistency and validity in previous studies, including convergent validity with structured interviews for personality disorders. It also tended to over diagnose personality disorders. The Personality Diagnostic Questionnaire-4th Edition (PDQ-4) is a 100 item, self-administered, true/false questionnaire that yields personality diagnoses consistent with the DSM-IV diagnostic criteria for the axis II disorders. It takes approximately 20-30 minutes to complete. It is widely used in clinical practice and in research projects throughout the U.S.A. and has been translated in several different languages. Though its principal use has been for screening for personality diagnoses, the newest version includes a Clinical Significance Scale to address the problem of false positive diagnoses.
Because the SCID and the PDQ-4 provide only categorical diagnoses, we will administer additional standard rating scales to quantify core symptoms of interest. The following instruments will be given at the clinic during the time slot allotted for questionnaires:
Self-Rating Depression Scale (SDS) (Zung, 1965): This self-report scale was designed to quantify the severity of current major depression in 20 items. The scale was tested for psychometric properties. Cronbach’s alpha ranged from 0.75 to 0.95. Various studies showed good to adequate results for validity. Most guidelines suggested that index scores of less than 50 are within the normal range, while scores of 50 to 59 indicate minimal or mild depression, 60 to 69 indicate moderate depression and scores above 70 indicate severe depression. It requires about 10 - 15 minutes to obtain the psychiatric status of subjects.
Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger, 1983): The self-report instrument was designed to assess levels of state anxiety and trait anxiety, through 40 items scored by a Likert-scale. State anxiety can be defined as a transient momentary emotional status that results from situational stress. Trait anxiety represents a predisposition to react with anxiety in stressful situations. For the Trait-anxiety scale the retest reliability coefficients ranged from .65 to .86, whereas the range for the State-anxiety scale was .16 to .62. The STAI requires approximately 10 – 15 minutes to complete.
Davidson Trauma Scale (DTS) (Davidson et al., 1997): The DTS was designed as a self-report scale measuring frequency and severity of PTSD symptoms in three clusters: intrusion, avoidance, and hyperarousal. For the 17 frequency and severity items, the Cronbach’s alpha was 0.99, for the frequency items alone it was 0.97 and for the severity items 0.98. The scale showed good convergent and discriminative validity. Davidson proposed a threshold of 40 for a total symptom score to predict the onset of a PTSD. The Davidson PTSD Scale requires approximately 5 – 10 minutes to complete.
6. Assessment of Early and Adult Life Experiences (Appendix 11)
The assessment of the stress history will focus on early and lifetime trauma experiences and their relationship to chronic fatigue. Because of the increasing evidence for a role of stress during development as a risk factor for CFS, and because of the evidence that symptoms of CFS are often exacerbated by acute life stress, we will include a detailed assessment of stress history in the present study. The following instruments will be given at the clinic during the time slot allotted for questionnaires:
Childhood Trauma Questionnaire (CTQ-SF) (Bernstein et al., 2003): This short-form of the Childhood Trauma Questionnaire was designed to assess retrospectively perceived childhood abuse and neglect. The 28 item self-report instrument consists of five clinical scales: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Cronbach’s alpha for the five scales ranged from 0.61 to 0.95. The short form also showed good evidence of criterion-related validity. It takes about 10 – 20 minutes to complete this instrument.
Traumatic Life Events Questionnaire (TLEQ) (Kubany et al., 2000): This self-report instrument assesses exposure to 21 types of potentially traumatic events that proceed gradually from stressors that are not highly personal (e.g., natural disasters, motor vehicle accidents) to events that are personally sensitive to many people (e.g., intimate partner abuse, sexual abuse). Subjects were asked to indicate the frequency of occurrence of each trauma and whether they experienced intense fear, helplessness or horror relating to the DSM-IV PTSD A2 criterion. The test-retest reliability for the occurrence of events ranged from 0.59 to 0.91. The questionnaire showed also good convergent and discriminative validity. The TLEQ requires approximately 10 – 15 minutes to complete.
Life Experiences Survey (LES) (Sarason et al., 1978): This self-report instrument was developed to evaluate major life events in the past year. The first section of the questionnaire contains 47 items and three blank spaces and was designed for the use in the general population. The format of the LES asks subjects to rate separately the desirability and impact of events that they have experienced in the last 12 months divided into two semesters. The questionnaire provides a positive change score by summing the impact ratings of events designated as positive by the subject and a negative change score summing the negative ratings. By adding these two values, a total change score can be obtained, representing the total amount of change. Test-retest reliability for the positive change score ranged from 0.19 to 0.88, for the negative change score from 0.56 to 0.88, and for the total change score from 0.63 to 0.64. Validity analysis revealed reasonable concurrent validity. The LES requires approximately 10 – 15 minutes to complete.
Parental Bonding Instrument (PBI-BC) (Klimidis et al., 1992): This instrument was designed to assess fundamental parental styles as perceived by the child. The measure is to complete for both mothers and fathers separately. The short form of the instrument consists of 8 items, including 4 items for the scale ‘care’ and 4 items for the scale ‘overprotection’. The PBI has been found to have good internal consistency and satisfactory construct and convergent validity. The PBI requires approximately 5 – 10 minutes to complete.
NEO Five Factor Inventory (NEO-FFI) (Costa and McCrae, 1992): The NEO-FFI is a widely used 60 item short form of the NEO Personality Inventory (Buckley et al., 1999; Fiedler et al., 2000; Taillefer et al., 2003). The instrument assesses five major domains of personality: Neuroticism (N), Extroversion (E), Openness to Experience (O), Agreeableness (A), and Conscientiousness (C), each represented by six lower level facet scale scores. Reliability coefficients for the domain levels range from 0.86 to 0.95 and for the facet scale scores from 0.56 to 0.90. The questionnaire showed excellent construct, convergent, and divergent validity.
7. Assessment of Stress and Coping (Appendix 12)
Complex psychological traits such as attribution styles, coping strategies and personality traits cannot be reliably assessed in the CATI phase of the study. We will therefore assess these traits in individuals that are invited to clinic. The following published instruments with demonstrated reliability and validity will be administered at clinic.
Trier Inventory for the Assessment of Chronic Stress (TICS-2-S) (Schlotz and Schulz, 2003): This short version was directly derived from TICS-2 and comprises nine scales with 30 items: work overload, social overload, overextended at work, lack of social recognition, work discontent, social tension, performance pressure at work, performance pressure in social interactions, social isolation, and worry propensity. Preliminary analysis reveals good reliability and validity coefficients. Cronbach’s alpha for the original version range from 0.76 to 0.90 and also the TICS-2 showed also good convergent and discriminative validity. This instrument requires approximately 10 – 15 minutes to complete.
Ways of Coping Questionnaire (WCQ) (Folkman and Lazarus, 1985): This instrument is a 66-item questionnaire containing a wide range of thoughts and acts that people use to deal with the internal and/or external demands of specific stressful encounters. There are four different factor solutions from three different authors. The WCQ requires approximately 15 – 25 minutes to complete.
Locus of Control: English Version of Fragebogen zu Kompetenz und Kontrollueberzeugungen (I-SEE) (Greve, Anderson and Krampen, 2001): The original self-report instrument was originally developed by Krampen (1991) in German. The present English version contains four primary scales each with eight items assessed: self-concept of own ability (SK), internality (I), social externality (P), and fatalistic externality (C). The secondary scales self efficacy (SKI) and overall externality (PC) were extracted from primary scales. Cronbach’s alpha for the four primary scales ranged from 0.62 to 0.70. The validity of the original German version is excellent. This instrument requires approximately 10 – 15 minutes to complete.
Marlowe-Crowne Social Desirability Scale- Short form C (MCSDS-C) The short form C of the Marlowe-Crowne Social Desirability Scale consists of 13 items. This scale appeared to have the best psychometric attributes in comparison with other short forms (Reynolds, 1982). This scale requires approximately 5 – 10 minutes to complete.
8. Assessment of Economic Impact (Appendix 13)
Economic Impact will be assessed by collecting data from clinic participants using a questionnaire developed by the CFS research group at CDC and Abt Associates. This instrument will be included in the packet of materials to complete at home, and collects information regarding health insurance, money spent on medical care, salary, employment status, and other items to assess direct and indirect costs of illness. We expect that some of the items in the questionnaire might require some extra time and thought. For this reason, we have not estimated a required time for completion of this instrument.
9. Assessment of Health Services Utilization (Appendix 14)
Utilization of health services will be assessed by collecting data from clinic participants using a questionnaire developed by the CFS research group at CDC. This instrument will be included in the packet of materials to complete at home, and collects information regarding number of visits to a health care professional, type of health care professional seen, purpose of visit, and diagnosis received. This instrument takes 10 to 15 minutes to complete.
10. Assessment of Residence History (Appendix 15)
Previous residence, metropolitan, urban, rural, in or outside of Georgia may be related to risk of CFS. We will assess residence history by a questionnaire developed by the CFS research group at CDC. This will be completed at home and requires approximately 5 minutes to complete.
C.2.f Emory GCRC Studies. Selected subjects classified clinically and, as necessary, selected subsets of Unwell and Well individuals who were interviewed in detail by telephone will be invited to participate in other clinical studies at the Emory University GCRC. These studies will be developed under separate protocols between CDC and Emory University. These studies will address specific hypotheses concerning mechanisms and pathophysiology of CFS. They will be developed to extend results from the ongoing clinical study in Wichita and from various CDC supported CFS modeling studies. GCRC studies will also be developed to evaluate specific treatment and intervention methods for CFS.
C.3 Work Plan
C.3.a Projected Duration of the Study. Depending on OMB and IRB approval, data collection will begin in February 2004 and will be completed by December 31, 2004. The total project, including lab analyses, data analyses and reporting of results should be completed by December 31, 2005.
C.3.b Staffing and Training Issues. Abt Associates, the primary contractor for this study, will provide three general categories of staff for data collection: Telephone Interviewing, Clinical Evaluation, and Data Processing. In addition to the data collection training described below, all project staff are required to complete a thorough training on confidentiality and data security procedures. All staff must also sign assurances of confidentiality statements before they may work on this study. These procedures are in accordance with the Abt Associates Information Protection and System Security (IPASS) Plan, approved by CDC for this project and task.
Telephone Interviewing. Abt Associates will provide two types of staff for the telephone interview portion of this study: telephone interviewers and occupation coders. Responsibilities and training are described below.
Telephone interviewers must complete sixteen hours of training on study procedures, including instruction on correctly obtaining informed consent, administering survey questionnaires, and documenting all contacts with study subjects. Training also includes modules about interviewer roles in maintaining confidentiality and data security. All new interviewers must also complete a course in basic interviewing techniques prior to attending the study-specific training. Interviewers are monitored throughout data collection to ensure adherence to all aspects of the data collection protocol. Refresher training is provided whenever interviewers require it.
Occupation coders assign 1998 Standard Occupational Classification (SOC) codes to employment information collected during detailed telephone interviews. Coders attend fifteen hours of training on interpreting and applying SOC codes. This training also includes a discussion of the importance of maintaining confidentiality and data security.
CDC staff work with Abt to prepare training agendas and materials.
Clinical Evaluation. Two clinics will be established—one in Atlanta the other in Macon. The clinic in Macon will serve a maximum of six subjects per day. This clinic will have a staff of ten—one clinic manager, two medical technologists, two clinic coordinators, three psychiatric interviewers, and two clinical examiners. The clinic in Atlanta will serve a maximum of four subjects per day. The Atlanta clinic will have a staff of seven—one clinic manager, one medical technologist, two clinic coordinators, two psychiatric interviewers, and one clinical examiner. Note that many of these staff will work part-time. In addition, case managers work from their homes to schedule subjects for clinic appointments.
Abt Associates will recruit case managers, clinic managers, and clinic coordinators from among their staff of experienced field managers and interviewers. The CDC/Emory University research team will work with Abt Associates to identify appropriate psychiatric interviewers and clinical examiners.
Responsibilities for each staff type are described below.
Case managers contact eligible subjects to schedule their clinic appointments. Case managers will review with subjects the clinic packets (including informed consent, self-administered questionnaires, and various instructions) that were mailed to subjects prior to their clinic visits.
Clinic managers oversee and supervise day-to-day clinic operations. Duties include scheduling staff and subjects, ordering/stocking supplies, overseeing clinic operations, and ensuring quality assurance procedures are always followed in data and specimen collection. Clinic managers assist in operations whenever they are needed, such as administering the CANTAB or witnessing physical examinations.
Clinic coordinators obtain informed consent, administer the CANTAB, and assist in other clinic operations as assigned by clinic managers. They also review self-administered psychiatric questionnaires while subjects are in the clinics and clarify incomplete answers or problem areas.
Medical technologists collect and process specimens, obtain data on medication usage and past medical history, and witness physical examinations.
Psychiatric interviewers administer psychiatric interviews and tests. These interviewers will be licensed psychiatric social workers, clinical psychologists, psychiatric nurse practitioners, or certified research nurses with experience in psychiatric assessments. They will be trained to handle patients who are suicidal. Abt Associates will be responsible for training psychiatric interviewers, but Emory University and CDC will coordinate standards. Each interviewer is trained to (1) provide a standardized introduction to each procedure; (2) correctly conduct each assessment; and (3) correctly follow standard operating procedures, established by the Emory University Department of Psychiatry, for all assessments, tests, as well as for any adverse events.
Clinical examiners conduct physical examinations. They also perform other operations as necessary (obtaining past medical history, reviewing medications, for example). Clinical examiners will be licensed MDs, rather than Physician Assistants or Nurse Practitioners. Because the state of Georgia requires that physicians supervise PAs or NPs, it is more effective and efficient to employ physicians to conduct physical evaluations. MDs will be able to definitively deal with any adverse events that might occur during clinical evaluations such as a subject in need of immediate medical attention.
Registered nurses will be staffed during the hours when psychiatric interviewers and clinical examiners are not on the clinic premises. They will respond to any medical or psychiatric emergency.
CDC staff have worked with Abt Associates to produce similar training materials for previous studies and will assure appropriately updated versions are produced for this study. We will conduct a single training for all clinic staff to control for bias and variation among data collectors and across sites. There will be an eight-hour training for clinic managers, case managers, medical technologists, and clinical examiners; a 24-hour training for clinic coordinators; and a 36-hour training for psychiatric interviewers. Abt Associates project staff will train case managers, clinic managers, and clinic coordinators. CDC/Emory clinicians will train on physical examinations as well as on CANTAB and psychiatric assessments.
In the week after training, clinical staff will practice and then demonstrate their proficiency to Abt Associates staff. Candidates who pass these proficiency tests will be scheduled for a “dry run” two weeks after training, when they will have the opportunity to integrate the training modules and apply them in a dress-rehearsal of a “typical” clinic day. During the role play, trainees will be expected to adhere to the protocols and conduct themselves as if the dress rehearsal were an actual clinical evaluation. The dress rehearsal benefits the staff in two important ways. First, it gives trainees an opportunity to apply their training in a controlled environment with subjects who have been hired to role-play typical study subjects. Second, carrying out the data collection protocols as designed will provide opportunities to discover any minor protocol flaws. Corrective action can be taken and information disseminated to staff before data collection begins.
Data Processing. Data preparation staff at Abt Associates receipt and prepare all paper questionnaires for data entry. These staff batch, pack and ship paper questionnaires to Abt Associates’ data entry vendor for entry. Data preparation staff must attend a 16-hour training on their tasks, including standard operating procedures for receipting data; rules for editing and preparing paper questionnaires for data entry; and maintaining data confidentiality and security.
C.3.c Data Handling. This section describes the software and processes for preparing the data for analysis.
Data collected during the telephone interviews (CATI) is entered directly into a computer database as it is collected
Data obtained during the clinical assessment will be handled as follows:
Laboratory Specimens Selected clinical laboratories will provide all laboratory results in specified electronic formats to CDC and Abt Associates.
Data Collected Using Paper-Pencil Forms These data will be reviewed on the premises while the subject is still present. Three times a week, the documents will be batch-shipped according to subject to Abt Associates’ Chicago, Illinois, facility. All forms will be date stamped and receipted into the study’s Information Management System. Each page of each form will be clearly labeled with the subject's identification number. Data entry will be 100 percent verified to ensure its accuracy.
Data Capture for Clinical Data Electronic data from the clinic (e.g., CANTAB) will be directly imported into the data management system.
C.3.d Information management and analysis software. All systems and procedures used by Abt Associates to handle and store data for the Georgia Survey of CFS are documented in detail in the Information Protection and Systems Security (IPASS) document submitted to the CDC’s Information Systems Security Officer. CDC has approved data handling and systems security procedures for the telephone survey and clinical evaluation components of the Georgia Survey of CFS.
The information and management software used by Abt Associates to conduct and track the data collection and perform data cleaning for the Georgia Survey of CFS are listed below.
The GENESYS sampling system, which is distributed and licensed by Marketing Systems Group (MSG). It will be used to generate the sample of randomly selected telephone numbers for the Georgia Survey of CFS.
Bellview CATI, commercial, off-the-shelf software, used to conduct telephone interviews and manage telephone survey data component of the Georgia Survey of CFS. Bellview is a product of PulseTrain, Limited.
A project-specific MS-Access system that will be used for coding subject occupations.
Sample Management System [using VisualStudio.net (written in C Sharp)], that tracks status of sample members who are eligible for clinical evaluation. This system also tracks the status of paper and electronic instruments.
CANTAB software which collects neurocognitive test data.
Proprietary software, developed by Abt Associates’ data entry vendor, DataShop, which will be used to key enter all paper instruments and will provide 100% verification of keyed items.
C.4 Data entry, editing, management, storage, and disposition. As noted above, CDC’s Information Systems Security Officer has approved the systems and procedures that will be used by Abt Associates to enter, edit, transmit, and store data for the Georgia Survey of CFS.
Each subject will be assigned a unique subject identification number. The contractor, Abt Associates, will maintain the electronic files that link the identification number to subject identifiers (name, address, and telephone); CDC will not have access to these records. The identification number will be affixed to all paper questionnaires and will be used to store and retrieve electronic data associated with the subject.
Data collected during the computer-assisted telephone interviews (CATI) are entered directly into a Bellview computer database under the subject’s identification number. Data are stored in real time as they are collected. Skip patterns and alternative versions of questions are programmed into the CATI system so that the interviewer sees only those questions appropriate for the current respondent. The system has been programmed with range and logic checks that alert interviewers to out-of-range or inconsistent responses so that they can correct them while subjects are still on the telephone.
The survey data will be housed in a central file that has a documented Bellview proprietary file format. Files are extracted, using a Bellview extraction program, into ASCII files. Any identifying information (name, address, telephone) will not be exported to CDC. After the ASCII data are reviewed, they are delivered to CDC as SAS data files. After final data delivery, Abt Associates will electronically archive the CATI data, including subject identifiers. All data are stored on the local area network (LAN) in access-restricted directories. Only staff authorized by the Abt Associates Project Director may access the data.
Data containing the responses to questions about current employment will be extracted periodically from the Bellview CATI system and loaded into an MS-Access database. An Access data form will be used to allow specially trained occupation coders to view the occupation data and assign codes from the 1998 Standard Occupation Classification code frame. This Access database is protected using industry standard encryption, 'strong password' authentication, and restricted access authorization.
Data obtained during the clinical assessment will be identified only by each subject’s identification number. Data handling and security are described below.
Laboratory Specimens. Selected clinical laboratories will provide all laboratory results on paper and in specified electronic formats to CDC and Abt Associates.
Data Collected Using Paper-Pencil Forms. Forms that are completed at home or at clinic will be labeled with the subject’s identification number. Each document will be edited for completeness and clarity at clinic before the subject leaves. This edit will identify most problems (such as missing answers and unclear or illegible responses) so that a coordinator can ask the subject to resolve the problem, while the subject is on the premises and can review the form(s). Afterwards, each page of each document will be stamped with the subject’s identification number. Three times per week, paper instruments are batched by subject and shipped overnight to the contractor’s office in Chicago for data entry. All forms are receipted into the receipt control component of the study’s Sample Management System (SMS). When not in use, they are secured in locked cabinets within the contractor’s office.
In Chicago, Abt Associates staff receipt, edit, and prepare completed questionnaires for data entry. Each week, edited clinical evaluation questionnaires are batched and sent to Abt Associates’ data entry vendor. Data entry will be 100-percent verified to ensure accuracy. The status of each questionnaire is updated in the SMS to indicate when the questionnaire was shipped to the data entry subcontractor and when it is returned.
Data Collected via Computer. Data collected using software (CANTAB) are uploaded to Abt Associates’ servers via a DSL line using 128 bit strong DES VPN creating a highly secure 'point-to-point tunnel' internet connection. Data packages are also compressed using 128 bit data encryption before transmitting. The subject’s identification number is the key field used to identify subject records. Weekly, the data are uploaded to Abt Associates’ VPN-secured servers via a DSL Internet connection. After data transfer, a test file will be created, and marginal frequencies will be run to ensure data completeness and accuracy.
All key-entered data are examined, mainly by review of data frequencies, and cleaned as necessary. The amount of data cleaning needed for the CATI interviews will be minimal because these data collection programs contain internal checks so that data problems can be resolved during actual interviews. Nevertheless, if an interviewer reports a key entry error that he/she could not correct, the data will be corrected during this stage of processing.
During the data cleaning process, only three versions of the data files are maintained on Abt Associates’ systems. The first is an archived source file, which preserves the data in its original state and is not used during the cleaning process. The second is a copy of the source file, which is used as the starting point for data cleaning (the “data cleaning copy”). The third is the most current, cleaned, version of the data (the “cleaned copy”), which is created from the data cleaning copy. This last file becomes the final data file at the conclusion of the cleaning process. All changes to the data cleaning copy, to create the cleaned copy, are made by computer programs (the “cleaning programs”). All differences between the data cleaning copy and the cleaned copy are documented in the cleaning programs and cleaning specification logs, so the history of any value of any variable can be traced from the collected data to the final, cleaned data. The data files, programs, and cleaning logs are date- and time-stamped by Windows 2000 and NT facilities.
Survey data are maintained at Abt Associates until the electronic data are verified and there is no longer need for reference to hard-copy documents (approximately six months after the end of data collection). At that time, the data will be moved to Abt Associates’ off-site storage facility. At the conclusion of the study, the survey data will be destroyed in accordance with the terms of the contract between CDC and Abt Associates.
C.5 Quality control/assurance:
Telephone interviews. Use of computer-assisted telephone interviewing (CATI) greatly reduces opportunities for error and increases data quality because the CATI system performs range and logic checks and ensures that questionnaire skip patterns are correctly followed. On every shift, telephone interviews are monitored by supervisory staff. Each Abt Associates’ Telephone Center is equipped with a monitoring system and separate monitoring rooms that allow for unobtrusive monitoring of interviewers. Interviewers do not know when they will be monitored. All interviewers are monitored, and monitoring occurs during every interviewing shift. The monitoring supervisor listens to the telephone interview while observing the interviewer’s CATI data entry screen on a computer monitor that mirrors the interviewer’s screen. After the monitoring session, supervisors give active coaching and immediate feedback, both positive and negative, on interviewer performance so that successful interviewing performance can be rewarded and reinforced, while interviewing problems are immediately identified and corrected. In addition, floor supervisors circulate through each interview room to ensure that interviewers are focused on their tasks; to answer questions; and to handle issues that may arise.
The monitoring system is also used to evaluate performance patterns across interviewers to identify any problem areas or items in the questionnaire. Specific problems are addressed in retraining for interviewing staff. In addition, data from the monitoring and CATI systems are reviewed each day to identify anomalies, inconsistencies, or inappropriate patterns across interviewers and for individual interviewers. All such issues are reviewed and resolved.
As an additional quality control measure, project staff review questionnaire item frequencies throughout data collection to correct errors or other anomalies in the data.
Clinical evaluations. At training, each clinical staff person is trained on and receives copies of standard operating procedures (SOPs) for each questionnaire or type of assessment. It is the responsibility of the clinic manager to see that all SOPs are closely followed by each staff person on each day of data collection. In addition, during clinic data collection, CDC, Emory University, and Abt Associates project staff visit the clinics to observe data collection activities, including SCID administration, CANTAB testing, physical examinations, and documentation of clinical events. Observers note any deviations from the protocol and retrain staff. Staff who cannot or will not follow SOPs are removed from the project.
One assurance of quality of the physical examination data, is hiring qualified medical doctors and technologists to complete these assessments. An accredited commercial laboratory will analyze blood, urine, and saliva specimens using industry-standard quality control procedures.
Quality checks are built into clinic operations. Clinic coordinators scan-edit completed questionnaires while subjects are still on the premises. Clinic coordinators check for missed items and incomplete answers and meet with individual subjects so that errors may be corrected and missing items completed. Psychiatric interviewers will be frequently assessed by CDC and Emory staff to ensure proper administration of the SCID.
Paper questionnaire data are 100 percent verified by two data entry operators. Any discrepancies in the data are adjudicated and corrected. Project staff also review frequencies for each item in each instrument to make sure skip patterns have been followed, to remove extraneous data, and to assign missing values when data are incomplete or missing.
Bias in data collection, measurement and analysis. Every effort will be made to eliminate potential sources of bias in the data gathered during the Georgia Survey of CFS. This section addresses the most common forms of bias in data collection, measurement, and analysis and the steps CDC and its contractor will take to reduce them. This discussion focuses on sampling bias, interviewer bias, sampling weights, and blinding.
Sampling bias may arise from completing interviews or clinical evaluations with a sample of subjects who are not representative of the population. Sampling bias has two main causes: 1) deliberate exclusion of a subgroup of the population being studied and 2) nonresponse. The effects of sampling bias can be reduced by careful implementation of the sample design, by minimizing unit (in this situation, subject) nonresponse during telephone data collection and clinical evaluation, and by adjusting for unit nonresponse.
The sample of telephone numbers for the Georgia Survey of CFS will be selected according to random-digit-dialing methods that are well accepted as a means of achieving a probability sample of the population under study. In this study, the population to be sampled (for clinical evaluations) is English-speaking adults, 18 to 59 years of age, living in certain counties of Georgia, with land-line telephone service. CDC recognizes that this definition excludes adults who use only mobile telephone service, as well as some ethnic adults and disabled adults from the study. However, CDC finds it prudent to restrict screening and interviewing for several reasons.
Land-line Telephone Service. While there is considerable information on numbers of mobile phones, there are few solid estimates about the current number of wireless-only households. The one source of government data on wireless-only households comes from the November 2001 Current Population Survey (CPS). In that survey, in addition to the traditional question on household telephone use, a followup question was asked regarding the type of telephone access that was available. Based on that question, the FCC estimated that 1.2 percent of households had only a wireless phone (Federal Communication Commission, 2003). However, the question measuring this topic was deemed to have an insufficient response rate for full reporting.2 Media reports on wireless-only households tend to rely on market research and telecommunication analysts for their data. We attempted to trace such data to sources, and found that the original reports were not publicly available. These estimates tended to be higher than the CPS estimate, but they are in line with what could reasonably be expected given that the CPS estimate is several years old. Typically, more current estimates place the number of people with wireless-only service between 5.8 and 7 million, or roughly 2 to 3 percent of the US population (Davidson, 2003; Chicago Tribune, October 21, 2003). Thus, it appears that, for the moment, the current number of wireless-only households is relatively small.
With regard to actually calling wireless phones, there are legal prohibitions against intentionally dialing wireless phones. The Federal Telephone Consumer Protection Act (TCPA) includes a provision that restricts unsolicited calls to wireless phones (Gillin, 2003). The regulation prohibits:
ALL calls made to a cellular phone, without the prior consent of the person called.
IF the call is made using an automatic telephone dialing system (defined as equipment which has the capacity to store or produce telephone numbers to be called using a random or sequential number generator and to dial such numbers) or an artificial or prerecorded voice.
IF the party is charged for the call.
Unlike many other regions of the world, virtually all US wireless providers charge for incoming calls. Despite a general increase in recent years in the number of “free” minutes included in most wireless plans, it is unlikely that respondents will willingly spend forty-five minutes of their plan to complete interviews.
In order to comply with the regulations noted above, we use a 1+ sampling procedure. That is, we sample only from exchanges where at least one residential phone number has been identified based on directory listings. We currently exclude, from any random-digit-dialing sample, exchanges that are identified as exclusively wireless. We do include exchanges identified as containing a mix of residential and wireless service. We estimate that about 2 percent of the exchanges sampled for a national random-digit-dialing survey contain both land-line telephone service and some type of wireless service.
A series of questions are included at the end of the screening interview to determine whether there are additional telephone lines in the household and what their uses may be (for example, fax or computer use). Weighting of the screening sample will include adjustment for the presence multiple telephone lines within households.
English
–speaking Households. The
CFS research group spent considerable time considering this issue
during the development of the protocol and concluded that we will
collect data from adults who speak English for these reasons:
It is costly and difficult to staff clinics with multilingual personnel.
The physical examination requires a provider-patient dialogue to collect accurate medical histories and to review current medical systems. Most of these instruments are available only in English.
We are reluctant to burden subjects by requiring them to provide their own translators.
It is possible that subject-supplied translators could bias the subject’s ability to provide reliable data.
The CFS research group examined county-level data from Census 2000 on the number of persons (aged 5 years and over) who speak Spanish and, among those persons, the number who speak English “very well” (a respondent would need considerable proficiency in English to understand the questions in the Detailed Telephone Questionnaire and answer them accurately). For DeKalb County and Fulton County combined, the percentage who speak English “very well” is less than 37 percent. This statistic reflects proficiency in both children and adults. It is plausible that, if similar data were available for persons aged 18 years and over, they would show an even greater degree of “linguistic isolation.” The desire to include Spanish-only speakers, however, must be tempered with the number of Hispanics in Georgia. As the following table indicates, Hispanics constitute less than 7 percent of the population of the metropolitan stratum and less than 2 percent of the urban and rural strata. Given the portion of Hispanics who are proficient in English, the percentages of Spanish-only speakers decrease even further. Such small numbers make translation of instruments and the hiring of bilingual interviewers costly compared to the benefits realized.
The contractor for data collection, Abt Associates Inc., tracks subjects who cannot participate due to language barriers by language type. If Spanish language-only subjects become significant in number, staff will use Spanish translators from the AT&T Language Line to conduct the screener and detailed interviews.
Selected Demographic Characteristics of Counties in the Georgia Telephone Survey
|
||||||||||||
County |
Population 2000 |
Persons under 18 yrs old, percent, 2000 |
Persons 60 yr old and over, percent, 2000 |
Female persons, percent, 2000 |
White persons, percent, 2000 |
Black or African American persons, percent, 2000 |
Persons of Hispanic or Latino origin, percent, 2000 |
White persons, not of Hisp/Lat origin, percent, 2000 |
HH, 2000 |
Persons per HH, 2000 |
Median HH money income, 1999 |
Persons below poverty, percent, 1999 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Atlanta Counties |
|
|
|
|
|
|
|
|
|
|
|
|
De Kalb |
665,865 |
24.6% |
10.9% |
51.5% |
35.8% |
54.2% |
7.9% |
32.2% |
249,339 |
2.62 |
$49,117 |
10.8% |
Fulton |
816,006 |
24.4% |
11.5% |
50.8% |
48.1% |
44.6% |
5.9% |
45.3% |
321,242 |
2.44 |
$47,321 |
15.7% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
1,481,871 |
24.5% |
11.2% |
51.1% |
42.6% |
48.9% |
6.8% |
39.4% |
570,581 |
2.53 |
|
13.5% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Macon and Surrounding Counties |
|
|
|
|
|
|
|
|
|
|
|
|
Baldwin |
44,700 |
21.7% |
14.5% |
46.0% |
54.2% |
43.4% |
1.4% |
53.5% |
14,758 |
2.50 |
$35,159 |
16.8% |
Bibb a |
153,887 |
26.6% |
16.4% |
54.0% |
50.1% |
47.3% |
1.3% |
49.6% |
59,667 |
2.49 |
$34,532 |
19.1% |
Bleckley |
11,666 |
26.6% |
18.2% |
51.8% |
73.2% |
24.6% |
0.9% |
72.9% |
4,372 |
2.52 |
$33,448 |
15.9% |
Crawford |
12,495 |
27.6% |
13.4% |
49.9% |
72.9% |
23.8% |
2.4% |
72.3% |
4,461 |
2.78 |
$37,848 |
15.4% |
Houston b |
110,765 |
28.2% |
13.1% |
50.8% |
70.6% |
24.8% |
3.0% |
69.0% |
40,911 |
2.65 |
$43,638 |
10.2% |
Jones |
23,639 |
27.1% |
14.7% |
51.2% |
75.0% |
23.3% |
0.7% |
74.7% |
8,659 |
2.69 |
$43,301 |
10.2% |
Macon |
14,074 |
27.6% |
16.5% |
50.4% |
37.4% |
59.5% |
2.6% |
36.8% |
4,834 |
2.71 |
$24,224 |
25.8% |
Monroe |
21,757 |
26.3% |
14.6% |
50.2% |
70.4% |
27.9% |
1.3% |
69.6% |
7,719 |
2.74 |
$44,195 |
9.8% |
Peach |
23,668 |
26.0% |
14.0% |
51.6% |
51.3% |
45.4% |
4.2% |
49.2% |
8,436 |
2.68 |
$34,453 |
20.2% |
Twiggs |
10,590 |
27.0% |
15.9% |
52.1% |
54.9% |
43.7% |
1.1% |
54.6% |
3,832 |
2.73 |
$31,608 |
19.7% |
Wilkinson |
10,220 |
27.2% |
17.7% |
52.5% |
58.0% |
40.7% |
1.0% |
57.7% |
3,827 |
2.65 |
$32,723 |
17.9% |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
437,461 |
26.6% |
15.0% |
51.5% |
59.3% |
37.7% |
1.9% |
58.4% |
161,476 |
2.65 |
|
15.7% |
|
|
|
|
|
|
|
|
|
|
|
|
|
a Contains the city of Macon
b Contains the city of Warner Robins (2000 population 48,804)
|
||||||||||||
Given this definition of the population and the nature of RDD surveys, the only selection bias arises from noncoverage of nontelephone households.
Recent research on noncoverage suggests that households with interruptions in telephone service (of one week or longer during the past 12 months) are more similar to households with no telephone service at the time of the survey than to households with uninterrupted telephone service [Keeter, 1995; Brick et al., 1999; Frankel et al., 2003]. CDC has included screener questions that address interruptions in telephone service and will compensate for noncoverage when creating sampling weights.
Bias in determining eligible subjects for clinical evaluations will be reduced through the objective application of the CFS case definition to each subject who completes the detailed interview. This goal will be accomplished by programming the case-definition criteria into the CATI interview so that certain responses (such as self-reported AIDS) will make the subject ineligible for further participation. Subjects who provide verbatim responses for “other-specify” conditions or recent surgeries will have those responses evaluated for eligibility by CDC medical staff without regard to subject race, ethnicity, or income.
Low response rates in either stage of the telephone or the clinical evaluation data collection can introduce bias if certain subgroups within the population systematically fail to complete the interview and evaluation process. CDC will minimize subject nonresponse by utilizing a data collection contractor experienced in RDD surveys and field data collection. The contractor, Abt Associates, will employ the following tactics to minimize unit nonresponse:
An automated CATI call-scheduling system where algorithms:
Increase the likelihood of resolving telephone numbers as business or household numbers, screening households, and conducting detailed telephone interviews.
Minimize respondent burden by carefully structuring answering machine messages.
Route refusals to specially trained interviewers, who specialize in gaining the cooperation of difficult subjects.
Deliver, on time, appointments and callbacks to interviewers.
Advance letters that inform the subject about the study before the first dialing attempt is made.
A toll-free number for subjects to call when they are ready to be interviewed.
Trained and practiced general telephone and field interviewers with experience in:
Gaining cooperation (addressing subject concerns and questions, averting refusals, using good voice skills to keep the subject engaged).
Navigating through complex questionnaires.
Locating subjects.
Refusal conversion. The term “refusal conversion” describes a process, defined by standard survey research criteria, in which an individual is contacted a second time concerning participation in a study that s/he initially could not consider. The intent is to assure that the potential subject has enough information to make an informed decision and choice about participation. A sizeable proportion of persons who are initially reluctant to participate will choose to participate once the study is explained in more detail. Refusal conversion is an effective and non-coercive tool when applied by a professional. To avoid possible coercion and assure that initially reluctant persons understand the reasons they have been asked to participate, the process is carefully managed and implemented. A telephone center (or field) supervisor assesses each initial refusal to identify those individuals defined as eligible for refusal conversion. For example, individuals who say they are currently too busy or those persons who immediately hang up would be eligible for refusal conversion. If a subject was hostile or asked to be removed from the call list, his or her refusal status is immediately finalized, and no further contacts are made. After this initial assessment, those individuals appropriate for recontact are assigned to refusal conversion specialists. These specialists are very well-trained and experienced interviewers with highly developed listening and response skills. The conversion specialist contacts the individual and explains the reason for the call. If the subject again declines participation, the conversion process is immediately terminated. The Record of Calls for each refusal is reviewed, by a supervisor, prior to each calling attempt. Clearly established rules (for example, a subject refuses twice) dictate when calling is immediately discontinued. Also, an unusual problem during a call (such as a subject noting that the call is coercive) are reviewed and documented.
Medical staff trained on study objectives and their specific duties.
Managers who track the sample by region, race, and ethnicity and who shift resources, as necessary, to achieve comparable response rates.
Interviewer bias will be minimized with training that includes practice in gaining subject cooperation, nondirective probing, probing for clarity and completeness, proper pacing, and reading questions verbatim. As described in the quality control section of this protocol, telephone interviewers are unobtrusively monitored by supervisors, who will provide feedback. As necessary, remedial training will be provided to help deficient interviewers improve their ability to complete their tasks and minimize refusals. CDC, Emory University, and Abt Associates staff will monitor clinic staff periodically. As with telephone interviewers, feedback and retraining will be provided.
If unit nonresponse occurs at random, it is not a source of bias; it simply reduces the size of the final sample. In adjusting the sampling weights for various forms of unit nonresponse (such as, unresolved telephone numbers, unscreened households, subjects not interviewed), CDC will take into account the possibility that, overall, nonresponse is non-random. Applying a standard approach, we will separate the population into disjoint subpopulations defined by characteristics that may be related to response (and to fatiguing illnesses): for example, sex, age group, race/ethnicity, fatigue status, and geographic stratum. Within each subpopulation the respondents and the non-respondents are substantially more likely to be comparable (than in the overall, unstructured sample), so it will be reasonable to treat nonresponse as random.
To reduce bias during data analysis, data users will be directed to apply household and subject sampling weights that account for unequal probabilities of selection among subjects and incorporate adjustments for unit nonresponse and for noncoverage of nontelephone households.
CDC plans to use blinding procedures for the subject’s fatigue status (CFS-like; chronic unwell, not CFS-like; well) on a need-to-know basis. Blinding is not an issue for telephone data collection, because fatigue status is determined at the conclusion of the Detailed Interview. Abt Associates staff (case managers and sample management staff) will know fatigue status in order to match subjects and coordinate clinical appointments. However, the medical staff (who collect the specimens, record medication and medical histories, and conduct the physical examinations) and psychiatric interviewers will not know the subject’s sample type.
C.6 Statistical Analyses
C.6.a Sample size justification. The sample size, 33,000 telephone numbers in each of the three strata, is designed to provide power of at least 0.8 for detecting, at a two-sided .05 level of significance, a 2-fold difference in the prevalence of CFS between white and black populations and between metropolitan/urban and rural populations (Specific Aim 1). (The prevalences used in the calculations, 200 per 100,000 in the white population and the metropolitan/urban population and 400 per 100,000 in the black population and the rural population, are consistent with estimates from the Sedgwick County Studies and the National Survey of CFS pilot survey.)
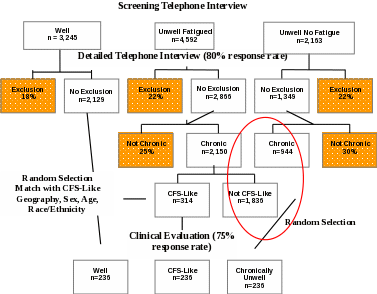
After allowing for telephone numbers that are non-working or non-residential, numbers whose residential status cannot be determined, and a small amount of nonresponse by households on the Screening Interview (estimated at about 3% from the pilot survey), the estimated numbers of screened households will be 6,056 in Atlanta, 6,644 in Macon and Warner Robins, and 6,644 in the rural counties. The estimated numbers of adults aged 18 to 59 in those screened households will be 10,108 in Atlanta, 9,950 in Macon and Warner Robins, and 11,028 in the rural counties. If the prevalence of prolonged fatigue (fatigue lasting for 1 month or longer) is as observed in the pilot survey (14.77%), these adults will include a total of 4,592 who have prolonged fatigue. The Survey will attempt to conduct a Detailed Interview with all of these subjects. In addition, 2,163 subjects will be randomly selected from among the adults who are reported as Unwell but not fatigued in the Screening Interview, and 3,245 subjects will be randomly selected from those who are reported as Well. Of this sample of 10,000, 8,000 are expected to complete the Detailed Interview.
Among the subjects who complete the Detailed Interview, the prevalence of CFS-like illness in the pilot survey (1.04% in urban areas and 1.67% in rural areas) yields an estimated 84 cases of CFS-like in Atlanta, 83 in Macon and Warner Robins, and 147 in the rural counties. These 314 subjects (who would not include subjects who have exclusionary conditions, by the definition used in the pilot survey) will all be eligible for clinical evaluation. An estimated 75%, or 236 subjects, will complete a clinical evaluation.
Two other groups of subjects who complete the Detailed Interview will be invited to undergo a clinical evaluation: 314 Well subjects (without exclusionary conditions), matched 1:1 to the subjects who are CFS-like on sex, age (+ or – 3 years), and race/ethnicity; and 314 randomly selected subjects who are Chronically Unwell but who are not CFS-like (and have no exclusionary conditions).
C.6.b General data analysis strategies
For Specific Aim 1, prevalence estimates will use weighted data and will be calculated according to methods described in Reyes et al. (2003). For Specific Aims 2 and 3, we will perform extensive descriptive analyses before we undertake formal statistical testing or modeling. Descriptive analyses will include calculation of frequency distributions, simple statistics and use of graphical methods, such as histograms, box-plots, and scatter plots. This approach is useful for identification of outliers and/or the need for data transformation to achieve normality, as well as determination of the most adequate parametric or non-parametric statistical test or model. Univariate analyses will be used to test and explore specific hypotheses. For example, among CFS persons, we will use traditional 2 tests or exact tests to assess the association of geographic area and categorical variables (e.g., symptoms, demographics, onset type), and we will perform analysis of variance or Kruskal–Wallis tests to assess significant differences in continuous variables (e.g., duration of illness, physical and psychiatric function measured by scales) by metropolitan, rural or urban areas. Different methods will be used for comparing outcomes among CFS cases, healthy controls and the Chronically Unwell comparison group. Because of the matching characteristic of the design, we will use paired t-tests or the Wilcoxon signed-rank to compare CFS cases with the Well subjects with respect to endocrine and immune parameters, as well as psychosocial factors and psychiatric co-morbidity scales. Unmatched tests will be applied for the comparisons between CFS cases and Chronically Unwell comparison subjects. We will use the McNemar’s test or traditional 2 tests to compare cases and comparison groups with respect to presence of binary outcomes. Multivariate analyses, such as unconditional and conditional logistic regression, as well as stratified analyses, will be performed to determine the association between the outcomes and CFS, adjusting for potential confounders. Odds ratios and confidence intervals will be estimated by these methods. Multivariate regression models will also be used to examine the relationship between continuous outcomes, CFS and other covariates. We will also perform multivariate analysis of variance for repeated measurements to compare cortisol awakening profiles between all groups. In the case of violation of sphericity of the repeated measurement variable, we will use either the Greenhouse-Geisser or the Huynh-Feldt adjustment. Alternatively, we will calculate the Area under the curve and a baseline to peak increase for the cortisol awakening profile. For Specific Aim 4 we will use factor analysis and cluster analysis. Finally, we will also use significantly contributing factors from previous analyses in a structural equation model for the development of CFS. We intend to identify an etiologic model for CFS and provide a basis for a revised CFS case definition.
C.6.c Reporting of results
Subsequent to data analyses, results will be prepared for presentation at scientific meetings and papers for publication of findings in internationally recognized journals will be prepared.
Study participants will be notified of any abnormal findings from the physical examination at the time of the examination. Study participants will also be mailed copies of their laboratory test results, accompanied by a cover letter alerting the participant if any of the results are outside the normal range. Additionally, a copy of the participant's laboratory test results will be sent to his or her personal physician, with the participant's consent.
Study participants who request information about study findings will be directed to the CFS page of CDC's website. This site summarizes the results of all recent CFS studies conducted by CDC.
D. Human Subjects
D.1 Proposed involvement of human subjects
As noted, the study will attempt to enumerate Well and Unwell persons in metropolitan Atlanta, Macon and Warner Robins, and rural counties surrounding Macon and Warner Robins areas of the Georgia population by random-digit-dialing. Identified Unwell and selected Well persons 18 to 59 years of age will be interviewed in detail by telephone. All CFS-like subjects and an equal number of randomly selected persons with Chronic Unwellness will be invited to participate in a clinical evaluation. In addition, selected Well subjects will also be invited, matched 1:1 with CFS-like subjects on geography, age, sex and race/ethnicity. Subjects with exclusionary medical/psychiatric conditions will not be invited to the clinic, or if identified at the clinic, will not complete the study.
D.2 Sex and minority inclusion
For the present study, subjects are identified in the Georgia communities by random-digit telephone dialing. Thus, the sample of study will reflect the actual distribution of sex and race/ethnicity in fatiguing illnesses in Georgia. We include English-speaking males and females of all races.
D.3 Sources of research material
The following research material is obtained from the human subjects:
a. For the telephone screener, research material is obtained in the form of answers to questions about symptoms and household information. The screener requires an average of 7 minutes to complete.
b. For the detailed telephone interview, research material is again obtained in the form of answers to questions. These questions cover demographic information, symptoms, medical history, and psychiatric history and take an average of 30 minutes to complete.
c. For the physical exam blood tests and cytokine measures, a total of 30 mL blood will be collected in the morning. Portions of the blood samples will be stored for genotype testing and for future, presently unidentified, research purposes. Subjects may give or give not consent to genetic testing and storage of their samples.
d. The neurocognitive test battery is computerized and monitors responses to specific tasks, including reaction times. The total duration of the battery is 40-45 minutes. The WRAT3 reading subtest takes 5 minutes to complete.
e. During the psychiatric screening, research material is obtained in the form of responses to items (question/answer). For the SCID, the number of items is variable and depends on the mental health status of the subject. The maximum duration of the SCID is estimated to be 60 minutes. The remaining questionnaires, self-administered at clinic, are expected to take 67 to 120 minutes to complete.
f. The self-administered questionnaires to complete prior to the clinic visit (economic impact, health care utilization, and residence history) are expected to take, on average, 15 minutes to complete.
D.4 Recruitment and consent procedures
As noted, all participants will be recruited from subjects identified in the population of the previously mentioned regions of Georgia through random-digit-dialing. Unwell and selected Well subjects will be interviewed in detail regarding fatigue and symptom criteria, and will then be classified as having a CFS-like illness, chronic unwellness that is not CFS-like, or wellness. Informed consent statements are included in both the screening and detailed telephone interview questionnaires. The consent statements for both of these interviews will be administered as soon as the respondent is on the telephone. Telephone interviewers will be prepared to discuss any aspect of the respondent’s participation at greater length, including respondent rights and the telephone numbers of staff who can be contacted for more or different information. The interview will not proceed until the informed consent statement has been read, exactly as written, to each respondent, and all of the respondent’s questions have been answered.
During a clinical evaluation, eligible subjects from these groups will be evaluated for classification and to gather other information and specimens as described in previous sections. Subjects will be completely informed about all procedures and will sign the informed consent prior to study entry (appended). The study protocol will be submitted for approval to the Institutional Review Boards of the CDC, Emory University, and Abt Associates.
D.5 Potential risks
D.5.a Risks for telephone portion. There may be risks associated with the telephone portion of this study. Portions of the telephone interview regarding psychiatric symptoms, stress, or life experiences may cause emotional distress.
D.5.b Risks for clinical evaluation. There may be risks or side effects associated with the investigational procedures: venipuncture can be associated with pain and bruising, and infection at the site of the needle entry. Interviews on psychiatric symptoms and questionnaires on symptoms, stress and life experiences may induce emotional distress.
D.6 Procedures for protecting or minimizing potential risks
D.6.a Identifying, managing and reporting adverse events:
The most common type of adverse event will be subjects who become upset by questions in the telephone or self-administered questionnaires. Several procedures are in place to deal with these situations.
First, subjects will be clearly told that they can skip any question that they do not wish to answer. Interviewers will be instructed to move immediately to the next question when requested without pushing the subject to respond, and they will be monitored to ensure compliance with this procedure. Second, subjects will be provided with the names and telephone numbers of CDC staff to call with questions or concerns about the study or their rights as a study subject. Third, interviewers will be instructed to report any incidents involving an upset/concerned respondent to their supervisor. Of course, interviewers and researchers are not in a position to provide referrals to medical professionals or counselors, but they will recommend that any distraught subjects contact their doctor or other trusted advisor for help. As noted below, local hotline numbers will be made available to respondents. Finally, study data for any subject will be destroyed after it is collected if the subject requests it.
Telephone interview: The interviews are conducted by trained research personnel. Interviewers are trained to seek assistance from supervisors if any portion of the interview appears to cause emotional distress to the respondent. The supervisor may contact the subject to discuss his concerns, and recommend that the subject contact a medical or mental health professional if necessary. Subjects will also be provided with a toll-free number to contact supervisors at Abt Associates with any questions or concerns that they have.
One part of telephone interviewer training will focus on the sensitive nature of questions in the detailed telephone interview. We will:
Teach interviewers to approach these questions in a calm and careful manner.
Provide guidance for interviewers to recognize early signs of concern or upset so that interviewers will repeat that respondents may refuse to answer any questions.
Prepare a list of Georgia and, where available, county hotline numbers which will be posted at every interviewing station. Interviewers will be trained to provide these numbers on request or when respondents express upset at sensitive questions.
Train interviewers to recognize signs of anguish or danger. An interviewer in this situation will alert a supervisor who will dial 911 and report the telephone number so that immediate assistance can be provided.
Clinical evaluation: A trained clinician will evaluate any evidence of psychological stress that might result from interviewing or psychometric tests. Subjects may decline to answer specific questions, and may withdraw from the study at any time. A registered nurse or physician will be on the premises during clinic appointments to respond to any medical or psychiatric emergencies. To minimize the risks of infection, and pain and bruising from the blood draw, blood samples will be taken by direct venipuncture in a sterile manner by experienced staff.
D.6.b Emergency care: The Georgia Survey of CFS does not involve medical care of study subjects. However, should a participant experience a medical or psychiatric emergency while at the clinic, a physician will be on site and appropriate referrals will be made.
D.7 Risk/benefit ratio
There may be no direct benefit to the subjects who participate in the study.
No treatments or interventions will be offered to study subjects. The results of the physical examination, laboratory tests and appropriate psychiatric evaluations will be made available to study subjects and their physicians if they so designate in writing. Study subjects will be alerted if any of these tests reveal potentially serious conditions of which the subject was not aware. However, any treatment for these conditions is outside of the bounds of this study, and must be handled by subjects with their private physicians.
D.8 Handling of unexpected and adverse events
CDC will review all test results. In the case of an unexpected finding, CDC will contact Abt Associates, who will in turn notify the subject and advise him/her to see a doctor for appropriate counseling and treatment. If the subject so requests, Abt Associates will also notify his or her physician of the findings. However, subjects will not be compensated for any treatment sought due to abnormal test results.
In the case of adverse events, emergency psychiatric treatment and referral will be provided by the on site psychiatric clinicians (Appendix 16). Prior to discharge from the clinic visit, an emotional well-being script will be performed. In the case that a patient appears suicidal, the patients will be sent to the nearest emergency room. Medical emergencies will be identified by the staff as supervised by on site nurses or physicians. Serious adverse events will be reported to the IRBs of CDC, Emory University and Abt Associates following the guidelines of each IRB.
D.9 Compensation
The clinics are frequently underused by 1 – 2 appointments due to cancellations or last minute no-shows without cancellations. If a subject prefers to book a clinic appointment on a day that is fully booked, the subject will be told of the unavailability of an appointment, and compensated with $40.00 if they choose to book the appointment, but actually are unable to be evaluated on that day due to unavailable clinic slots. If, for any reason, the subject is determined to be ineligible to participate in this study following the physical examination on the day of arrival, the subject will receive $40 compensation for his/her time. If an eligible subject chooses not to join the study after arrival at the clinic, the subject will receive $40. If the subject has been admitted to the study following the physical examination and completes the study, he/she will receive $250 to pay for the time the subject must take and any costs for being in the study. If the subject chooses to drop out before the end of the Clinic visit, the subject will receive $125.
Subjects may opt out of especially sensitive questions or tests, and decline consent or genetic testing and storage of specimens without penalty. This compensation schedule is appropriate for the demands placed on subjects by the protocol. Participation requires completing numerous questionnaires, providing blood, urine and saliva specimens. Some of these requirements are inconvenient and unpleasant, and none are related to more than minimal risk. Besides these demands, participation will require travel to the clinic (driving time, gasoline, and parking), and one day of work, which may represent a significant loss of revenue for some participants.
D.10 Concerns regarding genetic testing
This section addresses concerns regarding the genetic testing component of this study as outlined in the CDC’s Genetic Research Checklist.
Please refer to Background and Significance (section B.2.e/B.4.c, pages 12) for justification of the importance of genetic testing.
Please refer to sections B.2.e/B.4.c and C.2.e (pages X) for a description of the proposed genetic research.
There are two different types of tests we propose to perform on the genetic material present in the biological sample (white blood cells obtained from clotted blood or the pellet from anticoagulated blood) collected in this study. We will perform tests for genotypic polymorphisms in genes encoding proteins such as neurotransmitters and cytokines, mapping regions of the genome containing genes that might contribute to CFS by SNP mapping, or other methods that become available as technology advances after all subjects have been enrolled. Genetic information discovered by these methods may be used to estimate how many people have specific genetic variations that potentially contribute to development and persistence of CFS, or may in the future be suggested to play such a role. This may help us begin to learn why some people get CFS and others do not. We also want to know how factors such as early adverse life events, exercise, and exposure to various infectious agents affect people who have those genes. This will help the medical community decide if changing these factors will be effective in preventing or treating CFS.
Participants will be given the option to decline consent for genetic testing without penalty. Subjects will be advised that, should they withhold consent for genetic testing, they will still receive full compensation for their participation. Furthermore, subjects may agree to have their samples stored and later decide to withdraw them from storage. If this occurs, the sample(s) will be discarded but any data from testing of that sample(s) until that point will remain part of the research.
Although we hope that we will be able to use these tests to distinguish patients with CFS, they are not indicative of any known pathologies. Individual subjects will not get any direct benefit for providing a blood sample for this study, but they will be informed that the information and results from these kinds of studies may help prevent and treat CFS in the future. We will not create a mechanism for individual subjects to learn the results of our research on their specific samples.
The kind of information we will look for in this study is not expected to reveal anything specific about the current or future personal health of individual subjects. Even so, some people may have concerns about their blood being used for genetic testing. Genetic information could be misused in the hands of people other than the researchers. Every precaution will be taken to prevent this from happening. Blood samples will be assigned a code number, and patient names or any other identifying information will be separated from the sample. Only the Contractor will maintain files that link identifying information to the code number. Specific individual test results will be kept private and released only if ordered by a court of law. Patient names or identifying information will not appear when this study is presented or its results published. Although no one can absolutely guarantee confidentiality, using a code number greatly reduces the chance that someone other than the study staff will ever be able to link a subject’s name to their sample test results.
The results of these tests should not affect the future employment or insurability of the participants. Although patient names will not be with the sample, it will have other facts such as race, ethnicity, and sex. These facts are important because they will help us learn if the factors that cause CFS to occur, persist, or get worse are the same or different in men and women, and in people of different racial or ethnic backgrounds. However, it is also possible through these kinds of studies that genetic traits might come to be associated with a particular group. In some cases, this could reinforce harmful stereotypes. In our presentation and publishing of results from this study, we will consciously avoid language that might result in harmful stereotyping.
The aim of our research is to improve the public health. Sometimes, such research may result in findings or inventions that have value if they are made or sold. We may get a patent on these. We may also license these, which could give a company the sole right to make and sell products or offer testing based on the discovery. Some of the profits from this may be paid back to the researchers and the organizations doing this study, but individual research subjects would not receive any financial benefits.
When this study is completed, we will retain unused blood samples for future research on gene expression, as well as endocrine and immune systems. We do not have specific research plans at this time but, in the future, we will submit these studies to the IRB for review and approval. We will keep each blood sample frozen in a specimen bank at CDC and use it for as long as it lasts. Like the genetic testing samples, these blood samples will also be assigned code numbers. Patient names, or any other identifying information, will be separated from the sample identified by code numbers. Only the Contractor will maintain files that link identifying information to code numbers. This link is maintained to provide demographic or medical data that may inform future studies.
Specific individual test results will be kept private and released only if ordered by a court of law. Patient names or identifying information will not appear when this study is presented or its results published. Although no one can absolutely guarantee confidentiality, using a code number greatly reduces the possibility that someone other than the study staff will ever be able to link a subject’s name to their sample test results. We may share the samples with other researchers for future CFS research, but we will not give other researchers any information that would allow them to identify individual subjects. An Institutional Review Board will review and approve all future projects.
Although we hope that we will be able to use these tests to distinguish patients with CFS, they are not indicative of any known diseases and will not be used for the treatment or evaluation of study patients.
Some people may have concerns about their blood being used for genetic testing. The tests that we will be performing are looking only for differences in gene structure. We will not be testing for known genes that cause specific diseases. The results of these tests should not affect the future employment or insurability of the participants, and will be kept confidential to the extent legally possible. Participants are given the option to decline consent for genetic testing without penalty. Subjects are advised that, should they withhold consent for genetic testing, they will still receive full compensation for their participation.
E. Literature Cited
Afari N, Buchwald D: Chronic fatigue syndrome: a review. Am.J Psychiatry 160:221-236, 2003.
Ahern MM, Hendryx MS, Siddharthan K: The importance of sense of community on people's perceptions of their health-care experiences. Med Care; 34:911-923, 1996
Ax S, Gregg VH, Jones D: Chronic fatigue syndrome: sufferers’ evaluation of medical support. J R Soc Med 90:250-254, 1997.
Ax S, Gregg VH, Jones D: Coping and illness cognitions: chronic fatigue syndrome. Clin.Psychol.Rev. 21:161-182, 2001.
Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med; 130(11):910-21, 1999
Bates DW, Schmitt W, Buchwald D, et al. Prevalence of fatigue and chronic fatigue syndrome in primary care practice. Arch Intern Med 153:2759-2765, 1993.
Bergner L, Bergner M, Hallstron AP, Eisenberg M, Cobb LA. Health status of survivors of out-of-hospital cardiac arrest six months later. Am J Public Health. 1984;74:508-510.
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W: Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 27:169-190, 2003.
Bierl C, Nisenbaum R, Hoaglin D, et al. National pilot telephone survey of chronic fatigue syndrome: regional distribution of fatiguing illness. Population Health Metrics (submitted)
Bombardier CH, Buchwald D. Chronic fatigye, chronic fatigue syndrome, and fibromyalgia: disability and health-care use. Med Care 34:924-930, 1996.
Brick, J.M., Waksberg, J., and Keeter, S. (1996). "Using Data on Interruptions in Telephone Service as Coverage Adjustments," Survey Methodology, 22 (2): 185‑197.
Buchwald D, Herrell R, Ashton S, Belcourt M, Schmaling K, Sullivan P, Neale M, Goldberg J., A twin study of chronic fatigue, Psychosom Med. 63:936-43, 2001.
Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med.;101:364-370, 1996.
Buchwald D, Umali P, Umali J, Kieth P, Pearlman T, Komaroff Al. chronic fatigue and the chronic fatigue syndrome: prevalence in a Pacific Northwest health care system. Ann Intern Med 123:81-88, 1995.
Buckley L, MacHale SM, Cavanagh JT, Sharpe M, Deary IJ, Lawrie SM. Personality dimensions in chronic fatigue syndrome and depression. J Psychosom Res 46:395-400, 1999.
Butler JA, Chalder T, Wessely S: Causal attributions for somatic sensations in patients with chronic fatigue syndrome and their partners. Psychol.Med. 31:97-105, 2001.
Cannon JG: Cytokines in aging and muscle homeostasis. J Gerontol.A Biol Sci.Med.Sci.; 50 Spec No:120-123, 1995
Capuron L, Ravaud A, Dantzer R: Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med; 63:376-386, 2001
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science; 301:386-389, 2003
Ciccone DS, Busichio K, Vickroy M, Natelson BH: Psychiatric morbidity in the chronic fatigue syndrome. Are patients with personality disorder more physically impaired? J Psychosom.Res. ,54:445-452, 2003.
Cleeland C: Research in cancer pain. What we know and what we need to know. Cancer; 67:823-827, 1991
Costa PTJ, McCrae RR. NEO-PI-R. Professional manual: Revised NEO Personality Inventory (NEO-PI-R) and NEO-Five-Factor Inventory (NEO-FFI). Odessa, FL. Psychological Assessment Resources, 1992.
Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg M, Mellman T, Beckham JC, Smith RD, Davison RM, Katz R, Feldman ME: Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol.Med. 27:153-160, 1997.
Davidson, P: Cell users soon could take home digits along. USA Today, November 3, 2003, Money, B1.
Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab; 73(6):1224-34, 1991
Endicott NA., Chronic fatigue syndrome in private practice psychiatry: family history of physical and mental health . J Psychosom Res. 47:343-54, 1999.
Federal Communication Commission: Telephone Subscribership in the United States, April 2003.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS: Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am.J Prev.Med. 14:245-258, 1998.
Fiedler N, Lange G, Tiersky L, DeLuca J, Policastro T, Kelly-McNeil K, McWilliams R, Korn L, Natelson B: Stressors, personality traits, and coping of Gulf War veterans with chronic fatigue. J Psychosom.Res. 48:525-535, 2000.
First MB, Spitzer RL, Gibbon M, and Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute. 2002.
Fisher L, Chalder T: Childhood experiences of illness and parenting in adults with Chronic Fatigue Syndrome. J Psychosom.Res. 54:439-443, 2003.
Folkman S, Lazarus RS: If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers.Soc.Psychol. 48:150-170, 1985.
Frankel, M.R., Srinath, K.P., Hoalin, D.C., Battaglia, M.P., Smith, P.J., Wright, R.A., and Khare, M. (2003). "Adjustments for non-telephone bias in random-digit-dialing surveys.” Statistics in Medicine, 2003, 22: 1611-1626.
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Int Med 121:953-959, 1994.
Gillin, DL. Are you placing survey research calls to cellular telephones? If you are – beware!, Council for Marketing and Opinion Research (http://www.cmor.org/govt_affairs_new0802.htm), accessed November 3, 2003.
Greve W, Anderson A, Krampen G.: Self-efficacy and externality in adolescence: Theoretical conceptions and measurement in New Zealand and German secondary school students. Int J Theory Res. 1:321-344, 2001.
Gupta S, Aggarwal S, Starr A: Increased production of interleukin-6 by adherent and non-adherent mononuclear cells during 'natural fatigue' but not following 'experimental fatigue' in patients with chronic fatigue syndrome. Int J Mol Med; 3:209-213, 1999
Hardt J, Buchwald D, Wilks D, Sharpe M, Nix WA, Egle UT. Health-related quality of life in patients with chronic fatigue syndrome, an international study. J Psychosom Res. 2001;51:431-434.
Hart LG, Evans RW. The functional status of ESRD patients as measured by the sickness impact profile. J Chron Dis. 1987;40(Suppl 1):117S-136S.
Heim C, Bierl C, Nisenbaum R, Reeves WC. Decreased US national prevalence of fatiguing illness in the aftermath of the terrorist attacks of September 11, 2001. JAMA (submitted).
Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB: Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA; 284:592-597, 2000
Heim C, Nemeroff CB: The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry; 49:1023-1039, 2001
Hickie IB, Bansal AS, Kirk KM, Lloyd AR, Martin NG, A twin study of the etiology of prolonged fatigue and immune activation. Twin Res. 4:94-102, 2001.
Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 108:387-389, 1988.
Hyler SE, Skodol AE, Kellman HD, Oldham JM, Rosnick L: Validity of the Personality Diagnostic Questionnaire--revised: comparison with two structured interviews. Am.J Psychiatry 147:1043-1048, 1990.
Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Int Med 159: 2129-2137, 1999.
Jason LA, Taylor RR, Kennedy CL, Song S, Johnson D, Torres S. Chronic fatigue syndrome: occupation, medical utilization, and subtypes in a community-based sample. J Nerv Ment Dis 188:568 -76, 2000
Joyce E, Blumenthal S, Wessely S: Memory, attention, and executive function in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry; 60:495-503, 1996
Kang HK, Natelson BH, Mahan CM, Lee KY, Murphy FM: Post-traumatic stress disorder and chronic fatigue syndrome-like illness among Gulf War veterans: a population-based survey of 30,000 veterans. Am.J Epidemiol 157:141-148, 2003.
Keeter, S. (1995). "Estimating Telephone Noncoverage Bias with a Telephone Survey," Public Opinion Quarterly, 59: 196‑217.
Kirschbaum C, Hellhammer DH. Salivary cortisol. In Encyclopedia of Stress Volume 3. Academic Press. 379 – 383, 2000
Kitani T, Kuratsune H, Yamaguchi K. Diagnostic criteria for chronic fatigue syndrome by the CFS Study Group in Japan. Nippon Rinsho 50:2600-2605, 1992.
Klimidis S, Minas IH, Ata AW: The PBI-BC: A brief current form of the parental bonding instrument for adolescent research. Comprehensive Psychiatry 33(6): 374-377, 1992
Komaroff AL, Fagioli LR, Doolittle TH, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. 1996;101:281-290.
Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K: Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol.Assess. 12:210-224, 2000.
Kudielka BM, Kirschbaum C: Awakening cortisol responses are influenced by health status and awakening time but not by menstrual phase. Psychoneuroendocrinology; 28: 35-47, 2003
Larson SL, Fleishman JA: Rural-urban differences in usual source of care and ambulatory service use: analyses of national data using Urban Influence Codes. Med Care; 41:III65-III74, 2003
Levine PH, Whiteside TL, Friberg D, Bryant J, Colclough G, Herberman RB: Dysfunction of natural killer activity in a family with chronic fatigue syndrome. Clin Immunol Immunopathol. 88:96-104, 1998.
Lloyd AR, Hickie I, Boughton CR, Spencer O, Wakefield D. Prevalence of chronic fatigue syndrome in an Australian population. Med J Austr. 153:522-528, 1990.
Lloyd A, Pender H. The economic impact of chronic fatigue syndrome. Med J Australia 157:599-601, 1992
Lutgendorf SK, Antoni MH, Ironson G, Fletcher MA, Penedo F, Baum A, Schneiderman N, Klimas N: Physical symptoms of chronic fatigue syndrome are exacerbated by the stress of Hurricane Andrew. Psychosom.Med. 57:310-323, 1995
McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB: Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA 277:1362-1368, 1997.
McCauley LA, Joos SK, Barkhuizen A, Shuell T, Tyree WA, Bourdette DN: Chronic fatigue in a population-based study of Gulf War veterans. Arch.Environ.Health 57:340-348, 2002.
McCrone P, Barbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med 33:253-261, 2003.
Minowa M, Jiamo M. Descriptive epidemiology of chronic fatigue syndrome based on a nationwide survey in Japan. J Epidemiol 6:75-80, 1996.
Munck A, Naray-Fejes-Toth A: Glucocorticoids and stress: permissive and suppressive actions. Ann N Y Acad Sci.; 746:115-130, 1994
Nisenbaum R, Barrett DH, Reyes M, Reeves WC. Deployment stressors and a chronic multisymptom condition among Gulf War veterans. J Nerv Ment Dis 188:259-266, 2000.
Nisenbaum R, Jones JF, Unger ER, Reyes M, Reeves WC. A population-based study of the clinical course of chronic fatigue syndrome. BMC Hlth Quality Life Outcomes. 1:49, 2003.
Papanicolaou DA, Tsigos C, Oldfield EH, Chrousos GP: Acute glucocorticoid deficiency is associated with plasma elevations of interleukin-6: does the latter participate in the symptomatology of the steroid withdrawal syndrome and adrenal insufficiency? J Clin Endocrinol.Metab; 81:2303-2306, 1996
Parker AJ, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med; 31(8):1331-45, 2001
Parker G: Parental characteristics in relation to depressive disorders. Br.J Psychiatry 134:138-147, 1979.
Pennebaker JW, Susman JR: Disclosure of traumas and psychosomatic processes. Soc Sci Med ; 26:327-332, 1988
Pruessner JC, Hellhammer DH, Kirschbaum C: Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med; 61: 197-204, 1999
Radant A, Tsuang D, Peskind ER, McFall M, Raskind W: Biological markers and diagnostic accuracy in the genetics of posttraumatic stress disorder. Psychiatry Res; 102:203-215, 2001
Raison CL, Miller AH: When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry; 160:1554-1565, 2003
Reeves WC, Lloyd A, Vernon SD, et al. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Services Research (submitted].
Reuters: Cell phone directory planned. Chicago Tribune, October 21, 2003, Business, 1.
Reyes M, Dobbins JG, Mawle AC, Steele L, Gary HE, Malani H, Schmid S, Fukuda K, Stewart J, Nisenbaum R, Reeves WC. Risk factors for CFS: a case control study. J CFS 2:17-33, 1996.
Reyes M, Gary HE, Dobbins JG, et al. Surveillance for chronic fatigue syndrome: four US cities. MMWR Morb Mortal Wkly Rep 46:1-13, 1997.
Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Int Med 163:1530-1536, 2003.
Reyes M, Stanton PK: Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J Neurosci. 16:5951-5960, 1996.
Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Prev Chronic Dis (submitted).
Reynolds WM: Development of reliable and valid short forms of the Marlowe-Crowne social desirability scale. J Clin Psychol. 38 (1): 119-125, 1982.
Reid S, Chalder T, Cleare A, Hotopf M, Wessely S: Chronic fatigue syndrome. BMJ 320:292-296, 2000.
Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Lancet (submitted).
Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P: Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia; 5:266-281, 1994
Romans S, Belaise C, Martin J, Morris E, Raffi A: Childhood abuse and later medical disorders in women. An epidemiological study. Psychother.Psychosom. 71:141-150, 2002.
Sarason IG, Johnson JH, Siegel JM: Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin.Psychol. 46:932-946, 1978.
Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C: The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences; 64(18), 1653-1660, 1999
Schulz P, Kirschbaum C, Pruessner J, Hellhammer DH: Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med; 14: 91-97, 1998
Scott LV, Dinan TG. Urinary free cortisol excretion in chronic fatigue syndrome, major depression and in healthy volunteers. J Affect Disord; 47(1-3):49-54, 1998
Sharpe MC, Archard LC, Banatvala JE, et al. A report: chronic fatigue syndrome: guidelines for research. J R Soc Med 84:118-121, 1991.
Smets EM, Garssen B, Bonke B, De Haes JC: The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res; 39:315-325, 1995
Solomon L., Nisenbaum R., Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome by primary health care providers. Am J Epidemiol (submitted).
Solomon L, Nisenbaum R, Reyes M, Papanicolaou DA, Reeves WC. Functional status of persons with chronic fatigue syndrome in the Wichita, Kansas, population. BMC Hlth Quality Life Outcomes. 1:48, 2003.
Spielberger CD: State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press 1983
Steele L, Dobbins JG, Fukuda K, et al. The epidemiology of chronic fatigue in San Francisco. Am J Med 105:83S-90S, 1998.
Suraway C, Hackmann A, Hawton K and Sharpe M. Chronic Fatigue Syndrome: A Cognitive Approach. Behavioural Research Therapy. 33(5): 535-544. 1995.
Taillefer SS, Kirmayer LJ, Robbins JM, Lasry JC. Correlates of illness worry in chronic fatigue syndrome. J Psychosom Res 54:331-337, 2003.
Taylor RR, Friedberg F, Jason LA: A Clinician's Guide to Controversial Illnesses: Chronic Fatigue Syndrome, Fibromyalgia, and Multiple Chemical Sensitivities. Sarasota, Fla.: Professional Resource Press, 2001.
Taylor RR, Jason LA: Sexual abuse, physical abuse, chronic fatigue, and chronic fatigue syndrome: a community-based study. J Nerv.Ment.Dis. 189:709-715, 2001.
Torpy DJ, Bachmann AW, Grice JE, Fitzgerald SP, Phillips PJ, Whitworth JA, Jackson RV. Familial corticosteroid-binding globulin deficiency due to a novel null mutation: association with fatigue and relative hypotension, J Clin Endocrinol Metab. 86:3692-700, 2001.
Van Houdenhove B, Neerinckx E, Onghena P, Lysens R, Vertommen H: Premorbid "overactive" lifestyle in chronic fatigue syndrome and fibromyalgia. An etiological factor or proof of good citizenship? J Psychosom.Res; 51:571-576, 2001.
Walsh et al. A family history study of chronic fatigue syndrome. Psychiatr Genet. 11:123-8, 2001.
Ware JE, Jr., Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care; 30:473-483, 1992
Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet; 354(9182):936-9, 1999
Wesley S, Chalder T, Hirsch S, Wright D. the prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health. 87:1449-1455, 1997.
White C, Schweitzer R. The role of personality in the development and perpetuation of chronic fatigue syndrome. J Psychosom Res 48:515-524, 2000.
Wilkinson GS. Wide Range Achievement Test 3 Administration manual. Delaware: Wide Range Inc., 1993.
Wilson A, Hickie I, Hadzi-Pavlovic D, et al. What is chronic fatigue syndrome? Heterogeneity within an international multicenter study. Aust NZ J Psych 35:520-527, 2001.
Zung WW, Richards CB, Short MJ: Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch.Gen.Psychiatry 13:508-515, 1965.
F. Appendices
Note—these approved Phase 1 appendices are not included in this submission. Appendices in the following Phase 2 section include only those instruments that have been modified for Phase 2.
Appendix-1. Advance Telephone Survey Letter
Appendix-2. Telephone Interview Follow-up Letters (Screener non-contact; Screener reluctant, Detailed interview non-contact, Detailed interview reluctant)
Appendix-3. Screening Telephone Interview.
Appendix-4. Detailed Telephone Interview.
Appendix-5. Descriptive materials sent to subjects prior to clinical evaluation
Clinic Appointment Letter
Informed Consent
Saliva Collection Instructions
Clinic Refusal Conversion Letter
Appendix-6. Physical Examination (including Medical History and Medications)
Appendix-7. Saliva and Blood Collection
Appendix-8. Questionnaires Concerning Symptomatology
Symptom Inventory
Medical Outcomes Study 36-item short-form health survey (MOS SF-36)
Multidimensional Fatigue Inventory (MFI)
Sleep Assessment
Brief Pain Inventory (BPI)
Appendix-9. Neurocognitive Assessment
Cambridge Neuropsychological Test Automated Battery (CANTAB) is a computer administered test battery described in detail in the protocol. It cannot be appended.
Wide Range Achievement Test (WRAT3) Reading Subtest
Appendix-10. Psychiatric Evaluation
Structured Clinical Interview for DSM-IV (SCID) is a 112 page standard psychiatric diagnostic instrument and is not appended.
Personality Diagnostic Questionnaire-Revised (PDQ4)
NEO Five-Factor Inventory (NEO-FFI)
Zung Self-Rating Depression Scale (SDS)
Spielberger State-Trait Anxiety Inventory (STAI)
Davidson Trauma Scale (DTS)
Appendix-11. Assessment of Early and Adult Life Experiences
Childhood Trauma Questionnaire-Short Form (CTQ-SF)
Traumatic Life Events Questionnaire (TLEQ)
Life Experiences Survey (LES)
Parental Bonding Instrument (PBI-BC)
Appendix-12. Assessment of Stress and Coping
Trier Inventory for the Assessment of Chronic Stress (TICS-2-S)
Ways of Coping Questionnaire (WCQ)
Locus of Control
Marlowe-Crowne Social Desirability Scale (MCSDS-C)
Appendix-13. Economic Impact
Appendix-14. Health Services Utilization
Appendix-15. Residence History
Appendix 16. Adverse Event Protocol
Phase 2 (February 14, 2006 to May 8, 2008)
Summary
Chronic fatigue syndrome (CFS) is a complex medical and public health problem. It is estimated that approximately 700,000 adults in the U.S. suffer from CFS. They have been ill for 5-7 years, a quarter of them are unemployed or receive disability, yet fewer than 20 percent have received medical care for CFS. CFS is a Congressional and DHHS priority, and CDC is responsible for its control and prevention. The goal of the CDC CFS research program is to reduce morbidity associated with CFS in the United States and to devise prevention strategies. We accomplish this goal through an integrated program of health care provider education, hypothesis-driven population-based studies, in-hospital clinical studies, laboratory studies that evaluate genetics and gene transcription (genomics), gene translation (proteomics), and a bioinformatics effort that devises methods to identify biologic pathways involved in the illness (metabolomics).
The objectives of the hypothesis-driven cohort study presented in this protocol are to identify risk factors and biologic markers of CFS so as to improve identification, clinical evaluation, diagnosis and management of persons with CFS. This study builds on data collected during the 2004 to 2005 Phase 1 baseline survey of CFS in metropolitan, urban, and rural populations of Georgia (Baseline Survey of CFS and Chronic Unwellness in Georgia CDC IRB #4121). Subjects in the survey agreed to be recontacted for participation in this follow-up study. Because the current study focuses on time course of CFS, the majority of data collection instruments and procedures are unchanged from approved CDC protocol #4121.
The study concentrates on major gaps in current knowledge that must be clarified to understand CFS and devise, implement and monitor effective control and prevention strategies. CFS is defined by self-reported symptoms, because as yet there are no defining physical symptoms or diagnostic laboratory abnormalities and the pathophysiology remains unknown. This study will evaluate specific behaviors, clinical markers and biomarkers necessary to understand the pathophysiology of CFS. Socioeconomically disadvantaged minority racial/ethnic groups appear to disproportionately suffer from CFS and there is evidence of markedly different risks in metropolitan, urban, and rural populations. This study will identify risks in these populations so as to accurately target intervention efforts and measure their effects. The study will also identify the economic burden of CFS to permit cost-benefit analyses of control and prevention programs.
The current protocol describes Phase 2, the First Follow-up Study of CFS and Chronic Unwellness in Georgia. It is a follow-up of 3 cohorts (CFS, unwell — with or without fatigue, and well) identified from metropolitan, urban, and rural Georgia populations. This First Follow-up Study of CFS and Chronic Unwellness in Georgia involves detailed computer assisted telephone interviews of the 438 people with CFS-like Illness, 1,665 unwell (with or without fatigue) and 1,484 well participants from the first phase who volunteered to be recontacted. The Follow-up Study also includes a 1-day detailed clinical evaluation (as in the Baseline Survey). We will ask volunteers who participated in the Baseline Survey clinical evaluation to return to the clinic to evaluate improvement or worsening over time. We will also clinically assess persons who participated in the Baseline Survey telephone interview, but did not attend clinic at that time, and who subsequently developed severe fatiguing illness; a random sample of persons reporting other chronic unwellness; and a matched set of healthy controls. Clinical assessments are as in the Baseline Survey and will include evaluation of each subject’s medical and psychiatric status and psychosocial factors. We will obtain specimens for standard clinical laboratory testing, measures of allostatic load index, endocrine status, and samples for a biorepository. Findings from the Follow-up Study will be used to: 1) identify variables that predict the clinical course of CFS; 2) identify physiologic markers that characterize CFS and predict the clinical course; 3) evaluate the economic impact of CFS and access to/utilization of medical care of persons with CFS; 4) begin to evaluate the incidence of CFS; 5) and, generate specific hypotheses that will be further evaluated in laboratory and clinical studies.
Performance Sites
Centers for Disease Control & Prevention (CDC), Atlanta, GA (OHRP # FW A 00001413)
Department of Psychiatry & Behavioral Sciences, Emory University School of Medicine, Atlanta, GA (OHRP # MI426)
Abt Associates Inc., 640 North LaSalle Street, Suite 640, Chicago, IL 60610 (OHRP # IRB00001281)
Key Personnel
William C. Reeves, MD, MSc CDC Principal Investigator (PI)
Joann House CDC Project Officer
James F. Jones, MD CDC CDC Deputy PI
Urs Nater, PhD CDC Coordinator Psychology
Elizabeth Maloney, DrPH CDC Coordinator Epidemiology
Roumiana Boneva, MD, PhD CDC Coordinator Field Studies
Suzanne D. Vernon, PhD CDC Coordinator Molecular Epidemiology
Jin-Man (Sally) Lin, PhD CDC Biostatistician
Abt Associates Inc. CDC has contracted with Abt Associates to implement those tasks necessary to complete the study. This includes collaboration in study design and sampling strategies, detailed telephone surveys, and clinical evaluation.
Key CDC/Emory University Personnel Qualifications
Dr. Reeves is Chief of the Chronic Viral Diseases Branch (CVDB), Division of Viral and Rickettsial Diseases, NCID and has served as Principal Investigator for CDC’s chronic fatigue syndrome (CFS) research program since 1992. He is a medical epidemiologist with expertise in infectious and chronic diseases. He is an elected member of the American Epidemiologic Society, an elected Fellow in the American College of Epidemiology, and elected Fellow in the Infectious Diseases Society of America. He is a recipient of the Amador Guerrero Medal (the highest honor awarded by Panama in the field of science and equivalent to the U.S. Medal of Science) awarded by the President of Panama in recognition of his research and service to public health. Dr. Reeves has published 180 peer-reviewed articles of which 38 deal with CFS (encompassing the case definition, prevalence, incidence, outbreaks, risk factors, clinical aspects, laboratory measures, and stress).
Ms. House has been Administrative Officer and Deputy Director for VEHB since 1996. Prior to this (1990 to 1996) she was Program Specialist responsible for program management in the Division of Viral and Rickettsial Diseases. She is currently responsible for administrative management of the CVDB CFS and human papillomavirus research programs and serves as Project Officer for all CDC funded studies of CFS. Because optimal administrative management requires ongoing integrated analysis and interpretation of management information related to large epidemiology, clinical, and molecular biology laboratory studies, she participates actively in the CDC CFS Research Group to maintain a level of scientific competence sufficient to place management into an appropriate context.
Dr. Jones is a senior Medical Research Officer in CVDB and serves as Deputy PI to oversee clinical aspects of this study. He is board certified in Pediatrics and Allergy/Immunology. Prior to accepting a position at CDC in 2003, he was Professor of Pediatrics at the National Jewish Medical Center and University of Colorado School of Medicine. He was responsible for an active CFS research program involving clinical studies, studies of behavior and CFS, studies of immunology and CFS, studies of infectious agents and CFS, and studies of endocrinology and CFS. Between 1986 and 2002 he was PI on 4 NIH RO-1 grants investigating CFS. He has written 20 book chapters and 85 peer-reviewed publications.
Dr. Nater is a post-doctoral ORISE Fellow at the Centers for Disease Control. He has received his PhD in 2004, after completing a PhD program in psychology/neuroscience at the University of Zurich and the Federal Institute of Technology, Switzerland. He is the recipient of several scientific awards, such as the Young Investigator Award, International Society for Psychoneuroendocrinology (ISPNE), 2005, Young Scientist Award, Swiss Society of Psychology, 2005, Scholar Award, American Psychosomatic Society (APS), 2006, Early Career Award, International Society of Behavioral Medicine (ISBM), 2006. He has research interests in the psychological and physiological underpinnings of stress in chronic fatigue syndrome. Since 2005, he has authored 14 peer-reviewed papers and 4 book chapters.
Dr. Maloney is VEHB Epidemiology Team Leader. She has degrees in psychology, biostatistics, and epidemiology. She joined the CDC CFS research group in 2004, after 15 years as a staff scientist in the Viral Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. She is responsible for epidemiology components of the Georgia Survey and is designing a CFS Registry that will operate in parallel with population surveillance. At NCI, she was responsible for population-based studies of HTLV infection and disease in Jamaica, Panama, and the United States. She has an extensive bibliography that includes field, clinical, and laboratory studies of HTLV in Jamaica, Panama, and the United States.
Dr. Boneva is a Medical Epidemiologist who joined the CDC CFS research group in 2004. She served as an EIS Officer in the Division of Birth Defects and Developmental Disabilities, NCEH from 1996 to 1998 and then as a Medical Epidemiologist in HIV and Retrovirus Branch, NCHSTP. She is responsible for coordinating and managing medical aspects of field studies.
Dr. Vernon is a Research Microbiologist and a team leader of the CVDB Molecular Epidemiology Group. She has been a staff scientist at the Centers for Disease Control and Prevention in Atlanta, Georgia since 1992. For several years, Dr. Vernon conducted research on molecular interactions between human papillomavirus (HPV) and human immunodeficiency virus based on her observations that HIV-induced immunosuppression could not explain HPV-associated cervical cancer. Since 1997, Dr. Vernon has directed the Molecular Epidemiology Program in CVDB to characterize chronic fatigue syndrome at a systems biology level by using population-based epidemiology, genomics, proteomics, and physiology. To accomplish this, Dr. Vernon has been instrumental in building a multidisciplinary team of molecular biologists, mathematicians and computational biologists.
Dr. Lin is a statistician who recently joined CVDB. She has both a doctorate and masters degree in statistics, and a masters degree in applied mathematics. She has more than two years experience as the Director of the Biometrics Core at the Clinical Research Center at Meharry Medical College in Tennessee. During that time she provided consultative services to Meharry investigators on the design of their research studies from the point of conceptual formulation to completion, including sample size analysis, data analysis, interpretation of results and writing of manuscripts. She also assisted researchers in the design of data capture methods to insure quality control in the development of databases. Dr. Lin has taught statistics, data analysis and data management at Meharry Medical College. She is proficient in the use of several statistical software packages, including SAS, SAS Macro, S-Plus, R, SPSS, GLIM and IMSL subroutines for Fortran. She is also proficient in Mathematica and GIS software.
Key Abt Associates Personnel Qualifications
As noted above, CDC has contracted with Abt Associates Inc. to collect and deliver data for the First Followup Study of Chronic Fatigue Syndrome and Chronic Unwellness in Georgia. Abt Associates’ staff is experienced with CDC telephone surveys and clinical studies involving CFS research. Below, we briefly describe the qualifications and responsibilities of the senior-level Abt Associates’ project team.
Scott Royal, Ph.D., leader of Abt Associates' Public Health and Epidemiology Practice, is Abt Associates’ project director of all CDC CFS field studies. Dr. Royal has responsibility for, and authority over, the scientific, operational, and financial aspects of the task orders conducted under this master contract with CDC. Survey Director Rebecca Devlin has day-to-day responsibility (budget, schedule, and staff) for the telephone interview and clinical evaluation components. She has eight years of survey management experience and has extensive knowledge of CFS. She was Abt Associates study director for the telephone component of the Baseline Survey of CFS and Chronic Unwellness in Georgia (CDC IRB #4121) and task leader for psychiatric instrument data collection in the clinical evaluation component. Prior to those assignments, she trained and provided quality assurance for neurocognitive testing and psychiatric interviewing in the Clinical Research Center study of CFS in Wichita (CDC IRB #3504).
Senior statistician David C. Hoaglin, Ph.D. collaborated on study design and sampling strategies and will compute sample weights and calculate prevalence and incidence estimates and provide technical advice on project design and on analytic protocols. Dr. Hoaglin has provided statistical support for CDC projects since 1996.
Study Consultants
Dr. Phaedra Corsu is a health economist at Georgia State. She will collaborate with the Principal Investigator in the analysis of economic data.
Acknowledgment of all funding sources and substantial contributions:
As part of a coordinated Department of Health and Human Services (DHHS) program of chronic fatigue syndrome (CFS) research, CDC has been charged by the Congress to address CFS control and prevention. Congress has specified that CDC conduct community-based studies that target race/ethnicity-specific differences in the occurrence of CFS and that CDC use these studies to identify the causes, risk factors, diagnostic markers and economic impact of CFS. Monies appropriated by Congress for this purpose fully fund this study. As an ex-officio member of the Department of Health and Human Services CFS Advisory Committee, CDC has presented this research plan to the Committee, provided the Committee with regular updates as to the study’s progress and had extensive discussion with committee members regarding the study.
Statement of any conflict of interest:
None of the investigators have any conflict of interest with respect to this project.
A. Objectives and Specific Aims
A.1 Objective:
The objective of this study is to identify risk factors for, and biomarkers of, chronic fatigue syndrome (CFS) so as to improve identification, clinical evaluation, diagnosis, and management of the illness. Participants are from a population-based cohort surveyed 2004 to 2005 (Baseline Survey of CFS and Chronic Unwellness in Georgia (CDC IRB# 4121)) to describe baseline epidemiologic and clinical aspects of CFS. Participants at that time volunteered to participate in further studies.
A.2 Specific aims:
Specific Aim 1 – Identify clinical, psychosocial and environmental variables that characterize people with CFS and predict the clinical course of the illness. Unwell and well participants will serve as controls. This information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project.
Hypothesis – Persons with shorter duration illness/sudden onset CFS will have higher recovery rates than those with longer duration/gradual onset CFS and will have lower recovery rates than unwell participants.
Hypothesis – Persons who develop CFS associated with cumulative life-time stress history will have lower recovery rates than those whose CFS is not associated with stress history. Cumulative stress history will be similarly associated with clinical course of unwellness.
Specific Aim 2 – Identify physiologic markers that characterize people with CFS and predict the clinical course of the illness. Unwell and well participants will serve as controls. This information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project.
Hypothesis – CFS patients will be 2-times more likely to have a high allostatic load index compared to unwell and well controls. Allostatic load index will be determined by clinical and laboratory measures of metabolic and cardiovascular factors, and HPA axis neuroendocrine status.
Hypothesis – Among persons with CFS, increasing allostatic load index will be associated with significantly greater levels of symptom severity and impaired functioning (measured by the Symptom Inventory and the SF-36) compared to unwell and well controls.
Hypothesis – High level of baseline allostatic load index among persons with CFS will be associated with risk of persistent or progressing illness and with functional impairment in the one-year follow-up of patients.
Hypothesis –Women with CFS will have been in menopause longer than age- and sex- matched Well controls and use of hormone replacement therapy will modify this association.
Specific Aim 3 – Evaluate economic impact of CFS and access to/utilization of medical care of persons with CFS in metropolitan, urban, and rural Georgia. Unwell and well participants will serve as controls. This information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project. Finally, information concerning economic impact will be used to evaluate cost effectiveness of different control modalities.
Hypothesis – CFS will be associated with a substantial economic burden on affected individuals, their families, and the metropolitan, urban, and rural populations of Georgia. The economic impact of CFS will be substantially greater than that of unwellness.
Hypothesis – Persons with CFS who consult and are treated by a health care provider will have higher recovery rates than those who do not receive such attention.
Specific Aim 4 – Evaluate the incidence of CFS and unwellness in different racial/ethnic groups representative of metropolitan, urban, and rural Georgia populations. This will be the first year in a study of 4-year CFS incidence. The incidence of CFS will be determined among those categorized as unwell and well in the Baseline Survey. This information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project.
Hypothesis – this study will detect significantly higher CFS incidence rate than previous population-based studies.
Hypothesis – clinical, psychosocial, and laboratory variables (e.g., adverse life experiences, allostatic load index, personality and coping mechanisms) will be associated with incident CFS. Specifically, it is assumed that persons who were defined as unwell in the baseline survey or have experienced a triggering event within the past year have a higher likelihood of new onset CFS. This risk is enhanced by early-life adversity, increased allostatic load index, reactive personality and maladaptive coping styles.
Hypothesis – the incidence of CFS will differ between white and black populations.
Hypothesis – the incidence of CFS will differ between metropolitan/urban and rural populations.
Specific Aim 5 - Identify persons representative of the Georgia population with CFS, unwellness and a healthy comparison group to invite for future enrollment in General Clinical Research Center (GCRC) studies at Emory University. Select persons with CFS clinical profiles identified in Aims 1, 2 and 4 for enrollment in GCRC mechanistic studies.
Two GCRC protocols are being developed in collaboration with Emory University. One involves a stress diathesis model and the second involves cognition and brain function.
Specific Aim 6 - Maintain unlinked (anonymous) storage of samples (e.g., serum, plasma, white cells, urine, DNA, RNA) from each subject group (e.g., CFS, unwell, well) for validation of relevant findings and markers established in Aims 1 through 4, and findings from Wichita clinical study (CDC IRB #3504) (e.g., gene expression profiling, genetic polymorphisms, DNA methylation, and proteomics) and for future biomarker discovery.
B. Background and Significance
CFS is a complex medical and public health problem. CDC estimates that approximately 700,000 adults in the U.S. suffer from CFS. Their median duration of illness is 7 years, a quarter of them are unemployed or receiving disability, yet fewer than 20 percent have received medical care for CFS [Reyes, 2003]. Despite more than 3,000 articles in the peer-reviewed medical literature, the pathophysiology of CFS is not well understood. There are no diagnostic physical signs, laboratory abnormalities or clinical tests. There is no specific control or prevention strategy for CFS. CFS is a DHHS priority and CDC is the lead agency for research concerning its control and prevention. The goal of the CDC CFS Program is to reduce morbidity associated with CFS in the United States. Information collected in the study presented in this protocol will be applied to a national health care provider education program for CFS (Contract #200-2002-00793) and into a national CFS public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project. Finally, economic impact data gathered during this study will be used to evaluate cost effectiveness of different control strategies.
CFS is also a Congressional priority. Beginning in 1992, Congressional language has stressed legislators’ desire for CDC to develop control and prevention measures for CFS. Recently, Congressional language has directed that CDC should target race/ethnicity-specific differences in the occurrence of CFS and should accelerate its CFS research plan to identify the causes, risk factors, diagnostic markers, and economic impact of CFS. Congress has also directed CDC to accelerate its educational activities for health care providers and for public awareness.
The objective of this study is to identify risk factors for, and biomarkers of, CFS so as to improve identification, clinical evaluation, diagnosis, and management of the illness. Participants are drawn from a population-based surveillance cohort, representative of metropolitan, urban and rural populations, that was identified in 2004 and 2005. At that time they volunteered to participate in further studies. This study is a modification of that survey; it utilizes primarily the same instruments, with some modifications.
B.1 Aim 1. Clinical, Psychosocial and Environmental Variables that Characterize CFS.
CFS presents a challenge for patients, health care providers, health insurance groups, public health officials and other social services because of its incapacitating nature, poorly understood clinical course, unknown cause and lack of definitive treatment. The objective of this specific aim is to identify clinical, psychosocial and environmental variables associated with improving, stable and deteriorating CFS. This information will benefit health care providers and their patients. This information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project.
B.1.a. Hypothesis – Persons with shorter duration illness/sudden onset CFS will have higher recovery rates than those with longer duration/gradual onset CFS and will have lower recovery rates than unwell participants. A systematic review of prospective studies revealed that between zero and 37% (median 6%) of adult CFS patients recovered and between 6 and 63% (median 35%) improved over time [Joyce et al., 1997]. Shorter duration of illness predicted a higher likelihood of recovery and recovery was more likely among CFS patients seeing primary care physicians compared to those attending specialty clinics [Hill et al., 1999; Reyes et al., 1999]. Unfortunately, most published studies concerning the clinical course of CFS were conducted in clinical settings and thus involved people who were sick enough and had sufficient resources to seek and obtain medical care. The CDC Wichita surveillance study found that 40% of people with CFS identified from the general population experienced partial remission, 10% sustained total remission, and 20% had a medical or psychiatric illness, which caused the fatiguing illness, discovered during follow-up [Nisenbaum et al., 2003].
We designed this phase of the Georgia study to collect precise information regarding clinical parameters of CFS. Specifically, the clinical evaluation of CFS-like, unwell fatigued, unwell not fatigued and well controls assesses functional impairment by the SF-36 [Ware and Sherbourne, 1992], dimensions of fatigue by the MFI [Smets et al., 1995], occurrence and severity of symptoms by the CDC Symptom Inventory [Wagner et al., 2005], and psychiatric comorbidity by means of the Structured Clinical Interview for DSM-IV (SCID) (First et al., 2002).
B.1. b. Hypothesis – Persons who develop CFS associated with cumulative life-time stress will have lower recovery rates than those whose CFS is not associated with life-time stress history. Stress, such as acute disease, physical trauma and emotional trauma, has long been considered as an important factor in the development of CFS. However, few studies have evaluated the association between stress and CFS or other fatiguing illnesses. One study reported that Hurricane Andrew induced relapses of CFS and symptom exacerbations in a sample of 49 CFS patients living in South Florida [Lutgendorf et al., 1995]. The extent of individual emotional and behavioral stress responses was the single strong and significant predictor of the likelihood and severity of the relapse and functional impairment within 4-months after the hurricane. Our group has reported a strong and statistically significant association between major life-time stress in the year preceding onset of CFS [Reyes et al., 1996] and a similar association between Gulf War illness and self-reported exposure to chemical, emotional, and physical stressors during the conflict [Nisenbaum et al., 2000]. Other studies have reported elevated rates of CFS in Gulf War veterans, especially in association with combat-related post-traumatic stress disorder (PTSD) [McCauley et al., 2002; Kang et al., 2003]. It thus appears that severe stress in adulthood might be a precipitating factor of CFS, or at least in a subgroup of CFS patients.
However, stress is part of normal life and most individuals do not develop CFS. Currently attention is focused towards understanding the long-term effects of stress over the life span on health and functioning. Epidemiological studies provide compelling evidence for a strong and significant association between adverse childhood experiences (e.g., abuse or neglect, parental loss and household dysfunction) and unwellness later in life [McCauley et al., 1997; Felitte et al., 1998]. One study, involving almost 2000 women, reported that those who had been abused as children exhibited increased levels of fatigue and pain in adulthood, and additional symptoms of depression, anxiety, substance abuse and interpersonal sensitivity when compared to women not abused as children [McCauley et al., 1997]. A population-based study conducted in New Zealand reported elevated rates of chronic fatigue in women with childhood adversity [Romans et al., 2002]. Another population-based study reported an association between childhood abuse and fatiguing illnesses [Taylor & Jason, 2001]. A study in tertiary care patients with CFS found that those with CFS more frequently reported various types of abusive victimization starting in childhood and persisting throughout adulthood, as compared to controls [van Houdenhove et al., 2001]. Finally, our recent population-based in-hospital case control study of CFS in Wichita found exposure to childhood stress was significantly associated with 3- to 8-fold increased risk for CFS. There was a graded relationship between extent of stress exposure and risk for CFS. Further, childhood stress was associated with greater CFS symptom severity [Heim et al., 2006].
These findings that acute stress often precedes and exacerbates CFS in adults, combined with the strong association of childhood stress with CFS, support a stress-diathesis model, in which genetic liabilities interact with stressful experiences in determining individual vulnerability to disease, including CFS. This vulnerability likely reflects the combined effects of early experience and genes on the developing brain, resulting in a stable phenotype with different neurobiological expressions, which may determine perceptual thresholds and the set-point of neuroendocrine, immune and behavioral reactivity to the environment [Heim & Nemeroff, 2001], thereby contributing to the development of CFS upon further challenge. We hypothesize that CFS patients with a lifetime history of stressful experiences will be less likely to recover or improve compared to CFS patients who do not have a lifetime history of stressful experiences. We collected data on stress history during the Baseline Survey with the purpose of confirming this association in the Georgia population, and using the information as a framework for evaluating this study of clinical course.
B.2. Aim 2 – Physiologic Markers that Characterize CFS.
Clinical, psychosocial and environmental factors evaluated in Specific Aim1 are also reflected physiologically as physiologic markers that characterize CFS. As noted, stress is part of normal life and most individuals do not develop CFS. The pathophysiology of stress involves hypothalamic pituitary adrenal (HPA) axis-related function and encompasses autonomic nervous system, immunologic, endocrine and brain function. This information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop a targeted regional provider education project.
Many of the biomarkers of stress are included in allostatic load index, an overall measure of encompassing stress-related variables. Allostatic load was first described by McEwen and Stellar [McEwen & Stellar, 1993] as the cumulative wear and tear on the body and brain resulting from chronic over-activity or inactivity of the HPA axis in adaptation to environmental challenge, such as acute disease, physical and emotional trauma (i.e. stress). Elevated allostatic load is significantly associated with increased risk of mortality, heart disease, cognitive decline and decline in physical functioning [Karlamangla et al., 2002; Seeman et al., 2001; Seeman et al., 1997]. Allostatic load index includes laboratory measurements of metabolic, cardiovascular, inflammatory and HPA-axis factors; specifically, diastolic and systolic blood pressure, aldosterone, glycosolated hemoglobin, body mass index, triglycerides, HDL cholesterol, total cholesterol, albumin, C-reactive protein, interleukin-6 (IL-6), fibrinogen, creatinine clearance, homocysteine, DHEA, urinary cortisol, urinary norepinephrine, and urinary epinephrine. High/low risk categories for these factors will be determined based on either clinical reference values or lower/upper 25th percentile values among healthy controls. An allostatic load index will be computed by CDC for each individual based on the sum of factors in which an individual’s measurements fall in the high-risk category.
B.2a. Hypothesis – CFS patients will be 2-times more likely to have a high allostatic load index compared to healthy and unwell controls. Our recent clinical study of CFS in Wichita (CDC IRB #3504) found that CFS patients were 2-times more likely to have a high allostatic load than were controls and the likelihood of being a CFS case increased with increasing allostatic load index. This finding must be confirmed in a different population. The original Georgia Survey did not obtain all of the laboratory parameters that comprise the allostatic load index. This follow-up study will obtain all the required laboratory parameters and includes sufficient participants to identify a significant difference.
B.2.b Hypothesis – Among persons with CFS, increasing allostatic load index will be associated with significantly greater levels of symptom severity and impaired functioning (measured by the Symptom Inventory and the SF-36) compared to well and unwell controls. In preliminary analysis of data from the Wichita clinical study, high allostatic load index was associated with high levels of self-reported bodily pain and symptom severity, and reduced physical functioning among CFS patients but not controls. We will assess the relationship of allostatic load index with scores on the SF-36 scales in separate analyses by study group to confirm whether high levels of allostatic load index are associated with greater disability/illness severity among CFS cases but not controls. Additionally, we will examine the relationship of allostatic load to scores for the MFI scales to determine if the larger sample size afforded by this study allows us to detect an association that was not detected in the Wichita analysis.
B.2.c. Hypothesis – High level of baseline allostatic load index among persons with CFS will be associated with risk of persistent or progressing illness and with functional impairment in the follow-up study. Other prospective studies have documented increasing risk of mortality, cognitive decline and cardiovascular disease associated with higher levels of allostatic load index at baseline. We will measure changes in symptoms and functioning at follow-up by administering the same instruments used to assess these clinical parameters at baseline (i.e., MFI, SF-36 and Symptom Inventory). We are particularly interested in whether allostatic load index predicts clinical outcome in the acute versus gradual onset cases and whether the illness contributes to the allostatic load index.
B. 2d. Hypothesis –Women with CFS will have been in menopause longer than age- and sex- matched Well controls and use of hormone replacement therapy will modify this association. The majority of CFS patients are women and a large proportion of them are in perimenopausal age. Our preliminary analysis of the Wichita clinical sample shows that women with CFS have been in menopause, on the average, for 4 years longer than their age-matched Well controls. They were also more likely to have had ovariectomy/total hysterectomy. Some of the symptoms of CFS indicate an inflammatory process (joint and muscle aches, tender lymph nodes, sore throat) and others overlap with symptoms observed in menopause (e.g., sleep problems, tiredness, memory and concentration problems, joint and muscle aches). There is well-documented evidence for an inverse relationship between female hormones (estrogen, in particular) and inflammatory cytokines: (a) increase in the proinflammatory cytokines IL-1 and TNF-alpha is observed after surgical menopause (Pacifici, 1991); (b) low estrogen levels are associated with high TNF-alpha and IL-12 [Wilder, 1999]; (c) low estradiol and high FSH (follicle stimulating hormone) have been found in women with fibromyalgia [Neek G, 2000] - a condition that has overlapping symptoms with CFS; (d) dramatic increase in IL-6 occurs with decrease of sex hormones with age [Dijsselbloem et al., 2004]; (e) some evidence suggests that estrogen/progesterone triphasic hormone replacement therapy increases the CD4+ calls (inducer/helper T cells) and the CD4+/CD8+ ratio [Dogan et al., 2005], i.e., improves immune response. In addition, experimental and clinical data show that female sex hormones have neuroprotective effects. To test our hypothesis we will use information from questionnaires (medical history, a gynecological history, medication use (for HRT)) and will test stored serum samples for sex hormone levels and for FSH. Gene expression data from stored samples will be used where appropriate.
B.3 Aim 3 - Economic Impact of CFS — Access to/Utilization of Health Care.
Allocation of public and private health care and of disability resources requires accurate information concerning the economic burden of disease. Development and evaluation of public health control and prevention programs demands accurate information concerning the costs and benefits of interventions and information concerning access to and utilization of health care by those who are ill. Congress has directed CDC to accelerate its CFS research plan to identify the economic impact of CFS and to accelerate its educational activities for health care providers. In addition to the first two Specific Aims (which identify risk factors and biomarkers to improve identification, clinical evaluation, diagnosis and management of CFS), this population-based cohort study evaluates direct and indirect costs of CFS, compares access to/utilization of health care by those who suffer from CFS with well and unwell controls, and evaluates their changes over time. Information concerning access to/utilization of health care will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop an intensive regional provider education project. Information concerning economic impact of CFS will be used to tailor an intensive regional provider education project, develop public health strategies, and evaluate cost effectiveness of different therapeutic modalities.
B.3.a. Hypothesis – CFS will be associated with a substantial economic burden on affected individuals, their families, and the metropolitan, urban, and rural populations of Georgia and the economic impact of CFS will be substantially greater than that of unwellness. The ability of CFS patients to carry out productive lives can be severely limited. Several, clinic-based, studies have found CFS patients to have substantial functional impairment compared with both healthy controls and other chronically ill patient groups [Buchwald et al., 1996; Hardt et al., 2001; Komaroff et al., 1996]. Indeed, clinical studies have reported patients with CFS to be more severely impaired than those with end-stage renal disease [Hart et al., 1987], heart disease [Bergner et al., 1984], and multiple sclerosis [Komaroff et al., 1996].
However, scant empirical scientific work exists to quantify the economic impact of CFS on society. Previous studies have addressed consequences of CFS in terms of disability (16%) and unemployment (21%) [Jason et al., 1999; Nisenbaum et al., 2003; Solomon et al., 2003]. Three clinic-based studies have shown that people with CFS were more likely than other chronically ill patients to have lost their job or to be unemployed [Lloyd & Pender, 1992; Bombardier & Buchwald, 1996; McCrone et al., 2003]. Persons with CFS also pose a disproportionate burden on the health care system and their families because they are sick for long periods of time and there is no known cure for the illness [McCrone et al., 2003].
In addition to direct costs (associated with health services and products, diagnosis, assessment, and management of CFS) persons with CFS incur indirect costs irrespective of their health care. Indirect costs are affiliated with the loss in productivity – that is, forgone income due to a decrease in hours worked or required job change. Medical and nonmedical costs are usually described as resources expended, and productivity losses are described as resources foregone. The individual will experience a lower standard of living due to foregone resources stemming from increased morbidity and mortality. Additionally, the government foregoes tax revenue as well due to lost (reduced) earnings. Our preliminary estimates [Reynolds et al., 2004] indicate that CFS accounts for $9.1 billion annually in lost productivity and this disproportionately affects women (90% of the burden).
This population-based cohort study measures economic impact associated with CFS over time and in relation to the clinical course. We are measuring direct costs, indirect costs to society as a whole (costs related to decreased levels of output, time spent to obtain health care) and lost productivity (change in employment status as a result of morbidity or mortality).
B.3.b. Hypothesis – Persons with CFS who consult and are treated by a health care provider will have significantly higher recovery rates than those who do not receive such attention. The median duration of illness among persons with CFS identified in community studies varied between 2.5 and 7.3 years [Reyes et al., 2003; Jason et al., 1999]. In spite of this, only half of those with CFS in Chicago were under medical care [Jason et al., 2000] and only 16% of persons identified with CFS in Wichita had received medical care for CFS [Solomon et al., 2004]. Similarly, a clinic-based study in Seattle found that individuals with chronic fatigue, CFS, and fibromyalgia each saw multiple medical physicians, but that those with CFS or fibromyalgia saw alternative providers, such as chiropractors, more frequently than did the other subjects [Bombardier and Buchwald, 1996]. These observations, in the context of the preceding discussion, document the need to determine whether illness characteristics and utilization of health services differ between CFS and other study groups, and by duration of illness and type of onset within the CFS group. We designed the Georgia survey to collect additional information concerning access to and utilization of health care by persons with CFS, other forms of unwellness, and well controls, particularly in terms of timing of health care intervention during the course of the illness. Preliminary analysis of data from the Baseline Survey indicates that 74% of CFS subjects reported seeking medical care from a doctor for their fatigue and 15% reported being diagnosed with CFS by a medical doctor. Clinical follow-up of subjects with CFS will allow us to examine the relationship between provision of health care and recovery. This information will be used in other studies involving physicians in metropolitan, urban and rural Georgia and in CDC physician education and public awareness campaigns.
B.4 Aim 4 – Incidence of CFS in Different Racial/Ethnic Populations of Metropolitan, Urban, and Rural Georgia
An understanding of the occurrence of incident CFS is fundamental to focusing etiologic research, targeting health-care and educational programs, and developing prevention strategies. This will be the first year in a study of 4 year CFS incidence. The objective is to estimate the incidence of CFS among those categorized as unwell and well in the Baseline Survey. As with other specific aims, this information will be incorporated into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722) and will also be used to develop an intensive regional provider education project.
Essentially all published studies of CFS (both clinic and population-based) involve prevalent cases and most persons with CFS identified in such studies have been ill from 3 to 8 years [Lloyd et al., 1990; Reyes et al., 1997; Jason et al., 1999; Reyes et al., 2003] and this introduces substantial survival bias. Only two published studies, one in Chicago [Jason et al., 1999] and the other in Wichita [Reyes et al., 2003] have used population-based random samples to estimate the prevalence of CFS and describe its demographic and socioeconomic characteristics. Both studies reported that racial/ethnic minorities (Hispanic and black, respectively) had a greater risk for CFS than did Caucasians. Preliminary data from the Georgia Survey indicate that the prevalence of CFS is highest in Hispanics when considering metro and urban areas, but higher in non-Hispanics in the rural area. In addition, the CFS prevalence is generally higher among Caucasians in the Georgia Study. Only one published study [Bierl et al., 2004] has evaluated urban versus rural occurrence of CFS and found rural populations to be at almost twice the risk for CFS as urban. This finding is also suggested, albeit to a lesser extent, by preliminary data from Baseline Survey, whereby the weighted prevalence of CFS in rural areas was 4.5%, which was similar to that in urban areas (4.4%) but higher than the prevalence in metropolitan areas (3.6%).
Unfortunately, as noted previously, the average duration of prevalent CFS is about 6 years. Thus, differences in prevalence between the populations must be interpreted in conjunction with data concerning incident cases in order to determine whether differences reflect risk factors for developing illness or factors associated with its clinical course.
This cohort follow-up will be the first of 4 studies of incident CFS in racial/ethnic populations of metropolitan, urban and rural Georgia. At the end of 4 years we will have identified at least 20 to 72 incident cases. Cohort analysis methods will be used to determine the hazard ratios as estimates of relative risk associated with risk factors. These methods optimize the power to detect statistical significance, and also account for subjects who are lost to follow-up.
B.4.a Hypothesis – this study will detect significantly more cases of incident CFS than previous population-based studies. The published incidence rate of CFS is 180 per 100,000, or .0018 [Reyes et al., 2003]. Using this number, we would expect to detect 10 cases of CFS within 1 year among the 5,630 persons who were interviewed in detail in the Baseline Survey. Preliminary analysis of data from the Baseline Survey is in line with this; we identified 8 subjects with incident CFS (duration of fatigue 1 year). However, we believe various modifications to the Georgia study will increase the probability of detecting incident cases. First, the Georgia study includes fatigued and non-fatigued unwell individuals of whom up to 10% are at risk of progressing to CFS [White et al., 2001]. Second, we modified screening and clinical evaluation in the Georgia study and believe this modification will result in considerably more cases. For example, preliminary analyses indicate we have detected almost 10 times more CFS cases than in Wichita.
B.4.b. Hypothesis – psychosocial factors (e.g., adverse life experiences, allostatic load index, personality, and coping mechanisms) will be associated with incident CFS. Specifically, we assume that persons who have experienced a triggering event within the past year have a higher likelihood of new onset CFS. This risk is enhanced by early-life adversity, increased allostatic load index, reactive personality and maladaptive coping styles. As discussed and cited above (Specific Aims 1 and 2), stress (including physical insults such as childhood disease) or emotional traumas and their sequelae, and increased allostatic load index have been considered as major risk factors and precipitating factors in the development of CFS. Few epidemiological or clinical studies have evaluated the association between severe stress or trauma and development of CFS or other fatiguing illnesses.
B.4.c Hypothesis– the incidence of CFS will differ between white and black populations. Preliminary data obtained from the Baseline Survey indicate that CFS prevalence is higher among Whites compared to Blacks, suggesting that the incidence will be higher among Whites as well. We will compare the incidence of CFS by race (Whites/Others vs. Blacks) among those identified as unwell or well in the Baseline Survey by following them at yearly intervals for 4 years.
B.4.d Hypothesis – the incidence of CFS will differ between metropolitan/urban and rural populations. Preliminary data obtained from the Baseline Survey indicate that CFS prevalence is highest in rural and urban areas compared to metropolitan areas. We will examine whether the incidence of CFS follows this pattern by measuring incident CFS among those who were classified as unwell and well in the Baseline Survey and followed on an annual basis for 4 years.
B.5 Aim 5 - General Clinical Research Center (GCRC) Studies
The GCRC studies will be performed at Emory University on persons with CFS and control subjects identified in the Georgia study. We have submitted 2 new protocols for these studies. Subjects in the Baseline Survey who were evaluated clinically have volunteered to be contacted for future studies.
Since stress exacerbates CFS in adults, a stress-diathesis model postulates that genetic liabilities interact with stressful experiences in determining individual vulnerability to disease, including CFS. Based on results from our clinical study in Wichita (CDC IRB #3504), we have developed a protocol to evaluate the effect of stress on production of CFS by performance of baseline biological and psychological measurements in control and CFS subjects at rest and in selected “challenge” models.
Based on results of previous studies with Emory University (CDC IRB #2964) we have found differences in cognition and brain function among persons who develop CFS-like illness following immune challenge. We have developed a protocol to use functional magnetic resonance imaging (fMRI) to explore brain function at rest and in cognitive function and self-recognition paradigms in persons identified in the Georgia study.
B.6 Aim 6 – Maintain anonymous (unlinked) storage for biological samples for validation of relevant findings and markers established in Aims 1 through 4.
Blood and urine specimens must be collected during the clinical evaluation phase of this study to perform standard clinical laboratory tests to identify exclusionary and comorbid medical conditions. These tests must be conducted immediately. However other laboratory tests (e.g., those for allostatic load index) are not time sensitive and we will store specimens until batches can be run together. After laboratory tests specific to this protocol are completed, remaining samples will be stored for future testing and validation studies, which will be described in new protocols that will be forwarded for appropriate approvals.
The CDC CFS Molecular Epidemiology Group has conducted research to identify biomarkers of CFS including: the identification of transcripts that are differentially expressed in the peripheral blood of people with CFS compared to healthy controls, the identification of genetic polymorphisms (SNPs) that may be associated with an increased risk for CFS, and the identification of serum proteins and hormones that may differentiate people with and without CFS in selected populations. All previously identified biomarkers must be validated. We will collect and store biologic samples from subjects participating in this longitudinal study to validate biomarkers identified from other CFS study samples. The types of biomarkers to be potentially assayed include: single nucleotide polymorphisms (SNPs), hormone levels, cytokine levels, steroid family receptor expression, and gene-expression levels.
We propose a four-year longitudinal study design of which this protocol addresses the first year of follow-up to provide follow-up needed to assure precision in ascertaining incident cases of CFS and evaluate biomarker change over time.
C. Research Design and Methods
C.1 Design
C.1.a Study population.
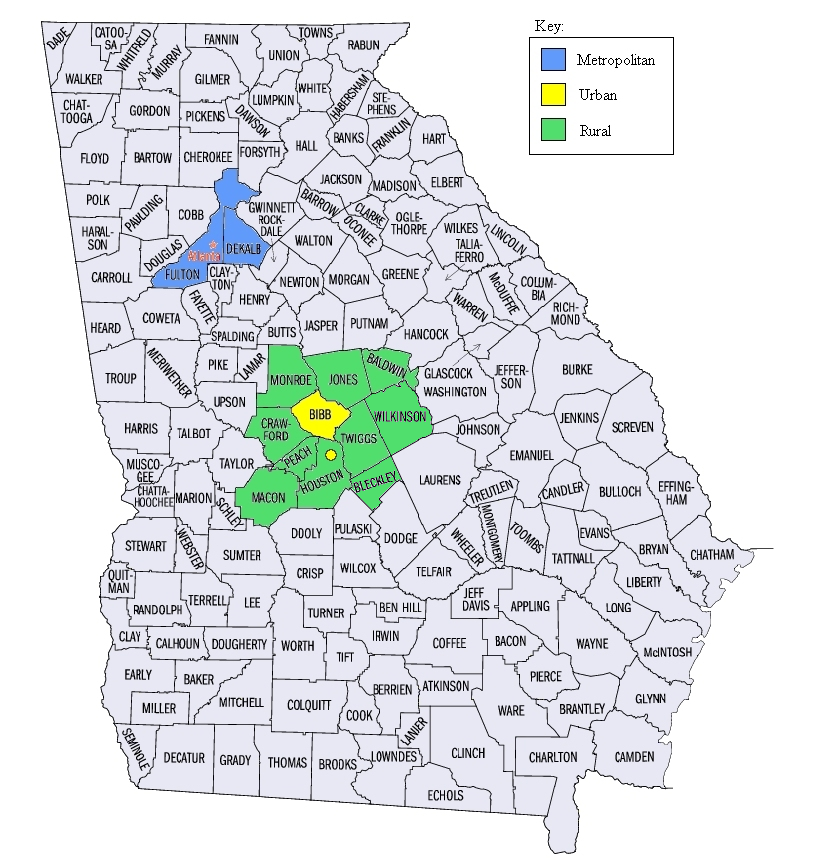 The
initial cross-sectional study conducted 2004 to 2005 used a
random-digit-dialing (RDD) survey to identify and enroll subjects
from metropolitan, urban, and rural Georgia populations. To identify
persons with CFS and a comparison group of unwell subjects with
similar symptoms, we enrolled Unwell
subjects. Unwell
subjects were
defined as reporting one month or longer duration of any of the
following CFS-defining symptoms (severe fatigue, unrefreshing sleep,
impaired memory or concentration, muscle or joint pain). Unwell
subjects were further evaluated to identify the subset with CFS.
Well
subjects, who did not report any of these CFS-defining symptoms for
one month or longer, composed the controls.
The
initial cross-sectional study conducted 2004 to 2005 used a
random-digit-dialing (RDD) survey to identify and enroll subjects
from metropolitan, urban, and rural Georgia populations. To identify
persons with CFS and a comparison group of unwell subjects with
similar symptoms, we enrolled Unwell
subjects. Unwell
subjects were
defined as reporting one month or longer duration of any of the
following CFS-defining symptoms (severe fatigue, unrefreshing sleep,
impaired memory or concentration, muscle or joint pain). Unwell
subjects were further evaluated to identify the subset with CFS.
Well
subjects, who did not report any of these CFS-defining symptoms for
one month or longer, composed the controls.
The metropolitan (Atlanta) population consists of the residents of DeKalb and Fulton counties. The urban population consists of residents of Bibb county (whose population is primarily in the city of Macon) and Warner Robins (in adjoining Houston County). The rural population includes residents of counties surrounding Bibb, specifically Houston (excluding Warner Robins), Monroe, Jones, Baldwin, Wilkinson, Twiggs, Peach, Crawford, Bleckley, and Macon counties. We limited the study to the Atlanta and Macon areas for logistic reasons (conducting clinical evaluations).
We used standard random digit dialing to sample the study population.
C.1.b Baseline Population Survey. The survey included four components:
A Screening Telephone Interview of a single household informant to explain the study and solicit participation, provide a household census (for deriving weights to be applied during analysis) and to identify Unwell household members, who had at least one of the core CFS-defining symptoms (fatigue, cognitive impairment, unrefreshing sleep or muscle/joint pain) for a month or longer, and Well residents (subjects that had none of these problems for at least a month).
Detailed Telephone Interview of 1) all respondents between 18 and 59 years of age identified in the screener as Unwell with fatigue; 2) a random sample of Unwell subjects who were not fatigued but had cognitive impairment, pain, or sleep problems and; 3) a random sample of Well residents. The objectives of the detailed telephone interview were to explain the study in detail, solicit participation, and:
determine classification as: 1) CFS-like (i.e., those who report fatigue characteristics and symptoms of CFS and report no exclusionary medical or psychiatric conditions) — these individuals require clinical evaluation to confirm the diagnosis of CFS; 2) Unwell-Fatigued but not reporting all characteristics of CFS; 3) Unwell-Not Fatigued; and 4) Well.
obtain information on residence, symptom characteristics, demographics, medical/psychiatric comorbidity, and economics.
screen for psychiatric comorbidity and life experiences.
Telephone numbers associated with prior refusals will be excluded from the Phase 2 Survey and no one who refused participation for the first phase will be contacted as part of the Follow-up Cohort Study.
Clinical Evaluation (one day) of:
all subjects classified, based on information from the Detailed Telephone Interview, as having CFS-like illness. Clinical evaluation is necessary to confirm classification as CFS.
comparison subjects for the purposes of identifying characteristics specific to CFS and to assess impairment and utilization of health services. These included a random sample of subjects who were Chronically Unwell (fatigued — not CFS-like and not fatigued), and a sample of Well subjects. The Well comparison group was matched to CFS-like subjects by geography (metropolitan/urban/rural), sex, age (within 3 years) and race/ethnicity. The Unwell comparison group (approximately equal in size to the CFS-like) was randomly selected from the same geographic area as CFS-like.
C.1.c Follow-up Cohort Study. The cohort follow-up study will involve three components.
A Detailed Telephone Interview of participants who completed detailed telephone interviews during the initial survey and who did not report any exclusionary medical or psychiatric conditions. All participants indicated their willingness to be recontacted for future studies. This includes all 438 identified as CFS-Like, the 1,665 Unwell (with or without fatigue) and the 1,484 who were Well. The detailed telephone interview will:
determine current status as CFS-like, Unwell and Well
obtain information on residence, symptom characteristics, demographics, medical/psychiatric exclusions and economics
screen for psychiatric comorbidity and life experiences
Clinical Evaluation (one day) of:
all 671 participants in the initial survey who underwent clinical evaluation and did not have a permanent exclusionary medical or psychiatric condition identified (164 classified as CFS, 366 unwell, and 141 well). Those who reported suicidal ideation during clinical evaluation in Phase 1 (reported as adverse events) will not be contacted for Phase 2. Clinical evaluation is necessary to identify incident cases, describe characteristics of the clinical course and obtain biological specimens for deposition in the biorepository.
all subjects identified during the Detailed Telephone Interview as having CFS-like illness. Clinical evaluation is necessary to confirm classification as CFS. Possible incident cases will be defined as those who were classified as well or unwell (not CFS) during the initial telephone survey.
new control subjects (as in the Baseline Survey) for comparison to incident CFS-like subjects to assess impairment and utilization of health services, and for derivation of an empiric case definition of CFS. This will be done as in the Baseline Survey and will include a random sample of Unwell telephone survey subjects equal in size to the number of incident CFS-like subjects in the same geographic area . It will also include a sample of Well telephone interview subjects matched 1:1 to incident CFS-like subjects by geography (metropolitan/urban/rural), sex, age (within 3 years) and race/ethnicity.
GCRC Studies. Finally, selected subjects participating in the Detailed Telephone Interview and those undergoing the Clinical Evaluations will be offered the opportunity to participate in future follow-up studies and in clinical research studies of fatiguing illness, which will be conducted at Emory University.
C.2 Methods
This section describes data collection from initial contact through GCRC studies. All subjects in this follow-up study participated in the Baseline Survey and gave permission to be recontacted for a follow-up. As noted, the majority of procedures, methods, and materials have been approved (CDC IRB #4121). Thus, only new or modified material is included in Phase 2 appendices. To facilitate communication throughout the study, we will provide material on the CDC CFS Internet website so subjects can learn more about the study, get answers to frequently asked questions, and ensure themselves of the study’s legitimacy we have included the web site material previously approved by the IRB (Appendix 1). If findings from the Baseline Survey indicate that changes are needed at the time the follow-up study is initiated we will submit these to the IRB for review. We will also provide local press releases discussing the study. Press releases were previously approved (Appendix 1), and specific press releases for this phase will be submitted to the IRB when ready.
C.2.a Advance and follow-up letters.
We will send advance letters to subjects prior to calling them for detailed telephone interviews. These letters will notify respondents of the survey, explain its purpose and sponsor, and alert respondents to expect a call from a telephone interviewer. (Appendix 2).
Potential respondents who are initially reluctant to cooperate may be sent follow-up letters, emphasizing the importance of the study and giving them a toll-free telephone number to call to schedule or complete an interview. We will use two of the conversion letters approved and used successfully in the Baseline Survey (Appendix 3). These have minor modifications indicating that the person took part in the Phase 1 study. The first letter will be sent to subjects reluctant to complete the Detailed Telephone Interview. The second letter will be sent to subjects we are having difficulty reaching to complete a Detailed Telephone Interview.
C.2.b Telephone interview overview.
As noted, all potential participants previously completed detailed telephone interviews and indicated their willingness to participate in this follow-up study. Professional interviewers trained to administer the detailed questionnaires will conduct all telephone interviews. All telephone interviews will be administered using computer-assisted telephone interviewing. The scripts that the telephone interviewers will use to contact respondents and conduct the interviews have been previously approved but some questions have been deleted and others added. Informed consent statements are included in the detailed telephone interview questionnaires and have been previously approved. Consent statements will be administered as soon as the respondent is on the telephone. Telephone interviewers will be prepared to discuss any aspect of the respondent’s participation at greater length, including respondent rights and the telephone numbers of staff who can be contacted for more or different information. The interview will not proceed until the informed consent statement has been read, exactly as written, to each respondent, and all of the respondent’s questions have been answered.
A waiver of documentation for the verbal consent for the detailed telephone interview questionnaire has been approved and we request its extension to this phase of the study for the following reasons. 1) These questionnaires involve no more than minimal risk to participants. The interview provides participants with a number to call (Dr. James F. Jones) if they think that they have been injured in this study. Dr. Jones (CDC Co-PI) has considerable clinical experience managing persons with CFS and other illnesses and is available at all times. He is backed up by Dr. Roumiana Boneva (CDC Co-investigator). Previous versions have been administered with verbal consent in previous CDC studies of CFS (the Longitudinal Study of CFS in Wichita – CDC IRB #1698) and the Pilot National Survey of CFS – CDC IRB #2936) and there have been no unexpected adverse events in these studies. In the Baseline Survey, there were two adverse events associated with telephone interviews in which participants reported thoughts of suicide. These adverse events were minimal considering that we completed screening interviews with 10,837 households and detailed interviews with 5,630 participants. 2) The waiver or alteration will not adversely affect the rights and welfare of the participants. The verbal consent explains the study, the reasons it is being conducted, the nature of the questions, discusses possible risk, and informs the participant that she/he can choose not to answer any question or terminate the interview at any time. The interview also informs participants that they can call the CDC Deputy Director for Science at a toll-free number if they have any questions about their rights in this study. Finally, participants are reminded at the beginning of the exclusionary medical conditions section that some questions are potentially sensitive , responses are completely voluntary, and that he/she is free to not answer any question. 3) Because of the nature of telephone surveys, it is not practical to obtain written informed consent. 4) Whenever appropriate, participants will be provided with additional pertinent information after participation. The interview closes informing participants that if they have any questions about this research study they can contact Dr. Jones.
C.2.c Detailed Telephone Interview.
The detailed telephone interview is included in Appendix 4 and has been slightly modified from that approved for the Baseline Survey. Specifically, we have added 7 standard questions on health perception (from CDC’s Behavioral risk Factor Surveillance Study and NHANES) (24-26). Based on results from the Baseline Survey, we eliminated 45 of the 69 questions concerning exclusionary medical conditions. Because all potential participants previously completed the 21-item life events scale, we replaced it with 4 questions targeting experiences over the last year (perceived stress scale). Finally, we have added 4 questions on socioeconomic status during childhood.
The detailed telephone interview has 5 objectives: 1) obtain information on symptoms of illness, psychosocial and environmental variables to compare the clinical course among those with CFS to that of persons with other forms of unwellness, stratified on psychosocial and environmental variables; 2) evaluate the incidence of CFS in different racial/ethnic groups representative of metropolitan, urban, and rural Georgia populations (in comparison with other forms of unwellness); 3) identify Unwell subjects who fulfill criteria of the 1994 CFS Research Case Definition (i.e., have CFS-Like illness) and recruit them for clinical evaluation to confirm whether they have CFS; 4) obtain data from Well and Unwell respondents for use in determining associations between life experiences and unwellness; and 5) obtain additional information needed for weighting during data analysis.
We will conduct detailed telephone interviews with all 1,251 fatigued Unwell subjects who were interviewed during the Baseline Survey (i.e., those at risk of developing CFS at follow-up), all 852 (originally randomly selected) not fatigued Unwell who completed baseline detailed telephone interviews, and the 1,484 (originally randomly selected) Well subjects who completed baseline detailed interviews.
The detailed telephone interview will:
Confirm the respondent is the individual who was interviewed at baseline.
Document current residence.
Document demographic characteristics (age, sex, race, and ethnicity).
Document status of fatigue and other symptoms by a modified version of the CDC Symptom Inventory used in previous population surveys of CFS conducted by CDC. This will record presence or absence of specific symptoms, and whether they have been present for at least 6 months.
Screen for medical conditions that exclude classification as CFS, by a modified version of the CDC screener used in the previous population surveys.
Screen for psychiatric conditions that exclude classification as CFS and comorbid psychiatric conditions and experiences thought to be associated with CFS.
We will screen for exclusionary psychiatric disorders by using the Structured Clinical Interview for DSM-IV (SCID) [First et al., 2002]. Exclusionary psychiatric conditions identified by this instrument include: bipolar disorder, psychotic disorders, eating disorders, and alcohol and substance abuse or dependence. During the clinic visit, melancholic (and other) subtypes of depression will be determined in a detailed psychiatric interview.
Screen for psychiatric comorbidity, chronic stress and major life events/trauma within the past year (i.e., triggers for incident CFS).
Because psychopathology is highly prevalent in patients with CFS and may be related to the clinical course and incidence of CFS, we will assess lifetime and current psychiatric disorders that are not exclusionary for CFS, such as depression. We will include a screening for mood and anxiety (e.g., PTSD) to determine associations between psychopathology and CFS and to identify persons to recontact for future clinical studies at Emory University. The screening questions were compiled from the SCID [First et al., 2002].
Because of the increasing evidence for a role of stress during development as a risk factor for CFS, and because of the evidence that symptoms of CFS are often exacerbated by acute life stress we included a detailed assessment of stress history in the initial study. This information does not need to be collected again. Rather, we will evaluate chronic stress. We are using the short version of the Perceived Stress Scale [Cohen et al., 1983], a 4-item scale designed to be used in telephone interviews to assess the degree to which situations in life experienced during the previous month were perceived as stressful. As discussed above, following the telephone interview, all 671 people who completed a clinical evaluation at baseline and did not have a permanent exclusionary medical or psychiatric conditions identified will be invited for a follow-up clinical evaluation. This includes 164 in whom CFS was diagnosed, 366 who were unwell but did not fulfill criteria for CFS, and 141 who were classified as well.
Using similar methods to the Baseline Survey, we use information from the detailed telephone interview to classify Well and Unwell as having or not having an exclusionary medical or psychiatric condition. Unwell participants in whom an exclusionary condition was not identified will be classified as CFS-Like if they fulfill criteria of the 1994 CFS research case definition. As in the Baseline Survey, all newly identified CFS-Like telephone interview respondents will be invited for a clinical evaluation. As in the original survey, we will randomly select an equal number of Unwell not CFS-like participants and invite them for a clinical evaluation and will randomly select Well subjects (matched 1:1 to CFS-Like subjects by geography, sex, age (within 3 years), race/ethnicity) to invite for clinical evaluation
At the conclusion of the detailed telephone interview, the telephone interviewer will read a script to subjects informing them that they may have been selected for the clinical evaluation component. Next, the telephone interviewer will be prompted to collect information to facilitate follow-up by clinics for scheduling purposes.
C.2.d Clinical Evaluation.
The objectives of the Clinical Evaluation are: 1) to classify subjects as CFS, Unwell not CFS, or Well; 2) identify clinical, psychosocial and environmental variables that characterize people with CFS and predict the clinical course of the illness; 3) identify biomarkers that characterize people with CFS and predict the clinical course of the illness; 4) evaluate the economic impact of CFS and access to/utilization of medical care of persons with CFS; 5) identify incident CFS cases; 6) identify persons with CFS, unwellness and a healthy comparison group to invite for future enrollment in GCRC studies at Emory University; and 7) obtain biologic samples for storage and later testing.
No one who refused to participate in the Phase 1 study will be contacted with reference to the clinical evaluation of Phase 2 and no one who indicated on telephone interview that they did not wish to be contacted again will be contacted with respect to the clinical evaluation or any other aspect of Phase 2 or future phases.
In summary, the following will be assessed during clinical evaluation: 1) physical and laboratory examinations for classification purposes; 2) saliva, urine and blood collection to screen for medical conditions, determine allostatic load index, and store for future testing; 3) questionnaires concerning symptomatology; 4)) psychiatric evaluation; 5) early and adult life experiences; 6) stress, coping and personality traits; 7) economic impact; and 8) health care utilization.
Each subject scheduled for Clinical Evaluation will receive a packet of materials prior to the appointment (Appendix 5). This will include a letter describing the study, for those reluctant to participate—a clinic refusal conversion letter, a sample clinic schedule, an informed consent document for review (8th grade reading level), and questionnaires to complete at home before arriving at clinic (included in other Appendices as detailed below). The packet will include 4 salivette devices and written instructions (Appendix 5) for the collection of saliva samples at home (discussed below). The packet will also include instructions (Appendix 5) and supplies for collecting an overnight urine sample (discussed below). Upon arrival at the clinic, the coordinator will explain the study, answer any questions, and confirm eligibility for clinical evaluation. A subject will be ineligible for clinical evaluation if his/her body mass index (BMI) is 40 or greater, if the subject is not between 18 and 59 years of age (if he/she did not complete a baseline clinical evaluation), or if the subject has been pregnant within the past 12 months. Also, if the registered nurse determines the subject is suffering an acute illness (based on basal temperature and observation) or uncontrolled high blood pressure, the subject will be temporarily deferred until he/she is no longer contagious or blood pressure is controlled. Finally, a matched Well subject may be ineligible if his/her matching criteria (such as age, race, or ethnicity) no longer match the CFS-like subject with whom he/she has been paired.
Clinic coordinators will administer the informed consent to eligible subjects. Descriptive materials sent to subjects prior to Clinical Evaluation are appended (research instruments are appended separately).
The planned clinic schedule is presented in a letter to all those invited to clinic (Appendix 5). Below we outline the clinic procedures. All new and modified instruments are appended. Some instruments will be administered using Computer-Assisted Self-Interviewing (CASI) on tablet personal computers (PCs). Subjects can use a stylus or keyboard to indicate their response to questions.
1. History, Physical Examination, Review of Medications and Laboratory Examinations
A past medical history, review of medications, physical examination, and screening laboratory tests are necessary to rule-out medical causes of illness and to classify a subject as CFS. The past review of medications, physical examination, and screening laboratory tests are as approved in the original CDC IRB protocol #4121 (the Baseline Survey clinical evaluation).
Study participants will complete the past medical and gynecologic history at home and on arrival at the clinic; a specifically trained interviewer will review it and clarify ambiguities (Appendix 6). Study participants will be asked to bring all current prescription and over the counter medications to clinic and these will be reviewed and recorded by a nurse. Each subject will undergo a complete physical examination by the local study physician.
Screening laboratory tests will be the same as approved in the original CDC IRB protocol #4121 (the Baseline Survey clinical evaluation). They include a complete blood count (CBC) with differential, C-reactive protein, alanine aminotransferase (ALT) (SGPT), albumin, alkaline phosphatase, asparatate aminotransferase (AST) (SGOT), total bilirubin, calcium, carbon dioxide, chloride, creatinine, glucose, lactate dehydrogenase (LDH), phosphorus, sodium, potassium, total protein, urea nitrogen (BUN), antinuclear antibodies, rheumatoid factor, TSH, FSH, free T4, and urinalysis.
Thirty ml of venous blood and a urine specimen will be collected between 8 and 9 am, after the subject has given informed consent. Subjects will have been instructed to be fasting for 10 hours before coming to clinic. These specimens will be used for screening laboratory tests, and for storage at CDC. The subject will be given breakfast following phlebotomy.
Subjects will collect a 12-hour overnight urine sample the morning of their scheduled clinic visit and this sample will be used to measure urinary cortisol, epinephrine and norepinephrine. This was not evaluated for approval in the original CDC IRB protocol #4121 (the Baseline Survey clinical evaluation). Subjects will be provided with instructions and collection equipment.
2. Assessment of Allostatic Load Index, HPA axis, and Biorepository Specimens
Allostatic load index. Collection of specimens to define allostatic load index and HPA axis activation was not evaluated for approval in the original CDC IRB protocol #4121 (the Baseline Survey clinical evaluation). To determine allostatic load index, we will measure waist and hip circumference, body mass index (BMI), serum homocysteine, C-reactive protein, fibrinogen, glycosolated hemoglobin (Hb), triglycerides, HDL and total cholesterol, DHEA, aldosterone, and 12-hour urinary specimens will be collected to measure creatinine, cortisol, epinephrine, and norepinephrine. The urine specimens will be collected during the night before coming to clinic in collection vessels supplied to the subjects (Appendix 5)
HPA axis. As described in the Background section, alterations of the HPA axis have been implicated in the pathophysiology of CFS. Specifically, decreased cortisol levels (relative to controls) have been postulated to play a causal role in CFS. As during the Baseline Survey, we will evaluate samples of salivary cortisol, which the patients collect at home before coming to clinic. Salivary cortisol assessment is a widely used method in psychobiology [see Kirschbaum & Hellhammer, 2000]. Salivary cortisol offers 2 major advantages to serum cortisol measures. First, sample collection is non-invasive and does not lead to HPA axis activation. Second, salivary cortisol concentrations represent the free fraction of circulating cortisol, which is not bound to CBG, and therefore reflect the biologically active fraction of cortisol. A number of recent clinical studies have provided evidence that there is a pronounced surge of cortisol secretion immediately after awakening. Measures of the cortisol response curve to awakening have been validated as a useful marker for HPA axis function in a variety of studies. This marker has also been shown to vary with clinical measures, such as chronic stress, pain and chronic health problems and CFS [Schulz et al., 1998; Pruessner et al., 1999; Kudielka & Kirschbaum, 2003]. It has also been shown that the cortisol response to awakening is highly correlated with cortisol responses to dynamic challenge of cortisol secretion with a low dose of ACTH1-24 [Schmidt-Reinwald et al., 1999].
The assessment of alpha-amylase in saliva is a new method for measuring autonomic activity in an ecological and non-invasive manner. Alpha-amylase has been repeatedly shown to be a sensitive marker for stress-related changes in the autonomic nervous system [Nater et al., 2005, 2006]. More specifically, alpha-amylase reflects sympathetic activation via alpha adrenergic mechanisms [Ehlert et al., 2005]. Alpha-amylase activity can be measured in the same samples as salivary cortisol.
Saliva collection was approved in the original CDC IRB protocol #4121 (the Baseline Survey clinical evaluation). As during the Baseline Survey, subjects will be instructed to collect saliva using Salivette devices (Sarstedt, Germany) at home on a regular workday within 3 days of their clinic visit. Saliva will be collected immediately after awakening (0 minutes) as well as 30, 45 and 60 minutes after awakening. The first sample will be taken while still lying in bed. Subjects will be instructed not to brush their teeth or consume coffee, food or cigarettes throughout the sampling period. Regular saliva sampling by the subjects is prone to measurement error due to a lack of compliance to take the sample at the exact time. Specimen collection compliance can be enhanced via electronic monitoring devices [Kudielka, Broderick, & Kirschbaum, 2003]. Electronic monitoring of saliva collection was not evaluated for approval in the original CDC IRB protocol #4121 (the Baseline Survey clinical evaluation). We will randomly evaluate compliance in 10% of participants by means of a compliance box (MEMS 6 TrackCap Monitor, Aardex Ltd., Switzerland). All other participants (90% of the total clinical sample) will receive a dummy compliance box, which will be handled the same way as the device with the electronic components. This electronic device records the time at which the container containing the cotton rolls was opened. Concurrently, the exact time when the salivette is used is noted on a prepared sheet. After chewing, the cotton rolls are placed in the plastic tube of the salivette. Subjects will store salivettes in the refrigerator and will bring the salivettes to clinic, where they will be stored at -20° C until assayed. Instructions that subjects will receive for saliva collection are appended with materials to be sent to them prior to the clinic appointment.
Specimen storage. We will collect blood for storage in an 8 ml CPT tube. These cells can then be used for methylation studies, DNA analyses, receptor analyses, and protein determination. We will also collect 5 ml of blood in a Tempest tubes (a collection system that requires no immediate processing and gives high quality RNA). This sample is a source of RNA for validation of gene expression experiments. Plasma will be harvested from the CPT tubes and serum from the routine lab clot tubes.
Finally, FTA blood cards will be collected (as in the Baseline Survey) to hold for genotyping studies.
For a summary of current blood, urine, and saliva collection and handling, see Appendix 7.
3. Questionnaires Concerning Symptomatology (Appendix 8)
Symptom Inventory. We will use the CDC Symptom Inventory [Wagner et al., 2005] to collect information on occurrence, frequency, and severity of symptoms common in CFS and other fatiguing illnesses. This symptom inventory was approved by the IRB for the Baseline Survey. This instrument takes 5 to 10 minutes to complete and is completed at the clinic.
Medical Outcomes Study 36-item short-form health survey (SF-36v2) [Ware & Sherborne, 1992]: a general indicator of health status (function and well-being) and includes primarily non-psychiatric health status questions. The SF-36v2 is a well-researched, reliable and valid broad-based, sophisticated instrument with population norms and normative data for a wide variety of medical conditions. The IRB approved use of this instrument in the Baseline Survey. The SF-36v2 assesses health-related quality of life in 8 areas: 1) limitations in physical activities because of health problems; 2) limitations in social activities because of physical or emotional problems; 3) limitations in usual role activities because of physical health problems; 4) bodily pain; 5) general mental health; 6) limitations in usual role activities because of emotional problems; 7) vitality (energy and fatigue); and 8) general health perceptions. This instrument takes 10 to 15 minutes to complete and is completed at the clinic using tablet computers.
Multidimensional Fatigue Inventory (MFI) [Smets et al., 1995]: a 20-item self-report instrument designed to measure fatigue, covering the dimensions General Fatigue, Physical Fatigue, Mental Fatigue, Reduced Motivation and Reduced Activity. The IRB approved use of this instrument in the Baseline Survey. This instrument takes 5 to 10 minutes to complete and is completed at the clinic using tablet computers.
Research Diagnostic Questions for Functional Gastrointestinal Disorders [Thompson et al., 1999]: This questionnaire was not used in the Baseline Survey; it was developed for the assessment of functional gastrointestinal symptoms in epidemiological surveys. It contains the Rome II criteria of more than 20 functional gastrointestinal disorders (FGD) in addition to other symptom-related items that can be used in clinical research. FGD are highly prevalent in the general population and share a substantial symptom overlap with CFS, resulting in high comorbidity rates. The questionnaire comprises a total of 66 items. To make a diagnosis, the questionnaire criteria must be fulfilled as indicated in the coding form. The Rome II criteria require that specific gastrointestinal symptoms must be present for at least 12 weeks (at least one day in that week) over the past year. This instrument takes 10 to 15 minutes to complete and is done at the clinic.
Illness Perception Questionnaire (revised version) (IPQ-R) [Moss-Morris et al., 2002]: This questionnaire was not used in the Baseline Survey. The IPQ-R is a recently developed and widely used quantitative measure of illness representations containing 70 items. The revised version stemmed from a need to deal with minor psychometric problems with two subscales, and to include additional subscales, assessing cyclical timeline perceptions, illness coherence, and emotional representations. Reliability analyses provided good evidence for both the internal reliability of the subscales and the short (3 week) and long term (6 month) retest reliability. The IPQ-R also demonstrated sound discriminant, known group and predictive validity. This instrument takes 10 to 20 minutes to complete. The IPQ-R will be only given to participants who are identified as CFS-like or unwell.
4. Psychiatric Evaluation
All subjects will undergo a general psychiatric evaluation administered by experienced licensed psychiatric social workers, clinical psychologists, psychiatric nurse practitioners, or certified research nurses with experience in psychiatric assessments. The objective of the psychiatric evaluation is to identify psychiatric conditions that would exclude diagnosis, identify psychiatric comorbidity, and measure psychologic characteristics postulated as associated with CFS. All of these instruments were approved by the IRB for clinical evaluation during the Baseline Survey. Some of the psychiatric evaluation interview forms administered to persons undergoing clinical evaluation during the Baseline Survey need not be repeated; they will only be administered to new participants in the clinical evaluation.
Structured Clinical Interview for DSM-IV (SCID) [First et al., 2002]: Administered to all clinic subjects. The SCID is a semistructured interview for making the major Axis I DSM-IV diagnoses. The SCID was approved by the IRB for use in the Baseline Survey. It includes an introductory overview followed by nine modules, seven of which represent the major axis I diagnostic classes: Screening module, mood episodes, psychotic symptoms, psychotic disorders, mood disorders, substance use disorders, anxiety disorders, somatoform disorders, eating disorders, and adjustment disorders. Because of its modular construction, it can be adapted for use in studies in which particular diagnoses are not of interest. Using a decision tree approach, the SCID guides the interviewer in testing diagnostic hypotheses as the interview is conducted. We will use the Research Version of the SCID for this study. The output of the SCID is a record of the presence or absence of each of the disorders being considered, for current episode (past month) and for lifetime occurrence. It requires about 30 – 120 minutes to obtain the psychiatric status of subjects.
Personality Diagnostic Questionnaire (PDQ-4) [Hyler et al., 1990]: Administered to new clinic patients only. The PDQ-4 was approved by the IRB for use in the Baseline Survey. The Personality Diagnostic Questionnaire-4th Edition (PDQ-4) is a 100 item, self-administered, true/false questionnaire that yields personality diagnoses consistent with the DSM-IV diagnostic criteria for the axis II disorders. It takes approximately 20 to 30 minutes to complete. It is widely used in clinical practice and in research projects throughout the US and has been translated in several different languages.
Because the SCID and the PDQ-4 provide only categorical diagnoses, we will administer additional standard rating scales to quantify core symptoms of interest. The following instruments will be given at the clinic during the time slot allotted for questionnaires:
NEO Five Factor Inventory (NEO-FFI) [Costa and McCrae, 1992]: Administered to new clinic patients only. The NEO-FFI was approved by the IRB for use in the Baseline Survey. The NEO-FFI is a widely used 60-item short form of the NEO Personality Inventory [Buckley et al., 1999; Fiedler et al., 2000; Taillefer et al., 2003]. The instrument assesses five major domains of personality: Neuroticism (N), Extroversion (E), Openness to Experience (O), Agreeableness (A), and Conscientiousness (C), each represented by six lower level facet scale scores. Reliability coefficients for the domain levels range from 0.86 to 0.95 and for the facet scale scores from 0.56 to 0.90. The questionnaire showed excellent construct, convergent, and divergent validity. Subjects will complete a computerized version of the NEO-FFI on tablet computers. The NEO-FFI takes 10 to 15 minutes to complete.
Self-Rating Depression Scale (SDS) [Zung, 1965]: Administered to all clinic patients. The SDS was approved by the IRB for use in the Baseline Survey. This self-report scale was designed to quantify the severity of current major depression in 20 items. Most guidelines suggested that index scores of less than 50 are within the normal range, while scores of 50 to 59 indicate minimal or mild depression, 60 to 69 indicate moderate depression and scores above 70 indicate severe depression. It requires about 10 to 15 minutes to obtain the psychiatric status of subjects. Subjects will complete the SDS on tablet computers.
Spielberger State-Trait Anxiety Inventory (STAI) [Spielberger, 1983]: Administered to all clinic patients. The STAI was approved by the IRB for use in the Baseline Survey. The self-report instrument was designed to assess levels of state anxiety and trait anxiety, through 40 items scored by a Likert-scale. State anxiety can be defined as a transient momentary emotional status that results from situational stress. Trait anxiety represents a predisposition to react with anxiety in stressful situations. The STAI requires approximately 10 to 15 minutes to complete.
Davidson Trauma Scale (DTS) [Davidson et al., 1997]: Administered to all clinic subjects who report a traumatic event during the SCID. The DTS was approved by the IRB for use in the Baseline Survey. The DTS was designed as a self-report scale measuring frequency and severity of PTSD symptoms in three clusters: intrusion, avoidance, and hyperarousal. For the 17 frequency and severity items, the Cronbach’s alpha was 0.99, for the frequency items alone it was 0.97 and for the severity items 0.98. The scale showed good convergent and discriminant validity. Davidson proposed a threshold of 40 for a total symptom score to predict the onset of a PTSD. The Davidson PTSD Scale requires approximately 5 to 10 minutes to complete.
5. Assessment of Early and Adult Life Experiences
The assessment of the stress history will focus on early and lifetime trauma experiences and their relationship to chronic fatigue. All were approved by the IRB for use in the Baseline Survey. Because of the increasing evidence for a role of stress during development as a risk factor for CFS, and because of the evidence that symptoms of CFS are often exacerbated by acute life stress, we will include a detailed assessment of stress history in the present study. The following instruments will be given at the clinic during the time slot allotted for questionnaires. Some questionnaires will be administered to returning clinic participants (who previously completed them) in order to evaluate change over time and others are administered only to new clinic participants because they need not be repeated by returning participants.
Childhood Trauma Questionnaire (CTQ-SF) [Bernstein et al., 2003]: Administered to new clinic patients only. The CTQ-SF was approved by the IRB for use in the Baseline Survey. This short-form of the Childhood Trauma Questionnaire was designed to assess retrospectively perceived childhood abuse and neglect. The 28-item self-report instrument consists of five clinical scales: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. The short form takes about 10 to 20 minutes to complete and will be completed using the CASI program on tablet computers.
Traumatic Life Events Questionnaire (TLEQ) [Kubany et al., 2000]: Administered to new clinic patients only. The TLEQ was approved by the IRB for use in the Baseline Survey. This self-report instrument assesses exposure to 21 types of potentially traumatic events that proceed gradually from stressors that are not highly personal (e.g., natural disasters, motor vehicle accidents) to events that are personally sensitive to many people (e.g., intimate partner abuse, sexual abuse). Subjects are asked to indicate the frequency of occurrence of each trauma and whether they experienced intense fear, helplessness or horror relating to the DSM-IV PTSD A2 criterion. The TLEQ requires approximately 10 to 15 minutes to complete.
Life Experiences Survey (LES) [Sarason et al., 1978]: Administered to all clinic patients. The LES was approved by the IRB for use in the Baseline Survey. This self-report instrument was developed to evaluate major life events in the past year. The first section of the questionnaire contains 47 items and three blank spaces and was designed for the use in the general population. The format of the LES asks subjects to rate separately the desirability and impact of events that they have experienced in the last 12 months divided into two semesters. The questionnaire provides a positive change score by summing the impact ratings of events designated as positive by the subject and a negative change score summing the negative ratings. By adding these two values, a total change score can be obtained, representing the total amount of change. The LES requires approximately 10 to 15 minutes to complete.
Parental Bonding Instrument (PBI-BC) [Klimidis et al., 1992]: Administered to new clinic subjects only. The PBI-BC was approved by the IRB for use in the Baseline Survey. This instrument was designed to assess fundamental parental styles as perceived by the child. The measure is to be completed for both mothers and fathers separately. The short form of the instrument consists of 8 items, including 4 items for the scale ‘care’ and 4 items for the scale ‘overprotection’. The PBI requires approximately 5 to 10 minutes to complete and will be completed on tablet computers.
6. Assessment of Stress and Coping (Appendix 9)
Complex psychological traits such as attribution styles, coping strategies and personality traits cannot be reliably assessed by telephone interview. We will therefore assess these traits in individuals that are invited to clinic. Except as noted, these instruments were approved by the IRB for use in the Baseline Survey clinical evaluation. The following published instruments with demonstrated reliability and validity will be administered at clinic. Again, subjects who have taken part in the first wave of this study have already completed some questionnaires and need not complete them again.
Trier Inventory for the Assessment of Chronic Stress (TICS-2-S) [Schlulz et al., 2004]: Administered to all clinic patients. The TICS-2-S was approved by the IRB for use in the Baseline Survey. This short version was directly derived from TICS-2 and comprises nine scales with 30 items: work overload, social overload, overextended at work, lack of social recognition, work discontent, social tension, performance pressure at work, performance pressure in social interactions, social isolation, and worry propensity. This instrument requires approximately 10 to 15 minutes to complete and will be completed on tablet computers using CASI.
Stress Reactivity Scale (SRS) [Schulz et al., 2005]: Administered to all clinic patients. Stress reactivity refers to the disposition of a person to respond to stressors with immediate, intense, and long lasting stress reactions. The SRS was not used in the Baseline Survey. The SRS measures general stress reactivity and stress reactivity in specific domains (social conflict, social evaluation, failure, work load). Two scales evaluate stress reactivity before and after stressful events in general. Validation of the SRS has been provided regarding the factorial validity and correlations with other construct-related personality traits, bodily complaints, sleep behavior, chronic diseases, and cortisol reactions to laboratory stress. This instrument contains 29 items and requires approximately 10 to 15 minutes to complete. Subjects will complete this questionnaire on tablet computers using the CASI program.
Illness Management Questionnaire (IMQ) [Ray et al., 1993]: Administered to unwell clinic patients only. The illness management questionnaire (IMQ) was designed to assess coping in CFS by the use of 45 items. The IMQ was not used in the Baseline Survey. The IMQ yields four factors: maintaining activity, accommodating to the illness, focusing on symptoms and information seeking. Scales based upon these factors together predicted 26, 27 and 22% of the variance in functional impairment, anxiety and depression, respectively (Ray et al., 1993). It is suggested that the IMQ may be employed to relate ways of coping to outcomes in CFS. This instrument requires approximately 10 to 15 minutes to complete and will be completed using the CASI program on tablet computers.
Ways of Coping Questionnaire (WCQ) [Folkman and Lazarus, 1985]: Administered to new clinic subjects only. The WCQ was approved by the IRB for use in the Baseline Survey. This instrument is a 66-item questionnaire containing a wide range of thoughts and acts that people use to deal with the internal and/or external demands of specific stressful encounters. There are four different factor solutions from three different authors. The WCQ requires approximately 15 to 25 minutes to complete and will be completed on tablet PCs using CASI.
Social Support Questionnaire (SSQ) [Sarason et al., 1983]: Administered to all clinic patients. The SSQ is designed to measure the number of social support sources available to individuals, as well as their satisfaction with available support by 27 items. The SSQ was not used in the Baseline Survey. Participants are asked (a) to list all of the people they can count on for support in different domains (e.g., Who do you know whom you can trust with information that could get you in trouble?) and their relationship with the individual, and (b) to rate on a 6-point scale how satisfied they are with each person’s support. Average satisfaction with spousal support can be calculated by summing the satisfaction scores for spouse support and dividing by the number of times the spouse is mentioned as a source of support. The SSQ was not included in the Baseline Survey and requires approximately 10 to 15 minutes to complete.
Ironson-Woods Spirituality/Religiousness Scale [Ironson et al., 2002]: This questionnaire was not administered in the Baseline Survey, and will be administered to all clinic patients in the follow-up study. The ISRS consists of 25 items grouped under 7 categories: (1) Comfort, strength, peace, (2) Feeling a connection, less alone, (3) Existential/afterlife, (4) View of God, (5) Somatic/illness recovery, (6) Religious behavior, (7) View of others, compassion for others. The items were developed from interviews with 60 medically ill patients, 20 with cancer, 20 with HIV and 20 with cardiac illness, who identified themselves as spiritual, religious or both (by Woods and Ironson in a separate study). The key purpose to development of this scale was to include items that were both pertinent to traditional religion and relevent for those who described themselves as spiritual only or as both religious and spiritual. Ironson has shown that longtime survival with AIDS was significantly associated with all 7 categories of the IRSRS, and that in HIV positive persons, the IRSRS score was associated with 24 hour cortisol output mediated by 'benefit finding' in the face of illness (Ironson G et al,. 2002).
The ISRS has been documented to have very good test-retest reliability (0.88) and the overall Cronbach alpha was .94 (with category alpha's ranging from .85 to .94). It was also tested against 3 other instruments that measured spirituality/religiousness and correlated highly (Hoge and Duke Religiosity Scales and the Use of Religion to Cope subscale from the COPE instrument (r = .66, .60, .70, respectively). This instrument will be administered using the CASI program on tablet computers, and should take no more than 5 minutes to complete.
7. Assessment of Economic Impact (Appendix 10)
Economic Impact will be assessed by collecting data from clinic participants using a questionnaire developed by the CFS research group at CDC and Abt Associates. The Economic Impact Questionnaire was approved by the IRB for use in the Baseline Survey. The questionnaire has been revised replacing the year 2003 with 2006, the year preceding this follow-up. This instrument will be included in the packet of materials to complete at home, and collects information regarding health insurance, money spent on medical care, salary, employment status, and other items to assess direct and indirect costs of illness. We expect that some of the items in the questionnaire might require some extra time and thought. For this reason, we have not estimated a required time for completion of this instrument.
8. Assessment of Health Services Utilization (Appendix 11)
Utilization of health services will be assessed by collecting data from all clinic participants using a questionnaire developed by the CFS research group at CDC. The Health Services Utilization Questionnaire was approved by the IRB for use in the Baseline Survey. This instrument will be included in the packet of materials to complete at home, and collects information regarding number of visits to a health care professional, type of health care professional seen, purpose of visit, and diagnosis received. This instrument takes 10 to 15 minutes to complete.
C.3 Work Plan
C.3.a Projected Duration of the Study.
Depending on approvals, data collection will begin in August 2007 and will be completed by May 2008. The total project, including lab analyses, data analyses and reporting of results should be completed by March 31, 2009.
C.3.b Staffing and Training Issues.
Abt Associates, the primary contractor for this study, will provide three general categories of staff for data collection: Telephone Interviewing, Clinical Evaluation, and Data Processing. In addition to the data collection training described below, all project staff are required to complete a thorough training on confidentiality and data security procedures. All staff must also sign assurances of confidentiality statements before they may work on this study. These procedures are in accordance with the Abt Associates Information Protection and System Security (IPASS) Plan, approved by CDC for this project and task.
Telephone Interviewing. Telephone interviewers must complete sixteen hours of training on study procedures, including instruction on correctly obtaining informed consent, administering survey questionnaires, and documenting all contacts with study subjects. Training also includes modules about interviewer roles in maintaining confidentiality and data security. All new interviewers must also complete a course in basic interviewing techniques prior to attending the study-specific training. Interviewers are monitored throughout data collection to ensure adherence to all aspects of the data collection protocol. Refresher training is provided whenever interviewers require it.
CDC staff work with Abt to prepare training agendas and materials.
Clinical Evaluation. Two clinics will be established—one in Atlanta the other in Macon. The clinic in Macon will serve a maximum of six subjects per day and the clinic in Atlanta will serve a maximum of four subjects per day. Clinic staff includes clinic managers, medical technologists, clinic coordinators, psychiatric interviewers and clinical examiners. Note that many of these staff will work part-time. In addition, scheduling coordinators work from their homes to schedule subjects for clinic appointments.
Abt Associates will recruit scheduling coordinators, clinic managers, and clinic coordinators from among their staff of experienced field managers and interviewers. Abt Associates will also be responsible for the hiring of appropriate medical technologists, registered nurses, psychiatric interviewers, and clinical examiners. Emory University and CDC will work with Abt Associates in coordinating standards for hiring.
Responsibilities for each staff type are described below.
Scheduling coordinators contact eligible subjects to schedule their clinic appointments. Scheduling coordinators will review with subjects the clinic packets (including informed consent, self-administered questionnaires, and various instructions) that were mailed to subjects prior to their clinic visits.
Clinic managers oversee and supervise day-to-day clinic operations. Duties include scheduling staff and subjects, ordering/stocking supplies, overseeing clinic operations, and ensuring quality assurance procedures are always followed in data and specimen collection. Clinic managers assist in operations whenever they are needed, such as witnessing physical examinations.
Clinic coordinators obtain informed consent, and assist in other clinic operations as assigned by clinic managers. They also review self-administered questionnaires while subjects are in the clinics and clarify incomplete answers or problem areas.
Medical technologists collect and process specimens, obtain data on medication usage, review past medical history for completeness and clarity, and witness physical examinations.
Psychiatric interviewers administer psychiatric interviews and tests. These interviewers will be licensed psychiatric social workers, clinical psychologists, psychiatric nurse practitioners, or certified research nurses with experience in psychiatric assessments. They will be trained to handle patients who are suicidal. Abt Associates will be responsible for training psychiatric interviewers, but Emory University and CDC will coordinate standards. Each interviewer is trained to (1) provide a standardized introduction to each assessment; (2) correctly conduct each assessment; and (3) correctly follow standard operating procedures, established by the Emory University Department of Psychiatry, for all assessments, tests, as well as for any adverse events.
Clinical examiners review recorded past medical history and medication data, interview the participants to collect targeted and more detailed medical history information in areas where the subject reported a positive medical history, and include these details as hand-written notes on the medical history form. The clinical examiners also conduct physical examinations. Clinical examiners will be licensed MDs, rather than Physician Assistants or Nurse Practitioners. Because the state of Georgia requires that physicians supervise PAs or NPs, it is more effective and efficient to employ physicians to conduct physical evaluations. MDs will be able to definitively deal with any adverse events that might occur during clinical evaluations such as a subject in need of immediate medical attention.
Registered nurses will be staffed during the hours when psychiatric interviewers and clinical examiners are not on the clinic premises. They will respond to any medical or psychiatric emergency.
CDC staff have worked with Abt Associates to produce similar training materials for previous studies and will assure appropriately updated versions are produced for this study. We will conduct a single training for all clinic staff to reduce bias and variation among data collectors and across sites. There will be an eight-hour training for clinic managers, scheduling coordinators, medical technologists, and clinical examiners; a 24-hour training for clinic coordinators; and a 36-hour training for psychiatric interviewers. Abt Associates project staff will coordinate training for all staff; Emory University and CDC will coordinate standards.
In the week after training, clinical staff will practice and then demonstrate their proficiency to Abt Associates staff. Candidates who pass these proficiency tests will be scheduled for a “dry run” two weeks after training, when they will have the opportunity to integrate the training modules and apply them in a dress-rehearsal of a “typical” clinic day. During the role-play, trainees will be expected to adhere to the protocols and conduct themselves as if the dress rehearsal were an actual clinical evaluation. The dress rehearsal benefits the staff in two important ways. First, it gives trainees an opportunity to apply their training in a controlled environment with subjects who have been hired to role-play typical study subjects. Second, carrying out the data collection protocols as designed will provide opportunities to discover any minor protocol flaws. Corrective action can be taken and information disseminated to staff before data collection begins.
Data Processing. Data preparation staff at Abt Associates receipt and prepare all paper questionnaires for data entry. These staff batch, pack and ship paper questionnaires to Abt Associates’ data entry vendor for entry. Data preparation staff must attend a 16-hour training on their tasks, including standard operating procedures for receipting data; rules for editing and preparing paper questionnaires for data entry; and maintaining data confidentiality and security.
C.3.c Data Handling.
This section describes the software and processes for preparing the data for analysis.
Data collected during the telephone interview is entered directly into a computer database as it is collected
Data obtained during the clinical assessment will be handled as follows:
Laboratory Specimens. Selected clinical laboratories will provide all laboratory results in specified electronic formats to CDC and Abt Associates.
Data Collected Using Paper-Pencil Forms. These data will be reviewed for completeness on the premises while the subject is still present; Abt staff will query subjects about missing data before they leave the clinic in order to assure completeness. Three times a week, the documents will be batch-shipped according to subject to Abt Associates’ corporate facility. All forms will be date stamped and receipted into the study’s Information Management System. Each page of each form will be clearly labeled with the subject's identification number. Data entry will be 100 percent verified to ensure its accuracy. Paper records (such as medical history and physical exam data) that have not been electronically entered by Abt Associates in the past will be photocopied and the originals will be sent to CDC on weekly basis.
Data Collected Using Computer Assisted Self-administered Interviewing (CASI). CDC will collect some questionnaire data via a CASI system. This mode of data collection allows the subject to work from a tablet computer in the clinic. Like CATI systems in telephone production centers, CASI reduces errors that can occur when completing a paper form. For instance, with a CASI system multiple answers for questions that require one answer are not possible. Routing errors are not possible because CASI can be programmed to obey skip instructions and follow the correct path through the questionnaire. Clinic staff will provide instructional support and, if necessary, the CASI system can be set up to so that the subject can have the questions and answer categories “read” aloud by the computer. Data collected from CASI questionnaires will be uploaded daily to Abt Associates.
C.3.d Information management and analysis software.
All systems and procedures used by Abt Associates to handle and store data for the First Follow-up Study of CFS are documented in detail in the Information Protection and Systems Security (IPASS) document submitted to the CDC’s Information Systems Security Officer. CDC has approved data handling and systems security procedures for the telephone survey and clinical evaluation components of the First Follow-up Study of CFS.
The information and management software used by Abt Associates to conduct and track the data collection and perform data cleaning for the First Follow-up Study of CFS are listed below.
Bellview CATI, commercial, off-the-shelf software, used to conduct telephone interviews and manage telephone survey data component of the First Follow-up Study of CFS. Bellview is a product of PulseTrain, Limited.
Sample Management System (using VisualStudio.net _ written in C#), that tracks status of sample members who are eligible for clinical evaluation. This system also tracks the status of paper and electronic instruments.
CASI questionnaires programmed in C# will be used at the clinic to capture questionnaire data.
Proprietary software, developed by Abt Associates’ data entry vendor, DataShop, which will be used to key enter all paper instruments and will provide 100% verification of keyed items.
C.4 Data entry, editing, management, storage, and disposition.
As noted above, CDC’s Information Systems Security Officer has approved the systems and procedures that will be used by Abt Associates to enter, edit, transmit, and store data for the First Follow-up Study of CFS.
Each subject will be assigned a unique subject identification number. The contractor, Abt Associates, will maintain the electronic files that link the identification number to subject identifiers (name, address, and telephone) and store them in a double locked box for security purposes; CDC will not have access to these records. The identification number will be affixed to all paper questionnaires and will be used to store and retrieve electronic data associated with the subject.
Data collected during the computer-assisted telephone interviews are entered directly into a Bellview computer database under the subject’s identification number. Data are stored in real time as they are collected. Skip patterns and alternative versions of questions are programmed into the interview system so that the interviewer sees only those questions appropriate for the current respondent. The system has been programmed with range and logic checks that alert interviewers to out-of-range or inconsistent responses so that they can correct them while subjects are still on the telephone.
The survey data will be housed in a central file that has a documented Bellview proprietary file format. Files are extracted, using a Bellview extraction program, into ASCII files. Any identifying information (name, address, telephone) will not be exported to CDC. After the ASCII data are reviewed, they are delivered to CDC as SAS data files. After final data delivery, Abt Associates will electronically archive the telephone interview data, including subject identifiers. All data are stored on the local area network (LAN) in access-restricted directories. Only staff authorized by the Abt Associates Project Director may access the data.
Data obtained during the clinical assessment will be identified only by each subject’s identification number. Data handling and security are described below.
Laboratory Specimens. Selected clinical laboratories will provide all laboratory results on paper and in specified electronic formats to CDC and Abt Associates.
Data Collected Using Paper-Pencil Forms. Forms that are completed at home or at clinic will be labeled with the subject’s identification number. Each document will be edited for completeness and clarity at clinic before the subject leaves. This edit will identify most problems (such as missing answers and unclear or illegible responses) so that a coordinator can ask the subject to resolve the problem, while the subject is on the premises and can review the form(s). Three times per week, paper instruments will be batched by subject and shipped overnight to the contractor’s office for data entry. Upon receipt, each page of each document will be stamped with the subject’s identification number. All forms will be receipted into the receipt control component of the study’s Sample Management System (SMS). When not in use, they will be secured in locked cabinets within the contractor’s office.
Abt Associates staff will receipt, edit, and prepare completed questionnaires for data entry. Each week, edited clinical evaluation questionnaires will be batched and sent to Abt Associates’ data entry vendor. Data entry will be 100-percent verified to ensure accuracy. The status of each questionnaire will be updated in the SMS to indicate when the questionnaire was shipped to the data entry subcontractor and when it is returned. Abt Associates will insure that all raw data collected from these questionnaires are transmitted to CDC as delivered data.
Computer-assisted self-administered (CASI) questionnaires will be used to collect and store questionnaire data under the subject’s identification number. Like CATI, data are stored in real time as there are collected. Skip patterns are enforced so that the subject stays on the correct path through the questionnaire. Subjects can change answers as necessary and the corrected path is rechecked and followed. CASI prevents multiple responses to questions that allow only one response. Daily, these data will be uploaded to a secured FTP site. Abt Associates staff will download the data and review it for completeness before sending files to CDC. Abt Associates will insure that all raw data is included in the files sent to CDC.
All key-entered data will be examined, mainly by review of data frequencies, and cleaned as necessary. The amount of data cleaning needed for the telephone interviews and CASI questionnaires will be minimal because these data collection programs contain internal checks so that data problems can be resolved during actual interviews. Nevertheless, if an interviewer reports a key entry error that he/she could not correct, the data will be corrected during this stage of processing.
During the data cleaning process, only three versions of the data files are maintained on Abt Associates’ systems. The first is an archived source file, which preserves the data in its original state and is not used during the cleaning process. The second is a copy of the source file, which is used as the starting point for data cleaning (the “data cleaning copy”). The third is the most current, cleaned, version of the data (the “cleaned copy”), which is created from the data cleaning copy. This last file becomes the final data file at the conclusion of the cleaning process. All changes to the data cleaning copy, to create the cleaned copy, are made by computer programs (the “cleaning programs”). All differences between the data cleaning copy and the cleaned copy are documented in the cleaning programs and cleaning specification logs, so the history of any value of any variable can be traced from the collected data to the final, cleaned data. The data files, programs, and cleaning logs are date- and time-stamped by Windows 2000 and NT facilities.
Survey data are maintained at Abt Associates until the electronic data are verified and there is no longer need for reference to hard-copy documents (approximately six months after the end of data collection). At that time, the data will be moved to Abt Associates’ off-site storage facility. At the conclusion of the study, the survey data will be destroyed in accordance with the terms of the contract between CDC and Abt Associates.
C.5 Quality control/assurance.
Telephone interviews. Use of computer-assisted telephone interviewing greatly reduces opportunities for error and increases data quality because the system performs range and logic checks and ensures that questionnaire skip patterns are correctly followed. On every shift, telephone interviews are monitored by supervisory staff. Each Abt Associates’ Telephone Center is equipped with a monitoring system and separate monitoring rooms that allow for unobtrusive monitoring of interviewers. Interviewers do not know when they will be monitored. All interviewers are monitored, and monitoring occurs during every interviewing shift. The monitoring supervisor listens to the telephone interview while observing the interviewer’s data entry screen on a computer monitor that mirrors the interviewer’s screen. After the monitoring session, supervisors give active coaching and immediate feedback, both positive and negative, on interviewer performance so that successful interviewing performance can be rewarded and reinforced, while interviewing problems are immediately identified and corrected. In addition, floor supervisors circulate through each interview room to ensure that interviewers are focused on their tasks; to answer questions; and to handle issues that may arise.
The monitoring system is also used to evaluate performance patterns across interviewers to identify any problem areas or items in the questionnaire. Specific problems are addressed in retraining for interviewing staff. In addition, data from the monitoring and systems are reviewed each day to identify anomalies, inconsistencies, or inappropriate patterns across interviewers and for individual interviewers. All such issues are reviewed and resolved.
As an additional quality control measure, project staff review questionnaire item frequencies throughout data collection to correct errors or other anomalies in the data.
Clinical evaluations. At training, each clinical staff person is trained on and receives copies of standard operating procedures (SOPs) for each questionnaire or type of assessment. It is the responsibility of the clinic manager to see that all SOPs are closely followed by each staff person on each day of data collection. In addition, during clinic data collection, CDC, and Abt Associates project staff visit the clinics to observe data collection activities, including SCID administration, physical examinations, and documentation of clinical events. Observers note any deviations from the protocol and retrain staff. Staff who cannot or will not follow SOPs are removed from the project.
One assurance of quality of the physical examination data is hiring qualified medical doctors and technologists to complete these assessments. An accredited commercial laboratory will analyze blood, urine, and saliva specimens using industry-standard quality control procedures.
Quality checks are built into clinic operations. Clinic coordinators scan-edit completed questionnaires while subjects are still on the premises. Clinic coordinators check for missed items and incomplete answers and meet with individual subjects so that errors may be corrected and missing items completed. Psychiatric interviewers will be frequently assessed by CDC and to ensure proper administration of the SCID.
Paper questionnaire data are 100 percent verified by two data entry operators. Any discrepancies in the data are adjudicated and corrected. Project staff also review frequencies for each item in each instrument to make sure skip patterns have been followed, to remove extraneous data, and to assign missing values when data are incomplete or missing.
CASI questionnaires have built-in edit checks and provide feedback to the subject with respect to the need to complete incomplete items before submitting the file. When the subject attempts to submit an incomplete questionnaire, the system automatically provides the unanswered question and prompts the subject to complete the item by answering the question. Only when completed questionnaires are successfully submitted to the computer system does the system provide the next questionnaire to be completed. Once all CASI questionnaires are completed, Abt Associates staff will review their completion status, as recorded by the system, before closing the computer session.
Bias in data collection, measurement and analysis. Every effort will be made to eliminate potential sources of bias in the data gathered during the First Follow-up Study of CFS. This section addresses the most common forms of bias in data collection, measurement, and analysis and the steps CDC and its contractor will take to reduce them. This discussion focuses on sampling bias, interviewer bias, sampling weights, and blinding.
Sampling bias may arise from completing interviews or clinical evaluations with a sample of subjects who are not representative of the population. Sampling bias has two main causes: 1) deliberate exclusion of a subgroup of the population being studied and 2) nonresponse. The effects of sampling bias can be reduced by careful implementation of the sample design, by minimizing unit (in this situation, subject) nonresponse during telephone data collection and clinical evaluation, and by adjusting for unit nonresponse.
The First Follow-up Survey requires sampling in selecting, for a clinical evaluation, the Unwell subjects for comparison with the subjects who have been newly identified as having CFS-like illness (Section C.1.c). This sample will be selected randomly (i.e., without bias) within each of the three geographic strata. In addition, a random selection may be necessary when two or more Well subjects satisfy the criteria for 1:1 matching with a CFS-like subject (and thus being invited to have a Clinical Evaluation). Thus, the potential for sampling bias will arise mainly from nonrandom nonresponse, either on the follow-up Detailed Telephone Interview or on the Clinical Evaluation.
Low response rates in either the telephone or the clinical evaluation data collection can introduce bias if certain subsets of subjects systematically fail to complete the interview and evaluation process. As in the Baseline Survey, CDC will minimize subject nonresponse by utilizing a data collection contractor experienced in telephone surveys and field data collection. The contractor, Abt Associates, will employ the following tactics to minimize unit nonresponse:
An automated telephone call-scheduling system whose algorithms:
Minimize respondent burden by carefully structuring answering machine messages.
Route refusals to specially trained interviewers, who specialize in gaining the cooperation of reluctant subjects.
Deliver, on time, appointments and callbacks to interviewers.
Advance letters that inform the subject about the study before the first dialing attempt is made.
A toll-free number for subjects to call when they are ready to be interviewed.
Trained and practiced general telephone and field interviewers with experience in:
Gaining cooperation (addressing subject concerns and questions, averting refusals, using good voice skills to keep the subject engaged).
Navigating through complex questionnaires.
Locating subjects.
Refusal conversion. The term "refusal conversion" describes a process, defined by standard survey research criteria, in which an individual is contacted a second time concerning participation in a study that s/he initially was reluctant to consider. The intent is to ensure that the potential subject has enough information to make an informed decision and choice about participation. A sizable proportion of persons who are initially reluctant to participate will choose to participate once the study is explained in more detail. (The number of such subjects is likely to be small in the First Follow-up Survey, because we will be recontacting subjects who completed the Detailed Telephone Interview in the Baseline Survey and indicated their willingness to be recontacted for future studies.) Refusal conversion is an effective and non-coercive tool when applied by a professional. To avoid possible coercion and ensure that initially reluctant persons understand the reasons they have been asked to participate, the process is carefully managed and implemented. A telephone center (or field) supervisor assesses each initial refusal to identify those individuals defined as eligible for refusal conversion. For example, individuals who say they are currently too busy or those persons who immediately hang up would be eligible for refusal conversion. If a subject was hostile or asked to be removed from the call list, his or her refusal status is immediately finalized, and no further contacts are made. After this initial assessment, those individuals appropriate for recontact are assigned to refusal conversion specialists. These specialists are very well-trained and experienced interviewers with highly developed listening and response skills. The conversion specialist contacts the individual and explains the reason for the call. If the subject again declines participation, the conversion process is immediately terminated. The Record of Calls for each refusal is reviewed, by a supervisor, prior to each calling attempt. Clearly established rules (for example, a subject refuses twice) dictate when calling is immediately discontinued. Also, any unusual problems during a call (such as a subject noting that the call is coercive) are reviewed and documented.
Medical staff trained on study objectives and their specific duties.
Managers who track the sample by region, race, and ethnicity and who shift resources, as necessary, to achieve comparable response rates.
Interviewer bias will be minimized with training that includes practice in gaining subject cooperation, nondirective probing, probing for clarity and completeness, proper pacing, and reading questions verbatim. Also, telephone interviewers are unobtrusively monitored by supervisors, who provide feedback. As necessary, remedial training will be provided to help deficient interviewers improve their ability to complete their tasks and minimize refusals. CDC, and Abt Associates staff will monitor clinic staff periodically. As with telephone interviewers, feedback and retraining will be provided.
If unit nonresponse occurs at random, it is not a source of bias; it simply reduces the size of the final sample. In adjusting the sampling weights for various forms of unit nonresponse (e.g., subjects not interviewed), CDC will take into account the possibility that overall, nonresponse is non-random. Applying a standard approach, we will separate the subjects into disjoint subsets defined by characteristics that may be related to response (and to fatiguing illnesses): for example, sex, age group, race/ethnicity, fatigue status, and geographic stratum. Within each subset the respondents and the non-respondents are substantially more likely to be comparable (than in the overall sample), so it will be reasonable to treat nonresponse as random.
The sampling weights resulting from the First Follow-up Survey will be based on the sampling weights from the Baseline Survey, which provide the link between the subjects and the population. The interview weights from the Baseline Survey reflect the probability of selecting the telephone number of the subject’s household, an adjustment for unit nonresponse on the Screening Telephone Interview, an adjustment for households without telephones, the probability of selecting the subject for the baseline Detailed Telephone Interview (for subjects who were identified as Unwell but not fatigued and subjects who were identified as Well), an adjustment for unit nonresponse on the Detailed Telephone Interview, and an adjustment to population control totals from the 2000 Census. The various adjustments aimed to reduce or remove forms of bias. In addition, subjects who completed a baseline Clinical Evaluation received a clinical-evaluation weight, which reflected the probability of being selected for a Clinical Evaluation (for Chronically Unwell subjects) and incorporated an adjustment for unit nonresponse on the Clinical Evaluation.
To reduce bias during data analysis, data users will be directed to apply sampling weights that incorporate adjustments for unit nonresponse.
CDC plans to use blinding procedures for the subject’s fatigue status (CFS-like; chronic unwell, not CFS-like; well) on a need-to-know basis. Blinding is not an issue for telephone data collection, because fatigue status is determined at the conclusion of the Detailed Interview. Abt Associates staff (scheduling coordinators and sample management staff) will know fatigue status to match subjects and coordinate clinical appointments. However, the medical staff (who collect the specimens, record medication and medical histories, and conduct the physical examinations) and psychiatric interviewers will not know the subject’s sample type.
C.6 Statistical Analyses
The Baseline Survey sample was obtained using random digit dialing within geographic strata that included a metropolitan, urban and rural area of Georgia. For the clinical evaluation portion of the study, each CFS-like subject was matched to a Well subject with regard to geography, age (within 3 years), sex and race/ethnicity. A random sample of subjects with Chronic Unwellness were also selected to participate. Weights have been calculated to reflect the probability of the subject’s participation at each stage of the study (screening telephone interview, detailed telephone interview, clinical evaluation). We plan to conduct both weighted and unweighted analyses of risk factors associated with CFS using logistic regression analysis to generate odds ratios (ORs) as estimates of risk, and 95% confidence intervals (CI) as estimates of the precision of the ORs. In all unweighted analyses we will control for all matching factors. If the ORs calculated from these two approaches do not differ by less than 20%, we will assume that there was no significant bias introduced by our sampling strategy and will report the unweighted ORs. If the ORs differ by 20% or more, we will report the results of the weighted analysis. All analyses will use two-tailed tests, and an alpha of 0.05 as criteria for statistical significance.
C.6.a Analysis addressing specific aim 1: Identify clinical, psychosocial and environmental variables that characterize people with CFS and predict the clinical course of the illness.
To examine whether persons with shorter duration illness/sudden onset CFS have higher recovery rates than those with longer duration /gradual onset CFS, we plan to conduct a ‘case-control’ analysis. Cases will be defined as CFS subjects with short duration (< 5 years) of illness, and will be compared to CFS subjects with long duration (> 5 years) of illness (controls) with respect to recovery. Recovery is a dichotomous outcome (yes/no), defined as an improvement in physical functioning, fatigue, or symptom frequency/severity during the period from baseline to follow-up, which results in re-categorization of the subject into either the insufficient symptoms/fatigue (ISF) or nonfatigued (NF) group. At baseline all CFS subjects scored below the cut-off on the 3 instruments that measured these recovery outcomes. For a case to be considered as recovered, their score on at least one of these 3 instruments measured at follow-up must be above the cut-off value. Based on published data (Nisenbaum et al., 2003), we expect 40% of CFS subjects to recover in one year. Given that we have identified 47 CFS subjects with short duration of illness and 47 CFS subjects with long duration of illness in the Baseline Survey, and expect to recall 80% of those subjects, we will have a sample size of 38 in each group. This sample size provides us with 80% power to detect a 2.3-fold difference in recovery between these two groups, assuming a 24% recovery rate among CFS subjects with long duration of symptoms. Logistic regression will be used to conduct this analysis, adjusting for age, sex, race in an unweighted analysis, and including fixed weights in a weighted analysis.
C.6.a Analysis addressing specific aim 1: Identify clinical, psychosocial and environmental variables that characterize people with CFS and predict the clinical course of the illness.
In order to examine whether persons with shorter duration illness/sudden onset CFS have higher recovery rates than those with longer duration /gradual onset CFS, we plan to conduct a ‘case-control’ analysis. Cases will be defined as CFS patients with short duration (< 5 years) of illness, and will be compared to CFS patients with long duration (> 5 years) of illness (controls) with respect to recovery. Recovery is a dichotomous outcome (yes/no), defined as an improvement in physical functioning, or fatigue, or symptom frequency/severity during the period from baseline to follow-up, which results in re-categorization of the subject into either the ISF or NF group. At baseline all CFS patients scored below the cut-off on the 3 instruments that measured these outcomes. In order for a case to be considered as recovered, their score on at least one of these 3 instruments measured at follow-up must be above the cut-off value. Based on published data (Nisenbaum R, 2003), we expect 40% of CFS patients to recover in one year’s time. Given that we have identified 47 CFS patients with short duration of illness and 47 CFS patients with long duration of illness in our baseline study, and expect to recall 80% of those subjects, we will have a sample size of 38 in each group. This sample size provides us with 80% power to detect a 2.3-fold difference in recovery between these two groups, assuming a 24% recovery rate among CFS patients with long duration of symptoms. Logistic regression will be used to conduct this analysis, adjusting for age, sex, race in an unweighted analysis, and including fixed weights in a weighted analysis.
C.6.b. Analysis addressing specific aim 2: Identify biomarkers that characterize people with CFS and predict the clinical course of the illness.
C.6.b.1. To examine whether CFS is associated with a high level of allostatic load, we plan to conduct a cross-sectional analysis using case-control methods to compare the relative prevalence of ‘high’ allostatic load between CFS cases and well controls enrolled in this follow-up study. High allostatic load is defined as an allostatic load index above the median value for well controls. Allostatic load index is a summary score determined for each subject based on 12 metabolic/cardiovascular/HPA-axis factors, as described in Section C.2.d.2. Assuming that we will be able to successfully recall 80% of the 94 CFS patients and 115 well controls identified in the Baseline Survey, we will have a sample size of 76 cases and 88 controls. This sample size provides us with 96% power to detect a 2-fold difference in risk of high allostatic load between cases and controls, assuming the prevalence of high allostatic load is 30% in controls (based on unpublished data). Logistic regression will be used to conduct this analysis, adjusting for age, sex, race in an unweighted analysis, and including fixed weights in a weighted analysis.
C.6.b.2. To determine whether allostatic load is associated with symptom severity and impaired functioning in our study population, and whether that association varies by case group, we will conduct separate cross-sectional analyses of the associations between allostatic load and symptom severity/impaired functioning among the CFS case group, the ISF group, and the nonfatigued controls. Allostatic load index will be determined at follow-up based on measurements of the 12 metabolic/cardiovascular/HPA-axis factors described in Section C.2.d.2, and dichotomized into high/low categories based on the median allostatic load index among controls. Symptom severity will be measured based on the summary symptom inventory (SI) score for the 8 CFS-related symptoms and the scales of the multi-dimensional fatigue inventory (MFI). Impaired functioning will be based on the scales of the SF-36. The SI and each of the scales of the MFI and SF-36 instruments will be scored on a scale of 0 – 100. We will compare the median scores for each of these scales by high/low levels of allostatic load using the median test in separate analyses conducted among the three study groups. Assuming an 80% recall rate of Baseline Survey subjects, we expect to have 76 CFS subjects, 196 ISF subjects and 88 non-fatigued controls. To estimate power for this comparison, we assume the following, based on unpublished data: 58% of CFS subjects have high levels of allostatic load; the mean score for the bodily pain scale of the SF-36 is 36 in those with high allostatic load and 49 in those with low allostatic load. We have 99% power to detect this difference in mean bodily pain scores as statistically significant, given our sample size.
C.6.b.3. To evaluate whether a high level of allostatic load determined at baseline predicts progression of CFS illness approximately one year later, we will conduct a ‘case-control’ analysis. Cases will be defined as CFS subjects with high levels of allostatic load (greater than the median value in non-fatigued controls), and will be compared to CFS subjects with low levels of allostatic load with respect to recovery. Recovery will be treated as a dichotomous outcome (yes/no), defined as an improvement in physical functioning, fatigue, or symptom frequency/severity during the period from baseline to follow-up, which results in re-categorization of the subject into either the ISF or NF group. At baseline all CFS subjects scored below the cut-off on the 3 instruments that measured these outcomes. For a case to be considered as recovered, their score on at least one of these 3 instruments measured at follow-up must be above the cut-off value. Given that we have identified 94 CFS subjects in the Baseline Survey, and expect that 80% of them will be recalled in the follow-up study, our study size for this analysis will include 76 CFS subjects. Based on a prior analysis of allostatic load and CFS, we expect that 58% of CFS subjects will be categorized as having high allostatic load (n = 44) and the remaining 32 will be categorized as having low allostatic load.
This sample size provides us with 76% power to detect a 2-fold difference in recovery between these two groups of CFS patients. Assuming 25% recovery occurs in CFS patients with low alloastatic load. Logistic regression will be used to conduct this analysis, adjusting for age, sex, race in an unweighted analysis, and including fixed weights in a weighted analysis.
C.6.c. Analysis addressing specific aim 3: Evaluate economic impact of CFS and access to/ utilization of medical care of persons with CFS in metropolitan, urban, and rural Georgia.
C.6.c.1. We will use the economic theory of human capital as the basis of the simulation model to estimate the economic impact of CFS. In this model, an individual’s productivity (in terms of employment and earnings) will be modeled as a function of human capital characteristics such as age, education, occupation and health status (Rice, 1967; Rice et al., 1985). Productivity loss will be estimated based on methods developed as part of the RAND Health Insurance Experiment microsimulation (Newhouse, 1993). These include a two-step microsimulation approach using logistic regression to predict employment and ordinary least squares regression to estimate expected income, conditional on employment, for the CFS and well groups. Direct costs of illness (healthcare services and products for the diagnosis, assessment and management of CFS) will also be considered in this model. Analyses of these data will be based on data collected in the follow-up visit, stratified by metropolitan, urban and rural geographic area.
C.6.c.2. In order to examine whether CFS patients who consult and are treated by a health care provider for their symptom of fatigue have higher recovery rates than those who do not receive such attention, we will create subgroups of CFS patients based on data collected in the baseline study regarding whether they did or did not consult a health care provider for fatigue. Based on baseline data, 75% of 86 CFS patients consulted health care providers for fatigue and 15% were diagnosed by a physician. Recovery will be a dichotomous outcome variable, defined as either improved or not improved. Improvement is defined as a change in fatigue, functioning or symptom severity to the extent that a CFS case at baseline is recategorized as a patient with insufficient symptoms/fatigue (ISF), or as a non-fatigued patient at follow-up. Assuming that 94 CFS patients identified at baseline participate in this follow-up study, we will be able to estimate risk of recovery among 70 CFS patients who consulted a physician and 24 who did not. Given this sample size, we have 80% power to detect a 4.5-fold difference in recovery, assuming that 11% of those who do not consult a physician recover. Exact logistic regression analysis will be used to obtain ORs associating recovery at follow-up with consultation/diagnosis-treatment at baseline, adjusted for age, sex, race in an unweighted model. Data will be reanalyzed in a logistic regression model that includes fixed weights to see if the estimates of ORs vary between these two methods.
C.6.d. Analysis addressing specific aim 4: Evaluate the incidence of CFS in different racial/ethnic groups representative of metropolitan, urban, and rural Georgia populations
We are planning on conducting 4 years of annual follow-up assessments of our baseline cohort of CFS subjects, subjects with Chronic Unwellness, and Well subjects. The Chronically Unwell and Well groups will be assessed annually for CFS, allowing for measurement of CFS incidence. Based on published incidence rates of .0018, we expect to identify between 5 – 18 CFS cases annually, accumulating 20 – 72 cases at the end of 4 years. Among the Baseline Survey groups of Chronically Unwell and Well subjects, cohort study methods will be used to determine the numerator (incident CFS case) and denominator (person-years of follow-up) to measure the incidence rate of CFS, and compare rates by categories of ‘exposure’. Cox proportional hazards analysis will be used to compute hazards ratios (HR) as measures of CFS risk associated with the various ‘exposure factors’, adjusting for age, sex, and race as appropriate. Exposure factors will be determined at baseline. Below are the individual analyses that will be conducted to examine differences in incidence by several factors.
C.6.d.1. Incidence of CFS will be compared between those who were originally considered ‘unwell with fatigue’ and those who were considered ‘well’ in the Baseline Survey.
C.6.d.2. Incidence of CFS will be compared by dichotomous categories of ‘exposures’ that include (1) adverse life experiences, (2) high/low allostatic load, (3) personality and coping mechanisms. All of these analyses will be conducted separately
A separate analysis will examine the association of incident CFS (or CFS risk) associated with the occurrence of a triggering event during the preceding year. The triggering event will be treated as a time-dependent variable in the Cox model. Separate stratified analyses will be conducted to determine if this association varies by history of a traumatic early life event, and the following factors measured at the first follow-up visit: level of allostatic load index, reactive personality and maladaptive coping style.
C.6.d.3. Incidence of CFS will be compared between Black and White populations.
C.6.d.4. Incidence of CFS will be compared between metropolitan/urban and rural populations.
Given a cohort design and an estimated accumulation of 50 CFS cases, our cohort analysis has 77% power to detect a 2-fold difference in CFS risk, assuming that the prevalence of the ‘low risk’ exposure in C.6.d.1 – C.6.d.4. is 10% among CFS cases.
C.6.c Reporting of results
As noted, a major objective of this study is to obtain clinical information for use in evaluation, diagnosis, and management of persons with CFS. We will immediately incorporate findings into a national health care provider education program (Contract #200-2002-00793) and into a national public awareness effort (Contract #200-2004-09722). It will also be used to develop an intensive regional provider education project.
In addition, subsequent to data analyses, results will be prepared for presentation at scientific meetings and papers for publication of findings in internationally recognized journals.
Study participants will be notified of any abnormal findings from the physical examination at the time of the examination. Study participants will also be mailed copies of their laboratory test results, accompanied by a cover letter alerting the participant if any of the results are outside the normal range. Additionally, a copy of the participant's laboratory test results will be sent to his or her personal physician, with the participant's consent.
Study participants who request information about study findings will be directed to the CFS page of CDC's website. This site summarizes the results of all recent CFS studies conducted by CDC.
D. Human Subjects
D.1 Proposed involvement of human subjects
As noted, this is a follow-up study of a sample of persons in metropolitan Atlanta, Macon and Warner Robins, and rural counties surrounding Macon and Warner Robins areas of the Georgia population earlier screened by random-digit-dialing for fatiguing illness and unwellness. Identified Unwell and selected Well adults will be interviewed in detail by telephone. All CFS-like subjects and an equal number of randomly selected persons with Chronic Unwellness will be invited to participate in a clinical evaluation. In addition, selected Well subjects will also be invited, matched 1:1 with CFS-like subjects on geography, age (within 3-years), sex and race/ethnicity. Subjects with exclusionary medical/psychiatric conditions will not be invited to the clinic, or if identified at the clinic, will not complete the study.
D.2 Sex and minority inclusion
This is a follow-up study of subjects who participated in the Baseline Survey. We include English-speaking males and females of all races.
D.3 Sources of research material
The following research material is obtained from the human subjects:
a. For the detailed telephone interview, research material is obtained in the form of answers to questions. These questions cover demographic information, symptoms, medical history, and psychiatric history and take an average of 20 minutes to complete.
b. During the physical exam, a total of 30 mL blood and a urine specimen will be collected in the morning. Blood will be used for clinical laboratory studies to identify medical exclusionary conditions, and to measure allostatic load index and selected steroid hormones. Portions of the blood sample will be used for storing blood cells and plasma for biomarker validation studies. A buccal swab will be collected and used as the source of DNA for genotyping studies. Subjects may give or not give consent to genetic testing and storage of their samples.
c. The night before the clinic date, subjects will collect all urine voided between 7:00 pm and 7:00 am, to be used for measuring 12-hour urinary cortisol, epinephrine, norepinephrine.
d. During the psychiatric screening, research material is obtained in the form of responses to items (question/answer). For the SCID, the number of items is variable and depends on the mental health status of the subject. The maximum duration of the SCID is estimated to be 90 minutes. The remaining questionnaires, self-administered at clinic, are expected to take 190 to 305 minutes. (Based on our baseline experience, we expect questionnaires to take about 3 hours for new subjects and 2 hours for returning subjects.)
e. The self-administered questionnaires to complete prior to the clinic visit (medical history, gynecological history, economic impact, and health care utilization) are expected to take, on average, 60 minutes to complete.
D.4 Recruitment and consent procedures
As noted, all participants will be recruited from subjects identified in the Baseline Survey. Eligible Unwell and selected Well subjects will be interviewed in detail regarding fatigue and symptom criteria, and will then be classified as having a CFS-like illness, chronic unwellness with fatigue that is not CFS-like, chronic unwellness (not fatigued), or wellness. Informed consent statements are included in the detailed telephone interview questionnaires. The consent statements will be administered as soon as the respondent is on the telephone. Telephone interviewers will be prepared to discuss any aspect of the respondent’s participation at greater length, including respondent rights and the telephone numbers of staff who can be contacted for more or different information. The interview will not proceed until the informed consent statement has been read, exactly as written, to each respondent, and all of the respondent’s questions have been answered.
During a clinical evaluation, eligible subjects from these groups will be evaluated for classification and to gather other information and specimens as described in previous sections. Subjects will be completely informed about all procedures and will sign the informed consent prior to study entry (appended). The study protocol will be submitted for approval to the Institutional Review Boards of the CDC, Emory University, and Abt Associates.
D.5 Potential risks
D.5.a Risks for telephone portion. There may be some minimal risks associated with the telephone portion of this study. Portions of the telephone interview regarding psychiatric symptoms, stress, or life experiences may cause emotional distress.
D.5.b Risks for clinical evaluation. There may be risks or side effects associated with the investigational procedures: venipuncture can be associated with pain and bruising, and, extremely rarely, infection at the site of the needle entry. Interviews on psychiatric symptoms and questionnaires on symptoms, stress and life experiences may induce emotional distress.
D.6 Procedures for protecting or minimizing potential risks
D.6.a Identifying, managing and reporting adverse events:
The most common type of adverse event will be subjects who become upset by questions in the telephone or self-administered questionnaires. Several procedures are in place to deal with these situations.
First, subjects will be clearly told that they can skip any question that they do not wish to answer. Interviewers will be instructed to move immediately to the next question when requested without pushing the subject to respond, and they will be monitored to ensure compliance with this procedure. Second, subjects will be provided with the names and telephone numbers of CDC staff to call with questions or concerns about the study or their rights as a study subject. Third, interviewers will be instructed to report any incidents involving an upset/concerned respondent to their supervisor. Of course, interviewers and researchers are not in a position to provide referrals to medical professionals or counselors, but they will recommend that any distraught subjects contact their doctor or other trusted advisor for help. As noted below, local hotline numbers will be made available to respondents. Finally, study data for any subject will be destroyed after it is collected if the subject requests it.
Telephone interview: Trained research personnel conduct the interviews. Interviewers are trained to seek assistance from supervisors if any portion of the interview appears to cause emotional distress to the respondent. The supervisor may contact the subject to discuss his concerns, and recommend that the subject contact a medical or mental health professional if necessary. Subjects will also be provided with a toll-free number to contact supervisors at Abt Associates with any questions or concerns that they have.
One part of telephone interviewer training will focus on the sensitive nature of questions in the detailed telephone interview. We will:
Teach interviewers to approach these questions in a calm and careful manner.
Provide guidance for interviewers to recognize early signs of concern or upset so that interviewers will repeat that respondents may refuse to answer any questions.
Prepare a list of Georgia and, where available, county hotline numbers which will be posted at every interviewing station. Interviewers will be trained to provide these numbers on request or when respondents express upset at sensitive questions.
Train interviewers to recognize signs of anguish or danger. An interviewer in this situation will alert a supervisor who will dial 911 and report the telephone number so that immediate assistance can be provided.
Clinical evaluation: A trained clinician will evaluate any evidence of psychological stress that might result from interviewing or psychometric tests. Subjects may decline to answer specific questions and may withdraw from the study at any time. A registered nurse or physician will be on the premises during clinic appointments to respond to any medical or psychiatric emergencies. To minimize the risks of infection, and pain and bruising from the blood draw, blood samples will be taken by direct venipuncture in a sterile manner by experienced staff according to current medical standards.
D.6.b Emergency care: The First Follow-up Study of CFS does not involve medical care of study subjects. However, should a participant experience a medical or psychiatric emergency while at the clinic, a physician or registered nurse will be on site and appropriate referrals will be made.
D.7 Risk/benefit ratio
There may be no direct benefit to the subjects who participate in the study.
No treatments or interventions will be offered to study subjects. The results of the physical examination, laboratory tests and appropriate psychiatric evaluations will be made available to study subjects and their physicians if they so designate in writing. Study subjects will be alerted if any of these tests reveal potentially serious conditions of which the subject was not aware. However, any treatment for these conditions is outside of the bounds of this study, and must be handled by subjects with their private physicians.
D.8 Handling of unexpected and adverse events
CDC will review all test results. In the case of an unexpected finding, CDC will contact Abt Associates, who will in turn notify the subject and advise him/her to see a doctor for appropriate counseling and treatment. If the subject so requests, Abt Associates will also notify his or her physician of the findings. However, subjects will not be compensated for any treatment sought due to abnormal test results.
In the case of adverse events, emergency psychiatric treatment will be provided until an emergency psychiatric or medical technician arrives, and referral will be provided by the on site psychiatric clinicians. The adverse events protocol remains as previously approved by the IRB. Prior to discharge from the clinic visit, an emotional well-being script will be performed. In the case that a patient appears suicidal, the patient will be sent to the nearest emergency room if he/she poses an immediate threat to himself/herself (that is, he/she expresses suicidal ideation with plans to carry out the suicide). Medical emergencies will be identified by the staff as supervised by on site nurses or physicians. Serious adverse events will be reported to the IRBs of CDC, Emory University and Abt Associates following the guidelines of each IRB.
D.9 Compensation
If, for any reason, the subject is determined to be ineligible to participate in this study following the physical examination on the day of arrival, the subject will receive $50 compensation for his/her time. If an eligible subject chooses not to join the study after arrival at the clinic, the subject will receive $50. If the subject has been admitted to the study following the physical examination and completes the study, he/she will receive $450 to pay for the time the subject must take and any costs for being in the study. If the subject chooses to drop out before the end of the Clinic visit, the subject will receive $225.
The table below provides a justification for the compensation amount of $450 for the full clinic day.
Mean weekly wage in Georgia in 2000 |
$6583 |
|
|
Assume inflation rate |
17.5%4 |
|
|
Adjusted weekly wage in 2007 |
$773 |
|
|
Working hours in one week |
40 |
|
|
Average hourly rate |
$19.33 |
|
|
|
|
|
|
Time off from work to participate in this study |
|
|
|
Preparation time (completion of at-home questionnaires) |
1.5 |
|
|
Travel (2.5 hrs round trip) |
2.5 |
|
|
Clinic stay @ 8 to 9 hours |
9.0 |
|
|
Total time |
|
13.0 |
|
Average reimbursement |
|
|
$251 |
|
|
|
|
Dependent Care Coverage |
|
|
|
$10 per hour @11.5 hours |
|
$115 |
|
Average reimbursement |
|
|
$115 |
|
|
|
|
Other Expenses |
|
|
|
Assume round trip mileage |
100 |
|
|
Mileage rate |
|
$0.485 |
|
Reimbursement for miles |
|
|
$49 |
Parking and Tolls |
|
|
$25 |
Light meal following clinic appointment |
|
|
$10 |
Average reimbursement |
|
|
$84 |
|
|
|
|
Total Reimbursement |
|
|
$450 |
Subjects may opt out of especially sensitive questions or tests, and decline consent or genetic testing and storage of specimens without penalty. This compensation schedule is appropriate for the demands placed on subjects by the protocol. Participation requires completing numerous questionnaires, providing blood, urine and saliva specimens. Some of these requirements are inconvenient and unpleasant, and none are related to more than minimal risk. Besides these demands, participation will require travel to the clinic (driving time, gasoline, and parking), and one day of work, which may represent a significant loss of revenue for some participants. Some subjects must also arrange for dependent (children and elderly) care coverage.
D.10 Concerns regarding storage of specimens for biomarker testing (including genetic testing)
This section addresses concerns regarding storage of specimens for biomarker and the genetic testing component of this study as outlined in the CDC’s Genetic Research Checklist.
Refer to Background and Significance (section B.6) for justification of the importance of genetic testing.
Refer to sections B.2, B.6, and C.2.d for a description of the proposed biomarker research.
There are two different types of tests we propose to perform on the genetic material present in the biological sample (white blood cells obtained from clotted blood or the pellet from anticoagulated blood) and the blood cards. We will perform tests for genotypic polymorphisms in genes encoding proteins such as hormone receptors, neurotransmitters and cytokines, mapping regions of the genome containing genes that might contribute to CFS by SNP mapping, or other methods that become available as technology advances after all subjects have been enrolled. Genetic information discovered by these methods may be used to estimate how many people have specific genetic variations that potentially contribute to development and persistence of CFS, or may in the future be suggested to play such a role. This may help us begin to learn why some people get CFS and others do not. We also want to know how factors such as early adverse life events, exercise, and exposure to various infectious agents affect people who have those genes. This will help the medical community decide whether changing these factors will be effective in preventing or treating CFS.
Participants will be given the option to decline consent for storage of samples for biomarker and genetic testing without penalty. Subjects will be advised that, should they withhold consent for biomarker and genetic testing, they will still receive full compensation for their participation. Furthermore, subjects may agree to have their samples stored and later decide to withdraw them from storage. If this occurs, the sample(s) will be discarded but any data from testing of that sample(s) until that point will remain part of the research.
Although we hope that we will be able to use these tests to distinguish patients with CFS, they are not indicative of any known pathologies. Individual subjects will not get any direct benefit for providing a blood sample for this study, but they will be informed that the information and results from these kinds of studies may help prevent and treat CFS in the future. We will not create a mechanism for individual subjects to learn the results of our research on their specific samples.
The kind of information we will look for in this study is not expected to reveal anything specific about the current or future personal health of individual subjects. Even so, some people may have concerns about their blood being used for genetic testing. Genetic information could be misused in the hands of people other than the researchers. Every precaution will be taken to prevent this from happening. Blood samples will be assigned a code number, and patient names or any other identifying information will be separated from the sample. Only the Contractor will maintain files that link identifying information to the code number but the Contractor will not know the genetic test results. Specific individual test results will be kept private and released only if ordered by a court of law. Patient names or identifying information will not appear when this study is presented or its results published. Although no one can absolutely guarantee confidentiality, using a code number greatly reduces the chance that someone other than the Contractor will ever be able to link a subject’s name to their sample test results.
The results of these tests should not affect the future employment or insurability of the participants. Although patient names will not be with the sample, it will have other facts such as race, ethnicity, and sex. These facts are important because they will help us learn if the factors that cause CFS to occur, persist, or get worse are the same or different in men and women, and in people of different racial or ethnic backgrounds. However, it is also possible through these kinds of studies that genetic traits might come to be associated with a particular group. In some cases, this could reinforce harmful stereotypes. In our presentation and publishing of results from this study, we will consciously avoid language that might result in harmful stereotyping.
The aim of our research is to improve the public health. Sometimes, such research may result in findings or inventions that have value if they are made or sold. We may get a patent on these. We may also license these, which could give a company the sole right to make and sell products or offer testing based on the discovery. Some of the profits from this may be paid back to the researchers and the organizations doing this study, but individual research subjects would not receive any financial benefits.
When this study is completed, we will retain unused blood (cell, serum, and plasma) samples for future research on gene expression, as well as the specific structure of polymorphisms, and measurement of hormones, cytokines, and other biomarkers. We do not have specific research plans at this time but, in the future, we will submit these studies to the IRB for review and approval. All samples will be stored in respective appropriate storage modes and use them for as long as they last. Like the genetic testing samples, these blood samples will also be assigned code numbers. Patient names, or any other identifying information, will be separated from the sample identified by code numbers. Only the Contractor will maintain files that link identifying information to code numbers. This link is maintained to provide demographic or medical data that may inform future studies.
Specific individual test results will be kept private and released only if ordered by a court of law. Patient names or identifying information will not appear when this study is presented or its results published. We may share the samples with other researchers for future CFS research, but we will not give other researchers any information that would allow them to identify individual subjects. An Institutional Review Board will review and approve all future projects.
Although we hope that we will be able to use these tests to distinguish patients with CFS, they are not indicative of any known diseases and will not be used for the treatment or evaluation of study patients.
Some people may have concerns about their blood being used for genetic testing. The tests that we will be performing are looking only for differences in gene structure. We will not be testing for known genes that cause specific diseases.
E. Literature Cited
Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W: Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 27:169-190, 2003.
Bierl C, Nisenbaum R, Hoaglin D, et al. National pilot telephone survey of chronic fatigue syndrome: regional distribution of fatiguing illness. Population Health Metrics. 2:1, 2004
Bombardier CH, Buchwald D. Chronic fatigue, chronic fatigue syndrome, and fibromyalgia: disability and health-care use. Med Care 34:924-930, 1996.
Brugha T, Bebbington PE, Tennant C. The list of threatening experiences a subset of 12 life event categories with considerable long-term contextual threat. Psychological Medicine 15:189-194, 1985.
Brugha TS, Cragg D. The list of threatening experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatrica Scandinavica 82:77-81, 1990.
Buchwald D, Herrell R, Ashton S, Belcourt M, Schmaling K, Sullivan P, Neale M, Goldberg J., A twin study of chronic fatigue, Psychosom Med. 63:936-43, 2001.
Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med.;101:364-370, 1996.
Buckley L, MacHale SM, Cavanagh JT, Sharpe M, Deary IJ, Lawrie SM. Personality dimensions in chronic fatigue syndrome and depression. J Psychosom Res 46:395-400, 1999.
Butler JA, Chalder T, Wessely S: Causal attributions for somatic sensations in patients with chronic fatigue syndrome and their partners. Psychol.Med. 31:97-105, 2001.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R: Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science; 301:386-389, 2003
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behavior 24:385-396, 1983
Costa PTJ, McCrae RR. NEO-PI-R. Professional manual: Revised NEO Personality Inventory (NEO-PI-R) and NEO-Five-Factor Inventory (NEO-FFI). Odessa, FL. Psychological Assessment Resources, 1992.
Davidson JR, Book SW, Colket JT, Tupler LA, Roth S, David D, Hertzberg M, Mellman T, Beckham JC, Smith RD, Davison RM, Katz R, Feldman ME: Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol.Med. 27:153-160, 1997.
Dijsselbloem N, Vanden Berghe W, De Naeyer A, Haegeman G. Soy isoflavone phyto-pharmaceuticals in interleukin-6 affections. Multi-purpose nutraceuticals at the crossroad of hormone replacement, anti-cancer and anti-inflammatory therapy. Biochem Pharmacol. 68(6):1171-85, 2004.
Dogan E, Erkoc R, Demir C, Sayarlioglu H, Dilek I, Sayarlioglu M. Effect of hormone replacement therapy on CD4+ and CD8+ numbers, CD4+/CD8+ ratio, and immunoglobulin levels in hemodialysis patients. Ren Fail. 2005;27:421-4.
Endicott NA., Chronic fatigue syndrome in private practice psychiatry: family history of physical and mental health . J Psychosom Res. 47:343-54, 1999.
Ehlert, U., Erni, K., Hebisch, G., & Nater, U. M. (2005). Effects of yohimbine challenge on salivary alpha-amylase secretion. Psychosom Med, 67(1), A-92.
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS: Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am.J Prev.Med. 14:245-258, 1998.
Fiedler N, Lange G, Tiersky L, DeLuca J, Policastro T, Kelly-McNeil K, McWilliams R, Korn L, Natelson B: Stressors, personality traits, and coping of Gulf War veterans with chronic fatigue. J Psychosom.Res. 48:525-535, 2000.
First MB, Spitzer RL, Gibbon M, and Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute. 2002.
Fisher L, Chalder T: Childhood experiences of illness and parenting in adults with Chronic Fatigue Syndrome. J Psychosom.Res. 54:439-443, 2003.
Folkman S, Lazarus RS: If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers.Soc.Psychol. 48:150-170, 1985.
Gillin, DL. Are you placing survey research calls to cellular telephones? If you are – beware!, Council for Marketing and Opinion Research (http://www.cmor.org/govt_affairs_new0802.htm), accessed November 3, 2003.
Greve W, Anderson A, Krampen G.: Self-efficacy and externality in adolescence: Theoretical conceptions and measurement in New Zealand and German secondary school students. Int J Theory Res. 1:321-344, 2001.
Hardt J, Buchwald D, Wilks D, Sharpe M, Nix WA, Egle UT. Health-related quality of life in patients with chronic fatigue syndrome, an international study. J Psychosom Res. 51:431-434, 2001.
Hart LG, Evans RW. The functional status of ESRD patients as measured by the sickness impact profile. J Chron Dis.40(Suppl 1):117S-136S, 1987.
Heim C, Nemeroff CB: The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry; 49:1023-1039, 2001
Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, Unger ER, Reeves WC. Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. Archives of General Psychiatry (in press).
Hickie IB, Bansal AS, Kirk KM, Lloyd AR, Martin NG, A twin study of the etiology of prolonged fatigue and immune activation. Twin Res. 4:94-102, 2001.
Hill NF, Tiersky LA, Scavalla VR, Lavietes M, Natelson BH. Natural history of severe chronic fatigye syndrome. Arch Phys Med Rehabil 80:1090-1094, 1999.
Hyler SE, Skodol AE, Kellman HD, Oldham JM, Rosnick L: Validity of the Personality Diagnostic Questionnaire--revised: comparison with two structured interviews. Am.J Psychiatry 147:1043-1048, 1990.
Ironson G., Solomon GF, Balbin EG, O'Cleirigh C, George A, Kumar M, Larson D, Woods T. The Ironson-Woods Spirituality Index is associated with long survival, health behaviors, less distress, and low cortisol in people with HIV/AIDS. Annals of Behavioral Medicine 24: 34-48, 2002.
Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Int Med 159: 2129-2137, 1999.
Jason LA, Taylor RR, Kennedy CL, Song S, Johnson D, Torres S. Chronic fatigue syndrome: occupation, medical utilization, and subtypes in a community-based sample. J Nerv Ment Dis 188:568 -76, 2000
Joyce J, Hotopf M, Wessely S. The prognosis of chronic fatigue and chronic fatigue syndrome: a systematic review QIM 90:223-233, 1997.
Kang HK, Natelson BH, Mahan CM, Lee KY, Murphy FM: Post-traumatic stress disorder and chronic fatigue syndrome-like illness among Gulf War veterans: a population-based survey of 30,000 veterans. Am.J Epidemiol 157:141-148, 2003.
Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. McArthur studies of successful aging. J Clin Epidemiol; 55:696-710, 2002.
Kirschbaum C, Hellhammer DH. Salivary cortisol. In Encyclopedia of Stress Volume 3. Academic Press. 379 – 383, 2000
Klimidis S, Minas IH, Ata AW: The PBI-BC: A brief current form of the parental bonding instrument for adolescent research. Comprehensive Psychiatry 33(6): 374-377, 1992
Komaroff AL, Fagioli LR, Doolittle TH, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. 101:281-290, 1996.
Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K: Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: the Traumatic Life Events Questionnaire. Psychol.Assess. 12:210-224, 2000.
Kudielka B M, Broderick J E, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med, 65:313-319, 2003.
Kudielka BM, Kirschbaum C: Awakening cortisol responses are influenced by health status and awakening time but not by menstrual phase. Psychoneuroendocrinology; 28: 35-47, 2003
Larson SL, Fleishman JA: Rural-urban differences in usual source of care and ambulatory service use: analyses of national data using Urban Influence Codes. Med Care; 41:III65-III74, 2003
Levine PH, Whiteside TL, Friberg D, Bryant J, Colclough G, Herberman RB: Dysfunction of natural killer activity in a family with chronic fatigue syndrome. Clin Immunol Immunopathol. 88:96-104, 1998.
Lloyd AR, Hickie I, Boughton CR, Spencer O, Wakefield D. Prevalence of chronic fatigue syndrome in an Australian population. Med J Austr. 153:522-528, 1990.
Lloyd A, Pender H. The economic impact of chronic fatigue syndrome. Med J Australia 157:599-601, 1992
Lutgendorf SK, Antoni MH, Ironson G, Fletcher MA, Penedo F, Baum A, Schneiderman N, Klimas N: Physical symptoms of chronic fatigue syndrome are exacerbated by the stress of Hurricane Andrew. Psychosom.Med. 57:310-323, 1995
McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB: Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA 277:1362-1368, 1997.
McCauley LA, Joos SK, Barkhuizen A, Shuell T, Tyree WA, Bourdette DN: Chronic fatigue in a population-based study of Gulf War veterans. Arch.Environ.Health 57:340-348, 2002.
McCrone P, Barbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med 33:253-261, 2003.
McEwen B, Stellar E. Stress and the individual. Arch Intern Med 153:2093-2101, 1993.
Moss Morris, R., Weinman, J., Petrie, K. J., Horne, R., Cameron, L. D., & Buick, D. (2002). The revised illness perception questionnaire (IPQ-R). Psychology and Health, 17(1), 1-16.
Nater U M, La Marca R, Florin L, Moses A, Langhans W, Koller M M, et al. Stress-induced changes in human salivary alpha-amylase activity-associations with adrenergic activity. Psychoneuroendocrinology, 31:49-58, 2006
Nater U M, Rohleder N, Gaab J Berger S, Jud A, Kirschbaum C, et al. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol, 55:333-342, 2005.
Neeck G, Crofford LJ. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin North Am. 26(4):989-1002, 2000
Newhouse JP. The Health Insurance Group. Free-for-all: health insurance, medical costs, and health outcomes: the results of the health insurance experiment Cambridge, MA: Harvard University Press; 1993.
Nisenbaum R, Barrett DH, Reyes M, Reeves WC. Deployment stressors and a chronic multisymptom condition among Gulf War veterans. J Nerv Ment Dis 188:259-266, 2000.
Nisenbaum R, Jones JF, Unger ER, Reyes M, Reeves WC. A population-based study of the clinical course of chronic fatigue syndrome. BMC Hlth Quality Life Outcomes. 1:49, 2003.
Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5134-8.
Pruessner JC, Hellhammer DH, Kirschbaum C: Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med; 61: 197-204, 1999
Radant A, Tsuang D, Peskind ER, McFall M, Raskind W: Biological markers and diagnostic accuracy in the genetics of posttraumatic stress disorder. Psychiatry Res; 102:203-215, 2001
Ray C, Weir W, Stewart D, Miller P, Hyde G: Ways of coping with chronic fatigue syndrome: development of an illness management questionnaire. Soc Sci Med; 37:385-91, 1993
Reyes M, Dobbins JG, Mawle AC, Steele L, Gary HE, Malani H, Schmid S, Fukuda K, Stewart J, Nisenbaum R, Reeves WC. Risk factors for CFS: a case control study. J CFS 2:17-33, 1996.
Reyes M, Dobbins JG, Nisenbaum R, Subedar N, Randall B, Reeves WC. Chronic fatigue syndrome progression and self-defined recovery: evidence from CDC surveillance system. J CFS 5:17-27, 1999.
Reyes M, Gary HE, Dobbins JG, et al. Surveillance for chronic fatigue syndrome: four US cities. MMWR Morb Mortal Wkly Rep 46:1-13, 1997.
Reyes M, Nisenbaum R, Hoaglin DC, et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Int Med 163:1530-1536, 2003.
Reynolds KJ, Vernon SD, Bouchery E, Reeves WC. The economic impact of chronic fatigue syndrome. Cost Effectiveness Resource Allocation. 2:4, 2004.
Reynolds WM: Development of reliable and valid short forms of the Marlowe-Crowne social desirability scale. J Clin Psychol. 38 (1): 119-125, 1982.
Rice DP: Estimating the cost of illness. Am J Public Health Nations Health 57:424-40, 1967.
Rice DP, Hodgson TA, Kopstein AN. The economic costs of illness, a replication and update. Health Care Financ Rev 7:61-80, 1985.
Romans S, Belaise C, Martin J, Morris E, Raffi A: Childhood abuse and later medical disorders in women. An epidemiological study. Psychother.Psychosom. 71:141-150, 2002.
Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 17:367-88,2000.
Sarason IG, Johnson JH, Siegel JM: Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin.Psychol. 46:932-946, 1978.
Sarason IG, Levine HM, Basham RB, Sarason BR: Assessing social support: The Social Support Questionnaire. J Pers Soc Psychol. 44:127–139.
Schulz P, Schlotz W, Becker P: Das Trierer Inventar zum chronischen Stress – Manua [The Trier Inventory for Chronic Stress – Manual]. Göttingen: Hogrefe, 2004
Schulz, P., Jansen, L., & Schlotz, W. Stressreaktivität: Theoretisches Konzept und Messung [Stress reactivity: theoretical concept and measurement]. Diagnostica. 51:124-133, 2005.
Schulz P, Kirschbaum C, Pruessner J, Hellhammer DH: Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med; 14: 91-97, 1998
Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA; 98:4770-4775, 2001.
Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation – Allostatic load and its health consequences. Arch Intern Med; 157:2259-2268, 1997.
Smets EM, Garssen B, Bonke B, De Haes JC: The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res; 39:315-325, 1995
Solomon L., Nisenbaum R., Reeves WC. Factors influencing the diagnosis of chronic fatigue syndrome by primary health care providers. Arch Int med 164:2240-2245, 2004.
Solomon L, Nisenbaum R, Reyes M, Papanicolaou DA, Reeves WC. Functional status of persons with chronic fatigue syndrome in the Wichita, Kansas, population. BMC Hlth Quality Life Outcomes. 1:48, 2003.
Spielberger CD: State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press 1983
Taillefer SS, Kirmayer LJ, Robbins JM, Lasry JC. Correlates of illness worry in chronic fatigue syndrome. J Psychosom Res 54:331-337, 2003.
Taylor RR, Jason LA: Sexual abuse, physical abuse, chronic fatigue, and chronic fatigue syndrome: a community-based study. J Nerv.Ment.Dis. 189:709-715, 2001.
Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller Lissner: Functional bowel disorders and functional abdominal pain. Gut, 45 Suppl 2:II43-47, 1999.
Torpy DJ, Bachmann AW, Grice JE, Fitzgerald SP, Phillips PJ, Whitworth JA, Jackson RV. Familial corticosteroid-binding globulin deficiency due to a novel null mutation: association with fatigue and relative hypotension, J Clin Endocrinol Metab. 86:3692-700, 2001.
Van Houdenhove B, Neerinckx E, Onghena P, Lysens R, Vertommen H: Premorbid "overactive" lifestyle in chronic fatigue syndrome and fibromyalgia. An etiological factor or proof of good citizenship? J Psychosom.Res; 51:571-576, 2001.
Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC. Psychometric properties of the CDC symptom inventory for the assessment of chronic fatigue syndrome. Populn Health Metrics 3:8, 2005.
Walsh et al. A family history study of chronic fatigue syndrome. Psychiatr Genet. 11:123-8, 2001.
Ware JE, Jr., Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care; 30:473-483, 1992
White C, Schweitzer R. The role of personality in the development and perpetuation of chronic fatigue syndrome. J Psychosom Res 48:515-524, 2000.
White PD, Thomas JM, Kangro HO, Bruce-Jones WDA, Amess J, Crawford DH, Grover SA, Cleare AW. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet 2001; 358: 1946-54.
Wilder RL, Elenkov IJ. Hormonal regulation of tumor necrosis factor- alpha, interleukin-12 and interleukin-10 production by activated macrophages. A disease-modifying mechanism in rheumatoid arthritis and systemic lupus erythematosus? Ann N Y Acad Sci. 1999, 876:14-31.
Zung WW, Richards CB, Short MJ: Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch.Gen.Psychiatry 13:508-515, 1965.
F. Appendices
Appendix 1. Press Release and Web Site
Appendix 2. Telephone Interview Advance Letter
Appendix 3. Telephone Interview Follow-up Letters
Detailed Interview Non-contact
Detailed Interview Reluctant to Participate
Appendix 4. Detailed Telephone Interview
Appendix 5. Descriptive materials sent prior to clinical evaluation
Clinic Appointment Letter
Clinic Refusal Conversion Letter
Sample Clinic Schedule
Informed Consent
Saliva Collection Instructions
Urine Collection Instructions
Appendix 6. Physical Examination (including Medical History, Gynecological History, and Medications)
Appendix 7. Summary of Specimen Collection (Saliva, Urine, and Blood)
Appendix 8. Questionnaires Concerning Symptomatology
CDC Symptom Inventory
Research Diagnostic Questions for Functional Gastrointestinal Disorders
Illness Perception Questionnaire (Revised) (IPQ-R)
Appendix 9. Assessment of Stress and Coping
Stress Reactivity Scale (SRS)
Illness Management Questionnaire (IMQ)
Social Support Questionnaire (SSQ)
Ironson-Wood Spirituality/Religiousness Index
Appendix 10. Economic Impact
Appendix 11. Health Services Utilization
1 The Council of American Research Survey Organizations (CASRO) response rate equals the product of the resolution and response rates, and assumes that the proportion of households among unresolved telephone numbers is equal to the proportion of households found among resolved numbers. For the screening interview, the resolution rate (percentage of telephone numbers resolved as residential, nonresidential, or nonworking) was 72.1 percent. The response rate (percentage of completed interviews among known households) was 97.1 percent. The CASRO response rate is 72.1%*97.1% or 70.0 percent.
2 According the FCC’s April 2003 Telephone Subscribership in the United States report. There does not appear to be documentation on this question on the CPS’s webpage.
3 source: US Department of Labor, Bureau of Labor Statistics: Georgia statewide, all industries, all ownerships, all establishment sizes, average weekly wage in 2000.
4 source: US Department of Labor inflation calculator.
-
| File Type | application/msword |
| File Title | Studies of Persons with Fatiguing Illnesses in the Atlanta Population |
| Author | William Reeves |
| Last Modified By | Joann House |
| File Modified | 2007-04-23 |
| File Created | 2007-04-23 |
© 2026 OMB.report | Privacy Policy