Attachment 13a - Study Protocol and Materials
Attachment 13a Study Protocols and Materials.doc
National Health and Nutrition Examination Survey (NHANES)
Attachment 13a - Study Protocol and Materials
OMB: 0920-0237
Attachment 13a.
Pubertal Maturation Study Protocols and Materials
PROTOCOL
Project Title: Validation of an Automated Computer-Assisted Self-Interview (A-CASI) Questionnaire to Assess Pubertal Maturation
Study Objective: The objective of the study is to validate an audio computer-assisted self-interview (A-CASI) method for children and teens to self-assess their pubertal maturation (PM) status. If this research demonstrates that valid self-assessment data can be collected from children and adolescents using this method, the module will be considered for use in future National Health and Nutrition Examination Surveys (NHANES). Pubertal maturation assessment information has not been collected in NHANES since NHANES III ended in 1994. Pubertal maturation information is used by researchers to interpret NHANES laboratory, anthropometry, body composition, and interview data.
I. Background and Justification for Collecting Pubertal Maturation Information
From a public health perspective, there are compelling reasons to assess pubertal maturation in NHANES. There is uncertainty about population trends in pubertal maturation among U.S. children. For example, medical textbooks state that approximately 1% of girls show signs of puberty (breast development or growth of pubic hair) before 8 years of age (Rocket et al. 2004). However, other studies have reported that a substantial percentage of American girls presented with one or both of these characteristics by age seven (Herman-Giddens, et al. 1997). No national data have been collected on pubertal maturation status since NHANES III, 1988-94.
The rate of growth for children and adolescents in the United States and other countries is significantly greater than in previous years (Freedman et al. 2000; Karpati et al. 2002). The phenomena of earlier puberty and increased height velocity are referred to as "the secular trend in growth." This trend may have existed for as many as 150 years in certain parts of the world (Samaras and Storms 2002), and has been attributed largely to better child health resulting from improved nutrition, increased food supply, and improved health and sanitation services. A second hypothesis is that the mechanism of precocious puberty could involve environmental exposure to estrogenic endocrine disruptors (Teilmann et al. 2002). This is based on findings that a relatively high proportion of children (primarily girls) who emigrated from developing to developed countries show onset of puberty before age 8 years (termed “precocious puberty), and that their blood serum contains elevated levels of estrogenic pesticides (Krstevska-Konstantinova et al. 2001).
Pubertal maturation information is needed to interpret linear growth, bone density, and laboratory data collected in NHANES. The endocrine changes manifested in secondary sexual characteristics underlie many physiological changes during puberty. Pubertal development correlates more closely with other physical changes such as height, weight, bone density and certain biochemical markers than chronological age and facilitates interpretations of physical growth data. Researchers have also examined environmental health information to determine if there are interactions between environmental exposures and pubertal maturation status.
Environmental lead exposure is associated with delayed puberty (Wu et al. 2003). EPA researchers examined the relationship between blood lead concentration and pubertal development among NHANES females 8 to 18 years of age (Selevan 2003). Blood lead concentrations of 3 µg per deciliter were associated with significant delays in breast and pubic-hair development in African-American and Mexican-American girls. Among African-American girls, the delays in reaching Tanner stages 2, 3, 4, and 5 associated with a lead concentration of 3 µg/dL vs. 1 µg/dL were 3.8, 5.3, 5.8, and 2.1 months, respectively, for breast development and 4.0, 5.5, 6.0, and 2.2 months, respectively, for pubic hair development. The associated delay in age at menarche was 3.6 months. In white girls, the delays in all pubertal measures associated with a blood lead concentration value of 3 µg/dL were not statistically significant.
The NHANES is the only national survey that collects physical and biochemical data on U.S. children and adolescents. The NHANES III data documented the variability that occurs in sexual maturation patterns during adolescence. During NHANES III, Tanner stage information was obtained on children and adolescents 8-18 years of age using a visual inspection method performed by physicians. The NHANES III results showed that non-Hispanic black girls had lower median age at onset for pubic hair and breast development compared to Mexican American or non-Hispanic white girls (Sun et al. 2002); the observed differences in Mexican American and non-Hispanic white girls were not statistically significant. Non-Hispanic black boys had earlier median and mean ages for sexual maturity stages than the non-Hispanic white and Mexican American boys. Although there were age differences in the onset of puberty among race/ethnic groups, U.S. children completed their sexual development at approximately the same ages.
The NHANES data constitute the most important source of normative reference data on the U.S. population. The research community supports assessment of pubertal development in NHANES. A review of research on pubertal development in girls was supportive of population data collection in studies such as NHANES (Herman-Giddens 2004). DHANES sponsored an expert meeting held on August 23-24, 2005 to discuss pubertal maturation self-assessment methods. The expert group included prominent pediatricians, pediatric endocrinologists, child growth experts, and survey methodologists, several of whom have used examination and self-assessment methods to assess sexual maturation (panel members listed on page 9). The expert group recommended that NHANES adopt a self-assessment method as a means of determining pubertal maturation status. The group also provided suggestions for the NHANES protocol and A-CASI presentation that were based on their experiences with self-assessment instruments. The NHANES module incorporated the expert group’s suggestions.
The proposed validation study will be conducted with non-NHANES subjects to compare results reported using the NHANES self-assessment method to results obtained using the “gold standard” physical examination method.
II. Benefits of the Study
The anticipated benefits of this research:
1) A valid self-assessment instrument will provide an alternative to physical examination when examinations are infeasible or unacceptable to children and/or parents. Once validated, the NHANES self-assessment instrument would be made available to researchers. The module could be used with mail, web-based, and school-based questionnaire projects involving children and adolescents.
2) The materials, particularly the drawings, for the module could be used to educate children and teens about the physical changes that occur during puberty.
3) Physical maturation status is related to emotional, behavioral, and social development changes that occur during adolescence. This self-assessment instrument could be used by non-clinicians such as social science researchers to obtain an approximation of pubertal maturation status.
III. Description of the Validation Study:
The validation study will be conducted under a contractual agreement with Children’s Hospital National Medical Center (CHNMC), Washington, DC. The CHNMC Adolescent Health Clinic (outpatients) will be recruited by CHNMC staff for this study. Self-assessed pubertal maturation ratings reported by 8-19 year old study participants will be compared to pubertal maturation determinations made by CHNMC staff physicians and nurse practitioners. The examiners will use the “gold standard” Tanner staging method (Marshall & Tanner, 1969; Marshall & Tanner, 1970) to determine pubertal maturation for each participant.
IV. Validation Study Protocol:
Participants will be recruited during well-child appointments for a physical examination. Parents and children will be informed about the study and the content of the questionnaire prior to giving parental permission and child assent.
Exclusions: Pregnant girls, girls who have given birth previously, and children who have medical conditions, physical constraints or cognitive problems that interfere with normal pubertal development or their ability to use the computer equipment will be excluded from the study.
Sample Size: The sample size, based on a power calculation for 70% power for the study is 240 participants, approximately half males and females. The sample will include immature and mature participants. It is desirable to include race/ethnic subgroups in the sample.
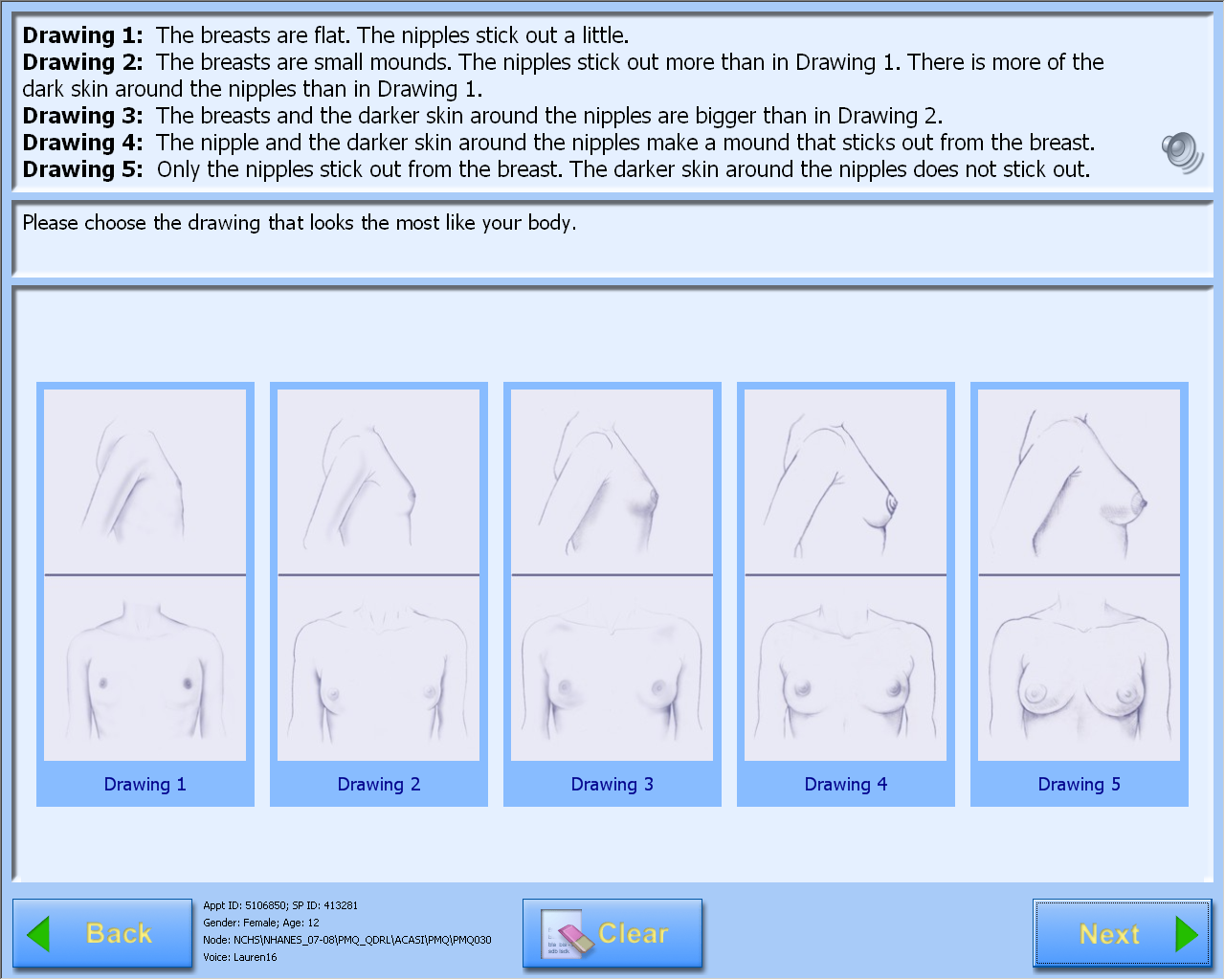
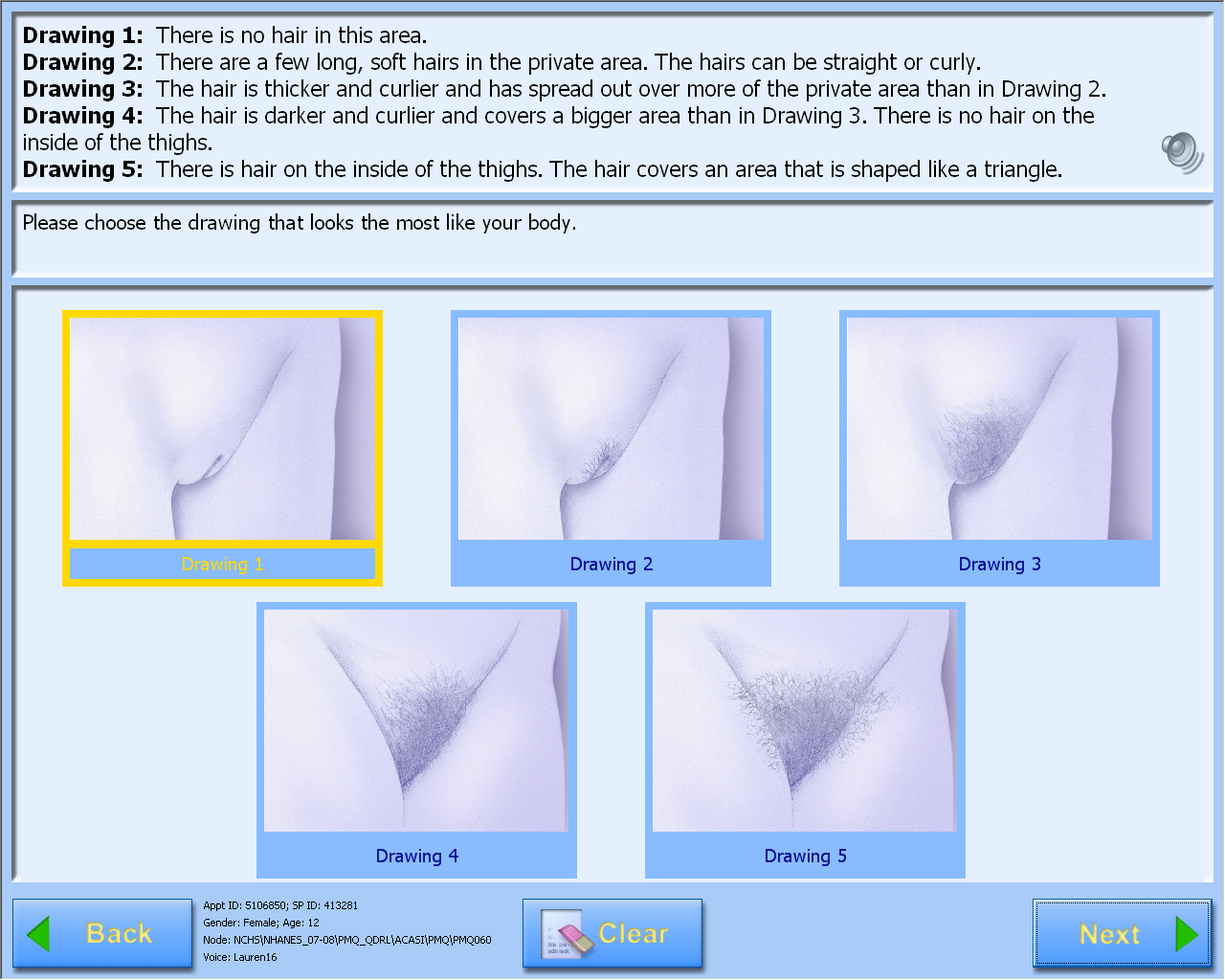
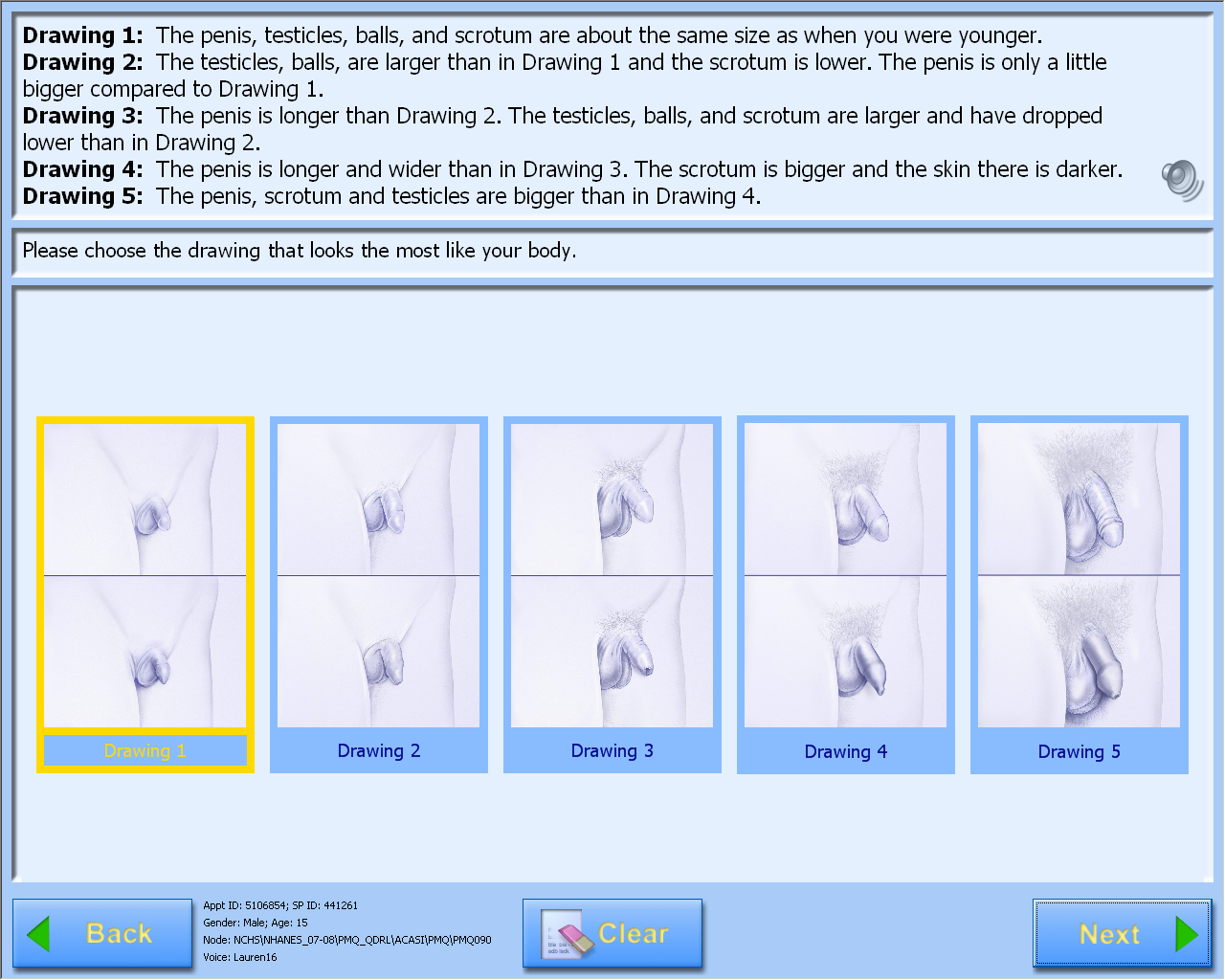
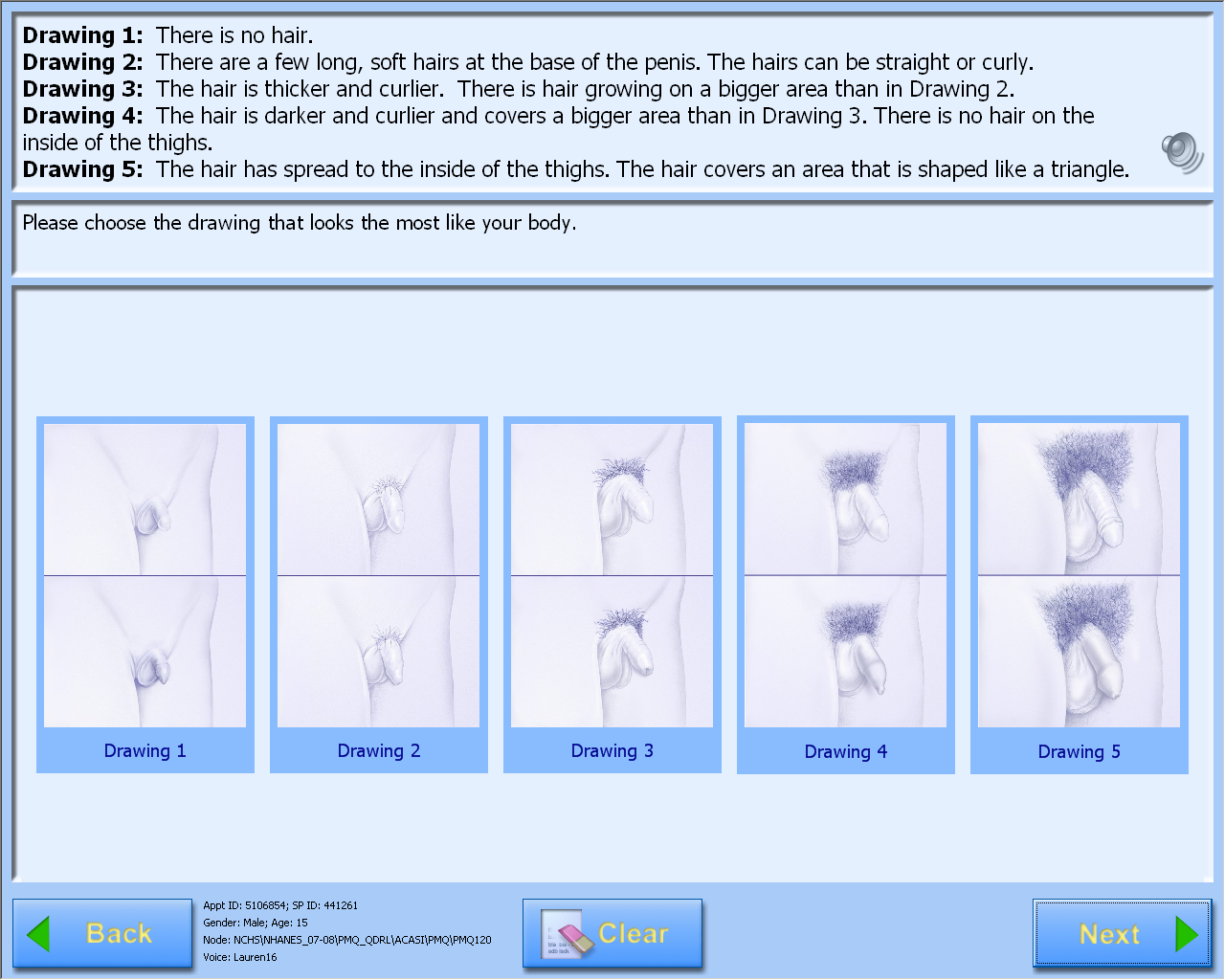
Data Collection: Participants will answer self-administered questions about pubertal maturation status in a private room using an audio-computer-assisted self-interview (A-CASI) methodology. The participants will view Tanner stage drawings on a computer screen and listen to descriptions of each stage through headphones. Participants will be asked to select the drawings that look the most like their body using a touch screen computer monitor.
Girls will select breast and pubic hair drawings.
Boys will select genital and pubic hair drawings.
The number of visuals shown to the participants varies by age, based on the expected maturation status of the participants. Children 8-9 years will view drawings of Tanner stages #1-4 on the computer screen and children 10-19 years will view drawings showing Tanner stages #1-5.
Respondent Burden: The computer instruction screen and entry of study ID number prior to questionnaire completion is two minutes; the estimated time to answer two (2) computer questions is less than 3 minutes. Total time: 5 minutes.
Examiners: Tanner examinations will be completed by trained physicians and nurse practitioners. The total number of examiners will be kept as low as possible (approximately 4-5). If feasible, same-gender examiners are preferable, given the sensitive nature of the examination.
Examiner Training: The examiners will be trained prior to data collection. The training will consist of a review of Tanner examination staging criteria, group discussions and certification. An assessment of inter-rater reliability is proposed; a subset of participants (n=50) will have paired-examiner assessments. A random assignment method for the repeat exams is preferable, but this might not be feasible due to clinic and client schedules. The replicate Tanner examination results will be used to determine inter-rater reliability for the gold standard method. Study examiners will be blinded to self-assessment ratings. Repeated measures by the examiners will be completed independently.
Data Collection: Each participant will have:
Self-assessed and examiner assessed Tanner ratings for breast (female), genital (male), and pubic hair (males and females).
All study data will be collected during a single visit.
In addition to the pubertal maturation determinations, measured stature and weight will be determined on all participants
Menstruation status will be ascertained for all female participants during their physical examination.
Remuneration: Study participants will receive$15 cash remuneration for their participation in the study.
V. Informed Consent:
Parental permission and child assent will be completed at the time of recruitment. Participants 18-19 years of age may consent for themselves. Consent, parental permission, and assent materials were drafted using the templates required by CHNMC. The parental permission, consent, and assent materials will be reviewed and approved by the CDC/NCHS Ethics Review Board (ERB) and the Children’s Hospital Institutional Review Board (IRB).
VI. Hardware and Software:
Workstation: A desktop computer workstation equipped with a touch screen monitor will be used by study participants to record responses to the A-CASI questions. CDC/NCHS will loan the computer equipment required for the study.
Software: CDC/NCHS will provide the A-CASI questionnaire and software required to collect the data. CDC will install and pretest the workstation prior to the main study implementation.
VII. Data Collection and Storage:
A numeric study identification number (ID) will be assigned to each participant. The study ID will be used on study records, including the printed form used to record the physical examination data required for the study.
Printed forms will be used because electronic data entry methods are not available in the examination rooms. The physical examination findings will be entered into a central study database at NCHS. The original hard copy records will retained until the study is completed and the data have been checked. All paper files will be destroyed at the end of the study.
Examination Data: Examiners will record Tanner stage ratings and other study information on height, weight, menses on a printed form (page 7). The recording forms will be collected at the end of each clinic session and taken to NCHS where the data will be keyed into the study database by CDC/NCHS staff.
Feedback Questions on the Use of the ACASI Method: After answering 2 questions related to pubertal maturation, study subjects will be asked questions about the use of the computer.
All data collected during the validation study will be downloaded daily and stored in a separate electronic database at CDC/NCHS. The files for the physician and ACASI records will be merged by study ID number. None of the participant data file will contain personal identifiers.
CNMC Principal Investigator:
Lee A. Savio Beers, MD
Director, Healthy Generations Program
Children's National Medical Center
Assistant Professor of Pediatrics
111 Michigan Avenue, NW
Washington DC 20010
202 884 3797 (office)
202 884 3386 (fax)
Project Timeline:
Children’s Hospital IRB Approval Granted for 1 year. Approval end 6/25/08
Data Collection: Data collection for the main study can begin as soon as the approvals are received. Data collection is expected to take six to eight weeks
Data Analysis: Data analysis is expected to take six to eight weeks to complete.
References
Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. 2002. Self-assessment of pubertal stage in overweight children. Pediatrics 110:743-747.
Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev 1987;58:829-41.
Dorn LD, Nottelman ED, Inoff-Germain G, Susman EJ, Chrousos GP. Perceptions of puberty: adolescent, parent, and health care professional. Dev Psychology 1990;26(2):322-29.
Freedman DS, Khan LK, Serdula MK, Srinivasan SR, Berenson GS. 2000. Secular trends in height among children during 2 decades: The Bogalusa Heart Study. Arch Pediatr Adolesc Med 154:155-161.
Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. 1997. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 99:505-512.
Herman-Giddens ME, Kaplowitz PB, Wasserman R. 2004. Navigating recent articles on girls’ puberty in Pediatrics: What do we know and where do we go from here? Pediatrics 113:911-917.
Kaplowitz PB, Oberfield SE. 1999. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics Executive Committee of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 104:936-941.
Krstevska-Konstantinova M, Charlier C, Craen M, Du Caju M, Heinrichs C, de Beaufort C, et al. 2001. Sexual precocity after immigration from developing countries to Belgium: evidence of previous exposure to organochlorine pesticides. Hum Reprod 16:1020-1026.
Morris NM and and Udry Jr. 1980. Adolescents’ self-assessment of sexual maturation. Pediatrics. 66:918-20.
Rockett JC, Lynch CD, Buck GM. 2004. Biomarkers for Assessing Reproductive Development and Health: Part 1--Pubertal Development. Environmental Health Perspectives, Volume 112, Number 1. Children’s Health Section.
Samaras TT, Storms LH. 2002. Secular growth and its harmful ramifications. Med Hypotheses 58:93-112.
Selevan SG, Rice DC, Hogan KA, Euling S, Pfahles-Hutchens A, Bethel J. 2003. Blood lead concentration and delayed puberty in girls. N Eng J Med. 348:1527-36.
Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS. 2002. National estimates of the timing of sexual maturation and racial differences among U.S. Children. Pediatrics. 110: 911-919.
Tanner JM. 1962. Growth at adolescence, 2nd ed. Oxford: Blackwell Scientific Publications.
Teilmann G, Juul A, Skakkebaek NE, Toppari J. 2002. Putative effects of endocrine disrupters on pubertal development in the human. Best Pract Res Clin Endocrinol Metab 16:105-121.
Wu T, Buck GM, Mendola P. 2003. Blood lead levels and sexual maturation in U.S. Girls. The Third National Health and Nutrition Examination Survey, 1988-94. Environ Health Perspect 111:737-741.
DHANES Expert Group Members
Marion
Balsam, M.D., F.A.A.P.
Research
Partnerships Program Director
The
National Children's Study
National
Institute of Child Health and Human Development
National
Institutes of Health, DHHS
6100
Executive Blvd., Suite 5C01
Bethesda,
MD 20892-7510
Phone:
301-435-7679
Fax:
301-480-1222
E-mail:
[email protected]
Paul Beatty, Ph.D
CDC/NCHS
Survey Statistician
Office of Research Methodology
3311 Toledo Road, Rm 3113
Hyattsville, MD 20782
Tel: 301-458-4090
Fax: 301-458-4031
Cameron Chumlea, Ph.D.
Fels Professor
Lifespan Health Research Center
Wright State University
School of Medicine
3171 Research Blvd.
Kettering, OH 45420
Tel: 937-775-1428
Fax: 937-775-1422
Email: [email protected]
Mary L. Hediger, Ph.D.
Human Biologist
Epidemiology Branch
Division of Epidemiology
Statistics and Prevention Research
National Institutes of Health
National Institute of Child Health and Human Development
9000 Rockville Pike
Bldg. 6100, Room 7B03
Bethesda, MD 20892-7150
Tel: 301-435-6897
Fax: 301-402-2084
E-mail: [email protected]
John H. Himes, PhD, MPH
Professor
Division of Epidemiology and Community Health
University of Minnesota School of Public Health
1300 South Second Street, Suite 300
Minneapolis, MN 55454 USA
t: 612-624-8210
f: 612-624-9328
Peter A. Lee, MD, PhD
Professor of Pediatrics
Penn State College of Medicine
The Milton S Hershey Medical Center
PO Box 850
Hershey, PA 17033-0850
Phone: 717-531-4751 #5
FAX: 717-531-6139
email: [email protected]
Joan Schall, M.D.
The Children’s Hospital of Philadelphia
The Joseph Stokes Jr. Research Institute
Leonard
& Madlyn Abramson Pediatric Research Center
3535 Market
Street
Philadelphia, PA 19104-4318
(215) 590-5688
Email: [email protected]
Ken Schoendorf, MD, NCHS
Chief, Office of Analysis, Epidemiology
And Health Promotion
NCHS
3311 Toledo Rd. Rm 6115
Hyattsville, MD 20782
Tel: 401-458-4486
Fax: 301-458-4037
PUBERTAL MATURATION VALDIATION STUDY
EXAMINATION STUDY DATA RECORDING FORM
(completed by CNMC health provider during physical examination)
Affix Study Group Sticker
Here
Study Group:
Subject’s Date of Birth (month/day/year): _______/________/______
Subject’s Gender (circle): Male Female
Date of CHNMC Physical: _____/_______/2007
Examiner ID Number (2-digit ID) ____________
Hispanic? (circle) Yes No Don’t know
Race (circle): White Black Other (includes more than one race)
Physical Exam Findings:
Weight at Exam (lb) _______ lb
Height at Exam (inches) _______ inches
Menstruation status (females only - Check one please)
Menses have begun ___ Non-menstruating: ___
Tanner Ratings (please indicate numeric rating 1-5 or Zero for “Not done”/refusal)
Girls Boys
Female breast stage: ___
Female pubic hair stage: ___
Male genital stage: ___
Male pubic hair stage: ___
Affix Study ID Sticker
Here
A-CASI Module Screens for
Girls (Girls 8-9 yr see drawings #1-4)
A-CASI Module Screens for Boys (Boys 8-9 yr see drawings #1-4)
)
Consent Materials
CHILDREN'S NATIONAL MEDICAL CENTER
Department of General and Community Pediatrics
111 Michigan Avenue, NW
Washington, DC 20010
(202) 884-5000
CONSENT TO PARTICIPATE
IN A CLINICAL RESEARCH STUDY AND AUTHORIZATION TO USE PROTECTED HEALTH INFORMATION
TITLE OF STUDY: Validation of an Automated Computer-Assisted Self-Interview (A-CASI) Questionnaire to Assess Pubertal Maturation
PRINCIPAL INVESTIGATOR: Lee Savio Beers, MD; Division of General and Community Pediatrics
"You" refers to "You" or "Your Child" throughout this document
INTRODUCTION: We would like to invite you to be part of a research study at Children’s National Medical Center. Before you decide if you would like to participate, we want you to know why we are doing the study. We also want you to know about any risks (anything unexpected that might happen) and what you will be expected to do in the study.
This form gives you information about the study. Your doctor will talk to you about the study and answer any questions you have. We encourage you to discuss this study with your family and anyone else you trust before making your decision. We will ask you to sign this form to show that you understand the study. If your child is seven years old or older, we may talk to your child about the study and ask your child to sign a form like this one but shorter. We will give you a copy of this form to keep. It is important that you know:
You do not have to join the study;
You may change your mind and stop being in the study any time you want. In some cases however, stopping the study medication early may cause harm to you. Your doctor will discuss this with you.
If we make any important change to the study we will tell you about it and make sure you still want to be in the study.
A. PURPOSE OF STUDY
We, in conjunction with the National Center for Health Statistics, Centers for Disease Control and Prevention, are testing some new questions about body changes that happen when children become teenagers and young adults. We want to know if children and teenagers can answer the questions on their own and how children’s answers compare to a doctor’s check-up. We are asking children and teens between 8 to 19 years of age to test the questions when they come to the clinic for their check-up. It will take about 5 minutes to answer the questions. Your child will be asked to answer two questions about their body development. A computer will be used to show some body drawings and the participants will listen to the questions using headphones. The participants will be in a private room. Nobody can see the participants in the room and nobody will ask them how they answered the questions.
Your child is being asked to be in the study because he/she is between the ages of 8 and 19 years of age. This information is being collected for statistical purposes to describe characteristics of groups rather than individuals.
B. PROCEDURE
If your child participates in the study, he/she will first have a check-up with the doctor. After the check-up, a clinic staff person will bring your child to a private room to answer the study questions. The room has a laptop computer. Your child will listen to the questions using the headphones. The computer will show the body development drawings. Girls will see 2 sets of drawings. Girls will see drawings of breast development and body hair in the private area. Boys will see 2 sets of drawings. Boys will see drawings genital development and body hair in the private area. Your child will be asked to pick the drawings that look the most like their body.
Nobody can see your child’s answers and we will not ask your child to tell us their answers. If your child changes his or her mind, they do not have to answer the questions. Your child can stop at any time.
There are no risks from being in the study. Your child will receive $15 for participating in the study. The study will help us to find out if children and teens can tell us about their body changes. We will compare what children say about their bodies to what the doctors find out when they do a check-up.
C. POTENTIAL RISKS/DISCOMFORT
We do not expect any bad things to happen. It is possible that your child might feel a little bit embarrassed or uncomfortable because the questions are personal. If your child feels upset or uncomfortable, he/she does not have to answer the questions. Your child can stop at any time.
D. VOLUNTARY PARTICIPATION
There will be no penalty or loss of benefits to which you are otherwise entitled if you decide to withdraw from the study.
E. POTENTIAL BENEFITS
Testing the questions is important because the questions were developed for children and teens to answer on their own. The study will help us to find out if children and teens can use the computer and answer the questions by themselves. We can also compare the answers that children and teens give to what doctors and nurses find out when they do a check-up. We hope the questions can be used in studies that are done to learn how children and teens grow and change when they become teenagers and young adults.
F. ALTERNATIVES TO PARTICIPATION
There are no alternatives to the interview for this study.
G. QUESTIONS – WHO TO CALL
We want you to ask questions about any part of this study or consent form either now or at any time in the future. If you have any questions about this study, call the Principal Investigator, Lee Savio Beers, MD at (202) 884 3797 If you believe you have been injured as a result of being in this study, you should call the Principal Investigator, Lee Savio Beers, MD at (202) 884 3797. If you have any questions or concerns about your rights in this research study at any time, please call Children’s National Medical Center’s Manager of Patient Relations, the Chief Academic Officer, or the Chair of the Institutional Review Board of the Children’s National Medical Center. All parties may be reached at (202) 884-5000.
H. CONFIDENTIALITY
We will keep the records of this study confidential. Only the people working on the study will know your name. They will keep this information in case we have to find you later to let you know of any new information that may affect your health. The federal government can review the study records and medical records to make sure we are following the law and protecting the children in the study. Your medical record is confidential, but just like any medical record; there are some exceptions under state and federal law.
HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY
In 1996 the government passed a law known as The Health Insurance Portability and Accountability Act (HIPAA). This privacy law protects your individually identifiable health information (Protected Health Information or PHI). The privacy law requires you to sign an agreement so researchers can use or share your PHI for research purposes. This describes to you how information about you may be used or shared if you are in a research study. It is important that you read this carefully and ask a member of the research team to explain anything you do not understand.
I authorize Lee Savio Beers, MD and her research staff to create, access, use, and disclose my PHI for the purposes described below.
Protected Health Information that may be used and shared includes:
x Information that identifies you such as name, address, telephone number, date of
birth, Social Security number, and other details about you
x Information that relates to your health or medical condition from your medical records
x Information obtained from the study procedures outlined in this consent form, for
example: things done to see if you can join the study such as physical exams, blood
and urine tests, x-rays and other tests, and any other medical information we learn
from you about your health history and family history
x Questionnaires or surveys you complete
x Interviews conducted with you by members of the research team
*Example: list any additional information that may be obtained from participants that is listed above such as information about financial and social circumstances, or educational level.
The Researchers may use and share my Protected Health Information, other than the answers to the private interview, with:
The Principal Investigator, and other Investigators in charge of doing work for the study;
Government agencies that have the right to see or review your PHI, including but not
limited to the Office of Human Research Protections and the Food and Drug
Administration;
Children's National Medical Center Institutional Review Board;
Audit Committee of the Children's National Medical Center Institutional Review
Board;
Quality Improvement Program Coordinator and other staff in the Office for the
Protection of Human Subjects at Children's National Medical Center.
In addition to the above people and organizations, the Researchers may also use and share my Protected Health Information with:
x Doctors and staff at other places that are participating in the study. The name(s)
of the other place(s) that are participating in this study are the Centers for Disease Control and Prevention, National Center for Health Statistics
x The Data Safety Monitoring Board (a group of people who examine the medical
information during the study)
x The Medical Monitor for the Study (a person who reviews medical information during
the study)
x The Patient Advocate or Research Ombudsman (person who watches out for your
best interest)
Also, your primary physician will be contacted if during the course of the study the researcher learns of a medical condition that needs immediate attention.
Should your health information be disclosed to anyone outside of the study, your information may no longer be protected by HIPAA and this Authorization. However, the use of your health information will still be regulated by applicable federal and state laws.
Storage of PHI in a Database:
We would like to store personal health information collected from you in this study in a database for future research. The database is maintained by the Centers for Disease Control and Prevention, National Center for Health Statistics
Please indicate your approval of any or all of the following by initialing next to the statement:
My personal health information may be stored in the above named database for future analysis related to Validation of an Automated Computer-Assisted Self-Interview (A-CASI) Questionnaire to Assess Pubertal Maturation. Yes No _______initials
You do not have to sign this Consent/Authorization. If you decide not to sign the Authorization, you will not be allowed to participate in the research study.
After signing the Consent/Authorization, you can change your mind and:
Revoke this Authorization. If you revoke the Authorization, you will send a written letter
to:Lee Savio Beers, MD; 111 Michigan Ave NW; Washington DC 20010; (202) 884 3797 to inform him/her of your decision.
If you revoke this Authorization, researchers may only use and disclose the PHI
that was collected for this research study before you revoked the Authorization.
If you revoke this Authorization your PHI may still be used and disclosed if you should
have an adverse event (unexpected side effect).
If you change your mind and withdraw the Authorization, you will not be allowed to
participate in the study.
If you have not already received a Notice of Privacy Practices from Children's National Medical Center, you may request a copy and will be given one. If you have any questions or concerns about your privacy rights, you may contact the Children's Hospital Privacy Officer at 202-884-4550.
I. COMPENSATION
You will receive a gift certificate to a local store for $15 in order to compensate you for your time in completing this study.
Children's National Medical Center cannot promise that the risks we have told you about or other unknown problems will not happen. If you think that something unexpected happened because you were in the study, please call the Chief Academic Officer of the Children’s National Medical Center at (202) 884-5000. We will give your child any emergency treatment needed.
J. ADDITIONAL ELEMENTS
Research Subject Advocate:
The National Institutes of Health supports a Research Subject Advocate or RSA for the research study that you are being asked to join. The RSA, Dr. Tomas Silber, is here to answer your questions or concerns about taking part in this research. Dr. Silber does not work for the doctors who are doing this research and they do not pay him. He is here only to help and protect you during any research.
You may contact Dr. Silber at any time. This can be done before you decide to take part in the research, during the study, or even after you finish the study. You can call Dr. Silber at 202-884-3066 or reach him by e-mail at [email protected].
CONSENT/AUTHORIZATION:
I am the participant or I am authorized to act on behalf of the participant. I have read this information and will receive a copy of this form after it is signed.
By signing this form, you agree that you have talked to your doctor about the study and understand it, and you want to be in the study. You agree that we have talked to you about the risks and benefits of the study, and about other choices. You may decide to stop being in this study at any time and no one will mind and nothing will change about your medical care other than not being in the study. Copies of this form will be:
(1) Kept in the study file by the Principal Investigator;
(2) Put in your medical record; and
Given to you to keep.
Please call the Principal Investigator, Lee Savio Beers, MD at (202) 884 3797 if you have any questions.
Printed Name of Participant:
Medical Record Number:
Printed Name of Parent(s)/Guardian(s):
Signature of Participant: Date:
(Participant must be 18 years of age or older)
Signature of Parent(s)/Guardian(s): Date:
Witness (to signatures): Date:
(may be investigator)
Translator’s Signature (if, applicable):
Language:
AFFIDAVIT OF PERSON OBTAINING CONSENT: I certify that I have explained to the above individual(s) the nature and purpose of the study, potential benefits, and possible risks associated with participation in this study. I have answered any questions that have been raised.
Printed Name of Individual Obtaining Consent:
Title: _______ Signature: Date:
Assent Materials
CHILDREN'S NATIONAL MEDICAL CENTER
Department of General and Community Pediatrics
111 Michigan Avenue, NW
Washington, DC 20010
(202) 884-5000
ASSENT (AGES 12 to 18) TO PARTICIPATE
IN A CLINICAL RESEARCH STUDY
TITLE OF STUDY: Validation of a Pubertal Maturation Self-assessment Questionnaire
PRINCIPAL INVESTIGATOR: Lee Savio Beers, MD; Division of General and Community Pediatrics
INTRODUCTION: We would like to invite you to be part of a research study at Children’s National Medical Center. Before you decide if you would like to participate, we want you to know why we are doing the study. We also want you to know about any risks (anything unexpected that might happen) and what you will be expected to do in the study. You can only be in the study if your parent(s) agree(s).
This form gives you information about the study. Your doctor or a research staff member will talk to you about the study and answer any questions you have. We encourage you to discuss this study with your family before making your decision. We will ask you to sign this form to show that you understand the study. We will give you a copy of this form to keep. It is important that you know:
You do not have to join the study;
You may change your mind and stop being in the study any time you want and no one will mind. In some cases however, stopping the study medication early may cause harm to you. Your doctor will discuss this with you;
If we make any important change to the study we will tell you about it and make sure you still want to be in the study.
A. WHAT IS THE REASON FOR THE STUDY?
We are testing questions about body changes that happen during puberty. Puberty is the time when your body changes into the body of a teenager or young adult. We want to know if the study participants can answer the questions.
We are asking children and teens between 8 to 19 years of age to test the questions when they come to the clinic for their check-up. It will take about 5 minutes to answer the questions. You will use a computer and headphones to answer two questions about body development. You will be in a room by yourself to give you privacy. We will not see or ask about your answers to the questions.
B. WHAT WILL HAPPEN IN THE STUDY?
If you decide to participate in the study, you will first have your check-up with the doctor. In accordance to hospital policy, you will be offered the presence of a chaperone. After your check-up, you will go to a private room to answer the study questions. The room has a laptop computer. You will listen to the questions using the headphones. The computer will show you drawings. You will pick the drawings that look the most like our body. You will answer the questions by touching the drawing shown on the computer screen. You will be asked to answer two questions about body development. Nobody can see your answers and we will not ask you about your answers. If you decide you do not want to answer the questions, you do not have to answer the questions. You can stop at any time.
C. WHAT POSSIBLE UNEXPECTED THINGS COULD HAPPEN?
We do not expect any bad things to happen. It is possible that you might feel a little bit embarrassed or uncomfortable because the questions are personal. If you feel upset or uncomfortable, you do not have to answer the questions. If you decide you do not want to answer the questions, it is okay. You can stop at any time.
D. WHAT POSSIBLE GOOD THINGS COULD HAPPEN?
Testing the questions with children and teens is important because we want to find out if teens can answer questions about their bodies. We hope the questions can be used in studies that are done to learn how children and teens grow and change as they become young adults.
Study participants will receive $15 gift cards.
WHAT OTHER CHOICES DO YOU HAVE IF YOU DO NOT WANT TO BE IN THE STUDY
You can decide if you want to participate in the study. It is your choice. If you decide that you do not want to be in the study that is fine.
F. HOW WILL WE KEEP YOUR RECORDS PRIVATE?
We will keep the records of this study confidential. Only the people working on the study will know your name.
ASSENT
By signing this form, you agree that you have talked to your doctor about the study and understand it, and want to be in the study. You also agree that you have been told about the risks (unexpected things) and benefits (good things) of the study, and about other choices. You may stop being in the study at any time and no one will mind and nothing will change about your medical care other than not being in the study. Please call the Principal Investigator, Lee Beers., at 202-884-3797 if you have any questions.
Printed Name of Participant: _______
Medical Record Number: _______________________
Signature of Participant:
Witness
(to signature):
Date:
(may
be investigator)
Translator’s Signature (if, applicable): Date:
Language:
AFFIDAVIT
OF PERSON OBTAINING ASSENT: I
certify that I have explained to the above individual(s) the nature
and purpose of the study, potential benefits, and possible risks
associated with participation in this study. I have answered any
questions that have been raised.
Printed Name of Individual Obtaining Consent: _______
Title: _______ Signature: Date:
| File Type | application/msword |
| File Title | Study Objective: The objective of the study is to validate a new audio-computer-assisted interview methodology used for sexua |
| Author | mxm7 |
| Last Modified By | vlb2 |
| File Modified | 2007-11-28 |
| File Created | 2007-11-25 |
© 2026 OMB.report | Privacy Policy