CMS-10246.Supporting Statement Part B
CMS-10246.Supporting Statement Part B.doc
Cost and Resource Utilization (CRU) Data Collection for the Medicare Post Acute Care Payment Reform Demonstration
OMB: 0938-1038
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
CENTERS FOR MEDICARE AND MEDICAID SERVICES
COST AND RESOURCE UTILIZATION (CRU)
DATA COLLECTION FOR THE MEDICARE
POST ACUTE CARE PAYMENT REFORM DEMONSTRATION
OFFICE OF MANAGEMENT AND BUDGET
CLEARANCE PACKAGE SUPPORTING STATEMENT – PART B
August 1, 2007
CONTENTS
B. COLLECTION OF INFORMATION EMPLOYING STATISTICAL METHODS 1
B.1 Respondent Universe 1
B.2 Procedures for the Collection of Information 1
B.3 Maximizing Response Rates 3
B.4 Tests of Procedures 3
B.5 Contacts 4
OFFICE OF
MANAGEMENT AND BUDGET
CLEARANCE PACKAGE SUPPORTING STATEMENT
B. COLLECTION OF INFORMATION EMPLOYING STATISTICAL METHODS
B.1 Respondent Universe
The CRU (Non-Therapist) Staff Activity Form or the CRU Therapy Staff Activity form will be completed daily by all clinical staff treating Medicare beneficiaries during the three two-week data collection periods in each of the institutional providers participating in the demonstration. Respondents will include nurses, nurse aides, therapists, therapy aides, social workers, discharge planners, pharmacists, and managers. The number of staff filling in the activity forms will depend on the size of the providers recruited to participate in the study and the provider-specific staffing patterns. Though the providers have not yet been recruited to participate, RTI and CMS estimate collecting approximately 56,496 Staff Activity Forms over the course of the data collection period at the 90 institutional providers that will be recruited to participate in the CRU data collection (3 Acutes, 11 LTCHs, 28 IRFs, and 48 SNFs). Similarly, the Home Health Patient Time Log will be completed by all home health staff treating Medicare beneficiaries during the three two-week data collection period in each of the 48 participating home health agencies. RTI and CMS estimate collecting approximately 4,935 Home Health Patient Time Logs during the study period.
The Patient Ancillary Service Log Form and the Consultant Log Form will be filled in by the site coordinators at each of the 90 participating institutional providers. This form will be filled out each day in order to track the number of high cost ancillary services provided to patients in each institutional provider and the number of off unit clinicians treating study patients.
The Study Patient Tracking Form will be filled out by the site coordinator at each of the 138 providers participating in the CRU data collection. One tracking form will be submitted for each of the three data collection periods to record patient study identifications and Medicare Health Insurance Claim numbers.
Prior to data collection at each of the participating providers, RTI and CMS will interviews selected staff members at each of the providers. Interviews will be conducted with the nurse manager, the therapy manager, the chief financial officer, and a senior administrator at each of the 138 providers participating in the CRU data collection. Interviews with a senior administrator and nurse manager will also be conducted at each 12 acute providers not collecting CRU data.
B.2 Procedures for the Collection of Information
Statistical Methodology for Stratification and Sample Selection
The Post-Acute Care Payment Demonstration features a hierarchical “clustered” design: beneficiaries within providers, providers within markets, and markets within the U.S. Ten markets will be selected based on the presence of the range of post-acute care providers, provider referral patterns, and provider willingness to participate in the demonstration. Other considerations in market selection include distribution of post-acute care ownership types (e.g., profit status, chain ownership, etc.), managed care penetration, census division, and rural/urban mix.
Once markets have been identified, providers will be recruited for participation. These will include providers volunteering to participate as well as those that CMS solicits for participation. Fifteen providers in each market will be recruited to participate. These providers will include at least one acute hospital as well as skilled nursing facilities, home health agencies, inpatient rehabilitation facilities, and long term care hospitals in the hospital’s referral network.
The CRU data collection will take place with all staff members treating Medicare patients during three two-week data collection periods over nine months. Interviews with therapy managers, nurse managers, administrators, and finance officers will take place at the participating providers prior to the data collection period.
Sample Size Estimation
We start from the premise that there will be 150 facilities in 10 markets (acute hospitals and post-acute providers) that agree to participate. Note that of the 15 acute providers recruited to participate in the demonstration, only 3 acute providers will participate in the CRU data collection. The notion of a “necessary” sample size is tied to the need for statistical power to identify as significant true differences in mean per diem costs of a subgroup from the overall average.
Because the analysis requires consideration of single-setting payment models as well as multiple-setting setting-neutral payment systems, we computed required sample sizes for each CRU collection setting (LTCHs, IRFs, HHAs, and SNFs) separately. Since Medicare patient days in LTCHs and IRFs comprise a relatively small share of all PAC facility (non-HHA) Medicare days, our approach effectively oversamples LTCHs and IRFs to increase the power for identifying case mix groups that may be disproportionately served in those settings.
We conducted a power analysis for a regression model based on an F-test of coefficient estimates (Taylor and Muller, 1995). For this power analysis we want to be at least 90 percent sure that we can detect true ten percent differences in cost between a group no smaller than 20 percent of the population and the cost of the overall average patient. This power analysis yielded estimates of the number of patient days of CRU data collection required for the analysis
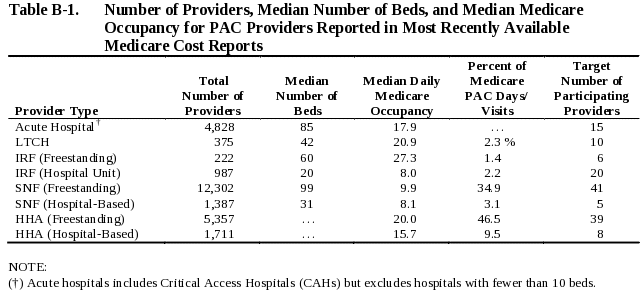
To translate patient days into data collection periods, we estimated the number of each type of facility we plan to recruit as well as the typical number of Medicare census in each setting. We examined Medicare Cost Reports to find the number of each type of provider and the median number of beds and daily Medicare occupancy reported in Fiscal Year 2004 or 2005. As a result of these analyses, we have defined three two-week data collection windows in each market. Markets will vary in terms of the availability of the range of post-acute providers, but all markets will be anchored by one or two acute hospitals. Ten or 11 other providers will be recruited to participate in the demonstration in each market area and will include LTCHs, freestanding and hospital based IRFs, freestanding and hospital-based SNFs, and freestanding and hospital-based home health agencies. The target number of each type of post-acute provider that will be recruited to participate is as shown in Table B-1.
B.3 Maximizing Response Rates
One person will be assigned as a project liaison in each participating provider to oversee the data collection process and provide feedback to the CMS and RTI team during the nine-month data collection. This person will be the study coordinator at each site and will ensure that CRU Forms are collected for all staff treating Medicare beneficiaries during the data collection periods. The site coordinators at institutional providers will also be responsible for monitoring the Ancillary Service Log Form , Consultant Log Form, and Patient Tracking Form. The site coordinator will be responsible for ensuring completeness of data prior to submission to RTI and CMS.
Data quality is a critical to meeting the goals of the Post-Acute Care Payment Reform Demonstration. RTI will review data as it is submitted to check for data quality issues. Any problems identified during the data collection period will be noted and RTI will retrain participating providers in order to ensure data quality. RTI will continue to monitor data quality throughout the data collection periods in each market and will contact providers when problems are identified. RTI will host a website for data coordinators to access and Frequently Asked Questions (FAQ) will be posted to address issues that arise during data collection. RTI will also support a help-desk for any questions that assessors have in filling out the CRU instruments.
The chief financial officer, senior administrators, nurse managers and therapy managers will be contacted as part of the provider recruitment process and interviews with individuals in these key positions will be scheduled prior to the start of data collection.
B.4 Tests of Procedures
Limited tests were already conducted.
B.5 Contacts
Shannon Flood, Project Officer, Post-Acute Care Payment Reform Demonstration
Office of Research Development and Information
Phone: 410-786-2583
Email: [email protected]
Barbara Gage, Project Director
RTI International
Phone: 781-434-1717
Email: [email protected]
Melvin Ingber, Associate Project Director
RTI International
Phone: 202-974-7868
Email: [email protected]
| File Type | application/msword |
| File Title | Supporting Statement for Administering the (to be named) Survey |
| Author | CMS |
| Last Modified By | CMS |
| File Modified | 2007-11-29 |
| File Created | 2007-11-29 |
© 2026 OMB.report | Privacy Policy