Significant New Alternatives Policy (SNAP) (40 CFR Part 82, subpart G)(Renewal)
Significant New Alternatives Policy (SNAP) Program (40 CFR part 82, subpart G) (Renewal)
SNAP FORM- Updated Guidance Documen04.30.08
Significant New Alternatives Policy (SNAP) (40 CFR Part 82, subpart G)(Renewal)
OMB: 2060-0226
U.S. ENVIRONMENTAL PROTECTION AGENCY
GUIDANCE DOCUMENT FOR THE SIGNIFICANT NEW ALTERNATIVES POLICY (SNAP)
PROGRAM INFORMATION NOTICE
EPA-1265-07May, 2008 Office of Atmospheric Programs Washington, DC 20460
The U.S. Environmental Protection Agency (EPA) has prepared this manual to assist you in submitting information on alternatives to ozone-depleting chemicals to the Significant New Alternatives Policy (SNAP) program under section 612 of the Clean Air Act Amendments (CAAA) of 1990. This manual provides instructions on submitting a SNAP notice, asserting confidentiality claims, and submitting test data and optional information. However, please note that in the event of any discrepancies between this Document and the Code of Federal Regulations (CFR), the CFR interpretation holds legal sway.
TABLE OF CONTENTS
1. INSTRUCTIONS FOR COMPLETING THE SNAP INFORMATION NOTICE 1
A. General Instructions 1
B. Confidentiality Claims 2
C. Test Data and Other Data 3
D. Who Must Submit a SNAP Information Notice 4
E. When to Submit a SNAP Notice 6
F. Completing the Notice Form 6
Part I - GENERAL INFORMATION 6
Part I, Initial Information 6
Part I, Section A - Submitter Identification 6
Part I, Section B - Alternative Identification (Page 4) 7
Part II - ALTERNATIVE-SPECIFIC INFORMATION 9
Part II, Section A - Physical and Chemical Properties (Page 6) 9
Part II, Section B - Atmospheric Information 10
Part II, Section C - Other Statutes 11
Part II, Section D - Cost and Availability of the Alternative 11
PART III - RELEASE AND EXPOSURE DATA 12
Part III, Section A - Toxicity and Hazard Information 12
Part III, Section B - Environmental Release and Disposal at Manufacture 13
Part III, Section C - Occupational Exposure at Manufacture 13
Part III, Section D - Environmental Release and Disposal in End-use 13
Part III, Section E - Occupational Exposure at End-use 13
Part III, Section F - Consumer Exposure 14
Part III, Section G - General Population Exposure 14
Part IV - LIST OF ATTACHMENTS (Page 14) 14
Part V - CERTIFICATION (Page 15) 14
2. SUPPLEMENTARY INFORMATION 15
A. Substitutes That Must Be Reported by Designated Submitters 15
B. Substitutes Excluded From Notification Requirements 15
C. Joint Review with Other EPA Offices 16
D. Consultation with EPA Concerning the SNAP Information Notice 18
E. EPA Contact Information 18
APPENDIX A: LIST OF END-USES WITHIN SECTORS INCLUDED IN THE SNAP PROGRAM 19
APPENDIX B: SECTOR-SPECIFIC DATA REQUIREMENTS 21
1. INSTRUCTIONS FOR COMPLETING THE SNAP INFORMATION NOTICE
A. General Instructions
The Significant New Alternatives Policy (SNAP) program is a regulatory program developed pursuant to section 612 of the Clean Air Act (CAA). SNAP is designed to :
$ Identify and evaluate substitutes for class I and class II ozone-depleting substances (ODSs)
$ Look at overall risk to human health and the environment of both existing and new substitutes
$ Publish lists of acceptable and unacceptable substitutes by end-use
$ Promote the use of acceptable substitutes for ozone-depleting compounds
To arrive at determinations on the acceptability of substitutes, the Agency performs a cross-media analysis of risks to human health and the environment from the use of various substitutes in different industrial and consumer uses. The SNAP program evaluates substitutes in the following sectors:
$ refrigeration and air-conditioning
$ foam blowing
$ solvent cleaning
$ fire suppression and explosion protection
$ tobacco expansion
$ adhesives, coatings and inks
$ aerosols
$ sterilants
For additional information on the SNAP program and a copy of the existing listing decisions, please call the Stratospheric Ozone Hotline at (800) 296-1996.
For the purposes of this document, EPA is using the word "substitute" as a synonym for "alternative". As defined in the final SNAP rule (59 FR 13044), a substitute is "any chemical, product substitute, or alternative manufacturing process, whether existing or new, intended for use as a replacement for a class I or II compound."
You must file a separate notice for each substitute you submit for evaluation. However, a single alternative may be submitted for multiple end-uses simultaneously provided that the appropriate information is submitted for each specific end-use. See Appendix A for a list of sector end-uses included under SNAP. If you submit a substitute for review under more than one sector, you may wish to complete a separate notice for each sector.
Please type the SNAP form or print legibly in black ink. All information must be in English. Provide all information requested on the notice form to the extent that you know or can reasonably ascertain it. If you do not know or cannot reasonably ascertain the information, enter "unknown" (for not known). However, if you cannot answer question that is needed for evaluation of the substitute within a given sector (see Appendix B), review of the substitute may be delayed. You may attach continuation sheets to any subsection or item on the form. You may photocopy the notice form, sections of the form, or this manual as frequently as you need. In fact, EPA suggests you make a clean copy of the form for future use before adding any information.
The SNAP staff includes specialists in each of the industrial use sectors for which substitutes must be submitted. EPA recommends that you contact the appropriate sector specialist before submitting your application to ensure completeness. Please call the Hotline at 1-800-296-1996 to be referred to the appropriate person.
Send your completed submission notice to the SNAP Document Control Officer (6205J), whose address appears on page 1 of the form. You must submit three copies of the submission to EPA. If information is claimed as confidential, it must be removed from one of the copies, which will be placed in the public docket; the other two copies must include all confidential material. If you do not claim any information as confidential, all copies must be identical. (See below for further guidance on handling of confidential information under SNAP.)
There is no application fee for submitting information to the SNAP program.
B. Confidentiality Claims
1. Asserting Claims
If you submit information for which you request Confidential Business Information (CBI) status, you must assert a claim of confidentiality at the time of submission. If you do not assert a claim of confidentiality at the time of submission, EPA may disclose the information to the public without further notice to you. Information which is publicly available (e.g., in journals, trade magazines, product literature, etc.) cannot be claimed as CBI. Requesting CBI status for such public information could delay review under section 612. All claims of confidentiality will be treated in a manner consistent with 40 CFR Part 2, Subpart B.
In addition, you should be aware that under CAA section 114(c), emissions data may not be claimed as confidential. There are other instances in which confidentiality assertions may later be reconsidered, even when confidentiality claims are originally received. This could occur, for example, when a Freedom of Information Act (FOIA) request on a particular substitute is received by the Agency. These circumstances and others are described in the provisions of 40 CFR Part 2, Subpart B. You will be contacted as part of this evaluation process if and when any reconsideration of the confidential status of your data occurs.
To claim information as CBI, circle or bracket the specific information you claim as confidential and check the box at the bottom of the page. Then provide substantiation of this claim on an attachment to the notice. EPA requires substantiation of all CBI claims under SNAP or a submission will be considered incomplete. For example, if you submit a study which describes a physical or chemical property, and it is only that property which you wish to claim as confidential, bracket only that property. Do not simply stamp "Confidential" on the page which contains that property.
If you claim the identity or formulation of a substitute as confidential, you must provide a generic description of this information. In addition to guidance on developing generic names that is provided in Section 1.F. of this document, EPA strongly recommends contacting the appropriate sector specialist for assistance in this area.
As stated above, you must provide three copies of your submission. If you do not claim any information as confidential, all copies must be identical. When portions of the submission are claimed as CBI, however, only two copies should include CBI material. To ensure that no confidential information is disclosed to the public, you must delete all CBI from the third "sanitized" copy, including attachments, which will become part of the public docket. The following special preparation is required for the third copy:
$ Remove from the body of the submission any information you claim as confidential. Replace with generic information if it is available.
$ Mark the third copy plainly on both its cover and its title page with the phrase "Public Docket Material -- contains no information claimed as confidential."
If you claim CBI but do not provide the sanitized copy with your submission, the submission will be incomplete and an acceptability determination on your product will not be published. EPA recognizes that substantial portions of submissions may be omitted from the public docket copy. However, all CBI claims must be substantiated to warrant restricting access to such information.
2. Substantiating CBI Claims
EPA requires substantiation of all confidentiality claims at the time of submission. In making these claims, the following provisions apply:
$ The specific information to which the claim applies must be clearly marked in the body of the study as subject to a claim of confidentiality;
$ A `Supplemental Statement of Data Confidentiality Claims' must be submitted, identifying each section claimed confidential and describing in detail the basis for the claim. (See below for specific points that should be addressed);
$ The `Supplemental Statement of Data Confidentiality Claims' must be signed and dated and must include the typed name and title of the official who signed it.
If you do not provide the required substantiation when submitting information claimed as confidential, EPA may make the complete submitted information available to the public without further notice.
Supplemental Statement of Data Confidentiality Claims
For any portion of a submission that you claim as confidential, the following information must be included within a Supplementary Statement of Data Confidentiality Claims:
$ Identify specifically by page and line number(s) each portion of the study for which you claim confidentiality.
$ Give the reasons why the cited passage qualifies for confidential treatment.
$ Indicate the length of time - until a specific date or event, or permanently - for which the information should be treated as confidential.
$ Identify the measures taken to guard against undesired disclosure of this information.
$ Describe the extent to which the information has been disclosed, and what precautions have been taken in connection with these disclosures.
$ Enclose copies of any determinations of confidentiality made by EPA, other Federal agencies, or courts concerning this information.
$ If you assert that disclosure of this information would be likely to result in substantial harmful effects to you, describe those harmful effects and explain why they should be viewed as substantial.
$ If you assert that the information is voluntarily submitted, indicate whether you believe disclosure of this information might tend to lessen the availability to EPA of similar information in the future, and if so, how.
3. Confidentiality Provisions for Toxicity Data
Toxicity or health and safety studies, to the extent that confidential treatment is prohibited under the Toxic Substances Control Act (TSCA) or the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), cannot be held confidential. However, other information can be maintained as confidential subject to the provisions of 40 CFR, Part 2, Subpart B, so long as it is: a) any information other than emissions data contained in the toxicity study, b) not health and safety data, and c) not relevant to the effects of a substance on human health and the environment (e.g., discussion of process information, proprietary blends). The Agency is therefore requesting that you do not identify the following information as confidential: all information concerning the objectives, methodology, results, or significance of any toxicity test or experiment performed on or with a substitute or its degradation products; any information concerning the effects of the substitute on any organism (e.g., fish, wildlife, humans and other mammals) or the environment (e.g., studies related to persistence, translocation, and fate); and pharmacokinetics/metabolism studies.
4. Contractor Access
Information submitted as CBI may be accessed by companies designated as Authorized Representatives of the United States Environmental Protection Agency (EPA) under an EPA contract for the purpose of assisting EPA in the development and implementation of national regulations for the protection of stratospheric ozone, including the development of the SNAP program. These Authorized Representatives may have access to any information received by the Stratospheric Protection Division within EPA's Office of Atmospheric Programs for use in reviewing the need for possible control of any substance, practice, process or activity that may reasonably be anticipated to affect stratospheric ozone. In general, this information will pertain to the feasibility, costs, and environmental and health impacts of using substitutes for class I and class II substances. Access to such information is necessary to ensure that these companies can complete the work required by the contract.
Authorized Representatives of EPA are subject to the provisions of 42 U.S.C. 7414(c) respecting confidential business information as implemented by 40 CFR 2.301(h).
C. Test Data and Other Data
You are required to provide test data on the health and environmental effects of alternatives, including data on physical/chemical properties, in your possession or control, and a description of any other health and environmental effects data on the substance known to or reasonably ascertainable by you. The Agency holds the position that data in the possession or in control of either a parent company or an affiliated subsidiary located outside the U.S. are considered data that should be known to or reasonably ascertainable by a submitter.
Complete test data, not summaries of data, must be submitted if they do not appear in the published literature. Incomplete reports (e.g. from ongoing studies) are exempt from full reporting. However, you must describe the nature and objective of any incomplete study, report, or test; the name and address of any laboratory developing the data; progress to date, type of data collected; significant preliminary results; and an anticipated completion date. If significant preliminary results or final results are obtained prior to the completion of the notice review period or any other additional information significant to the review of the notice becomes available to you, you must submit this information within 10 days of receipt. If information becomes available during the last 5 days of the review period, you should immediately inform EPA by telephone. Data must be submitted in English.
Examples of the types of test data you must submit for each sector are provided in Appendix B at the end of this manual. For additional information on health and safety studies and on submitting test data, see '82.172 and 82.178 of the SNAP Rule and Section 1.F. of these instructions.
D. Who Must Submit a SNAP Information Notice
1. Designated Submitters
As required by section 612(e) of the Clean Air Act Amendments (CAAA) of 1990, anyone who produces a substitute for a class I ozone-depleting substance must provide the Agency with that person's unpublished health and safety studies on the substitute, as well as notify the Agency at least 90 days before introducing the substitute or a product using the substitute into interstate commerce for significant new use as an alternative. Also, pursuant to sections 114, 301 and 612(c), producers of class II substitutes must abide by the same reporting requirements (see '82.176 of the SNAP Final Rule).
The Agency recognizes that a substitute can potentially pass through numerous steps prior to its introduction into interstate commerce. Therefore, the Agency considers responsibility for notification under SNAP to rest with the person who produces the substitute in its final form for interstate commerce for purchase by an end-user. Under this definition, the designated submitter could potentially be a manufacturer, formulator, or an end-user.
a. Chemical Manufacturers
Chemical manufacturers producing a substitute in its final form for direct commercial sale to the end-user are required to notify the Agency about the existence of that substitute. For instance, if a chemical manufacturer intends to market a chemical as a substitute foam blowing agent to companies that manufacture insulation products, the chemical manufacturer would be required to notify the Agency about the existence of the substitute. If the substitute is a new chemical, it must also undergo simultaneous review under EPA's TSCA Premanufacture Notice (PMN) program. For more information on this simultaneous review process, see Section 2.C of this document.
b. Formulators
A formulator is engaged in the preparation or formulation of a substitute, after chemical manufacture of the substitute or its components, for distribution or use in commerce. Formulators usually only sell substitutes based on existing chemicals, since they do not ordinarily possess chemical manufacturing capabilities. Chemicals used in such substitutes are frequently in common use and have already been approved for general use through other chemical review programs such as TSCA or FIFRA.
However, these formulators can be considered directly responsible for production of the substitute for an end-use. For example, by offering a specific solvent formulation for an industrial cleaning process, formulators would be subject to reporting requirements under SNAP. In such cases, the formulator is best suited to present information on how substitutes based on existing chemicals are or could be used. In cases where the manufacturer of a chemical is also the formulator of a blend, the manufacturer is responsible for meeting reporting requirements on the substitute.
c. End-users
In general, end-users of substitutes are not obligated to meet SNAP reporting requirements, except in rare cases where the end-user is also the substitutes=s producer that will be the first to introduce it into commerce While the Agency expects that this situation will occur infrequently, several large companies have developed substitutes for their own use and subsequently have notified EPA of their intent to offer those substitutes for commercial sale. Because EPA requires end-users to report only on those substitutes they introduce into interstate commerce, evaluating and listing such substitutes will not stifle research and development innovations by end-users.
2. Petitions
The SNAP program publishes lists of acceptable and unacceptable substitutes. However, section 612(d) of the CAA explicitly states that "any person may petition the Administrator to add a substance ... or to remove a substance from either of such lists." The petition provision serves two principal needs. The first is to give the public a way to appeal existing Agency determinations under the SNAP program. The second is to provide a mechanism for individuals and organizations to bring to the Agency's attention new information on substitutes that could affect existing listing determinations or result in new ones.
The opportunity for outside parties to comment on existing listing decisions is an important aspect of the petition process. As discussed in the section on notifications, companies that produce substitutes must submit specific data on the substitutes to the Agency for review. However, organizations and private citizens other than those required to submit SNAP notices may have additional information about existing substitutes or information on new substitutes not yet reviewed by the Agency. To ensure that the SNAP determinations are based on the best information on substitutes, it is essential that the Agency offer a means for such information to be incorporated into the SNAP analyses on a continuing basis.
Before individuals, organizations, or companies may initiate legal action against EPA for the purpose of changing the lists of acceptable or unacceptable substitutes, they must first exhaust all administrative remedies for receiving such relief, including remedies like the petition process described in this section.
a. Types of Petitions
Five types of petitions exist:
(1) Petitions to add a substitute not previously reviewed under the SNAP program to the acceptable list;
(2) Petitions to add a substitute not previously reviewed under the SNAP program to the unacceptable list;
(3) Petitions to move a substitute from the acceptable list to the unacceptable list or to move a substitute from the unacceptable list to the acceptable list;
(4) Petitions to add or delete use restrictions on an acceptability listing, and
(5) Petitions to grandfather general use of a substitute found "unacceptable" or "acceptable subject to narrowed use limits" in specified applications within an end-use.
Petitioners should note that the first type of petition is comparable to completing a SNAP submission, except that the latter is submitted by substitute producers. The first type of petition, by contrast, would generally be initiated by entities other than the company responsible for producing the substitute. Companies that manufacture, formulate, or use a substitute themselves and want to have their substitutes added to the acceptable list should submit information on the substitute under the 90-day advance notification review program.
b. Basis for Petition
A petitioner may submit a petition for several reasons, including:
$ Availability of new information on substitutes or end-uses not covered in the existing SNAP determinations;
$ Requests to extend the effective date for prohibitions on uses of an unacceptable substitute;
$ New technologies or practices that reduce exposure to a substitute previously unacceptable under SNAP due to toxicity or flammability concerns.
All of the above are examples of valid justifications for submitting a petition. Other bases for petitioning the Agency may exist as well, and all petitions with adequate supporting data will receive consideration under the SNAP program.
c. Content of the Petition
Petition types (1) and (2) must contain the information requested in the SNAP Information Notice. Information requirements for such petitions and 90-day notifications are the same, since the Agency will be applying the same scrutiny to petitions submitted by outside parties as to notifications received from the manufacturers themselves. For petition types (3) and (4), which request a reexamination of a substitute previously reviewed under the SNAP program, the submitter may reference the prior submission rather than submit duplicate information. In this case, the petitioner should specifically indicate any new or additional data and submit complete test reports.
As described in the SNAP Final Rule, EPA must consider a specific test to determine whether grandfathering should be allowed. This test involves balancing the results of four analyses, including whether the new rule represents an abrupt departure from previously established practice, the extent to which a party relied on the previous rule, the degree of burden which application of the new rule would impose on the party, and the statutory interest in applying the new rule immediately. Thus, petition type (5) requires information about the current level of use of the substitute and other information to allow EPA to conduct these analyses.
For all petitions, the Agency also requires the following information:
$ Action requested: A brief statement describing the type of petition; and
$ Rationale: A brief summary of the basis for the petition and the data that support the petition.
d. Nature of Response
EPA will only review and grant or deny petitions based on the sector and end-use identified in the petition. For example, the fact that a substitute is placed on the list of unacceptable substitutes for a particular end-use in the solvents cleaning sector does not mean the substitute is unacceptable for any specific end-use as a refrigerant. A similar caveat applies for petitions on end-uses within a sector. If a substitute, for instance, is found acceptable for a specific end-use within a sector, it will not automatically be deemed acceptable for any other end-use in that sector.
E. When to Submit a SNAP Notice
You must submit a SNAP notice at least 90 days before you begin sale of a new substitute for an ozone-depleting substance. A petition may be submitted any time adequate data exists for EPA consideration.
F. Completing the Notice Form
Please check the type of notice submitted on page 1 of the form. If this notice is being submitted as part of a petition to the Agency, indicate the type of petition. See Section 1.D for an explanation of the different types of petitions.
Part I - GENERAL INFORMATION
You must complete all of Part I for your submission to be accepted for review under SNAP. See below for sector-specific data requirements in this section and others.
Part I, Initial Information
Please check the boxes on page 2 indicating the sector(s) for which you are submitting information on the substitute and the types of test data included in your submission. Attach all test data to the notice form and reference them by page number in Part IV-List of Attachments (pg. 14).
Part I, Section A - Submitter Identification (Page 3)
1a. Person submitting notice - Enter information for the official who signs the certification on page 15.
b. Agent - Complete only if you authorize an agent to assist you in preparing this notice. The agent must also sign the certification as noted above.
c. Joint Submitter - Identify the joint submitter, if any, who is authorized by the primary submitter to provide some of the information required in the notice. A notice will not be considered complete until all information is received by the Agency. If information from multiple parties will not be sent together, contact the SNAP Document Control Officer (DCO) for information on submission tracking numbers to link multiple notices.
If you authorize another person (e.g. a foreign manufacturer or supplier) to provide information directly to EPA, indicate which information will be supplied by the other person. Identify that person by name, company, and address in a continuation sheet. Such a letter in support of your notice should be provided by the other person on company letterhead. An example of where this option could apply would be in situations where alternative formulation information is held confidentially by a foreign manufacturer. A notice will be considered incomplete until this information is provided. Whenever possible, use EPA's submission tracking number to link this information to your submission.
2. Technical Contact - Identify a person who can provide EPA with additional technical information on the substitute during the review period. The technical contact identified should be located within the U.S. and be available to be reached by telephone during normal business hours.
3. Provide information on any prior communication with EPA regarding this submission. Provide the EPA staff person's name, the date, and the form of communication (e.g. letter, phone). Mark the box in the lower right if no prior communication has occurred.
Part I, Section B - Alternative Identification (Page 4)
EPA must receive a complete and unambiguous identification of the new substitute. If the alternative is not adequately identified, we will consider the submission incomplete, and the 90-day notice review period will not begin. If you are an importer of an alternative and do not know the chemical identity of a substitute, you must contact the foreign manufacturer or supplier and have the specific chemical identity provided directly to EPA. In this way, foreign manufacturers can protect confidential business information. The same holds for U.S. manufacturers reporting substitutes using a generic or trade name to identify a component of the substitute. The submitter of the substitute must have the supplier provide chemical information directly to EPA before the notice can be considered complete. This information may be provided in a letter of support on company letterhead from the supplier or in a joint submission. Since a letter of support or a joint submission may be received separately by the Agency, EPA's submission tracking number should be used to link a submission or petition with information from a supplier or foreign manufacturer to avoid delay in the review process.
1. Commercial/Trade Name(s) of the Alternative - Indicate the name(s) under which the alternative is marketed.
2. Name of Chemical, Process, or Alternative Technology - Enter the specific name of the chemical substance, the Chemical Abstracts Service (CAS) registry number, and the molecular formula of the alternative. In describing chemical substances, EPA prefers that International Union of Pure and Applied Chemistry (IUPAC) nomenclature be used for identification purposes. If the substitute is a blend of chemicals, you must provide the exact composition and/or the range of percent composition of all components (where appropriate) of a substitute. In addition to active ingredients, you must also list other chemical substances, such as solvents, inhibitors, etc., that may also be present in the alternative.
For alternative technologies and/or processes, provide a detailed description and diagram of the technology or process and information on any chemical constituents.
Also, if you have applied for or hold a patent on the substitute, provide the patent name and number. If you are waiting for a response to a patent application, indicate the date you submitted the application and the expected time for a response. Provide a copy of all patents that have already been granted on the substitute as an attachment.
Sector-Specific Data Requirements
Solvents; Aerosols; Adhesives, Coatings, & Inks: Give the function of each constituent as well as the percent composition. For example, in an aerosol product, such a list may include:
HFC-134a (propellant)
dimethyl ether (propellant)
methanol (solvent)
silicone (active ingredient).
3. Generic name - If the identity of a substitute is claimed as confidential or if the formulation or process is such that it cannot be identified by its individual chemical components, you must provide a generic name that is only as generic as necessary to protect the confidential identity. The name should reveal the chemical identity or alternative process description to the maximum extent possible. The generic name will be published in the Federal Register notice and acceptability determination of the alternative. If the name seems more generic than necessary, EPA will contact you and assist you in developing an adequate name. EPA strongly prefers to avoid trade names or marketing information in listing substitutes.
EPA encourages you to contact the sector specialist to discuss the development of a generic name. This name should provide sufficient information for the public by indicating the classes of chemicals which the alternative contains without revealing specific information about the product's composition. For example, it may be necessary to reveal that a refrigerant blend contains an HCFC in order to allow users or importers to comply with regulations issued under sections 604or 608 of the CAA.
When reviewing the SNAP lists for the solvents, aerosols, and adhesives, coatings and inks sectors you will notice that many of the listings are grouped into general classes of substitutes. If your substitute is already included under one of these classifications, you need to submit information to EPA for review under SNAP only if your substitute contains a new chemical which is subject to review under the PMN program.
4. Specific End-use - Identify the specific end-uses in which the alternative is to be used. Be sure to provide a broad description (e.g. terpene-based semi-aqueous solvent cleaner of metal parts as an alternative for methyl chloroform, or HFC refrigerant as an alternative for CFC-12 centrifugal chillers.) See Appendix A to this document for a list of end uses in each industrial sector.
Specify the ozone-depleting substance (ODS) being replaced, and include an estimate of the quantity of alternative (lbs.) needed to replace the ODS for each end-use. This is known as the replacement ratio. For example, if a solvent formulation contained 25% CFC-113 and now requires 50% petroleum distillate instead of CFC-113 to achieve similar performance, the replacement ratio is 2:1. Similarly, if 100 pounds of a new refrigerant will replace 150 pounds of CFC-12, the replacement ratio is 1:1.5.
See Appendix A for a list of end-uses included under SNAP. If you are proposing a new end-use, please indicate why the substitute does not fit into an existing one.
Sector-Specific Data Requirements
Refrigerants: Indicate whether the alternative is a candidate for use in retrofits of existing equipment, for use in new equipment only, or both. If the alternative can be used both as a retrofit and in new equipment, these uses should be treated as separate end-uses throughout the notice. If the substitute is a chemical replacement, provide the size of the average charge used in each end-use. Describe the technolgy or the industry standard that will be used to recover the substitute.
Foams: If the alternative blowing agent can be used in several different types of foam, treat each end-use separately throughout the notice.
Fire Suppression: Provide information on the weight and storage volume equivalence replacement ratio for the substitute versus the ODS being replaced, using the method described in Appendix A.
Tobacco Expansion: Specify the base replacement ratio values on the amount of new expansion agent consumed per unit mass of tobacco compared to the amount of CFC-11 consumed per unit mass.
5. Impurities - Identify by name, weight percent, and CAS number (where available) each impurity that you reasonably anticipate will be present in the alternative as manufactured for commercial purposes. An impurity is any chemical substance that is unintentionally present in the alternative. List all impurities, regardless of weight percent. If the substance contains some unidentified impurities, also enter "unidentified". Do not include any substances that are mixed with the new substance after manufacture of the primary ingredients. If there are no impurities, enter "None."
6. Byproducts - Describe any byproducts or degradation products that you reasonably anticipate will result from the manufacture, processing, use, or disposal of the alternative both at sites you control and in end-use. Identify these byproducts or degradation products by specific name or class or range of structures (e.g., HF or other acid gases formed from the combustion of halocarbon compounds), CAS Registry number (where available), and the estimated amount formed. Also indicate where the byproduct or degradation product is formed (e.g., during manufacture, during end-use, following disposal).
Sector-Specific Data Requirements
Foams: Provide information on the catalyst used in manufacture and an analysis of breakdown products under different external conditions, such as temperature, during use.
Fire Suppression: Provide information on the degradation products of the alternative following discharge in a fire situation. Explain the conditions used in determining these products, such as the flame temperature, time required to extinguish the fire, amount of O2 present, and the combustible material.
Part II - ALTERNATIVE-SPECIFIC INFORMATION
EPA requires certain information on all substitutes. However, the data that are required may vary by sector. See Appendix B for more information on sector-specific requirements.
Part II, Section A - Physical and Chemical Properties (Page 6)
Include all data on the physical or chemical properties of the alternative that are reasonably available to you. Those properties included in this section are illustrative and are not an exhaustive list of potential data. See Appendix B for sector-specific data needs within this section.
If you are extracting this information from a public reference source (e.g., CRC Handbook of Chemistry and Physics, Merck Index), please indicate the reference in Question 27, "Other". If you have performed chemical analysis and testing on the substitute to derive the properties, attach copies of all test reports and specify the protocol used.
1-27. Complete the form as appropriate for the specific substitute. Many physical characteristics will apply to more than one sector.
28. Provide all test data regarding flammability of the substitute, including the procedures used for determining flammability and any other information on flammability concerns. If a substitute is flammable under the proposed end-use conditions, describe any abatement techniques being used to minimize the risks associated with use of a flammable substance. EPA recognizes that many flammable alternatives may be promising.
If an alternative is flammable (this applies to both blends and pure materials), you must analyze the risk of fire resulting from the use of the substitute in each proposed end-use. This assessment should include, but not be limited to, a description of typical scenarios in which the substitute is used, potential leak scenarios, sources of ignition, and probabilities of ignition. It should also assess the likelihood of injury within each scenario. Significant differences exist both in the design and in the ambient conditions for various end-uses. Thus, risk assessments are extremely sensitive to end-use. Low risk in one end-use does not, in general, imply low risk in another end-use. Describe measures taken to minimize any additional risk posed by the flammability of the alternative (e.g. equipment design modifications, alternative labeling).
Sector-Specific Data Requirements
Refrigerant alternatives: Information on the flammability of a blend as well as its individual constituents must be provided. Testing of all blends should identify the compositions for which the blend itself is flammable. Blends that contain flammable components should be tested during leaks in system components containing two phases to identify limits of fractionation. In addition, non-azeotropic blends must be tested during normal operation. For substitutes that will be used in consumer applications, documentation of testing safety results conducted by independent laboratories should be submitted. Also include both evaporator and condenser temperature and pressure conditions. See Appendix B for suggested tests and examples of potential scenarios to be examined in a risk assessment.
Sterilants: If the substitute is a blend with ethylene oxide (EtO), include information on the critical flammability ratios.
Aerosols: If the product contains an immiscible component, address the potential flammability of the product that could be expelled if the entire mixture is not shaken immediately before each use.
Part II, Section B - Atmospheric Information (Page 7)
1. Provide information on the predicted 100-year ozone depletion potential (ODP) of the alternative relative to CFC-11, if known. If the substitute is a blend, provide the ODPs of the individual constituents. You should also provide supporting documentation indicating how and by whom this value was calculated. This information may be provided as a citation from the published literature or by providing the background information used to develop the ODP.
For purposes of calculating ODP, EPA recommends the methodology used in the Scientific Assessment of Ozone Depletion: 2006 (Chapter 8) prepared for the United Nations Environment Programme (UNEP) by the World Meteorological Organization (WMO). The ODP refers to the amount of ozone destroyed by a gas over its entire atmospheric lifetime (e.g. at a steady state) relative to that due to emissions of the same mass of CFC-11. It is defined in modeling calculations as follows:
![]()
2. Provide information on the total global warming impacts (both direct and indirect effects) of a substitute for each end-use. Direct effects refer to the direct contribution to global warming from use of the substitute due to its radiative forcing. Indirect effects are contributions to global warming resulting from changes in energy consumption and corresponding changes in emissions of CO2 and other trace gases.
Required information includes the 100-year global warming potentials (GWPs) of a substitute relative to CO2. If they are available, you may also provide the 500- and 1000-year GWPs and the GWP relative to CFC-11, known as the halocarbon GWP (HGWP). If the substitute is a blend, provide the GWPs of the individual constituents. You should also include the data used to calculate these potentials such as atmospheric lifetime, infrared adsorption spectrum, and infrared absorption capacity. If known, provide information on the energy efficiency of the substitute relative to that of the substance being replaced and results of any testing or modeling done on the substitute. See Appendix B for a list of applicable tests. Data on energy efficiency is particularly of interest for alternative refrigerants and foam blowing agents.
If the alternative is captured as a byproduct of another manufacturing or industrial process, indicate the source of the alternative. This information is important in assessing the effects of the new use of the substitute versus those effects occurring strictly because of the release of a byproduct.
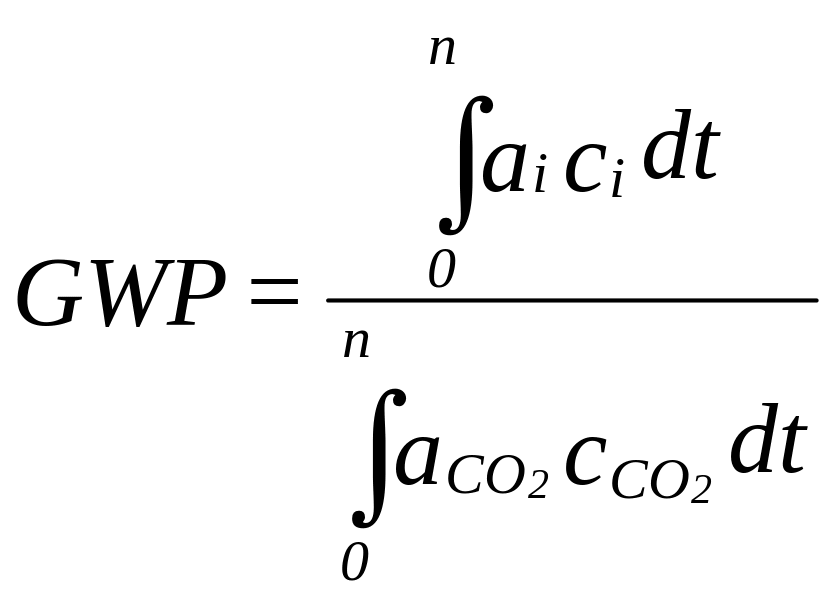
where:
ai = the instantaneous radiative forcing due to a unit increase in the concentration of trace gas, i
ci = the concentration of trace gas, i, remaining at time, t, after its release, and
n = the number of years over which the calculation is performed.
Corresponding values for CO2 are in the denominator.
Part II, Section C - Other Statutes (Page 8)
Please provide all information that is reasonably available regarding regulation of a substitute under other regulatory authorities. The Agency will evaluate substitutes under the SNAP program subject to existing regulatory constraints. This information allows EPA to coordinate regulatory efforts within EPA and among other authorities.
1. Environmental Statutes - Provide information on whether the substitute is regulated under any other environmental statutes such as:
$ other Titles of the Clean Air Act (CAA)
$ the Clean Water Act (CWA)
$ the Safe Drinking Water Act
$ the Resource Conservation and Recovery Act (RCRA)
$ the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA)
$ the Toxic Substances Control Act (TSCA)
$ the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA)
$ the Emergency Planning and Community Right-to Know Act (EPCRA or SARA Title III)
$ state and local laws
List any concentration-based or other numerical standards to which the substitute is subject under the above statutes or regulations.
For example, many alternative solvents are considered to be volatile organic compounds (VOCs) and are subject to emission restrictions under Title III of the CAA. If you know your alternative is either regulated or exempt as a VOC or Hazardous Air Pollutant (HAP), please indicate this.
2. Other Statutes - Indicate whether the substitute is regulated under other statutory authorities concerned with health and safety issues, such as those implemented by:
$ the Food and Drug Administration (FDA)
$ the Occupational Safety and Health Administration (OSHA)
$ the Department of Transportation (DOT)
$ and state and local laws.
Also include information on any other standard-setting bodies who will be evaluating the substitute, such as the National Fire Protection Association (NFPA) or the American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE). List any concentration-based or other numerical standards (e.g. permissible exposure limit (PEL), short-term exposure limit (STEL), safety classification) to which the substitute is subject under these statutes or organizations.
Part II, Section D - Cost and Availability of the Alternative (Page 9)
1. Estimate the cost per pound ($/lb) of the chemical substitute or, where appropriate, indicate the cost per unit volume or per packaging unit (e.g. aerosol spray can). If the cost is not per pound of material, specify the units. For alternative processes and technologies, describe the equipment costs and other costs such as those associated with installing the new substitute.
2. Provide information on the levels of production and market penetration that you expect for the substitute. Include values for total production in lbs/yr both at the start of production and in the future (e.g., 3 years from now), the number of years you anticipate until the substitute reaches its maximum market penetration, and the total production level that you anticipate for the substitute when it reaches the point of market saturation. Finally, estimate the percentage of the market held by the ODS being replaced that will be captured by this substitute. If the substitute is not currently available, indicate when you anticipate it will enter the marketplace.
If you are submitting the substitute for several end-uses, you must provide this information for each end-use.
3. Describe any new equipment and how the substitute or alternative technology is used. If retrofitting of existing equipment is possible, detail changes in technologies needed to use the substitute. Also provide information on materials compatibility and attach any available test results. Provide specific information on each different end-use, including cost of equipment, equipment lifetime, changes in labor, and changes in energy costs. For example, if the substitute is an alternative refrigerant that may be used both as a retrofit or in new equipment for chillers, information on the equipment changes should be provided for both end-use scenarios. This cost information may be provided as a range where equipment may be optimized to different levels of energy efficiency.
All claims that an agent is a "drop-in" for the ODS it is replacing must be substantiated, and qualified by a description of any necessary technical changes to existing equipment. Note, however, that not all sectors recognize drop-in replacements as a separate category.
PART III - RELEASE AND EXPOSURE DATA
The following sections describe the types of human health and safety and environmental exposure data used to evaluate substitutes under the SNAP program. For existing chemicals, specific data requirements may depend on what types of data are already available. Attach all complete test reports that are reasonably available to you. Also indicate all concentration-based exposure limits that have been set for the substitute, such as PELs, occupational exposure limits (OELs), or acceptable exposure limits (AELs) set by the manufacturer. You may submit references to studies that have already been submitted to the SNAP program. If you have done a literature search on the alternative or its constituents, please include it as well.
Decisions on data requirements will be determined by the exposure patterns of the sector(s) in which the substitute is to be used. For example, substitutes used only in settings which would generally preclude all but acute exposures (such as in fire extinguishing) may not require chronic toxicity data. For other sectors, such as solvent cleaning, where aqueous solvents are used, additional tests may be necessary depending on the means of discharge and disposal of the substitute.
If the substitute is a blend containing constituents not previously reviewed under SNAP for any end-use, additional data other than those specified below may be required.
Part III, Section A - Toxicity and Hazard Information (Page 10)
1. For chemical alternatives and for chemicals used with alternative processes, summarize information on the acute and chronic toxicity of the substitute and/or of its constituent chemicals on any organism (e.g. humans, other mammals, fish, wildlife, plants, etc.). In addition to those data mentioned specifically below, attach all supporting information such as test reports, if available. In addition, attach a copy of any hazard warning statement, label, material safety data sheet (MSDS) or other information that will be provided to any person who is reasonably likely to be exposed.
Sector-Specific Data Requirements
Refrigerants: A cardiosensitization study, usually measuring cardiotoxic effects in the dog, is required. This requirement also applies to blends of chemicals which have already been tested individually. Blends can exhibit unpredictable cardiotoxicity levels, and testing is necessary.
Foams: A study on the degradation products of the foam under different external conditions, including the catalyst used during manufacture and the temperature during use. EPA's assessment of potential hazards for insulation foam suggests that breakdown/degradation products of foam during use may be significant, and thus information on such processes is required.
Solvents: To characterize aquatic toxicity, both acute and chronic toxicity data for a variety of species may be required. The Agency requires a minimum aquatic data set to be submitted as described in "Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses," which is available through the National Technical Information Service (#PB 85-227049). If other regulatory limits such as effluent guidelines exist, these values may be used in lieu of compiling toxicity data.
Fire Suppression: A cardiosensitization study, usually measuring cardiotoxic effects in the dog, is required. For specialized halon use, such as in airline cabins, information on central nervous system (CNS) depression may also be required. An exposure assessment, such as personal monitoring testing, for evaluation of alternative streaming agents may also be required.
Aerosols: A cardiotoxicity study, usually measuring cardiotoxic effects in the dog, is required. Information on central nervous system (CNS) depression may also be required.
Adhesives, Coatings, & Inks: Depending on the end-use, data requirements for this sector may be similar to the solvents and/or aerosols sector. Submit all relevant information, to the best of your knowledge.
Sterilants: Concurrent review under FIFRA will cover toxicity issues and efficacy of such alternatives. The SNAP program does not require any additional toxicity testing of substitutes also reviewed under FIFRA. As stated above, attach copies of hazard warning statements, labels, and MSDSs that will be provided to any person likely to be exposed.
Part III, Section B - Environmental Release and Disposal at Manufacture (Page 10)
Identify by name and address all manufacturing site(s) (within the U.S. and worldwide) of each chemical or alternative technology. Also identify all site locations for manufacturing of products containing the substitute (e.g. refrigerators containing substitutes). If you are unable to identify all manufacturing sites, provide data on a typical facility. Identify all points of release and/or exposure during manufacture of the substitute and any products containing the substitute and the magnitude of the release. Also indicate the environmental media to which it is released (e.g. indoor air, outdoor air, water, land).
Part III, Section C - Occupational Exposure at Manufacture (Page 11)
Provide information on occupational exposure during manufacture of the substitute or alternative technology. This entry should include information on exposure during manufacture of the substitute itself and/or manufacture of products containing the substitute, where appropriate (e.g. refrigerators containing the alternative). Describe all activities in which workers may be exposed to the substitute, estimate the levels of exposure, and describe any protective measures taken to limit exposure.
Part III, Section D - Environmental Release and Disposal in End-use (Page 11)
Provide information in this section as it applies to the sector(s) in which the alternative will be used. For example, information applicable to wastewater treatment should be included for solvent cleaning alternatives but is not relevant to refrigeration substitutes. For each substitute, indicate the quantities of the alternative that are released directly to the environment and the environmental media to which it is released (e.g. air, water, land).
Describe any control technologies used to limit emissions and indicate the method and location of disposal and/or treatment of the substitute. For example, explain if disposal will be performed on site, by a contractor, by a vendor, or by other means. If the substitute is to be recycled, describe any necessary equipment and programs that may be set up to encourage recycling.
Identify the recovery technology that will be used to recapture the substitute for disposal in specific end use sectors. Is current technology available to recover the substitute? If yes, please describe the technology by providing specifications or the specific industry standard. However, if current recovery technology is not available please describe how the substitute will be recovered again by providing new equipment specifications, new industry standards or the new method.
Part III, Section E - Occupational Exposure at End-use (Page 12)
Provide information on occupational exposure during end-use (e.g., during use of an alternative solvent in an open-tank cleaning system). Describe all activities in which workers may be exposed to the substitute and estimate the levels of exposure. Also describe any protective measures taken to limit exposure.
Sector-Specific Information
Fire Suppression: Personal monitoring may be required for streaming agents, depending on the amount of agent required for fire suppression and the cardiotoxic level of the agent. For total flooding agents, indicate the extinguishing concentration using either a cup burner in heptane or full scale testing, and the design concentration of the substitute as defined by the National Fire Protection Association (NFPA) (cup burner plus 20 per cent). Indicate actual design concentration if it is likely to be higher, based on manufacturer recommendations.
Part III, Section F - Consumer Exposure (Page 12)
Provide information in this section as it applies to the sector(s) and end-use(s) in which the substitute will be used. If the alternative is intended for industrial use only (e.g. a refrigerant for cold storage warehouses) you need not provide information in this section.
Part III, Section G - General Population Exposure - OPTIONAL (Page 13)
EPA has performed risk screens on alternatives to ozone-depleting substances which indicate that risks to the general population during manufacture of these substitutes are lower, on an overall basis, than risks of continued reliance on ODSs. You do not need to provide the information requested in this section unless you choose to do so.
Part IV - LIST OF ATTACHMENTS (Page 14)
Attach any continuation sheets for sections of the form, test data, and other data that may assist EPA review of an alternative after the last page of the form. Clearly identify the attachment and the section to which it relates on page 14, where appropriate. Number consecutively the pages of each attachment and enter the inclusive page numbers on page 14. Enter the total number of pages in the notice, including attachments, on page 1 of the form.
Mark (X) the confidential box next to any attachment name that you claim as confidential. See Chapter I.B of this manual for information on how to claim any information in an attachment as confidential.
Part V - CERTIFICATION (Page 15)
The official named in Part I, Section A of the form as the person submitting the notice must sign the certification on page 15 of the notice form. This official is responsible for the truth and accuracy of each statement in the certification. If an agent assists you in preparing the notice, the agent must also sign the certification. All signatures must be original. If the submission is not signed, EPA will consider the submission incomplete and will not review the substitute.
2. SUPPLEMENTARY INFORMATION
A. Substitutes That Must Be Reported by Designated Submitters
Based on the language of section 612(a) of the CAA, EPA defines a "substitute" as any chemical, product substitute, or alternative manufacturing process that could replace a class I or class II substance. Section 612(e) makes clear that substitutes include both existing and new chemicals and technologies. Also, the language in section 612(c) clearly states that a new substitute may be currently or potentially available. EPA defines "potentially available" as indicating any alternative for which adequate information exists to make a determination under SNAP, and one the Agency reasonably believes to be technically feasible, even if all testing has not yet been completed and the alternative is not yet produced and sold.
EPA believes that the statutory language included in section 612 is written broadly to allow for an all-encompassing evaluation of substitutes that will be introduced as replacements for ozone-depleting chemicals. However, clarification is presented below to explain the Agency's definition of a "substitute" in specific circumstances based on section 612.
1. Chemicals Already Listed as "Existing" under TSCA
SNAP review is critical for such chemicals, given the differing statutory objectives of TSCA and the CAA.
2. Significant New Use of Existing Alternatives
Existing alternatives already being sold commercially within a SNAP sector (e.g., use of semi-aqueous cleaners in the electronics industry) are subject to review under SNAP.
3. Class I and Class II Chemicals as Substitutes
Class I and class II chemicals used as substitutes will continue to deplete stratospheric ozone and, thus, a SNAP review of these alternatives is necessary.
4. Substitutes for Class II Compounds
The same information reporting requirements and listing process apply to substitutes for class II compounds as for substitutes for class I compounds.
B. Substitutes Excluded From Notification Requirements
The Agency has identified several situations in which notification to the SNAP program is not required. These exemptions from reporting are discussed below.
1. Substitutes Already Listed Under SNAP
Lists of substitutes are available from EPA Air Docket A-91-42, online at http://www.epa.gov/ozone/snap/lists/index.html, and from the Stratospheric Protection Hotline at (800) 296-1996.
2. Small Sectors
The major industrial use sectors included under SNAP are: refrigeration; foam blowing; fire suppression and explosion protection; solvent cleaning; adhesives, coatings, and inks; aerosols; sterilization; and tobacco expansion. If an ozone-depleting compound is not being used under one of these sectors, substitutes need not be submitted under SNAP. Please submit information to EPA if you identify another large industrial use sector.
3. Small Volume Use within SNAP Sectors
Producers of substitutes for ozone-depleting compounds in annual quantities of 10,000 lbs per year or less for an end-use within a sector do not need to notify EPA of their activities under SNAP. However, EPA encourages producers to maintain documentation describing the basis for their view that any substitute being used meets this small use definition. This documentation could be necessary in the event the Agency receives a petition to evaluate such substitutes. Documentation should take the form of production volume and sales information which is typically maintained by many producers irrespective of any regulatory requirements.
4. Second-Generation Substitutes
EPA believes that as long as class I or class II chemicals are being used, any substitute designed to replace these chemicals is subject to review under section 612.
5. Formulation Changes
In general, formulation changes that accommodate replacement of class I and class II compounds are not subject to the provisions of section 612. Such changes may be necessary, for example, when a new foam blowing agent necessitates the replacement of the catalyst formerly used with the class I blowing agent. However, if a SNAP submitter has reason to believe such changes will significantly influence the environmental and human health risk characteristics associated with the use of any class I or class II substitute, this concern should be communicated to the Agency. EPA may also review any changes in formulation in connection with review of substitute compounds where the Agency has reason to believe the formulation change may affect the risk profile of a substitute.
6. Test Marketing
Use of alternatives for the sole purpose of test marketing is exempt from any reporting requirements under section 612. However, once you decide to sell an alternative as a class I or class II substitute, you must provide the Agency with notification at least 90 days prior to commercialization. Producers of new chemicals must still abide by the provisions of section 5(h)(1) of TSCA, which authorizes EPA to grant exemptions from TSCA-reporting requirements, provided that test marketing will not present an unreasonable risk to human health or the environment. When submitting the TSCA application, you are encouraged to notify EPA's Office of Air and Radiation; however, such notification is not required under SNAP.
7. Research & Development
Substitutes manufactured or imported solely for research and development are exempt from reporting requirements under section 612. Amounts used in research are assumed to be the minimum necessary for reasonable scientific experimentation. For new chemicals, the provisions of '720.36 of the PMN rule (40 CFR Part 720) are in effect.
8. Substitutes Produced for Export
In the final SNAP rule, substitutes that are produced solely for export and use by non-U.S. entities outside the U.S. were exempted from reporting requirements under SNAP. However, recent legal actions are pending resolution. Please contact the SNAP coordinator for the latest information on this topic.
9. Substitutes Used as Feedstock
Substitutes that could replace class I chemicals which are used solely as intermediates in the production of other chemicals are exempt from reporting to the SNAP program.
C. Joint Review with Other EPA Offices
1. Overlap with the TSCA PMN Program
New chemical substitutes must undergo review both under section 612 and section 5 of TSCA (the Premanufacture Notice program). Because of the overlap in statutory authority, the Agency has established a joint review process between the SNAP and TSCA Premanufacture Notice (PMN) programs. This process has been structured to minimize reporting burden and to ensure consistency in decisions between the two programs. The following sections describe the joint review and decision-making process in more detail.
a. SNAP and PMN Forms
In general, the Agency has identified only a few additional data elements that are not required by TSCA but are needed for SNAP review:
$ Ozone depletion potential
$ Global warming potential
$ Cost of using the substitute
$ Flammability testing results
Given this overlap, a submitter requesting review under both the SNAP and PMN programs should:
$ Complete the PMN form (EPA Form 7710-25) following the Instructions Manual available through the TSCA Assistance Information Service.
$ Indicate on page 11 of the PMN form, "Optional Pollution Prevention Information," that the chemical is also to be considered under the SNAP program.
$ Complete a SNAP TSCA addendum form, available from the SNAP document control officer.
The completed PMN form (EPA Form 7710-25) will remain the basis for all information needed to complete review of the new chemical under section 5 of TSCA. The completed PMN form and the SNAP addendum together will comprise the data submission for section 612 review and listing decisions for new chemicals. This approach is intended to minimize your reporting burden.
The Agency has modified the PMN Instructions Manual to provide you with more explicit direction on how to complete the SNAP addendum. Any questions regarding the completion of these forms can be directed to either the PMN pre-notice coordinator or the SNAP DCO.
b. Submission of Completed Forms
Both the PMN and SNAP programs have a review period of 90 days, subject to suspensions and extensions described in '82.180 for the SNAP program and in the PMN rule (40 CFR 720.75). To ensure that new chemical submissions are reviewed and decided on jointly, you are encouraged to provide both the PMN form and SNAP addendum to both programs. Failure to provide this information to both programs at the same time could result in delays in the review of your notice.
c. Procedures for Handling Confidential Business Information
EPA recognizes that information submitted to the PMN and SNAP programs may need to be held confidential. All CBI submitted as part of the joint PMN/SNAP review will be maintained and treated in a manner consistent with TSCA security procedures. Confidentiality claims will be processed and reviewed in a manner consistent with 40 CFR Part 2, Subpart B. Please note that while TSCA and CAA may have different language describing CBI handling procedures, there is no substantive difference in how CBI is maintained under the two statutes.
2. Overlap with the FIFRA Program
Any new pesticide or amendment of an existing formulation is already subject to Agency approval under provisions of the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA), P.L. 100-460, 100-464 to 100-526, and 100-532. In addition, as of April 18, 1994, new pesticides or formulation changes based on class I or class II substitutes will also be subject to review under section 612 of the CAA. These authorities apply in all cases where a manufacturer reformulates a pesticide product to replace class I or class II chemicals. Similarly, registrations of new pesticide products will also be subject to SNAP review if the new product contains chemicals functionally replacing class I or II compounds which would otherwise have been used in the new pesticide formulation.
a. Review Responsibilities Under FIFRA and CAA/SNAP
In general, review responsibilities for pesticide products under the SNAP program will focus on a substance's ozone depletion and global warming potentials. FIFRA reviews will address factors commonly examined during pesticide amendments and registrations. The two program offices responsible for these reviews will coordinate their efforts and share pertinent data to ensure appropriate technical consideration of the substitute.
b. Data Submission Requirements and Process
The Agency has found no significant overlap between FIFRA and SNAP review. Because there is so little overlap, a submitter requesting review under both programs must submit all information ordinarily required for the OPP process as well as a completed SNAP Information Form. A copy of the FIFRA form should be submitted to OPP, and a copy of the SNAP form should be submitted to the SNAP DCO.
If you are submitting an amendment to a product registration under FIFRA that currently contains a class I or II substance, you should note in Section II ("Amendment Information") of the FIFRA form that the amendment was filed in response to the CAA production phase-out. Similarly, if you are submitting an application for a new pesticide registration that would otherwise have been based on a class I or II compound, you should note in Section II of the FIFRA form that the registration includes a class I or II substitute. You should also identify both the substitute chemical and the class I or II compound it is replacing. Further, if you are aware that a particular chemical intended for use as a class I or class II substitute in a pesticide formulation has already been approved through earlier SNAP/FIFRA determinations, you should also reference the relevant part of the prior review.
D. Consultation with EPA Concerning the SNAP Information Notice
1. Contact Prior to Notice Submission
Copies of the SNAP rule, listing decisions, this Guidance Document, the SNAP Information form, and other materials relating to the rule are available online on EPA=s SNAP web page (http://www.epa.gov/ozone/snap/index.html) and through the Stratospheric Protection Hotline at (800) 296-1996. These materials are also available through EPA regional offices. You may contact the Stratospheric Protection Division of EPA in Washington, DC at (202) 343-9410. Written inquiries may be sent to:
SNAP Coordinator
U.S. EPA
Mail Code 6205J
1200 Pennsylvania Ave. NW
Washington, DC 20460
2. Contact During Notice Review
The 90-day notice review period begins when your notice is determined to be complete by the SNAP Document Control Officer (DCO). You will receive written notification within 15 days of receipt by EPA if your notice is declared "incomplete" as described in '82.180. If your notice is complete, you will receive an acknowledgment letter confirming the date EPA received your notification and the date EPA's 90-day review period begins. The letter will also include the document tracking number assigned to your submission. If your alternative has already been listed under the SNAP program for the proposed end-use, you will be notified that your substitute is not subject to SNAP review, and therefore you are free to begin manufacture immediately.
The appropriate SNAP sector specialist will contact you if the review period on the alternative will be extended under '82.180. However, following expiration of the initial 90-day review period, you are free to begin manufacturing your product for commercial sale, provided that EPA has not already listed the alternative as unacceptable through notice-and-comment rulemaking.
Review of petitions will occur by the same process and schedule as submissions from manufacturers of new substitutes.
E. EPA Contact Information
Stratospheric Ozone Information Hotline (800) 296-1996
Stratospheric Protection Division (202) 343-9410
Margaret Sheppard (202) 343-9163
SNAP Document Control Officer
APPENDIX A: LIST OF END-USES WITHIN SECTORS INCLUDED IN THE SNAP PROGRAM
All substitutes listed under the SNAP program are included in one of the following end-uses. A listing in an end-use does not imply EPA has reviewed the substitute for another end-use. Please specify all end-uses for which you would like to have your substitute evaluated.
Refrigeration and Air Conditioning
Note: All end-uses within this sector are further broken down into "Retrofit" and "New Equipment" subcategories
$ Commercial Comfort Air Conditioning (Centrifugal, Reciprocating, and Screw Chillers);
$ Industrial Process Refrigeration Systems;
$ Industrial Process Air Conditioning;
$ Ice Skating Rinks;
$ Uranium Isotope Separation Processing;
$ Cold Storage Warehouses;
$ Refrigerated Transport;
$ Retail Food Refrigeration;
$ Vending Machines;
$ Water Coolers;
$ Commercial Ice Machines;
$ Household Refrigerators and Freezers;
Household and Light Commercial Air Conditioning;
$ Residential Dehumidifiers;
$ Motor Vehicle Air Conditioning;
Motor Vehicle Air Conditioning for Buses and Passenger Rail (Substitute for Hcfc-22)
$ Residential Air Conditioning and Heat Pumps;
$ Non-mechanical Heat Transfer;
$ Very Low Temperature Refrigeration.
Foam Blowing
$ CFC-11 Polyurethane and Polyisocyanurate, Rigid Laminated Boardstock
$ CFC-11 Polyurethane, Rigid Appliance
$ CFC-11 Polyurethane, Rigid Spray, Commercial Refrigeration Foams, Spray Foams and Sandwich Panel Foams
$ CFC-11 Polyurethane, Rigid Slabstock and Other
$ CFC-12 Polystyrene, Extruded Boardstock and Billet
$ CFC-11, CFC-113 Phenolic, Insulation Board
$ CFC-11 Polyurethane, Flexible
$ CFC-11 Polyurethane, Integral Skin
$ CFC-12 Polystyrene, Extruded Sheet
$ CFC-12, CFC-114, CFC-11 Polyolefin
Solvent Cleaning
$ Metals Cleaning with Methyl Chloroform or Cfc-113
$ Electronics Cleaning with Methyl Chloroform or Cfc-113
$ Precision Cleaning with Methyl Chloroform or Cfc-113
Fire Suppression and Explosion Protection
$ Halon 1211 Streaming Agents
$ Halon 1301 Total Flooding Agents
Aerosols
$ CFC-11, HCFC-22, HCFC-142b as Propellants
$ CFC-11, CFC-113, MCF, HCFC-141b as Solvents
Sterilants
$ 12/88 Blend of EtO/CFC-12
Tobacco Expansion
CFC-11
Adhesives, Coatings, and Inks
$ Methyl Chloroform
APPENDIX B: SECTOR-SPECIFIC DATA REQUIREMENTS
The following lists give guidance about what particular characteristics are of interest within each sector, in addition to Ozone Depletion Potential and Global Warming Potential.
Refrigeration and Air Conditioning
Physical and Chemical Properties
$ Molecular weight
$ Physical state at room temperature
$ Boiling point
$ Vapor pressure (provide curve across a range of temperatures)
$ Critical temperature
$ Critical pressure
$ Flash point
$ Flammability limits (LFL, UFL)
$ Heat of combustion
$ Maximum pressure of combustion
$ Maximum rate of pressure increase during combustion
Suggested test data
$ ASTM E681 for flammability limits in air
$ Fractionation during leakage
Energy Efficiency
$ Laboratory testing of equipment using the alternative refrigerant vs. the CFC being replaced. Values should be given in kWh/day or a similar measure. Also address refrigerant/oil solubility.
$ Computer models should account for compressor efficiency, refrigerant transport properties and mass flow rates for given tubing geometry, capillary tube/suction line heat transfer, and liquid and vapor specific heats.
Toxicity
$ Cardiotoxicity
Foam Blowing
Physical and Chemical Properties
$ Thermal Conductivity
$ Flash Point
Energy Efficiency
Toxicity
$ Degradation Products
Solvents
Physical and Chemical Properties
$ Boiling Point
$ Specific Gravity
$ Odor Threshold
$ Solubility
$ Vapor Pressure
$ Dissociation constant
$ Volatilization from soil and water
$ pH
$ Flash Point
$ Flammability limits
Toxicity
$ List any PELs, STEL, AELs, etc.
$ Aquatic toxicity testing may be required
Fire Suppression Agents
Physical and Chemical Properties
$ Molecular weight
$ Boiling point
$ Specific gravity
$ Vapor pressure
$ Particle size distribution (applies to non-gaseous agents)
$ Vapor heat capacity
$ Heat of vaporization
$ Viscosity
$ Weight Equivalence
$ Storage Volume Equivalence
$ Extinguishment Concentration (specify method)
$ Design Concentration
Toxicity
$ Cardiotoxicity
$ Personal Monitoring (streaming agents)
Formula for determining Weight and Volume Equivalence:
NOTE: Weight and volume equivalents are calculated using a single, fuel-specific design concentration (heptane); therefore, they do not represent the exact weight or volume of the agent needed to protect any specific space against any specific hazard. The information used to calculate the equivalents is provided from agent manufacturers and NFPA 2001, "Standard on Clean Agent Fire Extinguishing Systems." Equivalents are included in SNAP rulemakings for general comparison and informational purposes only.
EPA understands that fire suppression agents must be evaluated in the context of the fire extinguishing system equipment with which they are used. Design concentration, and weight and volume equivalents are only meaningful when evaluated in specific system hardware configurations. This is especially important when comparing storage volume where storage container fill density varies with the equipment used. Agent fire suppression performance will vary with the system used and the detailed design of the system. Therefore, fire suppression agent manufacturers do not generally recommend design concentrations as these are also a function of the system hardware in which they are used. Hence, these data are provided for general guidance only and do not reflect a recommendation for system design or a basis for rigorous quantitative comparison.
(1) Weight and volume equivalent data should be presented relative to Halon 1301 at 120 per cent of cup burner as well as at 5 per cent, a typical use concentration;
(2) weight and volume equivalents should be based on agent concentrations at Standard Temperature and Pressure;
(3) weight and volume equivalents should be done at both the manufacturer's recommended design concentration and at 120 per cent of the cup burner value where the values are not the same;
(4) volume equivalents will be based on agent volume only (exclusive of container volume, fill density, etc.) at 70 degrees Fahrenheit and the storage pressure specified by the manufacturer since this varies widely and the required agent mass determined in item (5) below; and
(5) the required agent weight equivalents should be determined by the following equation:
W = V/S(C/100-C)
where C = design concentration (% volume)
V = one cubic foot
S = agent specific vapor volume at 70 degrees F (ft3/lb).
(6) Appropriate references to the technical literature on which the data are based should be provided.
Aerosols
Physical and Chemical Properties
$ Molecular weight
$ Boiling point
$ Odor threshold
$ Solubility
$ Vapor pressure
$ Flash point
$ Flammability limits
$ Explosive range
$ Viscosity
Adhesives, Coatings, and Inks
Physical and Chemical Properties
$ Molecular weight
$ Physical state
$ Melting point
$ Boiling point
$ Specific gravity
$ Odor threshold
$ Solubility
$ Volatilization from water and soil
$ pH
$ Flash point
$ Flammability limits
$ Explosive range
Sterilants
Physical and Chemical Properties
$ Molecular weight
$ Physical state
$ Vapor pressure
$ Specific gravity
$ Flash point
$ Flammability limits
$ Explosive range
Toxicity and efficacy issues are addressed under FIFRA and/or FDA reviews and may be summarized. Actual test data are not required.
Pesticides - Review under FIFRA will cover most data analysis
Physical and Chemical Properties
$ Provide what data are readily available
Ozone Depletion Potential
$ Provide if information is available
Global Warming Potential
$ Provide if information is available
Cost and Use Information
| File Type | application/msword |
| Last Modified By | oap |
| File Modified | 2008-04-30 |
| File Created | 2008-04-30 |
© 2026 OMB.report | Privacy Policy