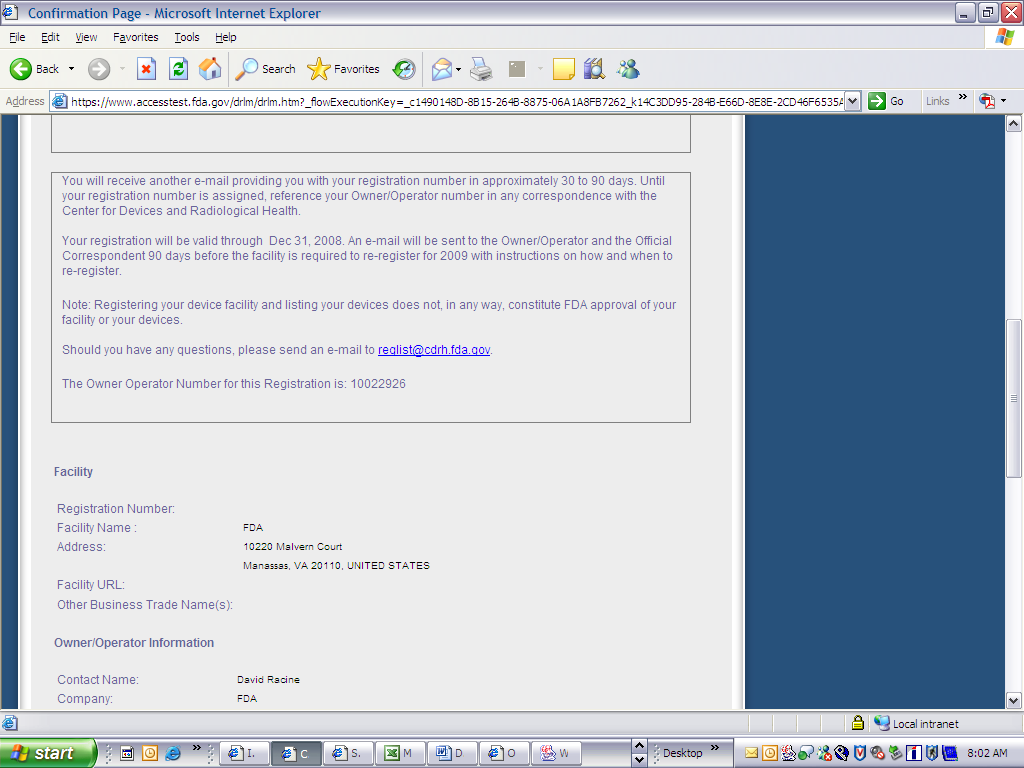
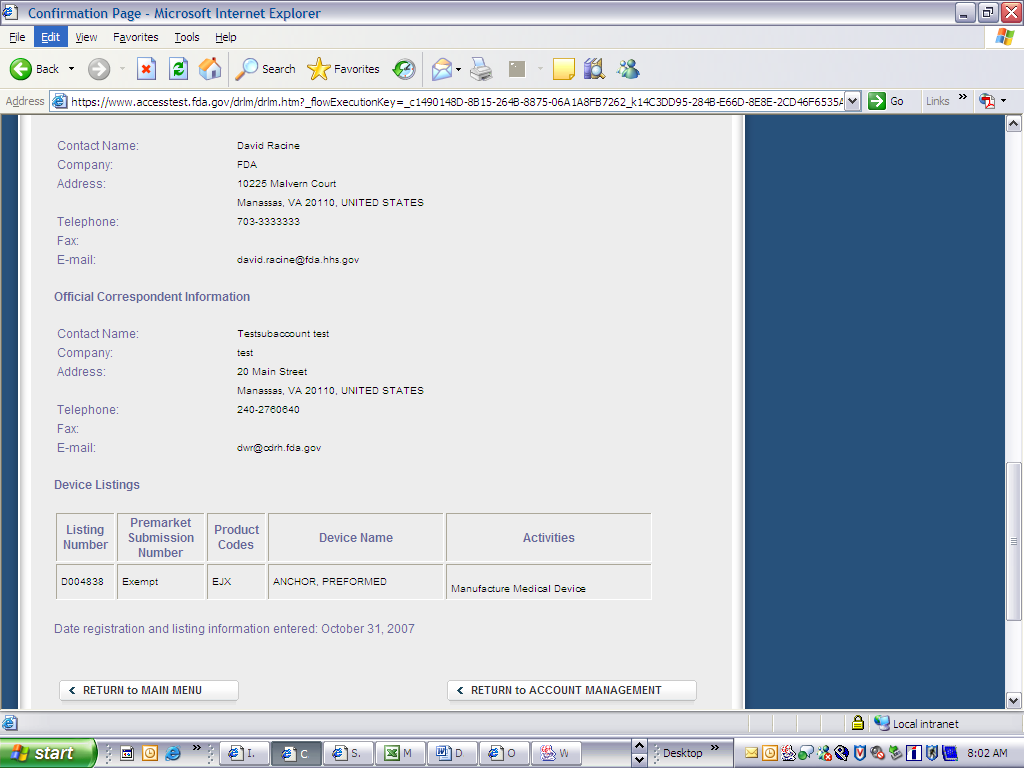
Form 3673 Device Registration & Listing Module
Agency Emergency Processing Under Office of Management and Budget Review; Implementation of Sections 222,223,and 224 of the Food and Drug Administration Act of 2007
FDA Form 3673 Device Regis. & Listing Module
Implementation of Sections 222, 223, and 224 of the Food and Drug Administratin Amendments Act of 2007
OMB: 0910-0625
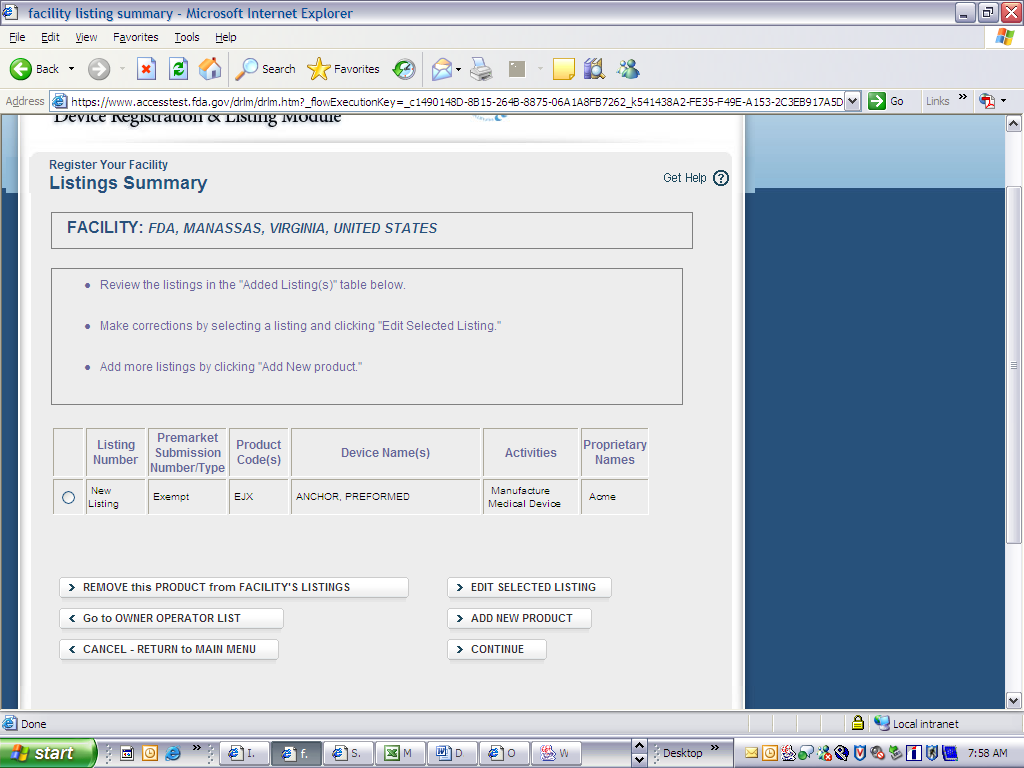
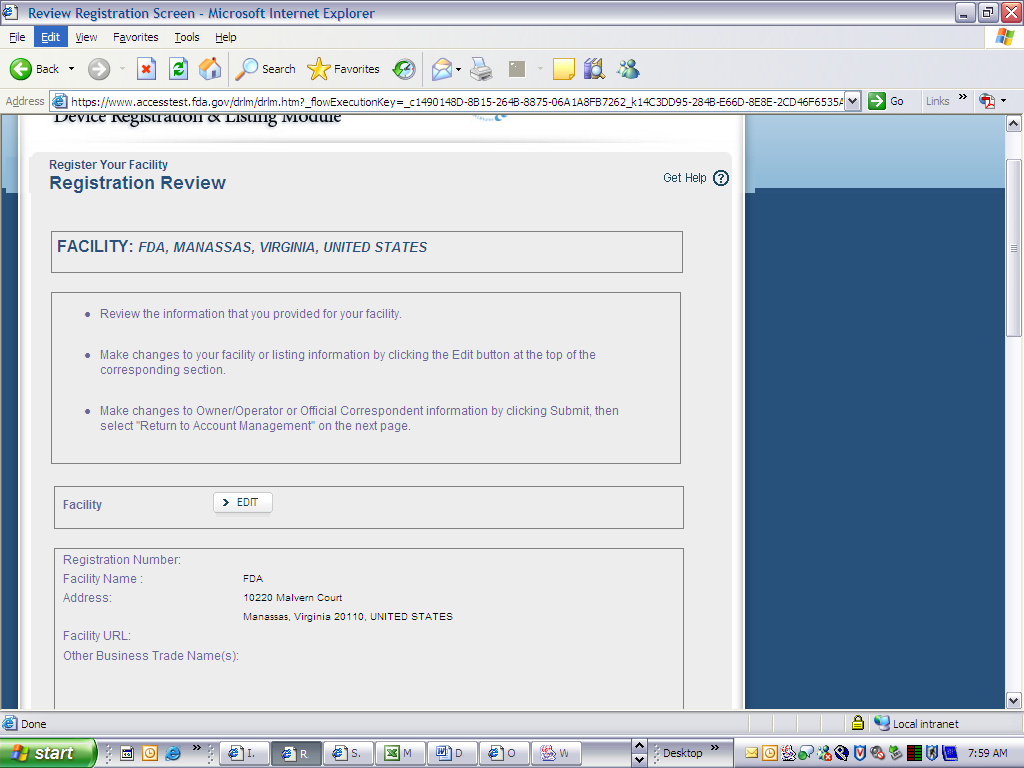
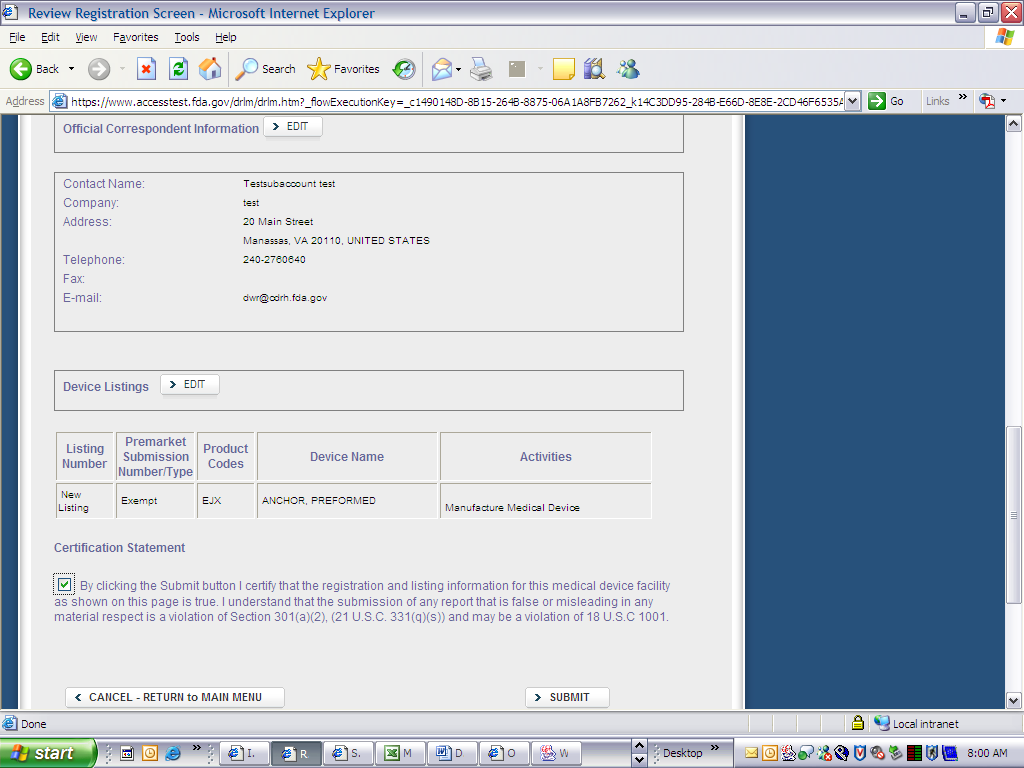
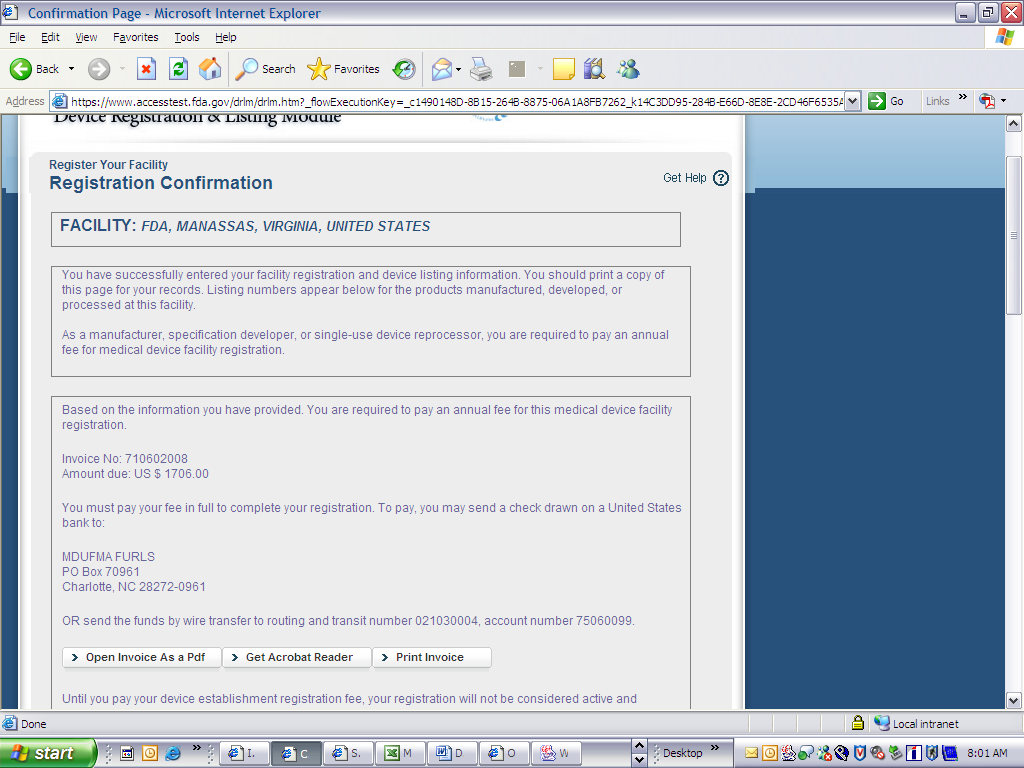
Device Registration and Listing Module
Form Number: FDA 3673(03/08)
OMB Number: 0910-XXXX
OMB Expiration Date:
OMB Burden Statement:
Public reporting burden for this collection of information on Form 3673 is estimated to be 0.50 hours per response for the purpose of firms annually registering their establishment and 0.25 hours per response for the purpose of firms annually listing their devices. These estimates are based on FDA's experience, data from the device registration and listing database, and our estimates of the time needed to complete other previously required forms.
Send comments regarding this burden estimate or another aspect of this collection of information, including suggestions for reducing this burden to:
Department of Health and Human Services
Food and Drug Administration
Office of the Chief Information Officer (HFA-250)
5600 Fishers Lane
Rockville,
Maryland 20857
An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid Office of Management and Budget (OMB) control number.


| File Type | application/msword |
| Author | David Racine |
| Last Modified By | DPresley |
| File Modified | 2008-05-08 |
| File Created | 2008-05-08 |
© 2026 OMB.report | Privacy Policy