NHDS Att V Sampling Manual for the Pretest new
NHDS Att V Sampling Manual for the Pretest new.doc
National Hospital Discharge Survey
NHDS Att V Sampling Manual for the Pretest new
OMB: 0920-0212
Attachment V Discharge Sampling Manual for the Pretest
National Hospital Discharge Survey
Pretest
D ischarge
Sampling Manual
ischarge
Sampling Manual
RTI International
P.O. Box 12194
3040 Cornwallis Rd.
Research Triangle Park, NC 27709
August, 2008
Table of Contents
B. Introduction to National Hospital Discharge Survey 2
C. National Center for Health Statistics 2
D. Privacy and Confidentiality 2
F. Important Information about Procedures for Completing and Handling Sampling Forms 2
Chapter II. Sampling Discharge Records 4
A. Purpose of Sampling Discharge Cases 2
B. Overview of Steps in Discharge Sampling 2
C. STAGE 1: Identifying and Assembling Lists of Inpatient and Observation Status Discharges 2
D. STAGE 2: Selecting Discharges (Completing the Sample Listing Sheets) 2
1. Completing Hospital Information, SLS-PART A (for Sampling Period 1) 2
2. Selecting Discharges from Each Group 2
3. Completing Hospital Information, SLS-PART A (for Sampling Period 2) 2
E. Instructions for Selecting Discharges for Data Abstraction 12
Chapter III. Special Situations 2
Chapter IV. Administrative Issues 2
Appendix A: SLS PARTS A and B 2
Appendix B: Instructions for Obtaining the Random Start Number 2
Appendix C: Summary of Discharge Grouping Information 2
List of Exhibits
Exhibit A. Steps and Definitions for Grouping Discharge Cases 2
Exhibit B SLS Part A Table 1 8
Exhibit C Number of cases to be targeted for selected sampling periods. 9
Exhibit D. Entering carry over number below Table 2, Part B 2
Exhibit E. Entering information into Table II, Part B 2
Exhibit F. Entering information into Table 1, SLS Part A (Sampling Period 2) 2
Chapter I. Overview
A. Purpose of this Manual
The purpose of this manual is to provide instructions on how to sample discharge records for the National Hospital Discharge Survey (NHDS) Pretest. For the pretest we will select samples from two sampling periods. Sampling Period 1 will include discharges for the months of June and July 2008; and Sampling Period 2 will include discharges for August and September 2008. This manual is intended for staff in hospitals who have agreed to participate in the NHDS pretest and to perform the discharge sampling themselves.
The NHDS Redesign includes four parts—the Pilot Study, the Pretest, the 2010 Survey, and the 2011 Survey. This version of the manual describes procedures to be used for the Pretest in which a major objective is to test discharge sampling procedures.
B. Introduction to National Hospital Discharge Survey
Since 1965 the National Hospital Discharge Survey (NHDS) has provided critical information on the utilization of the nation’s hospitals and on the nature and treatment of illness among the hospitalized population in short-stay, nonfederal hospitals in the United States. Data from the NHDS are used to examine important topics of interest in public health and for a variety of activities by governmental, scientific, academic, and commercial institutions. The NHDS is currently in the process of being redesigned to better reflect the types of care and services now provided in U.S. hospitals, the structure of the current health care delivery system, and the current distribution of the population. Redesign efforts to date have addressed what information is needed to inform emerging policy and research issues with particular focus on five areas: cost of care and resource usage; quality of care and patient safety; care delivered in the hospital; continuity of care; and disparities in care and access. This effort focuses on the final design and testing of the systems and processes recently tested during a pilot study to recruit a new panel of hospitals, collect a broader spectrum of data elements, and to implement a pc-based tool to collect the data. The proposed redesign will dramatically expand the survey’s value to public health analysts, providers, and policy makers.
C. National Center for Health Statistics
The NHDS is sponsored by the National Center for Health Statistics (NHCS). The mission of NCHS is to provide statistical information that will guide actions and policies to improve the health of the American people. NCHS is the statistical component of one of the Coordinating Centers in the Centers for Disease Control and Prevention (CDC). As the Nation’s principal health statistics agency, NCHS leads the way with accurate, relevant, and timely data. CDC’s mission is to promote health and quality of life by preventing and controlling disease, injury, and disability. Data collected in the NHDS is used to support these goals by describing the types of care and services provided in hospitals across the United States.
D. Privacy and Confidentiality
The National Center for Health Statistics has authority to collect data concerning the public’s use of hospital services under Section 306 (b) (1) (F) of the Public Health Service Act (42 USC 242k).
Any information which could identify the hospital or patient is held strictly confidential and seen only by those persons involved with the implementation of the National Hospital Discharge Survey. Assurance of confidentiality is provided to all respondents according to Section 308 (d) of the Public Health Service Act (42 USC 242m).
In addition, Section 513 of the Confidential Information and Protection and Statistical Efficiency Act or CIPSEA (Title 5 of Public Law 107-347) includes provisions for a felony conviction and/or fine of up to $250,000 if NCHS staff violates the confidentiality provisions.
The requirements of the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule on health information permit hospitals to make disclosures of protected health information without patient authorization for (1) public health purposes, or (2) research that has been approved by an Institutional Review Board. To assure compliance with the Privacy Rule, RTI will provide the hospital with a privacy notice to patients indicating that data may have been disclosed to NCHS for research or public health purposes. This notice will be placed in the patient’s record.
E. RTI International
RTI International is one of the world's leading research institutes, dedicated to improving the human condition by turning knowledge into practice. RTI has more than 3,800 professionals providing research and technical services to governments and businesses in more than 40 countries in the areas of health and pharmaceuticals, education and training, surveys and statistics, advanced technology, international development, economic and social policy, energy, and the environment. RTI International was awarded the contract to “Develop and Field the Redesigned National Hospital Discharge Survey.”
F. Important Information about Procedures for Completing and Handling Sampling Forms
The following procedures should be followed when handling and completing sampling forms during and after the process is completed:
Complete the forms using a blue or black ballpoint pen. If you make a mistake, cross it out, or start over on a new form. Our concern is not neatness (although readability is critical), but rather accuracy and completeness.
The Sample Listing Sheet (SLS) forms should be kept at the hospital and always stored in a secure place. Completed, original SLS-PARTS A and B must be given to the hospital primary contact before the RTI abstractor comes to the hospital to conduct abstraction. Please be sure to make photocopies of the SLS-PART A and B to keep with the discharge lists used for sampling. See Appendix A for copies of the SLS forms.
For the Pretest, we plan to conduct the abstractions of the sampled cases in January-February 2009. The pretest sample will be selected from discharges during June, July, August and September 2008. As mentioned above, in order to test our sampling procedures, we would like you to utilize two separate sampling periods, i.e. June/July and August/September 2008. Sampling must be performed before data collection is scheduled to begin. In addition, sampling should be performed only when all discharges for the sampling months (June-September 2008) are entered in your hospital’s record-keeping system.
The RTI abstractor will call the hospital primary contact in advance to make arrangements to pick up SLS Parts A and B when s/he comes to the hospital to conduct the abstractions. It is extremely important that SLS Parts A and B be given to the hospital primary contact before the abstractor arrives to perform the abstractions.
Although we are requesting that you sample discharges from the months of June-September, we plan to abstract (collect data) only for the discharges selected from the month of September. Instructions will be provided to your hospital primary contact regarding gathering information from the UB-04, as well as from medical and billing records for these discharges. Assembling this information must be done before the abstractor’s scheduled visit to collect data.
Chapter II. Sampling Discharge Records
A. Purpose of Sampling Discharge Cases
For the Pretest, we are asking you to select samples from 2 time periods: Sampling Period 1 (June/July 2008) and Sampling Period 2 (August/September 2008). The following instructions provide specifications for you to follow to create discharge lists and to sample cases from those lists for each sampling period. Depending on whether your hospital serves different types of inpatients, you will be selecting discharges from 1 to 5 different groups of patients. These groups will be created according to specific definitions that are described below. The goal is to sample a total of about 32 inpatients and 8 observation status cases over the two, 2-month sampling periods, although these numbers may vary based on special situations in your hospital.
For statistical purposes, we only need a representative sample of discharges from each hospital. By sampling just a small number of records, we limit the amount of time and effort needed to collect the information from your hospital. If hospitals selected discharge records without following specific sampling procedures, there would be bias in the selection. An example of improper sampling would include selecting convenience samples, such as the most recent or the most accessible discharge records.
It is important for all hospitals to follow standard sampling procedures so as to ensure an unbiased and representative sample of discharges. A major goal of the Pretest is to detect and correct problems in discharge sampling before the procedures are implemented in the larger, national sample of hospitals.
B. Overview of Steps in Discharge Sampling
In the following sections of this manual, we describe the steps to be followed in conducting the discharge sampling. There are two major stages in discharge sampling. The first stage is to create discharge lists for each group to be sampled, and the second stage is to follow the given procedures to randomly select the discharges (or observation status cases). You will create separate lists for each group of discharges for each of the two sampling periods. So essentially, you will have up to 5 lists for Sampling Period 1 and up to 5 lists for Sampling Period 2, potentially totaling 10 lists. The steps for each stage include:
STAGE 1: Creation of Lists (performed separately for each sampling period)
Identify all inpatient discharges and observation status cases for the months of June/July, 2008 (and then separately for August/September 2008).
Assign each discharge to one and only one group according to the definitions provided. There will be 1 to 5 group lists for each sampling period.
Sort the discharges in each of the groups by the date of discharge within the sampling period.
Number the discharges in each group sequentially from #1 to the last discharge.
These steps create the lists from which a sample will be selected.
STAGE 2: Select/Sample Discharges
Record the total number of discharges for each group for the sampling period on a Sample Listing Sheet, Part A.
Calculate the sampling interval according to the instructions provided below.
Select discharges to be in the sample using the method described below.
Record relevant information about the sampled discharges on a Sample Listing Sheet, Part B.
Keep a copy of all materials used in the sampling process in a secure location, and transfer all original completed sampling forms to your hospital primary contact, who will give them to the RTI abstractor.
Before the arrival of the RTI abstractor to perform data collection, the hospital primary contact will coordinate with other hospital staff in order to assemble UB-04, medical and billing records for the discharges whose information will be collected. For the pretest, these discharges will primarily be from the month of September 2008.
C. STAGE 1: Identifying and Assembling Lists of Inpatient and Observation Status Case Discharges
You will first need to identify and assemble a list of all inpatients and observation status cases discharged during June/July, 2008 (and then August/September 2008). You may need to use various sources to assemble complete lists. For example, in addition to an inpatient discharge list, it may be necessary to obtain observation status cases from lists maintained by the Emergency Department. Next, you will separate the master lists into the following distinct groups of discharges for each sampling period:
Group 1: Observation status cases, not including those admitted as inpatients
Group 2: Normal newborn infants
Group 3: Discharges with Acute myocardial infarctions (AMI)
Group 4: In-hospital deaths (not in Groups 1, 2 or 3)
Group 9: Other inpatient discharges (not in Groups 1, 2, 3, or 4)
REMEMBER: If your hospital does not serve one of these groups, then there will not be a listing for that group. In total, you will assemble from 1 to 5 discharge lists per sampling period depending on the groups that your hospital serves.
Exhibit A below describes the steps to follow when creating the lists of discharges for each sampling period, as well as the specific definitions for each of the groups of discharges.
EXHIBIT A. Steps and Definitions for Grouping Discharge Cases
STEP 1: For Sampling Period 1 (then again for Sampling Period 2), identify, list, sort by date, and number Group 1, Observation Status Cases.
These are discharges in which the patient was assigned to a hospital bed for a period of time (usually less than 24 hours) under medical supervision, without formal admission into the hospital.
The inpatient discharge list may or may not include all of these cases. It may be necessary to obtain a complete listing of these cases by combining lists of Emergency Department, outpatient and/or ambulatory surgery visits.
STEP 2: For Sampling Period 1 (then again for Sampling Period 2), identify, list, sort by date, and number Group 2, Normal Newborn Infants.
These are discharges of infants admitted by birth to the hospital, but having no complicating conditions necessitating medical treatment and not included in Group 1.
Included in Group 2 are discharges having one of the following ICD-9-CM diagnosis codes as the first-listed or principal diagnosis: V30.00, V30.01, V31.00, V31.01, V32.00, V32.01, V33.00, V33.01, V34,00, V34.01, V35.00, V35.01, V36.00, V36.01, V37.00, V37.01, V38.00, V38.01, V39.00, or V39.01. No other secondary diagnoses may be present in the record. Exclude newborns having any other diagnosis codes from this group.
Inpatient discharge lists will be used to create Group 2.
STEP 3: For Sampling Period 1 (then again for Sampling Period 2), identify, list, sort by date, and number Group 3, Discharges with Acute Myocardial Infarction (AMI)
These discharges must include at least one of the following ICD-9-CM diagnosis codes listed as any discharge diagnosis (i.e., principal or secondary): (410.00, 410.01, 410.02, 410.10, 410.11, 410.12, 410.20, 410.21, 410.22, 410.30, 410.31, 410.32, 410.40, 410.41, 410.42, 410.50, 410.51, 410.52, 410.60, 410.61, 410.62, 410.70, 410.71, 410.72, 410.80, 410.81, 410.82, 410.90, 410.91, 410.92, 411.0, 411.1, 411.81, 411.89).
These discharges are not already included in Group 1 or Group 2.
Inpatient discharge lists will be used to create Group 3.
STEP 4: For Sampling Period 1 (then again for Sampling Period 2), identify, list, sort by date, and number Group 4, In-hospital Deaths.
These include discharges whose status at discharge is dead.
These discharges are not already included Group 1, Group 2, or Group 3.
Inpatient discharge lists will be used to create Group 4.
STEP 5: For Sampling Period 1 (then again for Sampling Period 2), identify, list, sort by date, and number Group 9, Other Inpatient Discharges.
These include all other discharges, excluding those already listed in Groups 1, 2, 3, or 4, and excluding outpatients, emergency department visits, and ambulatory surgery visits.
Inpatient discharge lists will be used to create Group 9.
It is important when assigning the discharges to groups that you do so in order—first Group 1, second Group 2, etc.—so as to ensure that Groups 1, 2, 3, 4 and 9 are non-overlapping (mutually exclusive). For this reason, the definition for Group 4, in-hospital deaths, for example, includes discharges whose status at discharge is dead, and who are not included in Group 1, Group 2, or Group 3.
As indicated above, the groups will be created using information based on the medical diagnoses and discharge status for each discharge. Each discharge on the list must also include the date of discharge and a discharge identifier, which is a unique number assigned by the hospital to identify the hospitalization. It could be the medical record number, the Patient ID number, the visit number, the encounter number or the billing number. If no number exists in your hospital that identifies a unique hospitalization, please provide the medical record number and the date of discharge.
If your hospital does not provide services for one or more of these 5 groups, then you will reallocate the discharges from that group to Group 9, All other inpatient discharges, as described under Chapter III – Special Situations.
As outlined in Exhibit A, each group listing must be sorted in chronological order by date of discharge. All identified discharges and observation status cases included in the group lists must have been discharged between June 1 and July 31 for Sampling Period 1 and between August 1 and September 30 for Sampling Period 2.
Also, for each list that has been ordered by discharge date, a number must be assigned to each discharge, starting with #1, until all discharges have been enumerated sequentially. This assigned number is called the sequence number. Refer to Appendix E for an example of a numbered discharge list.
Finally, you will create either a paper copy or an electronic file (i.e. EXCEL, csv or txt) of each group listing that will be used for sampling. Because most hospitals will perform the process of assembling discharge listings electronically, we recognize that it may be easier to create and manipulate electronic files rather than paper copies of listings. We do ask, however, that you keep these files so that they can be retrieved and examined at a later time, if the need arises to check the sampling process.
D. STAGE 2: Selecting Discharges (Completing the Sample Listing Sheets)
After creating, sorting and numbering the group listings for each sampling period (up to 5 lists for each), you will then complete the Sample Listing Sheet (SLS) for each sampling period and select the discharges to be abstracted. You will complete the SLS sheet for Sampling Period 1 first. This is important as some figures calculated in Sampling Period 1 will be used to calculate figures in Sampling Period 2.
There are 2 parts to the SLS:
Part A: Hospital Information, Sampling Interval and Random Start Number.
Part B: Discharge Sampling Information to record identifying information on the sampled discharge cases.
The information that you record on the paper copy of the Sampling Listing Sheet, Parts A and B, will be entered by the abstractor into a computerized tool that s/he will use during the abstraction of the medical records.
1. Completing Hospital Information, SLS-PART A (for Sampling Period 1)
Parts A and B of the Sampling Listing Sheet must be completed separately for each sampling period. Again you will complete the process for Sampling Period 1 before doing Sampling Period 2. The following information is required on SLS, Part A:
The Hospital ID # will be filled in for you in advance.
Sampling performed by: Check the appropriate box if necessary- however, the abstractor may pre-fill this for you.
Your name (the person performing the sampling) and the date you are conducting the sampling.
The sampling period from which the discharge records will be sampled. For the Pretest the sampling period is June/July 2008 or August/September 2008.
Number of Months in Sampling Period. For this Pretest, you will enter “2” here.
EXHIBIT B. SLS Part A Table 1

Table 1 in Part A of the SLS requires information for each sampling period: (June/July, 2008 or August/September 2008). The information to be collected includes:
Column a. Did the hospital serve this group of inpatients during the year? This will be pre-filled by the RTI abstractor, based on your hospital’s response to items in the Facility Questionnaire. If the answer is “No” for the year, you do not need to complete the items to the right in the same row for that group. This information is only relevant for Sampling Period 1, because it determines how to compute the sampling interval.
Column b. Total number of discharges for this group during sampling period (zero if ‘No’ to a). This is the total number of discharges on each list for each group served by the hospital for the given sampling period.
Column c. Number of cases to be targeted in this sampling period.
i. During Sampling Period 1 (June/July, 2008), this is to be calculated using the answers to column a (Did hospital serve this group of patients during the year) and Number of Months in Sampling Period (2 for pretest). The target number of discharges to be sampled within each 2-month sampling period is known (20 for each sampling period). If a hospital has discharges in all 5 groups, Exhibit C below shows the target number of discharges per group, for 1- to 6-month sampling periods. For the pretest, we will use 2-month sampling periods, but in later surveys there may be longer or shorter periods.
If a hospital answers “no” to the question of whether a hospital serves a certain group, the discharges that would have been allocated to that group should be reallocated to the “All other inpatient discharges” group.
For example, given a 2-month sampling period, if a hospital does not have an obstetrics (labor and delivery) service, then it would not have newborn infants, as defined in this survey. Thus, the 2 records allocated to Group 2, Normal Newborn Infants, would be reallocated to Group 9, “All other inpatient discharges”. The resulting number of targeted cases for Group 9 would be 10, instead of 8 for the 2-month sampling period.
EXHIBIT C. Number of cases to be targeted for selected sampling periods
Group or Type of discharge |
Total for a 1 month sampling period |
Total for a 2 month sampling period |
Total for a 3 month sampling period |
Total for a 4 month sampling period |
Total for a 5 month sampling period |
Total for a 6 month sampling period |
1. Observation status cases |
2 |
4 |
6 |
8 |
10 |
12 |
2. Normal Newborn Infants |
1 |
2 |
3 |
4 |
5 |
6 |
3. Acute Myocardial Infarction (AMI) |
2 |
4 |
6 |
8 |
10 |
12 |
4. In hospital deaths |
1 |
2 |
3 |
4 |
5 |
6 |
9. All other inpatient discharges |
4 |
8 |
12 |
16 |
20 |
24 |
Total |
10 |
20 |
30 |
40 |
50 |
60 |
NOTE: Be sure to use the column that represents the correct length of the sampling period. For the Pretest, this is 2 months.
Column d. Sampling Interval for this group (b divided by c). This is to be calculated using the Total number of discharges for this group (column b) from Sampling Period I and column c (Number of cases targeted for sampling in this sampling period) for Sampling Period I to give you the sampling interval (column d). The sampling interval (column d) is the same throughout the remainder of the sampling periods for the year. You will calculate the sampling interval for each subsequent group in the same manner.
For example, if Hospital A, has 250 discharges in Group 1 for Sampling Period 1 (column b) and we know the number of targeted discharges for this sampling period is 4 (column c) then our formula would be:
250 = 62.5
4
Note: you would drop the decimal and use just the whole number (i.e., 62); this would be our sampling interval for Group 1. This same formula would be applied for each group to calculate the sampling interval to complete column d for each group the hospital serves.
Column e. Actual number of cases sampled for this group is calculated by, subtracting the random start number(column f) from the total number of discharges then dividing this result by the sampling interval. “1” is added to the quotient and the total is rounded down. The formula is:
[Total discharges – Random Start ] +1
Sampling Interval
For example, if Hospital A, has 250 discharges in Group 1 for Sampling Period 1 (column b) and we know the random start number (column f) and the sampling interval (column d), then our formula would be:
[250 – 40 ] +1 = 210 + 1 = 4.38
62 62
Note you would drop the decimal and use just the whole number (i.e., 4); this may differ from the “targeted” number due to the rounding down to a whole number and variations in hospital volume across months of the year.
Column f. Random Start Number. The random start number may be obtained by any method that generates random numbers. Alternatively, you can also follow the instructions in Appendix B. It must be number between 1 and the value of the sampling interval (column d) for that group. It is used only in Sampling Period 1 to determine the case selected first.
2. Selecting Discharges from Each Group
At this point you have completed the following activities in the sampling process:
Identified and grouped the discharges for the sampling periods of June/July, 2008 and August/September 2008 into up to 5 groups each, based on the definitions in Exhibit A. Groups 1, 2, 3, 4 and 9 must be mutually exclusive, that is, a discharge can only appear in one group only based on the priorities defined in Exhibit A.
Sorted groups 1, 2, 3, 4, and 9 by date of discharge.
Numbered each discharge case in each group.
Generated a hard copy listing or an electronic file for each group sorted by date of discharge, including the SEQUENCE NUMBER, DATE OF DISCHARGE, and DISCHARGE IDENTIFICATION NUMBER (e.g. Medical Record Number, Billing Number, etc) , and an alternate identifier, if needed.
You should have one SLS sheet (Part A) completed for Sampling Period 1 only.
The next step is to select the required number of discharges from each group. We will walk through the selection process and then you will complete Table 2 on SLS-PART B (the second page of the SLS sheet), for Sampling Period 1, including information for each group of discharges. Note: Be sure to enter the Hospital ID as it appears on the SLS-PART A and the dates of the sampling period at the top of the SLS sheet.
To select discharges for each group you will need the following:
The hard copy or electronic file containing all discharges for each group sorted by date of discharge, and numbered sequentially.
The sampling interval (k) that you calculated and documented in Table 1, column d for each group.
The random start number that you obtained from the computer or from the suggested website and documented in Table 1, column f for each group.
To sample discharge cases for a group, follow these steps for the first sampling period:
Step 1: Circle or highlight the sequence number on the numbered list that equals the random start number (Table 1, column f on SLS-PART A). This is your first discharge record selection, that is, your first sampled discharge. NOTE: If the number of sample records desired is 1, then this is the only discharge you will select.
Step 2: To make the next selection, add the value of the sampling interval (k) (Table 1, column d on SLS-PART A) to the sequence number of the discharge record selected in Step 1 (i.e. the discharge corresponding to the random start number) and the result is the number of the next discharge to be sampled. Circle or highlight that sequence number on the numbered list. This is your second selection. Continue this procedure until you have selected the actual number of cases for each group (column e). This will be the same as sampling discharges in this fashion until adding the sampling interval to the last sampled sequence number would result in a number greater than the total number of discharges for that group in the sampling period.
Example: If there are 250 observation status cases (Group 1) in the sampling period, the sampling interval (k) is 62 (250 observation status cases divided by 4, the number of targeted observation status cases to be sampled- column c on SLS-PART A). The random start number will be a number between 1 and 62. If, for example, the random start number is 40 (obtained from the website in Appendix B, or generated by computer), the first case selected would be the 40th discharge on the list (or sequence # 40). The next case selected would be 40+62, or the 102nd discharge listed (or sequence # 102). The third case selected would be 102 + 62 or the 164th discharge listed (or sequence # 164). The fourth case selected would be 164 + 62 or the 226th discharge listed (or sequence # 226). Since we only need to sample 4 observation status cases (Sequence numbers: 40, 102, 164, 226), you are finished with this group. As a check, adding 62 again would result in 288, which is greater than the total number of discharges in the group (250) for the sampling period.
Step 3: Once you have selected the actual number of cases for each group you will see that there may be additional cases in the listing after the last selection was made (the last sequence # selected).
To account for these remaining cases, you will calculate what is called a “carry over number” using this last sequence number. This carry over number will be used to determine (a) where to begin sampling in Sampling Period 2, and (b) the targeted number of cases for each group for Sampling Period 2 (August/September 2008).
First, list the information on the cases you have selected in Table 2 of the SLS-PART B for the group you are sampling. Include the group number, sequence number, a discharge identifier (medical record number, the Patient ID number, or the billing number) and the discharge date for each sampled discharge. Also, in the column under “alternate identifier” record any other number associated with the discharge that would facilitate locating the discharged patient’s medical record, billing information or laboratory information. (Detailed steps for entering information into Table 2 are below.)
Next, Calculate the Carry-over number for each group. This number is calculated by subtracting the last sequence number selected (Table II, first column) from the Total number of discharges for the sampling period (Table I, column b), or
Total number of discharges for the sampling period for group k
minus
the last sequence number selected for group k
Example: Continuing with our example above. For Group 1 (observation status cases) if your sequence numbers were 40, 102, 164, and 226 your last sequence number would be 226. Our total number of discharges is 250. Therefore the formula to calculate the carryover number is:
250-226 = 24
Repeat this calculation for each group, after you do steps 1 and 2 for each group, and enter the carry over number on the bottom of page two of the SLS sheet, below Table II, as shown in Exhibit D below.
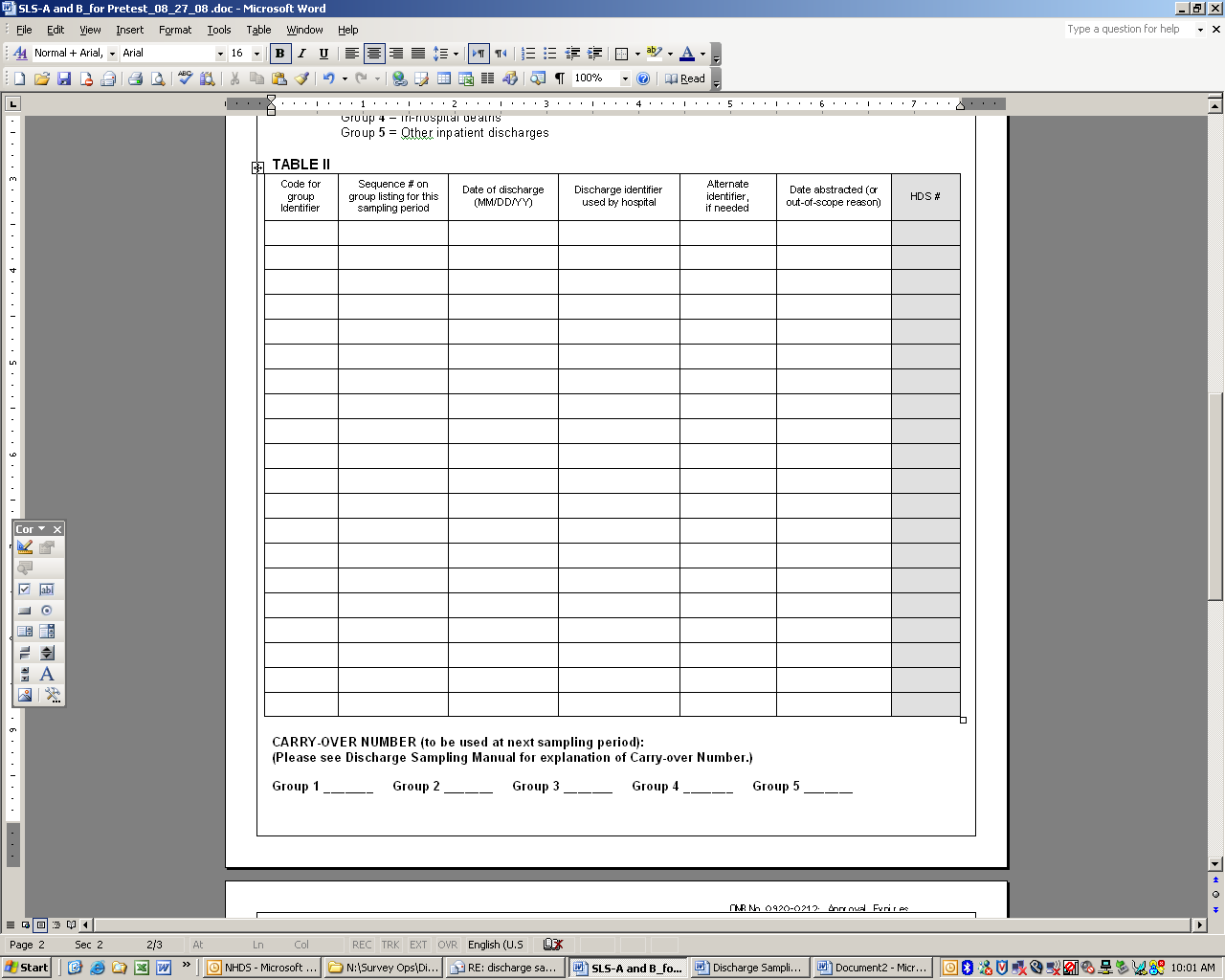
EXHIBIT D. Entering carry over number below Table 2, Part B

STEPS 1-3 NEED TO BE REPEATED FOR EACH GROUP THAT YOU ARE SAMPLING FOR THE SAMPLING PERIOD.
Details for entering information into Table 2 of the SLS Sheet:
Enter information into Table 2 for each sampling period (one SLS sheet per sampling period) starting with Sampling Period 1:
EXHIBIT E. Entering information into Table II, Part B

Column a: Enter the group code for the first discharge case you have selected, ideally starting with Group 1 all the way through Group 9. The number of discharges selected for each group in Sampling Period 1 should equal the value in Column e of SLS, Part A.
Column b: Enter the sequence numbers that you circled or highlighted to identify sampled discharges for each group as they appear on the list of discharges.
Column c: Enter the date of the discharge as it appears on the list of discharges for that group
Column d: Enter the discharge identifier used by your hospital for each selected discharge.
Column e: Enter the Alternate Identifier, if needed
Column f: NOTE- You do not need to complete this column. The date this discharge case is abstracted (or reason if this discharge has been determined as out of scope) will be completed by the RTI abstractor.
Column g: NOTE- You do not need to enter the HDS #. The abstractor will enter this number into the table when they come to abstract.
Once you have completed the selection of discharges for Sampling Period 1, and have filled in columns a through e, you will need to enter the “Carry-Over Number” to be used for the next sampling period in the designated area just below Table II as described in Step 3 above.
You have completed the discharge sampling process for the Sampling Period 1. You will now complete the discharge sampling process for Sampling Period 2.
At this point you have completed the following activities in the sampling process:
Identified and grouped the discharges for the sampling periods of June/July, 2008 and August/September 2008 into up to 5 groups each, based on the definitions in Exhibit A. Groups 1, 2, 3, 4 and 9 must be mutually exclusive, that is, a discharge can appear in one group only based on the priorities defined in Exhibit A.
Sorted and numbered each discharge in groups 1, 2, 3, 4, and 9 by date of discharge.
Generated a hard copy listing or an electronic file for each group sorted by date of discharge, including the SEQUENCE NUMBER, DATE OF DISCHARGE, and DISCHARGE IDENTIFICATION NUMBER (e.g. Medical Record Number, Billing Number, etc)
You should have one SLS sheet (Part A) completed for Sampling Period 1 only.
You should have one SLS sheet (Part B) completed for Sampling Period 1 only.
3. Completing Hospital Information, SLS-PART A (for Sampling Period 2)
You are now ready to complete Parts A and B of the Sample Listing Sheet for Sampling Period 2. The same information is required on SLS, Part A:
The Hospital ID # will be filled in for you in advance.
Sampling performed by: Check the appropriate box if necessary- however, the abstractor may pre-fill this for you.
Your name (the person performing the sampling) and the date you are conducting the sampling.
The sampling period from which the discharge records will be sampled: August/September 2008.
Number of Months in Sampling Period. For the Pretest, you will enter “2” here.
EXHIBIT F. Entering information into Table 1, SLS Part A (Sampling Period 2)

For Sampling Period 2, the only information required is for column b, because the information in column a was needed only in Sampling Period 1 for use in computing the sampling interval, which is constant throughout the data collection period.
Column a. Did the hospital serve this group of inpatients during the year? Do not enter for Sampling Period 2. Needed only in Sampling Period 1.
Column b. Total number of discharges for this group during sampling period (zero if ‘No’ to a). This is the total number on each discharge list for each group served by the hospital for Sampling Period 2.
Column c. Number of cases to be targeted in this sampling period.
i. This is the same as for Sampling Period 1, because Sampling Period 2 also has 2 months (August/September, 2008), and the targeted sample number is based only on the yearly totals desired and the number of months in the sampling period.
Column d. Sampling Interval for this group. As noted previously, the sampling interval (column d), calculated previously for Sampling Period 1, should remain the same throughout the remainder of the sampling periods for the year, regardless of the number of months in the sampling period. Enter the sampling interval calculated for Sampling Period 1 for each group.
Column e. Actual number of cases sampled. For Sampling Period 2 (August/ September, 2008), column c (Number of cases actually sampled in this period) is to be calculated using:
the carry-over number from Sampling Period 1
column d (sampling interval for this group)
column b (Total number of discharges for this group during Sampling period 2).
This will be done by adding the carry over number for the group to the total number of discharges for the group, and then dividing by the sampling interval.
Actual # of cases = (Carry-over # + Total # of discharges)
Sampling Interval
To obtain the actual number of discharges to be sampled in Sampling Period 2, drop the decimal and use just the whole number. This number is recorded in column e. Again, due to rounding down and variations in hospital volume across months of the year, this number may differ from the “targeted” number.
Example. If Hospital A, has 129 discharges in Group 2 for Sampling Period 2 (column b) and we know from Parts A and B of the SLS for Sampling Period 1 that the sampling interval is 39 (column d) and the carry-over number is 17 for this group, then our formula would be:
17 + 129 = 146 = 3.74 or 3
39 39
Column f. Random Start Number. Do not calculate for Sampling Period 2. The random start number is NOT calculated for Sampling Period 2.
For Sampling Period 2, follow these steps to sample discharge cases for a group:
Step 1: Use the list of discharges for Sampling Period 2 (August-September 2008). Circle or highlight the sequence number on the group list, that has already been numbered, that equals the sampling interval minus the carry-over number from Sampling Period 1. This gives you the sequence number of the first case, i.e., your first sampled discharge for Sampling Period 2.
First Selected Discharge = Sampling Interval minus
Carry-over # from Sampling Period 1
NOTE: If the number of sampled records desired is 1, then this is the sampled discharge.
Example: Group 1 from Sampling Period 1 had a sampling interval of 62 and a carry over of 24.
62-24= 38
Thus 38 is the sequence number of the first discharge selection for Group 1 for the Sampling Period 2.
Step 2: To make the next selection, if the number of records to be sampled for this group is greater than one, then add the sampling interval (table I, column d) to the sequence number for the first case you have already selected. Circle or highlight that sequence number on the numbered list of discharges. This is your second selection. Continue this procedure until the last possible sequence number is reached that is less than the total number of discharges for the sampling period (Table I, column b).
Step 3: List the information on the cases you have selected in Table 2 of the SLS-PART B for the group you are sampling. Include the group number, sequence number, a discharge identifier (medical record number, the Patient ID number, or the billing number) and the discharge date for each sampled discharge. Also, in the column under “alternate identifier” record any other number associated with the discharge that would facilitate locating the discharged patient’s medical record, billing information or laboratory information.
STEPS 1-3 NEED TO BE REPEATED FOR EACH GROUP THAT YOU ARE SAMPLING FOR THIS SECOND SAMPLING PERIOD.
After you have completed the discharge sampling process you will have selected approximately 40 discharges total (about 20 for each sampling period), you should make a copy of the completed SLS sheets for your records and give the original copy to the hospital primary contact for the abstractor when s/he comes to conduct abstraction.
F. Instructions for Selecting Discharges for Data Abstraction
For the purposes of the pretest, we plan to perform abstractions (collect data) for 10 discharges only. These records will be from Sampling Period 2 (August/September 2008) and will be selected starting with the last discharge and progressing backwards, until the number needed for each group is obtained. Most of these discharges will be from the month of September 2008. The hospital primary contact will arrange to have the UB-04, as well as the medical and billing records of these 10 discharges available for the abstractor when s/he arrives to do the data collection. This is why it is so important for the SLS Parts A and B to be completed and given to the primary contact as soon as the sampling is finished.
If your hospital serves each of the 5 groups of discharges listed on Part A of the SLS, then the 10 discharges chosen for abstraction will distributed as shown in Exhibit G.
EXHIBIT G. # of records in each group chosen for abstraction |
||
Group Code |
Group Description |
# of records to be abstracted |
1 |
Observation status cases, not including those admitted as inpatients |
2 |
2 |
Normal newborn infants |
1 |
3 |
Discharges with Acute myocardial infarctions (AMI) |
2 |
4 |
In-hospital deaths (not in Groups 1, 2 or 3) |
1 |
9 |
Other inpatient discharges (not in Groups 1, 2, 3, or 4) |
4 |
If your hospital does not serve all of the groups, remember you will reallocate the # targeted for the missing groups to the total for Group 9.
Appendix C shows a table that summarizes information for each group for your quick reference. Appendix D shows examples of completed SLS-PART A and PART B forms.
Chapter III. Special Situations
If your hospital does not provide service to one or more of Groups 1, 2, 3, or 4, then you must reallocate the number of targeted cases for the missing group(s) to Group 9, “All other inpatient discharges”. This must be done before you calculate the sampling interval for Sampling Period 1.
EXAMPLE: If a hospital does not have newborn infants (Group 2) and a sample of 2 normal newborn infants is required for Sampling Period 1, you would reallocate and select an additional 2 cases in Group 9, “All other inpatient discharges.” The resulting number of targeted cases for “All other inpatient discharges” would be 10, instead of 8 for a 2-month sampling period. This new total number of “All other inpatient discharges” would be recorded in Table 1, column c (SLS-PART A), and used to determine the sampling interval (k) in Table 1, column d (SLS-PART A). Repeat this procedure for any other group that your hospital does not serve, by reallocating the targeted number for each group that is missing to Group 9, “All other inpatient discharges”, then calculate the sampling interval as described.
2. What if my hospital does provide services for one or more of the groups but it is not possible to separate those cases into distinct groups?
In this case also, you would reallocate the targeted number of discharges from the given groups to Group 9, “All other inpatient discharges” and compute the sampling interval as directed.
The extreme case would be if the hospital is not able to create any of the groups. In this situation, you would proceed as if there were only 1 group, compute the sampling interval based on the total targeted number of discharges, then follow sampling instructions as you would for each separate group.
3. What is my hospital serves observation status cases, but it is not possible to obtain administrative or medical information on them from existing databases?
In this case, you would proceed as in #1 above, by reallocating the targeted cases for the observation status cases, and then call Tiffany King, Ph.D. at RTI International (Telephone: 312-777-5207. Email: [email protected]).
4. What if the discharge list at my hospital includes ambulatory surgery, emergency department or outpatient visits, and these cannot be taken out ?
If these types of cases cannot be separated out from your inpatient discharges, then contact Tiffany King, Ph.D. at RTI International (Telephone: 312-777-5207. Email: [email protected]). She will need to know the total annual number of such cases served in your hospital, and will provide assistance on sampling from an ungrouped list.
Chapter IV. Administrative Issues
Since the Pretest is being conducted to test the procedures of the NHDS redesign, several activities will be implemented to inform NCHS and RTI on what is working and which procedures need revisions or enhancements. For example, we will hold a debriefing with hospital staff to obtain feedback on what worked well and what we can improve to make hospital involvement in the NHDS as smooth as possible. Likewise, it is important for the person responsible for conducting the sampling of discharges (you) to note any issues related to the process. This would include such issues as:
The clarity, thoroughness, and accuracy of the sampling instructions;
The functionality and completeness of the SLS;
The amount of time it takes to conduct the sampling; and
Any issues within the hospital infrastructure that makes the sampling task difficult or impossible.
If you have any questions regarding the Pretest sampling procedures, call Tiffany R. King, Ph.D. at RTI International. Telephone: 312-777-5207. Email: [email protected].
Appendix A:
SLS PARTS A and B
N
OMB No.:
0920-0212
Approval Expires 08/31/2008
SAMPLE LISTING SHEET
PART A: Collecting Group Statistics and Determining Sampling Interval
Notice - Public reporting burden for this collection of information is estimated to average 14 minutes per response, including time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing burden to: CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road, MS D-24, Atlanta, GA 30333, ATTN: PRA (0920-0212).
Assurances of Confidentiality –All information which would permit identification of any individual, a practice, or an establishment will be held confidential, will be used only by NCHS staff, contractors, and agents only when required and with necessary controls, and will not be disclosed or released to other persons without the consent of the individual or the establishment in accordance with section 308(d) of the Public Health Service Act (42 USC 242m) and the Confidential Information Protection and Statistical Efficiency Act (PL-107-347).
For detailed instructions and definitions of terms used in this form, please see Discharge Sampling Manual.
Hospital ID #: |____|____|____|____|
Sampling Performed by: (check only one) □ RTI Abstractor □ Hospital Staff □ NHDS Project Staff
Dates of Sampling Period: Start date: ___/___/___ End date: ___/___/___ Number of Months in Sampling Period: _____
Date Sampling Performed: |____|____| - |____|____| - |____|____| Name of person performing sampling: ________________________
MM DD YY
TABLE I
|
a. Did the hospital serve this group of patients during 2008? |
b. Total Number of Discharges in this group during this sampling period (zero if ‘No’ to a.) |
c. Number of cases targeted for sampling in this sampling period |
d. Sampling Interval for this group ( b divided by c ) |
e. Number of cases actually sampled in this sampling period |
f. Random start number |
Group 1: Observation status cases |
□ Yes □ No |
|
|
|
|
|
Group 2: Normal Newborn Infants |
□ Yes □ No |
|
|
|
|
|
Group 3: Discharges with Acute Myocardial Infarction |
□ Yes □ No |
|
|
|
|
|
Group 4: In-Hospital Deaths |
□ Yes □ No |
|
|
|
|
|
Group 9: All other Inpatient Discharges |
□ Yes □ No |
|
|
|
|
|


Appendix B:
Instructions for Obtaining the Random Start
Number
INSTRUCTIONS FOR OBTAINING THE RANDOM START NUMBER
To get the random start number, go to the website: www.random.org. Click on Integer Generator. You will then see a screen that looks exactly like this:
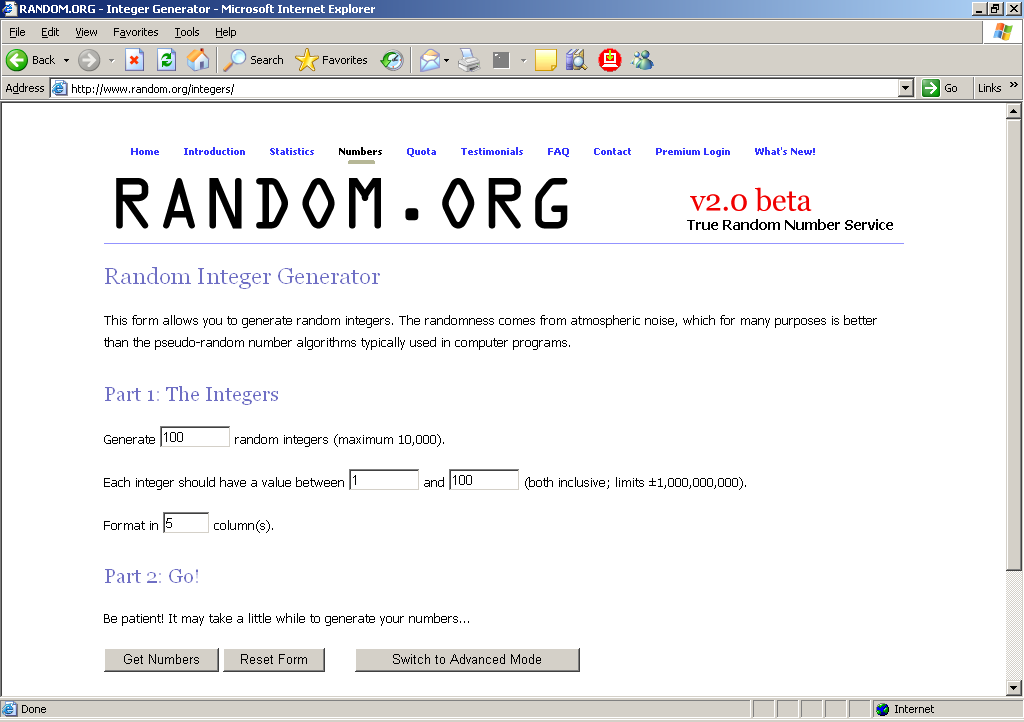
In the 1st field, enter “1”, meaning that you want to generate 1 random integer.
Leave the 2nd field as “1.”
In the 3rd field, enter the sampling interval from Column d of the table in the Sampling List Sheet. From the earlier example where the sampling interval was 62, you would enter 62 into the third field so you are requesting a value between 1 and 62.
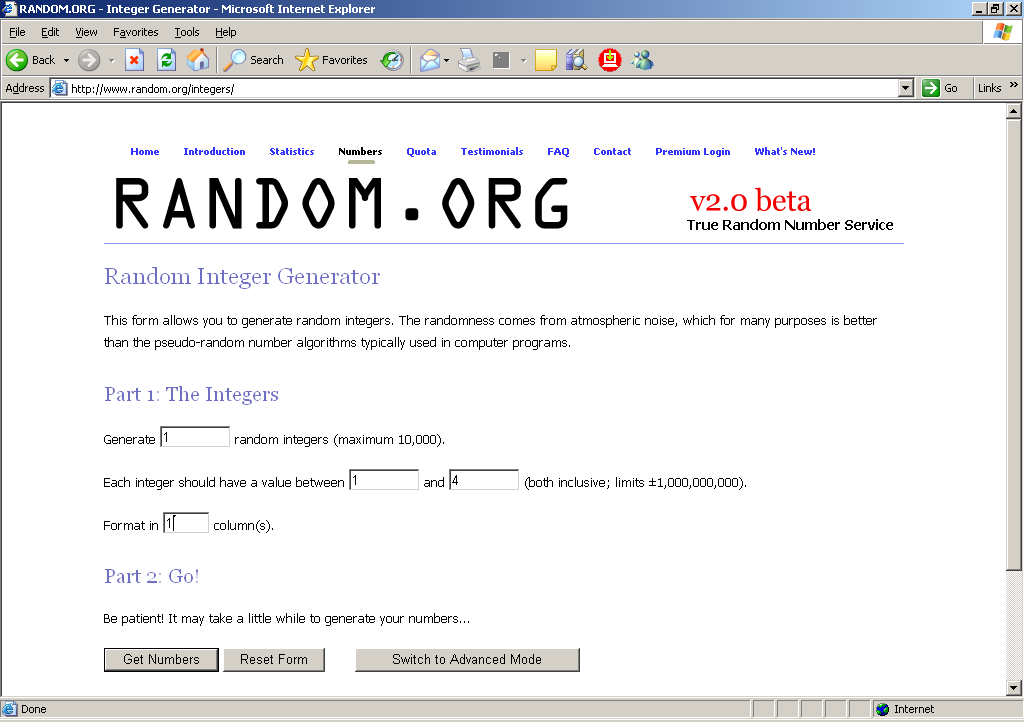
In the 4th field, enter “1” for the format.
In the example below, the request is for random start number between 1 and 4 (4 is the sampling interval in this example).
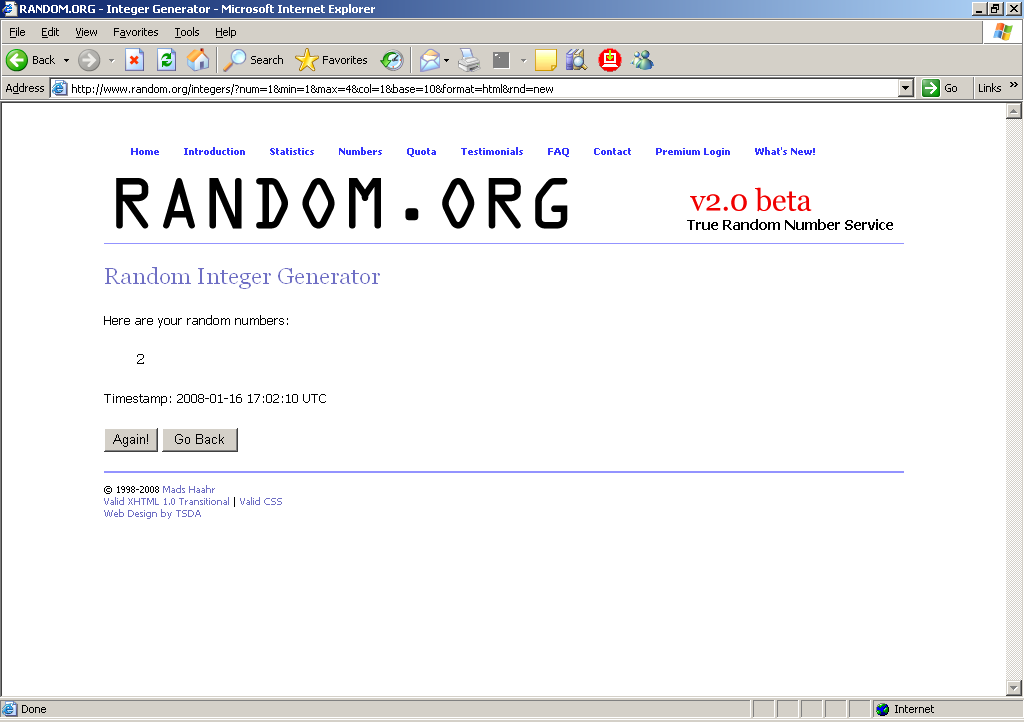
Click on “Get Numbers.”
You will then see a screen that looks like this, indicating that the random start number, in this example, is 2. Record the random start number in Column f of Table 1 on the SLS-PART A.
Appendix C:
Summary of Discharge Grouping Information
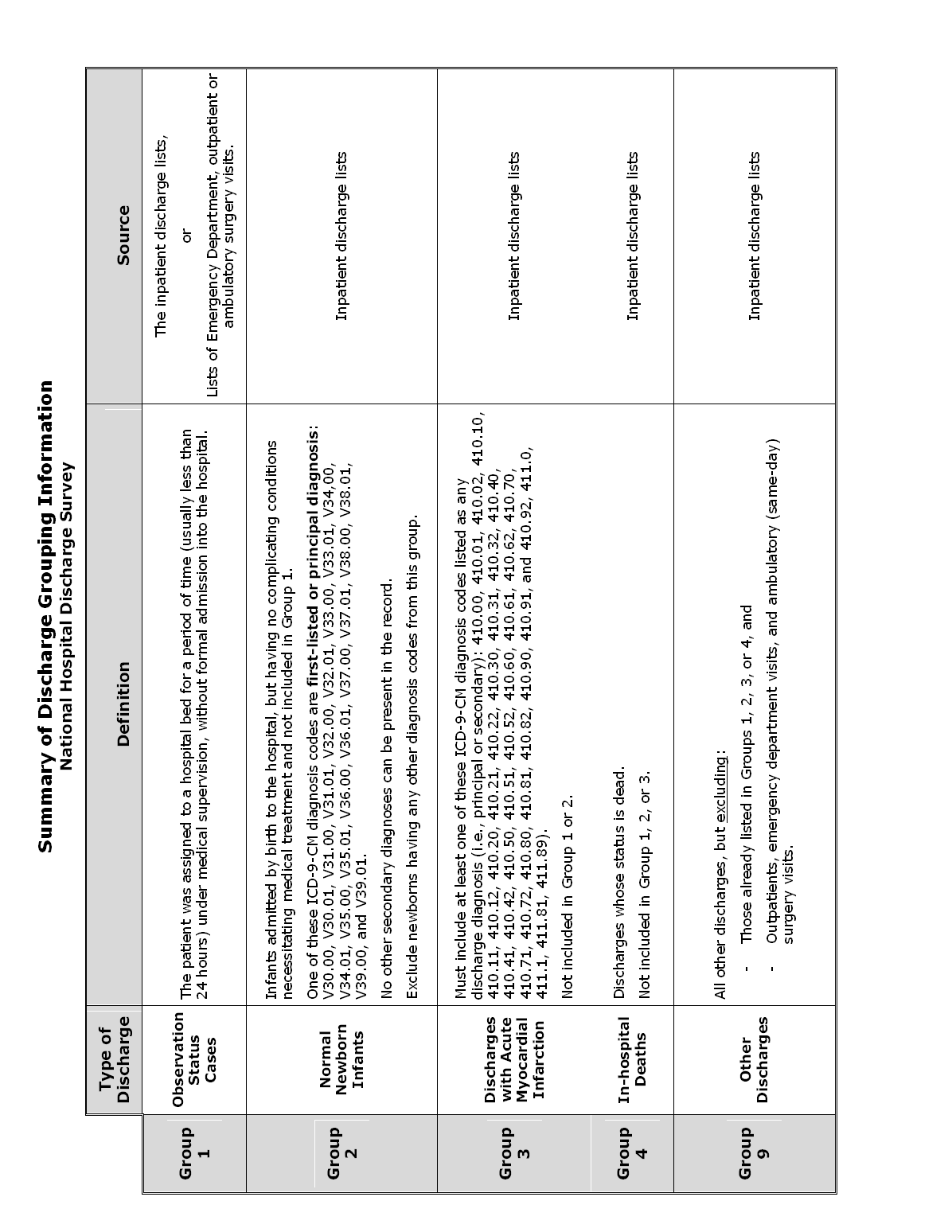
Appendix D:
Completed SLS PARTS A and B
Insert SLS – example page 1
insert SLS example page 2 here
insert example 2 here –page 1
insert example 2 – page 2 here
Appendix E:
Example of Discharge Lists
| File Type | application/msword |
| File Title | National Hospital Discharge Survey |
| Author | jps |
| Last Modified By | mxm3 |
| File Modified | 2008-10-02 |
| File Created | 2008-10-02 |
© 2026 OMB.report | Privacy Policy