Revised_Supporting Statement A_CLIC_11-25-08
Revised_Supporting Statement A_CLIC_11-25-08.doc
NIH-AARP Comprehensive Lifestyle Interview by Computer (CLIC) Study (NCI)
OMB: 0925-0594
SUPPORTING STATEMENT A FOR:
The NIH-AARP COMPREHENSIVE
LIFESTYLE INTERVIEW BY COMPUTER STUDY (clic)
nATIONAL CANCER INSTITUTE (NCI)
November 25, 2008
Arthur Schatzkin, M.D., Dr.P.H
Chief
Nutritional Epidemiology Branch
Division of Cancer Epidemiology and Genetics
National Cancer Institute/NIH
Executive Plaza South, Room 3040
6120 Executive Boulevard, MSC 7242
Bethesda, MD 20892-7335
Voice: 301-594-2931
FAX: 301-496-6829
Email: [email protected]
TABLE OF CONTENTS
Table of Contents ii
List of Attachments iii
A. Justification 1
1. Circumstances Making the Collection of Information Necessary 1
2. Purpose and Use of the Information Collection 10
3. Use of Improved Information Technology and Burden Reduction 12
4. Efforts to Identify Duplication and Use of Similar Information 13
5. Impact on Small Businesses or Other Small Entities 13
6. Consequences of Collecting the Information Less Frequently 13
7. Special Circumstances Relating to 5 CFR 1320.5 14
8. Comments in Response to the Federal Register Notice and Efforts to
Consult Outside the Agency 15
9. Explanation of Any Payment or Gift to Respondents 15
10. Assurance of Confidentiality Provided to Respondents 15
11. Justification for Sensitive Questions 17
12. Estimates of Annualized Burden Hours and Costs 18
13. Estimates of Other Total Annual Cost Burden to Respondents and Record Keepers 23
14. Annualized Cost to the Federal Government 23
15. Explanation for Program Changes or Adjustments 24
16. Plans for Tabulation and Publication and Project Time Schedule .24
17. Reason(s) Display of OMB Expiration Date is Inappropriate 24
18. Exceptions to Certification for Paperwork Reduction Act Submissions …. 24
References 25
LIST OF ATTACHMENTS
Attachment 1 NIH-AARP Diet and Health Study Original Study Summary
Attachment 2 Bibliography of Manuscripts Published in Peer-Reviewed Scientific Journals
Attachment 3-1 Invitation Letter to Current NIH-AARP Cohort Memebers
Attachment 3-2 Invitation Letter to New NIH-AARP Cohort Members
Attachment 3-3 30-Day Follow-Up Invitation Letter to Current NIH-AARP Cohort Members
Attachment 3-4 30-Day Follow-Up Invitation Letter to New NIH-AARP Cohort Members
Attachment 3-5 Invitation Letter from AARP to all invitees
Attachment 3-6 Enrollment Instruction Card for all invitees
Attachment 4-1A Screenshot of Burden Statement for ASA24 Instrument
Attachment 4-1B Screenshots of the ASA24 Instrument -- Automated Self-Administered 24-Hour Dietary Recall Instrument
Attachment 4-2A Screenshot of Burden Statement for ACT-24 Instrument
Attachment 4-2B Screenshots of the ACT-24 Instrument -- Activities Completed by Time in 24
Hours Instrument
Attachment 4-3A Screenshot of Burden Statement for LHQ Instrument
Attachment 4-3B Screenshots of the LHQ Instrument -- Lifestyle and Health History Questionnaire
Attachment 4-4A Screenshot of Burden Statement for DHQ
Attachment 4-4B Paper Version of DHQ Instrument - Diet and Health Questionniare
Attachment 5 NIH-AARP CLIC Study Summary
Attachment 6 Pre-Enrollment Screenshots
Attachment 7 Enrollment Screenshots
Attachment 8 Re-Entry Screenshots
Attachment 9 Evaluation Screenshots
Attachment 10 NIH-AARP Diet and Health External Working Group and Steering
Committee Members
Attachment 11 NIH Privacy Act Officer's Letter
Attachment 12 Westat's Procedures for Keeping Data Confidentiality
Attachment 13 National Cancer Institute Institutional Review Board Approval
Attachment 14 CLIC Study Consent
Attachments 15-1 Email messages sent to participants enrolled in the CLIC Study
to 15-5
A. JUSTIFICATION
A.1 Circumstances Making the Collection of Information Necessary
History and Background. The Public Health Service Act, Section 412 (42 USC § 285a-1) and Section 413 (42 USC § 285a-2) authorizes the Division of Cancer Epidemiology and Genetics (DCEG) of the National Cancer Institute (NCI) to establish and support programs for the detection, diagnosis, prevention and treatment of cancer; and to collect, identify, analyze and disseminate information on cancer research, diagnosis, prevention, and treatment. The goal of the Nutritional Epidemiology Branch (NEB) within the DCEG is to clarify the nutritional etiology of cancer by conducting independent and collaborative research intended to raise the level of evidence of the association between nutritional factors and cancer. More recently, researchers have included investigations on the relationship of cancer to total energy expenditure to more completely assess the impact of nutritional factors on the development of cancer.
In 1995, the Nutritional Epidemiology Branch of NCI fielded the Prospective Study of Diet and Cancer in members of the American Association of Retired Persons (AARP) (OMB# 0925-0423). The “public friendly” name of this study is the National Institutes of Health (NIH)-AARP Diet and Health Study. The study cohort consisted of men and women who were members of the American Association of Retired Persons (AARP). Screening questionnaires (food frequency questionnaires) were initially mailed to 3.5 million AARP members who were 50 to 69 years of age, and who resided in the eight geographic areas selected for this study. The eight states or areas that were selected for this study were chosen on the basis of: 1) having a population-based cancer registry with adequate coverage and quality, and 2) having a sizeable minority population.
The current cohort consists of 566,402 persons, (60% men and 40% women), including both live and deceased cohort members. This study is the largest cohort study capable of prospectively examining the relation between diet and major cancers in a cohort of early-to late-middle aged men and women in the U.S. In the early stages of the study, recruited cohort members completed and mailed back a food frequency questionnaire (FFQ) and a follow-up endpoint and exposure assessment questionnaire. More recently, another mailed follow-up questionnaire obtained information on disease outcomes and lifestyle factors. Cancer diagnosis and cause-of-death follow-up has been conducted over time by obtaining data from established population-based cancer registries and the National Death Index.
The study’s original summary (Attachment 1) addressed three methodological problems impeding epidemiologic investigations of diet and cancer: 1) the prospective cohort eliminated recall bias by assessing diet prior to cancer diagnosis; 2) the large size of the cohort compensated for dietary measurement error; and 3) a two-stage cohort construction strategy allowed for enrichment of study population with persons at the extremes of intake to reduce the potential problem of homogeneity of dietary intake. In fact, enrichment was not necessary as both men and women in this cohort were found to have the desired wide distributions of intake of foods groups and nutrients of interest.
Numerous analyses have been performed using the data collected. The data collected at the beginning (1995-96) of the NIH-AARP Diet and Health Study has been used to examine the diet and cancer relationship, and scientific papers with important public health messages have been published. To date, over 30 papers describing the study’s findings have been published in scientific journals; many more are currently in press or have been submitted for review (Attachment 2). However, rapidly growing public health problems, especially obesity, physical inactivity, and several medical conditions, demand more research on the associations between these health problems and cancer.
We propose building upon this research and recent developments in computer technology to conduct a feasibility study of four web-based instruments to better assess dietary intake, physical activity, lifestyle and behavioral factors, and self-reported health conditions. We plan to invite participation (Attachments 3-1 to 3-6) from 5,000 members of the current cohort, along with 10,000 current AARP members ages 50 and older from the same eight states as well as sixteen additional states known to have high-quality cancer registries. The four questionnaires are described below.
1. One of the instruments, to be used, ASA24 (Automated Self-Administered 24-hour Dietary Recall), is a newly-developed computerized questionnaire that assesses an individual’s diet during the previous 24-hour time-interval at multiple time points. This instrument has been developed in order to measure diet more accurately and in more detail than the traditional paper-based methods that assess an individual’s diet during the previous year. The most commonly used existing self-administered questionnaires that assess diet are only based on a relatively small set of questions (e.g. 124 food items consumed), which is likely contributing to measurement error. Investigators are concerned that error in the measurement of diet using food intake over the past year may be compromising the ability to detect important but modest associations between nutrition and cancer.
Researchers have determined that greater accuracy in assessment of dietary intake is possible using 24-hour dietary recall interviews administered at multiple time periods over a year.1-3 This method of data collection allows for reports on variation in a respondent’s diet through different seasons of the year and demands less estimation and recall by the respondent regarding his or her diet. However, this instrument has required the use of highly trained interviewers to conduct the interview, and subsequent complex coding of dietary and nutritional components, resulting in an extremely high cost per instrument completion. ASA24 translates this successful means of dietary data collection into a self-administered, low-cost dietary intake collection instrument. It also enhances the interviewer-led administration of the 24-hour dietary recall by providing pictures of foods reported as being consumed and associated portion sizes, permitting the respondent to visualize and more accurately indicate the food consumed.
The test version of the ASA24 can be viewed online at the following URL: http://asa24test.westat.com. The username for this questionnaire is “john” and the password is “bagelbites”. If you have a pop-up blocker you will need to turn it off, or “accept pop-ups”, for this website to work. The burden statement (Attachment 4-1A) and screenshots of the ASA24 instrument have also been provided (Attachment 4-1B). The food items reported in the screenshots are only examples of what a participant might report. The actual food items reported will be unique for each participant given the instrument uses a calendar or diary format. Additional information about the ASA24 instrument can be found at the National Cancer Institute’s Risk Factor Monitoring and Management website: http://riskfactor.cancer.gov/tools/instruments/asa24.html
2. Another web-based instrument to be used, ACT-24 (Activities Completed by Time in 24 hours), assesses common daily physical activities or categories of physical activities performed on the day before the questionnaire is completed. This instrument addresses the need to obtain more detailed reports of physical activity in order to detect the potential associations between physical activity and cancer. While research has demonstrated an association between energy expenditure and health conditions such as cardiovascular disease and all-cause mortality,4 associations between physical activity and cancer are less clear. Some questions in each of the NIH-AARP Diet and Health Study questionnaires have a physical activity component; however they are limited to 5-10 broad questions about an individual’s physical activity over the past year or over the course of their life. Other epidemiologic studies include 2-4 questions regarding the amount of time spent in moderate or vigorous physical activities over the period of a week.5 No other instrument is known to us as having been developed to measure physical activity over the past 24 hours at multiple time points throughout a year.
Other measurement devices such as doubly labeled water, considered the gold standard measure of free-living activity energy expenditure,6-7 or motion sensor devices such as accelerometers and heart rate monitors, used to measure the movement of a person over time, have been employed in studies. However, measurement by these means requires physical supplies, direct respondent contact in many cases, and sufficient funding associated with the cost of the supplies and labor involved. Such methods are not feasible at this time in large prospective cohort studies.
In order to obtain self-reported responses of physical activity, we have developed a simple instrument that will measure physical activity on the day before the questionnaire is completed. The questionnaire will be completed online and will allow the respondent to report specific activities or categories of activities over a 24 hour time period. Like the ASA24, this instrument is designed to obtain measurements at multiple time points over a year in order to account for seasonal variation in activity and to obtain the most complete report of activities over time.
The test version of the ACT-24 can be viewed online at the following URL: http://pa24prd.westat.com. The username for this questionnaire is “john” and the password is “bagelbites”. The burden statement (Attachment 4-2A) and screenshots of the ACT-24 instrument have also been provided (Attachment 4-2B). The activities reported in the screenshots are only examples of what a participant might report. The actual activities reported will be unique for each participant given the instrument uses a calendar or diary format.
3. It is also important to consider how lifestyle and behavioral issues, medical conditions, and health practices influence or confound the associations observed between diet and physical activity exposure and cancer. Some questions regarding lifestyle factors have been included in previous NIH-AARP Diet and Health Study questionnaires; however they do not represent a comprehensive assessment of lifestyle and behavioral issues, medical conditions, and health practices. To measure these in a comprehensive and accurate way, we propose using a computerized questionnaire, the LHQ (Lifestyle and Health History Questionnaire) that will assess an individual’s lifestyle factors through their entire lifespan. This questionnaire is designed as a traditional online instrument with imbedded skip patterns and edit checks that allow the presentation of questions based on previous responses or information.
The test version of the LHQ can be viewed online at the following URL: http://staging4.spss-asp.com/mrIWeb/mrIWeb.dll?I.Project=LHQV05A. The ID for entering the site can be any number between 1 and 200. The burden statement (Attachment 4-3A) and the screenshots of the LHQ instrument have also been provided (Attachment 4-3B). The paper version of the LHQ has only been used for developing the computer-based LHQ questionnaire and will not be viewed by participants. The questions contained in the paper version of the LHQ are identical to the questions found in the computer-based version.
4. The DHQ (Diet and Health History Questionnaire) is a food frequency questionnaire developed by NCI and is widely used in the assessment of dietary intake over the year prior to the questionnaire completion date. The initial baseline questionnaire for the NIH-AARP Diet and Health Study used these questions, with additional questions added. This study will use the web-based version of the questionnaire that has been available to researchers online for a number of years. This questionnaire will be used to compare dietary intake responses on this questionnaire to dietary intake responses to the ASA24.
The demo version of the DHQ can be viewed online at the following URL: http://riskfactor.cancer.gov/DHQ/. You will need to click on the DHQ*Web Demo link found on the right-hand side of the screen in order to access the demo version of the questionnaire. The burden statement (Attachment 4-4A) and the paper version of the DHQ instrument have also been provided (Attachment 4-4B). The Web-based version is identical in content to the original DHQ.
The NEB has planned the current CLIC study (Attachment 5) to evaluate the feasibility of using these three new computerized questionnaires and the DHQ in a population of early-to-late-middle-aged men and women as described above. The specific objectives of this evaluation study are:
to determine response rates to an invitation to participate in a study evaluating the four computerized questionnaires;
to determine eligibility rates of those responding to the invitation to participate in the evaluation study;
to assess the completion rates for each pathway assignment, which is based on four different combinations of questionnaire ordering and timing;
to evaluate the performance and configuration of the technical design of the computerized questionnaires;
to evaluate the range of dietary intake, especially the extreme categories of dietary intake (in terms of fat, fiber, and other nutrients), using the ASA24 and to compare it to the dietary information collected using the DHQ;
to identify the range of physical activity using the PA24 to ensure adequate reporting of daily physical activities; and
to evaluate lifestyle and behavioral issues, medical conditions, and health practices, and the range of dietary intake and physical activity reporting associated with them.
To address these objectives, a random selection of 5,000 current cohort participants (aged 62-83) and 10,000 current AARP members (aged 50 and over), who are not current cohort participants, will be asked to participate in this evaluation study. The AARP organization and investigators from the NCI will jointly sponsor and mail an invitation letter (printed on letterhead containing both the NCI and AARP logos) and an enrollment instruction card (Attachments 3-1 to 3-6) to potential participants residing in the eight states including two metropolitan areas that comprise the current cohort, as well as 16 new states (Arizona, Colorado, Connecticut, Illinois, Iowa, Kentucky, Massachusetts, Nevada, New York, Oklahoma, Oregon, South Carolina, Texas, Utah, Washington, and Wisconsin). The original eight states and the 16 new states were selected based on the quality and availability of cancer outcome data from population-based state cancer registries. In this evaluation study, participants will not be followed for cancer outcomes. However, the data required for linking to cancer registries, such as date of birth, gender, and social security number will be collected in this study, in order to evaluate participant willingness to provide the information, which will be important for planning large-scale studies in the future that will rely on data linkages to cancer registries.
Once enrolled in the study, the type and frequency of computerized questionnaire assigned to each participant will be determined by systematically assigning them to one of four pathways. Pathway assignments have been determined in advance to ensure that participants are distributed to Pathway 1 or Pathway 2 15% of the time, and to Pathway 3 or Pathway 4 35% of the time. Each new participant that enrolls in the study is automatically assigned to the next pathway; the sequence of pathway assignments has been predetermined in advance. The participant will not know ahead of time to which pathway they will be assigned. The details of each pathway are described in the table below.
Table 1-1 Description of each pathway assignment.
Assignments |
Baseline Questionnaires |
30 Day |
60 Day |
90 Day |
Pathway 1 |
ACT-24, LHQ |
---- |
ACT-24, DHQ |
---- |
Pathway 2 |
ASA24, DHQ |
---- |
ASA24, LHQ |
---- |
Pathway 3 |
ACT-24, ASA24, LHQ |
---- |
ACT-24, ASA24, DHQ |
---- |
Pathway 4 |
ACT-24, DHQ |
ASA24, LHQ |
ACT-24 |
ASA24 |
This evaluation study comprises the necessary performance and feasibility tests for the new computerized questionnaires, which will provide an opportunity to assess the possibility of administering computerized questionnaires in future large prospective cohort studies. This evaluation study will also provide an opportunity to characterize participants’ diet, physical activity, and lifestyle factors by using more detailed diet and health questionnaires.
A.2 Purpose and Use of Information
The diet, physical activity, and lifestyle information obtained in the computerized questionnaires will be an improvement over traditional paper-based questionnaires. The format of the questions in the computerized questionnaires will eliminate potentially confusing skip patterns by presenting to the participant only those questions that pertain to them, since skip patterns will automatically run behind the scenes (for example men will not need to skip through questions pertaining to women’s health because they will not be presented with those questions). Data collection time and data entry errors will be diminished since the information collected from computerized questionnaires will be stored directly into a database and not transferred from paper to electronic format. This process will be more efficient and cost-effective compared to using traditional paper-based questionnaires.
The ASA24 will ask participants to identify specific food items they ate in the past 24-hour time period by selecting various items from a list. Participants will identify the portion size of each food item by choosing a photograph that most closely resembles it. The DHQ will also ask participants about what they ate over the past 12 months versus the past 24-hours. The ACT-24 will ask participants to enter the type of physical activity, the duration, and the time of day the physical activity was performed during the past 24-hour time period by choosing from a list of either daily activities performed (e.g. showering, cooking and preparing meals, driving a vehicle, working, using the computer, shopping, etc) or general activities (e.g. exercise and sports, routine chores, home/yard maintenance, quiet/leisure activities, etc). Depending on which activity is selected, the participant will enter more detail about the activity. The LHQ will ask the participant questions about lifestyle and behavioral issues, medical conditions, and health practices. The questionnaire is divided into sections including general health, family health history, smoking and alcohol use, reproductive health, body shape, oral health, vitamin use and medications, sleeping habits, and demographic information.
Information will also be collected from a comprehensive Web Survey Management System (WSMS). WSMS will be used to consent, enroll participants in the study, assign pathways, manage questionnaire schedules, and route participants to each questionnaire. The test version of WSMS can be viewed online at the following URL: http://aarpwsmsdemo.westat.com. Please enter a unique study code of “1” in the “First Visit” box in order to view the study consent, burden and confidentiality statements, as well as the enrollment questions. In addition to the URL, screenshots are provided of the pre-enrollment (Attachment 6) and enrollment activities (Attachment 7) that display how information will be collected by WSMS.
Additionally, data collected by WSMS will be used to determine the response rates to the invitation to participate in the evaluation study, the eligibility rates of those responding to the invitation, the completion rates for each series of questionnaires based on four different pathway assignments, and the performance and configuration of the technical design of the computerized questionnaires. If this were a large prospective cohort study and not an evaluation study, the information collected for each instrument would be used by researchers to examine the relationship between diet, physical activity, and lifestyle factors with major cancers in a population of early to late middle-aged men and women.
The data collected in this evaluation study will be reviewed by the Principal Investigator, External Working Group, and the Steering Committee members (Attachment 10).
A.3 Use of Improved Information Technology and Burden Reduction
Developments in computerized questionnaire technology provide an opportunity to collect diet, physical activity, and lifestyle information using computerized questionnaires, which are fast, cost-effective, non-intrusive, and convenient. The 24 hour-recall instruments (ASA24 and ACT-24) will help reduce the burden to the participants by allowing them to log only the food items they ate or the physical activities they performed the day before the questionnaire completion. The LHQ computerized questionnaire will help reduce the burden to participants by improving the flow of the questions. The instrument is designed to follow a traditional skip pattern without the participant having to scroll through questions that do not apply. This will make it easier for the participant to understand which questions need to be answered as well as reduce the amount of time required to complete the questionnaire. A participant will experience no burden if he/she chooses not to respond to the questionnaire.
This data collection will be associated with an IT system to collect, use, store, maintain, disclose and possibly transmit data if necessary. Prior to starting data collection, a Privacy Impact Assessment (PIA) will be undertaken with NCI’s Privacy Act Coordinator and Information Security Officer to assess privacy and security risks of the IT system.
A.4 Efforts to Identify Duplication and Use of Similar Information
This evaluation study is unique in that it will be used in a population including younger AARP members who are more likely to have greater access to and knowledge of the internet. This study will be using newly designed computerized questionnaires to collect more detailed, targeted information about dietary intake, physical activity, and lifestyle factors. The dietary intake and physical activity instruments are based on a 24-hour recall, which has not been conducted before in this population or any other large scale cohort study.
The computerized questionnaires and accompanying Web Survey Management System represent a novel approach to conducting diet, physical activity, and lifestyle factor research. The computerized questionnaires can be applied to observational studies (whether small or large in scope), case-control studies, cohort studies, as well as randomized controlled trials that are evaluating the effects of diet, physical activity, and lifestyle factors with cancer outcomes. This study will evaluate the use of computerized questionnaires in a small sample of participants in order to assess the feasibility of using these computerized questionnaires in a large cohort study.
A.5 Impact on Small Businesses and Other Small Entities
No small businesses or other small entities will be involved in this study.
A.6 Consequences of Collecting the Information Less Frequently
Participants will be asked to complete certain computerized questionnaires at either two or four time-points during a three month time-period, depending on their pathway assignment. Participants are being asked to complete the 24-hour recall instruments (ASA24 or ACT-24) more than once because evidence suggests that alternative data collection methods, such as 24-hour recalls or multi-day diet records, are more accurate compared to data collection methods that are administered once and require participants to recall their diet or physical activities over the course of the past year (such as the DHQ).
Pathway 1 and Pathway 2 will focus on the quality of the information the participants provide by completing a 24-hour instrument (ACT-24 or ASA24) in conjunction with a history questionnaire (LHQ or DHQ) at only two time points (60 days apart). Pathway 3 and Pathway 4 will focus on the participants’ ability and willingness to complete all of the questionnaires, some more than once (ASA24 and ACT-24 only) in a given time frame (either 60 days apart or at 30, 60, and 90 day intervals). Both types of pathways will evaluate the response rates, eligibility rates, and completion rates of the computerized questionnaires as well as evaluate the range of dietary intake, physical activity, and lifestyle and behavioral factors in this population of early-to-late-middle-aged men and women.
The formative information collected in this evaluation study will be used to improve the accuracy of the data collected, reduce respondent burden, and decrease the costs of administration. Collecting the information less frequently would result in measurement error for diet, physical activity, and lifestyle factors that may compromise the ability to detect important albeit modest associations between diet, physical activity, and lifestyle factors with cancer.
A.7 Special Circumstances Relating to 5 CFR 1320.5
No special circumstances are anticipated.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
The 60-Day Federal Register notice soliciting comments on this evaluation study prior to initial submission to OMB was published on June 10, 2008 (Vol. 73, No. 112, p. 32717). In response to the notice, no public comments were received.
The NIH-AARP Comprehensive Lifestyle Interview by Computer (CLIC) evaluation study was developed with consultation from a number of scientists throughout the development period. The study maintains a Steering Committee that meets monthly to discuss the design, conduct, and analyses for the study (Attachment 10). The committee provides overall scientific direction for the study and serves as the major decision-making body for operations. Additionally, NCI maintains an External Working Group advisory committee composed of external scientists well-prepared to advise NCI regarding the overall benefit of the study and its future direction (Attachment 10). The External Working Group meets every six months (alternating meetings conducted in-person and by teleconference), with the last in-person meeting held on January 11th, 2008.
A.9 Explanation of Any Payment of Gift to Respondents
Participants responding to this evaluation study will not receive remuneration for their participation.
A.10 Assurance of Confidentiality Provided to Respondents
The information collected in this evaluation study is covered by NIH Privacy Act System of Records 09-25-0156, “Records of Participants in Programs and Respondents in Surveys Used to Evaluate Programs of the Public Health Service, HHS/PHS/NIH/OD” (see Attachment 11 for NIH Privacy Act Officer’s Letter) and the Confidentiality Certificate (#NCI 01-009A) issued by the Department of Health and Human Services.
The participant’s name, home address, and email address will be collected and retained throughout the active study period in order to maintain contact with the respondent. Personally Identifiable Information (PII) such as Social Security Number and date of birth will also be collected, although passive follow-up of participants’ cancer outcomes will not be conducted in this study. The data required for linking to cancer registries, such as date of birth and social security number will be collected only to evaluate the participants’ willingness to provide the information, which is important for future large-scale prospective studies that will rely on linkages to state cancer registries.
Study data will be identified and retrieved by a study number only. Investigators will not have access to personal identifiers such as SSN, name, or address and will not follow up with participants except in response to communications initiated by the participants. All data collected in this study will be captured electronically, therefore avoiding concerns of hard-copy storage of materials that contain PII. Updated contact information will be noted in study databases to aid in future contact on continuing study participants.
Westat Inc. of Rockville, MD, a contractor for this evaluation study, is responsible for storing the identifiers in a secure, password protected, and locked file according to Department of Health and Human Services ADP Systems Security Policy as described in DHHS ADP Systems Manual, Chapters 6-30 and 6-35. All computerized data is maintained in a manner that is consistent with the Department of Health and Human Services ADP Systems Security Policy as described in DHHS ADP Systems Manual, Chapters 6-30 and 6-35. No reports or data files will contain PII. A complete list of the procedures Westat will take to keep the study data confidential are found in Attachment 12.
Furthermore, all contract staff members working on the study are required to sign a statement pledging to keep confidential all study data. Access to study data is limited to the staff working on the study. All respondents are made aware in writing that the information they provide will be kept confidential. The data collected will be maintained until the completion of the study or until it is no longer required for research purposes.
The National Cancer Institute’s Special Studies Institutional Review Board (IRB) reviewed and approved the NIH-AARP Comprehensive Lifestyle Interview by Computer (CLIC) study on 5/30/08, in accordance with 45 CFR 46. A copy of the IRB approval is found in Attachment 13.
A.11 Justification of Sensitive Questions
Participants will be asked to provide their date of birth, gender, and social security number as part of the enrollment process solely for the purpose of evaluating the participants’ willingness to provide the information, which will be important for planning large-scale studies in the future that will rely on linkages to state cancer registries. Participants will not be followed for cancer outcomes in this evaluation study.
The computerized questionnaires generally do not contain questions that are highly sensitive in nature (e.g., religious, cultural, sexual practices, or selected health conditions). However, when conducting epidemiologic studies, it is important to be able to capture data on medical conditions, medical procedures, health behaviors and physical characteristics. There may be exceptions and item sensitivity cannot always be predicted. The purpose of evaluating the computerized questionnaires is to determine the best possible way for fashioning sensitive questions so that the respondent’s comfort level is maximized and the responses are valid.
Respondents may choose where and when they would like to complete the questionnaire and they have the right not to answer any question without any consequence. Participants are informed that the information they provide is voluntary and will be kept confidential.
A.12 Estimates of Annualized Burden Hours and Costs
The study invitation letters and enrollment instruction card (Attachments 3-1 to 3-6) will be mailed to 15,000 potential participants, as described earlier, of which 2070 are expected to enroll in the study. A participant will be considered enrolled in the study if they agree to consent to participate in the study (Attachments 6 and 14), answer the required eligibility questions, and create a user identification code and password for accessing the study website that contains their assigned questionnaire schedule (Attachment 7). Of the 2072 participants that are expected to enroll in the study, approximately 312 will each be assigned to Pathway 1 or Pathway 2, and approximately 724 will each be assigned to Pathway 3 or Pathway 4. For additional details, please refer to the Flowchart 1 illustrating the distribution of the 15,000 invited participants during the two years of data collection.
Flowchart 1: Distribution of 15,000 invited study participants during
2-year data collection phase.
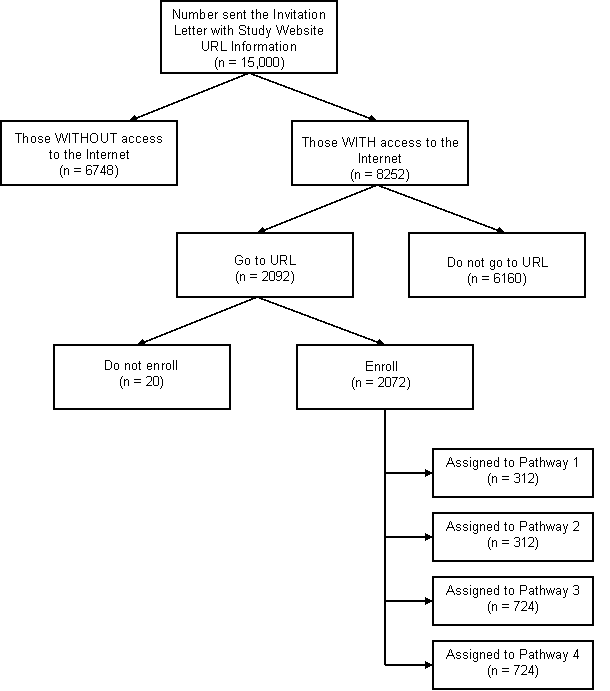
The estimate of hour burden varies based on the participant’s pathway assignment. Therefore, the hour burden has been estimated for each pathway as shown in Table 12-1. It is estimated that approximately 7500 participants will spend 1 minute each to read their invitation letter (Attachments 3-1 to 3-5). Of those participants, 1046 will continue to pre-enrollment following the steps on the enrollment instruction card (Attachment 3-6), which will account for the amount of time it will take a participant to access the study website, and read and agree to the consent statement. Answering the eligibility questions is considered part of the enrollment process. An hour burden has also been estimated for completing an optional evaluation survey, which will assess the participants’ experiences during the study.
The annualized hour burden listed in Table 12-1 is estimated at 2992, which amounts to a total burden of 5984 hours over the course of 2 years. However, it must be noted that this is truly an estimate for this feasibility study. A goal of this study is to determine how long it will take participants to complete the questionnaires given the participants' age, internet access and connection, and willingness to complete all surveys in the participants' assigned pathway.
Similarly, the potential annualized cost to respondents will vary based on their pathway assignment and their level of participation. The estimated annualized costs are listed in Table 12-2. At $17.68 per hour, the total estimated annualized cost to respondents for reading the invitation letter and instruction card, pre-enrollment (including navigating to the study website and reading and agreeing to consent) and answering the eligibility questions is approximately $6817. The annualized cost to respondents to complete Pathway 1 is $4,597, to complete Pathway 2 is $5,976, to complete Pathway 3 is $17,067, and to complete Pathway 4 is $18,134. The total annualized respondent cost is estimated at $52,897, which amounts to a total of $105,794 over two years.
An additional annualized cost for re-entry (Attachment 8) into the study website has been accounted for based on the frequency of questionnaire assignments. Also, an annualized cost for completing the optional evaluation survey has been included (Attachment 9).
Table 12-1. Estimates of Annual Burden Hours
-
Type of Respondents
Instrument(s)
TestedFrequency of Response
Average Time per Response (Hours)
# of Respondents/
PathwayAnnual Hour Burden
Senior Adults
Read Invitation
1
1/60
7500
125.000
Pre-Enrollment
1
10/60
1046
174.333
Enrollment Process
1
5/60
1036
86.333
Assigned Pathway 1
ACT-24
2
15/60
156
78.000
LHQ
1
20/60
156
52.000
DHQ
1
45/60
156
117.000
1 Web Re-entry
1
5/60
156
13.000
Assigned Pathway 2
ASA24
2
30/60
156
156.000
DHQ
1
45/60
156
117.000
LHQ
1
20/60
156
52.000
1 Web Re-Entry
1
5/60
156
13.000
Assigned Pathway 3
ACT-24
2
15/60
362
181.000
ASA24
2
30/60
362
362.000
LHQ
1
20/60
362
120.667
DHQ
1
45/60
362
271.500
1 Web Re-Entry*
1
5/60
362
30.166
Assigned Pathway 4
ACT-24
2
15/60
362
181.000
ASA24
2
30/60
362
362.000
LHQ
1
20/60
362
120.667
DHQ
1
45/60
362
271.500
3 Web Re-entries**
3
5/60
362
90.500
Evaluation Survey
1
1/60
1036
17.250
Totals
2991.91
Table 12-2. Annualized Cost to Respondents
Type of Respondents |
Instrument(s) |
Annual Hour Burden |
Hourly Wage Rate |
Annual Respondent Cost |
Total for Different Steps in the Process |
Senior Adults |
Read Invitation |
125.000000 |
$17.68 |
2,210.00 |
2,210.00 |
Pre-Enrollment |
174.333333 |
17.68 |
3,082.21 |
3,082.21 |
|
Enrollment Process |
86.3333 |
17.68 |
1,526.37 |
1,526.37 |
|
Assigned Pathway 1 |
|||||
ACT-24 |
78.0000 |
17.68 |
1,379.04 |
4,596.80 |
|
LHQ |
52.0000 |
17.68 |
919.36 |
||
DHQ |
117.0000 |
17.68 |
2,068.56 |
||
Web Re-entry |
13.000 |
17.68 |
229.84 |
||
Assigned Pathway 2 |
|||||
ASA24 |
156.0000 |
17.68 |
2,758.08 |
5,975.84 |
|
DHQ |
117.0000 |
17.68 |
2,068.56 |
||
LHQ |
52.0000 |
17.68 |
919.36 |
||
Web Re-Entry |
13.000 |
17.68 |
229.84 |
||
Assigned Pathway 3 |
|||||
ACT-24 |
181.0000 |
17.68 |
3,200.08 |
17,067.09 |
|
ASA24 |
362.0000 |
17.68 |
6,400.16 |
||
LHQ |
120.6667 |
17.68 |
2,133.39 |
||
DHQ |
271.5000 |
17.68 |
4,800.12 |
||
Web Re-Entry |
30.166 |
17.68 |
533.33 |
||
Assigned Pathway 4 |
|||||
ACT-24 |
181.0000 |
17.68 |
3,200.08 |
18,133.79 |
|
ASA24 |
362.0000 |
17.68 |
6,400.16 |
||
LHQ |
120.6667 |
17.68 |
2,133.39 |
||
DHQ |
271.5000 |
17.68 |
4,800.12 |
||
Web Re-entry |
90.500 |
17.68 |
1,600.04 |
||
Evaluation Survey |
17.2500 |
17.68 |
304.98 |
304.98 |
|
Totals |
|
|
|
$52,897.08 |
$52,897.08 |
A.13 Estimates of Other Total Annual Cost Burden to Respondents and Record
Keepers
There are no capital costs, operating costs, or maintenance costs to report.
A.14 Annualized Costs to the Federal Government
The estimated total cost to the government for the services of the study contractor(s) over the duration of the study will be $137,000 with an annualized cost of $68,500. These costs include all management of the pilot study including response tracking, coding and processing the data, and delivery of final data files.
NCI staff time required to participate in planning and design activities, monitoring the study, and in analysis of this data is estimated to average 0.05 FTE for scientific staff over the 24-month study period. This figure corresponds to a total of $10,000 over 24 months, or an average annualized cost of $5000. Finally, there are costs associated with data analysis, which total $7500. The average annual cost to the government over the 12-month period is approximately $77,250. The overall government distribution is summarized in the following table:
Table 14-1 Annual Cost to the Federal Government
|
TOTAL |
ANNUAL AVERAGE |
Contractor Costs |
$137,000 |
$68,500 |
NCI Personnel Subtotal |
$10,000 |
$5,000 |
Analysis |
$7,500 |
$3,750 |
Grand Total |
$154,500 |
$77,250 |
A.15 Explanation for Program Changes or Adjustments
This is a new collection of information.
A.16 Plans for Tabulation and Publication and Project Time Schedule
MILESTONE |
MONTHS AFTER OMB APPROVAL |
Invitation Letter Wave #1 |
1 Month |
Invitation Letter Wave #2 |
2 Months |
Completion of Pilot Study Questionnaires |
5 Months |
Data Processing and Analysis |
5 Months – 8 Months |
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate
All instruments will display the OMB expiration date.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
No exceptions to the Certification for Paperwork Reduction Act Submissions are requested.
References
1 Willett W: Nutritional Epidemiology (ed 2nd). New York, Oxford University Press, 1998
2 Kristal AR, Peters U, Potter JD. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev 14:2826-8, 2005.
3 Schatzkin A, Kipnis V, Carroll RJ, et al. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol 32:1054-62, 2003.
4 Manini TM, Everhart JE, Patel KV et al., Daily Activity Energy Expenditure and Mortality Among Older Adults. JAMA, Vol 296, No. 2, July 12, 2006.
5 Macera CA, Ham SA, Yor MM, Jones DA, Ainsworth BE, Kimsey CD., et al. Prevalence of physical activity in the United States: Behavioral Risk Factor Surveillance System, 2001. Prev Chornic Dis [serial online] 2005 Apr [April 30, 2008].
6 Lamonte MJ, Ainsworth BE. Quantifying energy expenditure and physical activity in the context of dose response. Med Sci Sports Exerc. 33(6suppl): S370-S378, 2001.
7 Schultz Y, Weinsier RL, Hunger GR. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obes Res. 9-368-379, 2001.
| File Type | application/msword |
| File Title | SUPPORTING STATEMENT |
| Author | Ann Truelove |
| Last Modified By | Vivian Horovitch-Kelley |
| File Modified | 2008-11-26 |
| File Created | 2008-11-25 |
© 2026 OMB.report | Privacy Policy