Attachment 4 - Brazil HIV Protocol
Attachment 4_Brazil_HIV_Protocol.doc
The Prevalence and Incidence of HIV Molecular Variants and Their Correlation with Risk Behaviors and HIV Treatment in Brazilian Blood Donors (NHLBI)
Attachment 4 - Brazil HIV Protocol
OMB: 0925-0597
Principal Investigator: Busch, Michael P. ATTACHMENT 4
THE PREVALENCE AND INCIDENCE OF HIV MOLECULAR VARIANTS, AND THEIR CORRELATION WITH RISK BEHAVIORS AND HIV TREATMENT IN BRAZILIAN BLOOD DONORS
Abstract.
Establishing and monitoring viral prevalence and incidence rates, and identifying behavioral risk behaviors for HIV incidence among donors, are critical steps to assessing and reducing risk of HIV transmission through blood transfusion. Identifying donation samples from donors with recent HIV infection is particularly critical as it enables characterization of the viral subtypes currently transmitted within the screened population and hence most likely to "break-through" routine screening measures (i.e., peri-seroconversion window period donations). In addition to characterizing genotypes of recently infected donors for purposes of blood safety, molecular surveillance of incident HIV infections in blood donors enables documentation of the rates of primary transmission of anti-viral drug resistant strains in the community, and serves a public health role in identifying new HIV infections for anti-retroviral treatment. We propose both prospective surveillance and a case-control design to enroll all eligible HIV seropositives detected at our three blood centers plus our satellite center in Rio de Janeiro, and compare their epidemiological risk profiles with a group of randomly selected seronegative donors. Aim A. We will perform laboratory studies (LS-EIA testing and sequencing of pol region) on linked specimens from all enrolled HIV cases, which will allow for estimation of HIV prevalence and incidence relative to genotype and putative route of infection. Aim B. A case control study will yield self-reported data on HIV risk behaviors among prospective donors that will be used 1) as covariates in the molecular surveillance analyses described above; and 2) to suggest modifications to current operational donor screening questionnaire. Finally, data from Aim C on molecular genotype, drug resistance genotype, will be provided, along with counseling, to all enrolled HIV positive donors to facilitate their clinical care via referral to the Brazilian national HIV treatment system. Our findings will be compared to trends in prevalence, incidence and molecular variants from studies of the general population and high risk populations in Brazil, thus allowing for broad monitoring of the HIV epidemic in Brazil and assessment of the impact of donor selection criteria on these parameters.
Specific Aims.
Aim A. Determine the prevalence and incidence of HIV among blood donors according to demographic and donation characteristics, and use incidence data to project residual risk of transfusion-transmitted HIV and impact of implementation of NAT screening.
Hypotheses: Prevalence and incidence rates previously documented among Sao Paulo donors will be similar in other regions of Brazil, including increased HIV rates and transfusion risk for community relative to replacement donors. The low predicted yield of NAT screening will be confirmed on a national level, supporting decisions to focus on improved donor selection and serological screening rather than implementing NAT with its very high cost and poor cost effectiveness.
Aim B. Determine risk factors associated with HIV infection among volunteer and replacement blood donors in Brazil.
Hypothesis: Male-to-male sex, having multiple heterosexual partners, and to a lesser extent injection drug use (IDU), are the predominant risk factors for HIV in Brazil.
Aim C. Determine HIV subtype, drug resistance profile, among HIV positive donors according to HIV infection status (recent vs long-standing), year of donation and site of collection, and risk behaviors, and provide results to the subjects as part of their referral to HIV clinical care.
Hypothesis: There will be clinically relevant increases in the diversity of HIV subtypes and increasing rates of primary drug resistance among recently infected donors in all three study regions. Non-B subtypes and drug resistant strains will be seen in recently infected persons from all risk factor categories.
Background.
AIDS epidemic in Brazil.
Brazil had 362,364 reported cases of AIDS as of June 2004, the second highest number of cases in the Americas, exceeded only by the United States.1 The Brazilian National Program on HIV-1/AIDS estimates that 597,000 people in Brazil are infected with HIV-1. Since 1996, the AIDS incidence rate has stabilized at around 15/100,000 inhabitants/year. The World Bank classifies the Brazilian epidemic as “concentrated”, defined as prevalence greater than 5% in one or more specific sub-populations. São Paulo, Rio de Janeiro and Minas Gerais States are responsible for 230,399 (64%) of the Brazilian cases. The figure below shows the cumulative number of AIDS cases reported in Brazil , by year and region.
D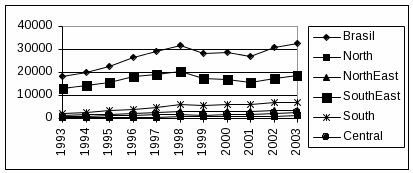
ata
on gender, level of education and area of residence of newly reported
AIDS cases indicate that the epidemiological profile of the AIDS
epidemic in Brazil has been changing dramatically over time.2,3
In the initial years of the epidemic, nearly 100% of the cases
occurred among men who have sex with men (MSM) and among individuals
who had an education and income level above the national average.1
In the last decade, however, the epidemic has been disseminating,
with an increasing impact on women and the poor throughout the
country.2
Heterosexual transmission is now the major route of infection,
accounting for 57% of cases, followed by transmission through sexual
activities among MSM and injection drug use.
Regarding clinical relevance of the proposed research, the Brazilian public health authorities have been responsive to the HIV epidemic, with prevention campaigns, provision of condoms, alternative testing sites, and most notably, the implementation of universal access to anti-retroviral treatment (see more below).4 These actions are considered to have forestalled a more significant epidemic among a young, low socioeconomic status population. However spread of the HIV epidemic into the heterosexual population makes identification of newly infected persons more difficult. Blood banks can play an important role in this effort due to HIV testing of large numbers of a young, otherwise healthy population.
Blood safety regarding HIV in Brazil.
Until 1980, blood transfusions in Brazil were mainly performed by private blood centers. Paid donations were a common practice, and there was no federal law regulating blood bank operations. With the onset of the AIDS epidemic, blood collection practices became an important political issue and the Brazilian Ministry of Health initiated a program to develop an infrastructure for the supervision of Brazilian blood banks.5,6 Universal screening of blood donors for HIV antibody was instituted in Brazil in 1988.7 Public blood centers were created in all States of Brazil, and although they were under State administration, Federal funds were made available to them. Periodic updates to transfusion safety regulations were promulgated.8
Other measures to safeguard the blood supply, including increasing the proportion of volunteer donors and individual interviews and deferral of donors with HIV risk factors have been put in place. At Fundacão Pro Sangue/Hemocentro Sao Paulo (FPS/HSP) the percentage of repeat donors increased from 22% to 48% from 1995 to 2001, and altruistic donors increased from 20% to 57% from 1995 to 2001.9 While considered a successful response to WHO and PAHO blood safety recommendations, no study has yet been performed to understand the composition of the newly recruited volunteer donors. Indeed, their higher HIV prevalence compared to contemporary replacement donors raised questions about HIV test-seeking. Understanding the HIV risk behaviors among these altruistic voluntary donors may allow other blood banks in Brazil to develop more focused intervention strategies to preventing unnecessary expenditures in blood donation processing.
Although technically recommended by the Brazilian Government, lack of funds and the poor cost-benefit ratio of HIV nucleic acid testing (NAT) have delayed its implementation in this low to middle income country. Even if NAT were to be implemented, the residual risk of HIV infection would still be substantially higher than in the USA. Additional measures towards safe donor recruitment and deferral are therefore essential in further reducing the risk of transfusion-transmitted HIV infection. The Brazilian Health Ministry does recommend a number of HIV risk factor questions in the standard blood donor questionnaire,8 but the effectiveness of these questions, and the extent to which HIV test-seeking donors may circumvent these questions, are unknown. Only preliminary studies of the HIV risk factor profiles have been accomplished among Brazilian blood donors.10.11
HIV subtypes and drug resistance in Brazil.
Monitoring the genetic diversity of HIV-1 in Brazil is important to understand the molecular epidemiology and spread of the epidemic. The genetics of the virus can be used to track the movement of HIV-1 into new groups or new at-risk populations. It is also conceivable that the diversity of the virus may impair the immune response to candidate vaccines, cause false negative results in blood screening, diagnostic and monitoring laboratory tests (e.g. especially viral load assays targeting nucleic acids),12,13 and lead to differences in the observed disease progression14,15 and therapeutic response.16
The predominant HIV-1 clade in Brazil is B (~70%), but unlike the U.S., Brazil also has appreciable numbers of HIV-1 infections caused by clades F (~15%), C (~3%), and recombinant forms (~12%).17-20 Furthermore, approximately 50% of the clade B strains circulating in Brazil comprise a cluster of genetically and antigenically distinct strains relative to the US clade B strains.21
HIV/AIDS health care in Brazil is provided by the state to all HIV infected individuals. As early as 1991, the Brazilian Ministry of Health provided antiretroviral (ARV) drugs through its extensive public health system. In 1996 a law was enacted guaranteeing free access of antiretroviral therapy to all Brazilians who required treatment, according to Brazilian guidelines. The widespread use of ARV has led to a decline in AIDS related mortality in Brazil.22,23 However, it is expected that the proportion of patients experiencing virologic failure and consequently harboring resistant strains will increase in time. Depending on the behavioral characteristic of these individuals, transmission of drug resistant strains may occur with increased frequency.24 The transmission of resistant variants to uninfected individuals raises serious clinical and public health consequences and may dramatically impair the capacity of treating HIV in the near future.57,58
Studies evaluating the prevalence of drug-resistant virus among recently infected individuals are thus extremely important in Brazil. In a recent survey, developed by the Brazilian Ministry of Health Program and including 535 patients from the entire country, the frequency of primary resistance was low, 4.42% for nucleoside reverse transcriptase inhibitors (NRTI) and non-nucleoside reverse transcriptase inhibitors (NNRTI) and 2.24% for protease inhibitors 20 (Dr Sabino was a co-investigator on this project). More recently, Sucupira and colleagues analyzed 75 drug naïve HIV positive individuals in the city of Santos and found 21 (28%) harboring resistant strains, raising grave concern that in certain areas of Brazil drug resistant strains may be rapidly increasing.27
Significance.
Defining prevalence and incidence in blood donors and residual risk of HIV transmission by transfusions in Brazil may lead to new regulatory laws for introduction of new blood safety initiatives in Brazil. The data can be used to project the yield, safety impact and cost effectiveness of implementing enhanced testing strategies such as combination antigen-antibody assays and/or NAT. Determination of HIV risk factors in donors (first time versus repeat donor status; volunteer versus replacement status; demographics and risk behaviors) will support policy discussions over strategies to recruit the safest possible donors in Brazil, and will also yield significant information for HIV surveillance in Brazil when combined with prevalence and incidence data derived from general populations and high risk surveillance studies. The identification of incident HIV infections allows for clinical identification of recently transmitted strains of virus in donor settings in the different cities of Brazil. This surveillance will monitor the trafficking of non-B subtypes and rates of transmission of drug resistant viral strains in low risk blood donors that can be compared with data from similar studies in high risk populations. Monitoring drug resistance strains is extremely important in a country that provides free ARV therapy for HIV infected individuals, many of whom have low level educations and modest resources making compliance with drug regimens and hence resistance a serious problem. The findings from this project will also complement similar monitoring of HIV prevalence, incidence, transfusion risk and molecular variants in the US and other funded international REDS-II sites, thus allowing direct comparisons of these parameters on a global level.
Approach.
All blood donations are routinely screened by two HIV-1 antibody assays in parallel, as mandated in Brazil. These assays have been selected to have comparably high sensitivity to early seroconversion and diverse clades, as well as excellent specificity with non-overlapping populations of false reactivity. Samples reactive by both EIAs will be considered positive for prevalence calculations. In Brazil, only subjects who return for counseling will have a follow-up sample obtained that will be tested by western blot (WB) (~80% of HIV reactive subjects return and WB is performed, of which 95% confirm as WB positive). To detect recently infected donations, samples from all HIV dual-EIA reactive donations will be tested according to the Standardized Testing Algorithm for Recent HIV Seroconversion (STARHS), which is based on use of a sensitive/less-sensitive enzyme immunoassay ("detuned" EIA).28 STARHS testing will be performed by BSRI (the REDS-II Core laboratory) because this kit is not registered for use in Brazil and would be difficult for the local laboratory to import. For enrolled subjects, this testing will be performed on linked index and enrollment specimens from WB-confirmed donors, whereas for subjects who do not return for counseling we will anonymize the dual EIA reactive samples prior to S/LS EIA testing. False positive samples will be identified by performing sensitive EIA and WB on samples lacking reactivity on the LS-EIA (i.e., standardized optical density <0.1). Incidence data derived using the STAHRS methods will be used to project risk and yield of enhanced donor screening strategies. Subtype and drug resistance profiles will be determined in Dr. Sabino’s laboratory on all donors who returned for counseling and consented and enrolled into the interview study (see below). Risk factors and donor demographics will be correlated with recent infection status, as well as with clade and acquisition of drug resistant virus.
This study will be conducted at all 3 participating REDS II centers in Brazil. In addition, HemoRio in Rio de Janeiro will participate. The inclusion of HemoRio greatly improves the ability to achieve the desired sample size and will also provide data to see whether risk factors for HIV acquisition are different in different geographic regions of Brazil.
To study HIV risk behaviors, we shall use a case control design because it is most efficient for a rare condition like HIV infection among blood donors. Because of operational screening for HIV antibody, we shall have ready access to at about 250 HIV seropositive donors annually at our three Brazilian blood centers (plus an additional 100 HIV seropositive donors from Rio), and estimate that we shall enroll about 200 annually. In our preliminary study, we have previously demonstrated the ability to enroll at least three quarters of similar seropositives when they return for HIV notification and counseling. We now propose a larger study to provide greater statistical power to analyze infrequent risk factors, and to allow analyses within subsets of donors as well as by duration of infection defined by LS-EIA.
Methods.
Study Design. Surveillance of all HIV positives, with serological confirmation and STAHRS, will allow calculation of HIV prevalence and incidence. In addition, the case control study of HIV risk factors will enroll approximately 200 HIV seropositives as well as 400 x 2 (see Subject Enrollment section below) seronegative controls per year, matched by collection site. A detailed risk factor questionnaire will be administered in the same fashion to all cases and controls, and testing for HIV genotype, drug resistance will be done on all consented HIV cases. Data analysis will compare the frequency of reported risk behaviors between the cases and controls. Analyses on the cumulative data will be repeated at annual intervals to assess secular trends in HIV risk factors.
Study Population. All identified HIV EIA positives will be included in the surveillance project. From these, we shall select cases at our three centers found to be confirmed HIV seropositive during routine blood donor testing. From the 210 available subjects (525,000 donations at all three centers x 0.0004 HIV prevalence), we estimate that about 75% (150) per year may be enrolled at the time of notification and counseling. We will also fund the Rio blood center to enroll 50 additional HIV-confirmed seropositive donors per year for a total of 200 HIV seropositive donors per year Two types of controls matched by collection site will be selected from seronegative donors at a ratio of 2:1 (see Subject Enrollment section below).
Inclusion & Exclusion Criteria. Confirmed HIV-positive by EIA and Western blot (cases) or negative by HIV EIA (controls); controls will also have to be negative on all other infectious markers that blood banks test for in Brazil, subjects must otherwise be eligible to donate blood (age 18 to 65, in good general health, passed donor deferral screening, adequate hemoglobin level), and can be volunteer or replacement blood donors. Autologous blood donors will be excluded.
Subject Enrollment. Subjects will be enrolled for a two-year time period from September 2008 – August 2010. All samples positive by both screening HIV-1/2 EIAs will be considered presumptive positive for HIV and saved for further testing. The study will be described to all potential subjects, and written informed consent will be obtained.
Cases: Donors who return for counseling and confirmatory testing (as is routinely performed in Brazil) will be invited to participate as cases and if consent is obtained a questionnaire about risk factors and motivations to donate will be administered. About 60% of donors who are HIV on the index donation return for counseling and additional testing. In order maximize the return of HIV positive donors for counseling and follow-up testing (and potentially for participating in this study), we will contact such donors by letter and/or telephone to encourage them to return to the blood center, indicating that we need to retest a sample of blood. If we are unable to get these donors back this may represent a potential bias. We will be able report basic demographic characteristics for donors who do and do not return to see if there are differences by age and gender. Of these we expect to be able to recruit 75% for study participation. We plan to enroll 50 cases per year at each center or approximately 1 case per week. For these subjects, in addition to the mandated Western blot testing, we will perform linked genotype and drug resistance testing on the sample obtained at the time of counseling. For those individuals who do not return for confirmatory testing, the samples will be anonymized and sent to BSRI to perform STAHRS.
Controls: Two control groups will be used in the study.
Control Group 1 - The first set of controls will be recruited by telephone and mail approximately 2 weeks after their recent disease marker negative blood donation – this will be the offsite or recall recruitment control group. Offsite control recruitment is designed to parallel the experience of cases who return for counseling. The goal here is to make each control’s interaction with the blood center including the time frame as similar to that which HIV cases will experience. Controls will also be asked to return to the blood center, but at a different location than the HIV counseling department to avoid unnecessary anxiety. In pilot studies we only had a 10% recruitment rate using offsite controls. In order to obtain the required number of controls it will be necessary to enroll on average across all centers 2 offsite controls per week for the proposed 2-year enrollment period. At each center there are between 250 and 400 donations per day. A list of eligible donors from a randomly selected day in the previous week pre-determined using the study management system will be used to determine a random sample of 30 potentially eligible controls (divided in 3 batches of 10). In batches of 10, we will contact these potential controls by telephone call and/or letters, until a total of 2 controls per week are enrolled. The controls will not be matched to specific cases but will be matched by blood center. Offsite recruitment will occur on an ongoing weekly basis over the entire enrollment period. To enroll a total of 100 offsite controls per year at each blood center, we may have to use as many as 1,500 call and letters under the worst-case recruitment assumption. Using the batch control recruitment approach this number is more likely to be 1,000 or 20 potential control donors contacted by letters and calls per week (first 2 batches). The same approach will be used for the second year of the study for offsite controls with a new random sample draw conducted to identify the days of week and donors to contact for recruitment at each site for the year.
Control Group 2 - As indicated above, preliminary recruitment efforts indicated that eligible donors with a recent donation may be reluctant to return for the study. Thus, a second group of controls will be used to ensure that a comparison group of sufficient size is obtained during the study period. The second control group will be recruited onsite at the time of blood donation. On average, approximately 2 onsite controls are expected to be enrolled per week at each site. With ongoing case and control interviews occurring over the study enrollment period, we believe that on average the dates of the interviews for the controls will be frequency matched to the interview dates for the cases. This second group of control donors will be directed to the same location as the offsite control group for the purpose of completing the risk factor questionnaire.
To recruit onsite controls a sampling frame that comprises a roster of all morning and evening blood collection shifts at each of the 4 blood centers on all working days of each week over the course of the year will be developed in advance using the study management system. A random sample of 50 shifts from each site from which to recruit donors for the questionnaire will be drawn. The clock time to begin recruitment at during each shift will be pre-determined with the list of shifts and times for the year distributed to each center at the start of the study. For each recruitment day, a “number of the day” will be defined based on a randomly selected last digit of each donor’s unique donor identification number. The last digit can take any value from 0 to 9. A value of 0 to 9 will be selected for each recruitment shift. At the selected shifts, we will look at each donor’s ID number and will consecutively approach eligible donors if the last digit is equal to the “number of the day”. We will explain the study and ask them to participate. We will keep records of how many donors we approach and how many refuse to participate. Once 2 onsite controls have consented to participate in the study we will end recruitment for that shift. At each site, recruitment will result in an estimated two subjects per shift from the 50 shifts for a total of 100 subjects per year. The same approach will be used for the second year of onsite controls with a new random sample draw conducted to identify the 50 shifts for recruitment at each site for the year.
At approximately 6 months after initial of study subject accrual, we will evaluate whether we are able to successfully enroll offsite controls at the required ratio 1:2 (cases:controls). If we are able to enroll offsite controls and believe that we will continue to be able to do so, we will consider dropping the onsite control recruitment group in order to reduce work load.
Procedures
Questionnaire.
A detailed HIV risk factor questionnaire will be administered to all subjects. A self-administered audio computer-assisted self-interview (ACASI) on a laptop computer will be used in order to maximize reporting of stigmatized behaviors. A research assistant or nurse will provide the ACASI laptop (including earphones to be able to listen to the questions confidentially) to each subject at the blood center. The study subject will be shown how to use the computer to complete the interview by entering basic demographic data with the help of the nurse, but will be given privacy to complete the rest of the questionnaire. The research assistant or nurse will remain available to answer questions and provide help as necessary. We chose ACASI to maximize reporting of stigmatized risk behaviors and to streamline the interview (built in skip patterns depending on initial responses so that donors are only prompted to answer questions about the details of a specific risk factor if they report having the risk). The ACASI format also uses electronic data capture which will reduce data entry errors. We anticipate that young Brazilian subjects will adapt easily to the computer interview, while older or illiterate donors will rely more heavily on the audio component and/or assistance from the research assistant. The questionnaire will be based upon an instrument previously utilized and validated by the CDC in its HIV surveillance at U.S. blood banks with modifications appropriate to the Brazilian setting (see Appendix).
Phlebotomy for Clinical Testing.
In addition to blood saved from their index blood donation, for cases 30 ml of blood will be drawn at the time of the enrollment and interview. Specimens will be sent for genotype and STAHRS test, and the remaining specimens will be processed into aliquots and saved in the study repository for future testing, including repeated genotyping and drug resistance, if necessary.
Detection of Recent Infections by LS-EIA Testing.
All eligible cases will previously have had dual HIV EIA testing and HIV Western blot to confirm their HIV seropositivity, per core procedures at our Brazilian central lab. Recently infected individuals will be defined through the Standardized Testing Algorithm for Recent HIV Seroconversion (STAHRS) protocol. STARHS is based on the detection of low avidity and/or titer antibodies by a less sensitive (LS)-EIA protocol (Vironostika®HIV-1 Microelisa , bioMérieux Industry, Raleigh, NC).29 Samples that tested weakly positive and did not have follow up results will be confirmed in a sensitive (S) EIA (Vironostika®HIV-1 Microelisa ) and Western blot (Calypte Biotech, Oakland, CA) to exclude false positives.
HIV-1 Clade Typing and Drug Resistance Testing.
Subtype and resistance analysis will be performed at FPS/HSP as previously described20,30 Sequencing of the entire HIV-1 protease gene (99 amino acids [aa]) and of the RT gene through amino acid 240 will identify all mutations known to confer resistance to protease, nucleoside and non-nucleoside RT inhibitors.30 The only class of approved anti-HIV-1 drug resistance not detected by this analysis will be envelope gene mutations known to confer resistance to the more recently introduced HIV-1 fusion inhibitor FuzeonTM, a drug that is not yet in use in Brazil. Following phylogenetic analysis, sequencing of the pro-RT region will also identify the subtype of the recently transmitted HIV-1 strain. RNA will be isolated using QIAamp Viral RNA Mini kit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. Complementary DNA will be obtained using Superscript reverse transcriptase (Invitrogen, Carlsbad, CA) and random primers (Pharmacia, Uppsala, Sweden). A nested PCR will be used to obtain one fragment containing the protease gene and approximately 700 base pairs of the RT gene. In the first round the primers K1/K2 will be used followed by DP10 and F2 primers.31 Three other sets of primers, RT4/DP16, F1/F2 and DP10/DP11, will be used for samples that are not amplified using the initial primers. Conditions for both rounds of PCR will be 94°C for 1 minute followed by 35 cycles at 94°C for 45 seconds, 55°C for 45 seconds and 72°C for 2 minutes, with a final elongation step at 72°C for 10 minutes All amplification products will be analyzed on a 1% ethidium bromide-stained agarose gel. PCR products will be purified using QIAquick PCR purification kit (Qiagen Inc.). To obtain sequence results for the entire amplified segment in both strands we will use at least 6 primers for sequencing each sample, including F1, F2, DP10 and DP11 primers and a new pair of primers - GABO 1 (sense -5' -CTC ARG ACT TYT GGG AAG TTC- 3') and GABO-2 (antisense - 5' -GCA TCH CCC ACA TCY AGT ACT G-3' ). Sequence data will be obtained using the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction kit (Applied BioSystems Inc., Foster City, CA, USA), according to manufacturer's protocol in an automated sequencer (ABI 377 Sequencer-Applied Biosystems). To detect possible PCR contamination, sequences will be compared to each other using a web interface that uses the Blast program and which highlights any pair of sequences that have a percentage of similarity higher that a specific threshold (we will use 99% for pol gene).32,33
Counseling and Medical Referral.
Prior to enrollment, all subjects will have received counseling regarding their HIV infection by trained personnel at the blood centers, per operational protocols. The genotype result will be sent to the donor by mail and they will be counseled to take it to their physician. If desired by the subject a new visit will be provided to discuss the results of genotype testing.
Data Analysis.
Analysis of Incidence, Residual Risk and NAT Yield.
Data analysis will be performed by the REDS data coordinating center, Westat, in Rockville, MD. Data from this study will be merged with the large Brazilian donation database to allow calculation of incidence and univariate and multivariate analysis of correlates of HIV incidence and calculation of residual risk and yield of NAT. Since Clade B is responsible for more than 80% of the infections in Brazil, we will assume the window period corresponding to the time from seroconversion by sensitive EIA and Western blot to seroconversion by the LS-EIA would be similar to that reported for U.S. clade B infected persons, i.e., 170 days (95% confidence interval [CI] 145-200 days). We will further assume that the detection window periods (period from infectivity by blood transfusion to initial detection by the respective markers) for viral RNA by ID-NAT, MP-NAT, p24 antigen and antibody EIAs were 5.6, 9.0, 15.0 and 20.3 days, respectively, as described by Busch et al.29 Based on our observed incidence rates and the published WP estimates, the predicted yield and associated residual risk (per 10,000 per year) for each test will be calculated using the formula: (incidence rate) ∕ (365) (detection window period). The statistical calculations will use the method of Busch, et al as follows. Confidence intervals for prevalence rates will assume prevalent cases are binomially distributed. Logistic regression will be used to assess differences in prevalence rates by year, type of donation, gender, and age. Confidence intervals for incidence rates will assume incident cases are Poisson distributed. Poisson regression will be used to assess differences in prevalence rates by year, type of donation, gender, and age. Wald type 95% confidence intervals around residual risk estimates and yield estimates will be computed using a Taylor series approximation to the residual risk standard error estimates and yield standard error estimates. These standard errors are a function of the standard errors of the Poisson distributed incidence rates and the standard error of the window periods.
Analysis of Risk Behaviors.
The dependent variable will be case vs. control status. The responses from the two control groups will be analyzed separately to see if there are any differences between onsite and recall (offsite) control recruitment. Independent variables will be HIV risk factors ascertained by questionnaire, including male-to-male sex, number of lifetime male and female sexual partners, number of male and female sexual partners within the past year, use of condoms, sex with a prostitute or sex work by the donor him/herself, IDU, sex with an IDU, and sex with an individual known to be HIV-positive. Secondary predictors will include demographics, socioeconomic status, and drug or alcohol use. Demographic and blood donation characteristics of the cases and controls will be presented to describe the study population and comparability of cases and controls. Univariate associations of specific risk factors with case/control status will be assessed using contingency tables with significance tests using Chi squared or Fisher's exact tests. Variables with significant or borderline univariate associations (p<0.10) with HIV seropositivity will be entered into a logistic regression model to assess independent associations and potential confounding. Data from this Aim's questionnaire will also be used in analyses of HIV subtypes and drug resistance, below.
HIV Subtype and Drug Resistance Analyses.
To define the subtype of each strain we will use interface software that automatically submits the sequences to two programs: RIP 34 and Blast 33.In the RIP program, the sequence is cut in fragments of 200 bp in 1 bp steps. Each fragment is compared to a set of reference strains and for each fragment the program determines which reference sequence has the highest similarity rate to that fragment. In the Blast approach, the sequence is cut into non-overlapping fragments of 200 bp. Each fragment is submitted to a bank of 10,000 sequences previously subtyped in the Los Alamos databank. The program detects which databank sequence is most similar to each fragment. If the results of both analysis agreed, the sample will be considered subtyped. Otherwise the sequence will be manually reviewed using SIMPLOT.35
Interpretation of results identifying possible Protease and RT mutations that have been associated with reduced antiretroviral-drug susceptibility will be based on the International AIDS Society classification:36 Protease: D30N; M46I; M46L; G48V; I50V; V82A; V82S; V82F; V82T; I84V and I90M. Reverse Transcriptase: M41L; A62V; K65R; D67N; T69D; 69 insert; K70R; L74V; V75I; V75T; V75M; V75S; V75A; F77L; A98G, L100I; K103N; V106A; V108I; Y115F; F116Y; Q151M; Y181C; Y181I; M184V; M184I; Y188C; Y188L; Y188H; Y188C; G190A; G190S; L210W; T215Y; T215F; K219Q; K219E; P255H; P230L and P236L. Reverse transcriptase mutations that are different from wild-type T215 and T69A/N/S will be included as well.
Sample Size Calculations. After two years of cumulative data collection there will be approximately 400 cases. Presuming the offsite control group is successfully recruited, there will be 800 controls in the offsite control group, and it will be used for data analysis. If not, the onsite control group (and its 800 controls) will be used for data analysis. While the sample design is stratified by site, the power calculations in the table below are for an unstratified design. The odds ratios of interest are not expected to be related to site, so the impact of the stratification variable on power is expected to be minimal.
Aim B lists the predominant risk factors as male-to-male sex, multiple heterosexual sex partners, and IDU. The male-to-male sex prevalence among controls is expected to be about 2%. As indicated in the table below, the study will then have 93.1% power in a one-tailed 0.05 level test to detect an odds ratio of 3.0 for male-to-male sex. The number of heterosexual sex partners can be dichotomized by the median among controls. The study will then have 94.3% power in a one-tailed 0.05 level test to detect an odds ratio of 1.5 comparing high number of sex partners with low number of sex partners. The IDU prevalence among controls is expected to be about 1%. The study will then have 90.9% power in a one-tailed 0.05 level test to detect an odds ratio of 3.0 for IDU.
Power. Statistical power for
various risk factor prevalences in controls and various odds ratios,
given 400 HIV+ cases and 800 controls in the case control study,
assuming alpha=0.05 and one-sided test. Risk
factor %
in Controls Odds Ratio Power 1% 4 90.9% 2% 3 93.1% 40% 1.5 90.3%* 50% 1.5 94.3% *
two-sided test; to reflect uncertainty whether volunteer donors are
more or are less likely to be HIV positive.
Expected Results. Based upon our preliminary data, we expect to find that male-to-male sex and multiple heterosexual partners remain risk factors for HIV. The male-to-male sex prevalence among controls is expected to be about 2%, and the odds ratio is expected to be greater than 3 (table in previous section shows power then greater than 90%). The number of heterosexual partners can be dichotomized by the median among controls. The power is high for such risk factors at 50% prevalence among controls. Given their age and socioeconomic status, we hypothesize that unrecognized heterosexual exposure to HIV or IDU may explain the unexpectedly high HIV prevalence in volunteer donors. Preliminary data shows 40% of donations are from volunteer donors. The table of the previous section shows power to be greater than 90% to detect whether volunteer donors are more or are less likely to be HIV positive. The power is also high for risk factors with low prevalence among controls (e.g. prevalence of IDU may be about 1%).
Human Subjects Considerations.
This project will be approved by the institutional review boards in Brazil and the U.S. before implementation. The main risks of this study are: 1) possible lost confidentiality regarding HIV status or risk behaviors; 2) possible discomfort due to the personal nature of the questionnaire; and 3) possible negative information risk of drug genotype test. Benefits of the study include: 1) HIV positives will receive additional HIV counseling as part of the study; 2) their clinical treatment may be improved by providing drug genotype test; and 3) benefits to Brazilian society as a whole by virtue of potential improvement blood safety and control of the HIV epidemic. We will attempt to minimize risks by stringent privacy protection of the subjects’ data (see elsewhere in this application), by using trained and empathetic research personnel, ACASI interviews, and by providing counseling and medical referral for HIV infection. Informed written consent will be obtained from all subjects prior to enrollment.
LITERATURE CITED
1. Boletim Epidemiologico - AIDS. Brasilia: Ministerio da Saude do Brasil / Coordenacao Nacional de DST/AIDS, 2004:26-35.
2. Brito AM, Castilho EA, Szwarcwald CL. [AIDS and HIV infection in Brazil: a multifaceted epidemic]. Rev Soc Bras Med Trop 2001;34(2):207-17.
3. Fonseca MG, Bastos FI, Derrico M, et al. [AIDS and level of education in Brazil: temporal evolution from 1986 to 1996]. Cad Saude Publica 2000;16((Suppl 1)):77-87.
4. Levi GC, Vitoria MA. Fighting against AIDS: the Brazilian experience. Aids 2002;16(18):2373-83.
5. Brazil, Lei 7.649 de 25 de janeiro de 1988. Dispõe sobre a obrigatoriedade do cadastro dos doadores de sangue, bem como a realização de exames laboratoriais laboratoriais no sangue coletado, visando prevenir a propagação de doenças e dá outras providências Diário Oficial da União. Brasília, 1988:52.
6. Brazil, Decreto n. 95.721, 11 de fevereiro de 1988. Regulamenta a Lei n. 7.649, de 25 de janeiro de 1988, que estabelece a obrigatoriedade do cadastramento dos doadores de sangue, bem como a realização de exames laboratoriais no sangue coletado, visando prevenir a propagação de doenças. Diário Oficial da União. Brasília, 1988:143-7.
7. Brazil, Portaria Interministerial MPAS/MS 14 de 18 de maio de 1987. Dispõe sobre a realização de testes para detecção de anticorpos do vírus da Síndrome da Imunodeficiência Adquirida (AIDS). Diário Oficial da União. Brasília, 1987:7536-7.
8. Brazil. Resolução RDC nº 153, de 14 de junho de 2004: Determina o Regulamento Técnico para os procedimentos hemoterápicos, incluindo a coleta, o processamento, a testagem, o armazenamento, o transporte, o controle de qualidade e o uso humano de sangue, e seus componentes, obtidos do sangue venoso, do cordão umbilical, da placenta e da medula óssea D.O.U. - Diário Oficial da União; Poder Executivo, de 24 de junho de 2004: ANVISA - Agência Nacional de Vigilância Sanitária.
9. Barreto C, Sabino E, Gonçalez T, et al. Prevalence, incidence and residual risk of human immunodeficiency virus among community and replacement first-time blood donors in São Paulo Brazil. Transfusion, 45:1709-14, 2005.
10. Reiche E, Vogler I, Morimoto H, et al. Evaluation of surrogate markers for human immunodeficiency virus infection among blood donors at the blood bank of "Hospital Universitario Regional Norte do Parana", Londrina, PR, Brazil. Rev Inst Med Trop Sao Paulo 2003 Jan-Feb;45(1):23-7.
11. Andrade Neto JL, Pintarelli VL, Felchner PC, et al. HIV prevalence among blood donors in a blood bank in Curitiba (Brazil). Braz J Infect Dis 2002;6(1):15-21.
12. Burgisser P, Vernazza P, Flepp M, et al. Performance of five different assays for the quantification of viral load in persons infected with various subtypes of HIV-1. Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2000;23(2):138-44.
13. Apetrei C, Loussert-Ajaka I, Descamps D, et al. Lack of screening test sensitivity during HIV-1 non-subtype B seroconversions. Aids 1996;10(14):F57-60.
14. Kanki PJ, Hamel DJ, Sankale JL, et al. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis 1999;179(1):68-73.
15. Kaleebu P, Ross A, Morgan D, et al. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. Aids 2001;15(3):293-9.
16. Accetturi CA, Pardini R, Novaes Pinto GH, et al. Effects of CCR5 genetic polymorphism and HIV-1 subtype in antiretroviral response in Brazilian HIV-1-infected patients. J Acquir Immune Defic Syndr 2000;24(4):399-400.
17. Morgado MG, Sabino EC, Shpaer EG, et al. V3 region polymorphisms in HIV-1 from Brazil: prevalence of subtype B strains divergent from North American/European prototype and detection of subtype F. AIDS Res Hum Retroviruses 1994;10(5):569-76.
18. Sabino EC, Diaz RS, Brigido LF, et al. Distribution of HIV-1 subtypes seen in an AIDS clinic in Sao Paulo City, Brazil. Aids 1996;10(13):1579-84.
19. Soares MA, De Oliveira T, Brindeiro RM, et al. A specific subtype C of human immunodeficiency virus type 1 circulates in Brazil. Aids 2003;17(1):11-21.
20. Brindeiro RM, Diaz RS, Sabino EC, et al. Brazilian Network for HIV Drug Resistance Surveillance (HIV-BResNet): a survey of chronically infected individuals. Aids 2003;17(7):1063-9.
21. Hendry RM, Hanson CV, Bongertz V, et al. Immunoreactivity of Brazilian HIV isolates with different V3 motifs. Mem Inst Oswaldo Cruz 1996;91(3):347-8.
22. Teixeira PR, Vitoria MA, Barcarolo J. Antiretroviral treatment in resource-poor settings: the Brazilian experience. Aids 2004;18 Suppl 3(S5-7.
23. Marins JR, Jamal LF, Chen SY, et al. Dramatic improvement in survival among adult Brazilian AIDS patients. Aids 2003;17(11):1675-82.
24. Sethi AK, Celentano DD, Gange SJ, et al. High-Risk Behavior and Potential Transmission of Drug-Resistant HIV Among Injection Drug Users. J Acquir Immune Defic Syndr 2004;35(5):503-10.
25. Wensing AM, Boucher CA. Worldwide transmission of drug-resistant HIV. AIDS Rev 2003;5(3):140-55.
26. Tang JW, Pillay D. Transmission of HIV-1 drug resistance. J Clin Virol 2004;30(1):1-10.
27. Sucupira M, Caseiro M, Alves K, et al. High Levels of Primary Antiretroviral Genotypic Resistance and B/F Recombinants in Santos SP, Brazil Submitted.
28. Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. Jama 1998;280(1):42-8.
29. Busch MP, Glynn SA, Stramer SL, et al. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion 2005;45(2):254-64.
30. Barreto CC, Nishyia AA, Araujo LV, et al. Trends in Antiretroviral Drug Resistance and Clade Distributions among HIV-1-infected Blood Donors in São Paulo, Brazil. J Acquir Immune Defic Syndr. 41(3):338-41, 2006.
31. Frenkel LM, Wagner LE, 2nd, Atwood SM, et al. Specific, sensitive, and rapid assay for human immunodeficiency virus type 1 pol mutations associated with resistance to zidovudine and didanosine. J Clin Microbiol 1995;33(2):342-7.
32. Brindeiro RM, Diaz RS, Sabino EC, et al. Implementation of the quality control program (QC) for HIV-1 resistance genotyping testing network - RENAGENO of Brazilian STD/AIDS program. In: MedGenMed., ed. The XV International AIDS Conference. Thailand, 2004:MoPeB3126.
34. Siepel AC, Halpern AL, Macken C, Korber BT. A computer program designed to screen rapidly for HIV type 1 intersubtype recombinant sequences. AIDS Res Hum Retroviruses 1995;11(11):1413-6.
33. Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol 1990;215(3):403-10.
35. Salminen MO, Carr JK, Burke DS, McCutchan FE. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res Hum Retroviruses 1995;11(11):1423-5.
36. D'Aquila RT, Schapiro JM, Brun-Vezinet F, et al. Drug Resistance Mutations in HIV-1. Top HIV Med 2002;10(5):21-5.
37. Centers for Disease Control. EpiInfo Software, version 3.3, 2004.
| File Type | application/msword |
| File Title | PROJECT 1 |
| Author | P62RBJ |
| Last Modified By | curriem |
| File Modified | 2009-02-11 |
| File Created | 2009-02-04 |
© 2026 OMB.report | Privacy Policy