FAA Civil Aerospace Medical Institute INSTITUTIONAL REVIEW BOARD APPLICATION FORM
2120-Flight Attendant Field Study_Emergency_CAMI_Protocol.doc
National Flight Attendant Duty/Rest/Fatigue Field Study
FAA Civil Aerospace Medical Institute INSTITUTIONAL REVIEW BOARD APPLICATION FORM
OMB: 2120-0736
APPENDIX A
FAA Civil Aerospace Medical Institute
INSTITUTIONAL REVIEW BOARD APPLICATION FORM
Version 2.0
This form serves as the cover sheet for IRB applications. The purpose is to facilitate the review by providing salient information to the reviewers and telling them where to find other central items. Please answer every question even if the entry is "N/A" or "none."
I. THE RESEARCH PROPOSAL
Principal Investigator: Steven R. Hursh
Degree: Ph.D.
Lab/Routing Symbol: Institutes for Behavior Resources, Human Performance Center
Phone: 410-752-6080, ext 150
Title: President, Institutes for Behavior Resources, Inc.
Co-Principal Investigator: None Degree: N/A
Lab/Routing Symbol: N/A
Phone: N/A
Title: N/A
Collaborating Investigators (give identifying info for each): Melissa M. Mallis, Ph.D. and Peter G. Roma, Ph.D., Institutes for Behavior Resources; John A. Caldwell, Ph.D., Archinoetics
Project Title: Flight Attendant Work/Rest Patterns, Alertness, and Performance Assessment (Cooperative Agreement/Grant # 08-G-006)
Sponsor: Civil Aerospace Medical Institute (CAMI)
PLANNED STARTING DATE AND ANY CONSTRAINTS ON START DATE:
Planned start date for data collection is November 3, 2008. Constraints on start date include IRB approval and sufficient development of data collection equipment.
II. STUDY POPULATION
Age Range:18-85
Gender: __Males __Females _X_ Both
Special Qualifications: Must be active US-based Flight Attendant
Source of Subjects: Active US-based Flight Attendants will be informed of the study by Airlines and Union representatives through written letters, an email, and advertising in industry-relevant print media.
Number of Subjects: 210
Exclusion Criteria (if any): 1) Worked one or more flights longer than 8 hrs within the 30 days prior to the start of the study, or 2) Plan on working one or more flights longer than 8 hrs within the 30-day study period. The exclusionary criteria are designed to reduce the overlap of this study with another study of flight attendants that focuses on long range and ultra-long range routes.
Mark any of the following subject groups that are included:
Children__ Pregnant Women__ Mentally Disabled__
Elderly__ Prisoners__ Federal Employees__
If any of these groups are included you might consult with the IRB Chair prior to submitting this application.
V. Does this study involve the use of any investigational drugs or devices?
____YES __X__NO
VI. Does this study involve the use of ionizing radiation? ___YES _X_NO
VII. REQUEST FOR EXEMPT STATUS OR EXPEDITED REVIEW.
I request this application be considered as: Exempt____ Expedited_X_
(See Attachment 1 for explanation of why expedited criteria are met.)
VIII. PROTOCOL REFERENCES CONSENT FORM REFERENCES
Page No. Topic Page No. Topic
_____ Purpose _____ Purpose
_____ Background _____ Description of Study
STUDY POPULATION _____ Risks
_____ Inclusion/Exclusion Criteria _____ Benefits
_____ Duration of Participation _____ Compensation/ Injury
_____ Early Termination Criteria _____ Contact Point for
Subject's Questions EXPERIMENTAL PLAN _____ Subject's Assurances
_____ Facilities
_____ Confidentiality
_____ Methods/Procedures
_____ Withdrawal With Impunity
_____ Risk Analysis
_____ Medical Monitoring (if any)
DATA
_____ Collection/Analysis
_____ Statistical Justification for
Number of Subjects/Sessions Used
_____ Confidentiality
_____ LIST OF REFERENCES
LIST OF ATTACHMENTS TO PROTOCOL:
Attachment 1: Explanation for Expedited Review Request
Attachment 2: Notification of Flight Attendant Survey with Subject Recruitment Solicitation
Attachment 3: Informed Consent Form
VIII. CERTIFICATION/SIGNATURE
I certify that the information contained herein (application, research protocol, consent form if required) is true and correct, and that I have received approval to conduct this research project from all persons named as collaborating investigators and from my division management.
P.I. SIGNATURE: _____________________________ DATE:________________
APPENDIX C
PROTOCOL REQUIREMENTS FOR IRB APPLICATION
Version 2.0
Identifying information:
Title: Flight Attendant Work/Rest Patterns, Alertness, and Performance Assessment
Principal Investigator: Steven R. Hursh, Ph.D.
Associate Investigators: Melissa M. Mallis, Ph.D, Peter G. Roma, Ph.D., and John A. Caldwell, Ph.D. Consultants: None
Contractor(s): Traci H. Downs, Ph.D. and J. Hunter Downs III, Ph.D., Archinoetics LLC; Harold P. Greeley, Ph.D., Response Applications LLC
Organization conducting the research: Institutes for Behavior Resources, Inc. (IBR)
Purpose and Objectives:
In the interest of serving the general public, we propose to assist CAMI with a comprehensive analysis of fatigue in flight attendants across a range of operational conditions. The specific goals of this project are to systematically assess activity patterns, fatigue, and performance on- and off-duty in 210 flight attendants of various levels of seniority from US-based network, low-cost, and regional carriers embarking on domestic and extended international flights. The risks associated with participation in this research are minimal.
Description of the Research:
Hypotheses:
The specific research questions to be addressed by this project include:
How much activity, rest, and sleep do US-based flight attendants engage in over the course of a typical 30-day period?
Do the activity patterns identified in (1) significantly differ between flight attendants from Network, Low-Cost, and Regional carriers?
Do the activity patterns identified in (1) significantly differ between flight attendants of Senior, Mid-Level, and Junior seniority status?
Do the activity patterns identified in (1) significantly differ between Network flight attendants engaged Domestic versus International flights?
How do the activity patterns identified in (1) affect attention, subjective sleepiness, and mood?
Do the effects of the activity patterns identified in (1) on attention, sleepiness, and mood vary as a function of carrier type, seniority status, or international flight assignment?
Correlate sleep, activity, and performance data with predictions from models of human sleep and alertness to assess validity of such systems for flight attendant alertness and fatigue.
Historical Background:
Flight attendants serve a number of safety and customer service functions aboard commercial aircraft. These safety functions are critical to protect the general public in the event of medical or operational emergencies. In particular, flight attendants review and enforce a wide range of safety procedures on every flight, can assist passengers in the event of an emergency evaluation, and can render first aid to passengers in the event of a medical emergency. In addition, flight attendants are an important component in the onboard security system in the event of a passenger disruption or security threat.
Operational fatigue is a persistent problem in commercial aviation and has been repeatedly cited by the National Transportation Safety Board (NTSB) as a critical problem requiring additional research and regulatory attention. Flight attendants who have excessive fatigue may not be able to carry out their safety and security duties in a timely, efficient, and effective manner and this could jeopardize the safety of the general public. Despite the important role flight attendants play in commercial airline passenger safety and service, very little scientific data exist on the work/rest patterns of flight attendants and potential effects of operational fatigue. Recognizing the potential risks to public safety, the US Congress recently allocated funding to CAMI specifically for the study of these issues.
This study will be the first to provide the evidence necessary to assess and address potential fatigue challenges faced by flight attendants and is critical to developing necessary training and operational mitigations of this potential threat to public safety.
Experimental Methods and Procedures:
Each volunteer will be scheduled for 30 consecutive days of participation in the project protocol.
The Archinoetics’ SleepBand (a wrist-watch actigraph device requiring no interaction with the subject, see below), will be worn on their non-dominant wrist continuously throughout the duration of the study to document sleep/wake cycles and activity levels of the volunteers. A commercially available pedometer will also be used while on-duty to assess physical activity levels and provide an estimate of workload levels.

Participants will also be issued a cell phone or portable digital assistant (PDA) device appropriately programmed to deliver tasks routinely used in operational fatigue research, including the 5-minute Psychomotor Vigilance Task (PVT) for assessing attention/reaction time, the Profile of Mood States (POMS) for assessing mood, Visual Analog Scale (VAS) assessments of subjective sleepiness and alertness, the NASA Task Load Index (TLX) for assessing perceived workload, and voice recording software for detecting physical signatures of fatigue. Completion of the PVT, POMS, VAS, TLX, and voice recording assessments typically takes no more than 8 minutes to complete.
The PVT, POMS, VAS, and voice recording assessments will be made within 45 minutes after waking and immediately before bed every day throughout the study period. In addition, during duty days, the PVT, POMS, VAS, and voice recording tasks will be performed one hour before each duty period and again along with the TLX within 15 min after each duty period. On duty days, if the pre-duty or post-duty test occurs within two hours of the tests associated with waking or sleeping, then the second of the two redundant tests would be skipped. Subjects will be instructed to perform all assessments on the PDA-type device in a relatively isolated space with minimal distractions, but never during active duty or while engaged in other activities where performing these tasks could compromise safety (e.g., driving). In addition to the above quantitative assessments, all participants will also use the PDA device to log their sleep/wake schedules throughout the entire protocol. On duty days, participants will continue using the log to record sleep/wake cycles, as well as time spent commuting, time spent on-duty, time spent in-flight, time spent in on-duty breaks, and time spent in off-duty personal activities. Subjects will be instructed to log these data preferably as soon as the information is available, but at least on the day they occur, and never during active duty or while engaged in other activities where diverted attention could compromise safety.
The data collection phase of the project is scheduled to take place from November 3, 2008 until March 1, 2009. No individual volunteer subject will be asked to participate in the study for more than 30 consecutive days. No medical monitoring beyond that provided by the employers during duty will be offered.
Data Collection, Analysis, and Anonymity:
All pedometer, actigraphy, PVT, POMS, VAS, TLX, audio, and logbook data will be stored on the respective electronic devices used to generate and record said data. Data collected via the cell phone device will be stored on a memory card (e.g., microSD) in the unit itself. However, to allow for periodic assessment of data collected, subjects will be requested to transfer their data via a normal cell phone protocol (or potentially) an internet connection to Archinoetics for data upload. Subjects will be asked to do this transfer no less than weekly but no more than daily. SleepBand and pedometer data will be transferred on the same schedule using identical or similar technology (at present, the SleepBand data may be restricted to internet-based transfer, but alternatives are being evaluated). Information from both devices will be keyed with participant identification information so that they can be associated with one another and with the Fatigue Avoidance Scheduling Tool (FAST) analysis which will be performed subsequent to the PDA and SleepBand data analysis.
Archinoetics will launch and maintain a secure website to which the subjects can upload the data from their devices if necessary. Regardless of transfer methodology, all raw data will be stored, maintained, and protected in a central server at Archinoetics. In order to ensure anonymity, individual subjects will be assigned a number in the database, and all data will be de-identified in all databases used for analysis and reporting. Only persons directly involved with the research will have access to the raw data, and under no circumstances will any individually identifiable data be published or made available to persons not directly associated with the research without explicit, prior written approval from the individual(s) in question. Employers will not have access to any individually identifiable data. All participants will be assured the rights and protections mandated by the United States government under TITLE 45 — PUBLIC WELFARE, Department of Health and Human Services, PART 46, PROTECTION OF HUMAN SUBJECTS (http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm).
Data from the cell phone/PDA devices will be analyzed via mixed-factorial analyses of variance (ANOVAs) for Carrier Group (network, low-cost, or regional), Seniority (senior, mid, or junior), Time (pre-duty and post-duty), and Day (1-30). Separate analyses will also be conducted within the network carrier group to assess the effects of Flight Length (short/domestic versus long/international). SAFTE-based fatigue risk data from the SleepBands will be analyzed for carrier group (network, low-cost, regional) and day (1-30). In addition, since the SleepBands calculate average sleep quality and quantity measures for the duration over which they are worn, these data will be analyzed in a between-groups ANOVA (across the three types of carrier groups). These analyses will indicate whether or not cognitive skills, mood and task effectiveness (as determined by voice parameters) deteriorate to a statistically-significant degree across consecutive duty periods, whether fatigue-related risk levels increase significantly across duty periods, and the degree to which observed changes are attributable to sleep restriction or sleep disruptions as opposed to time-on-duty. These variables will also be examined in relation to workload estimates as extracted by the pedometer data. In addition, the analyses will determine whether there are differences in the difficulties experienced by cabin crew operating domestic flights versus international flights (< 8hrs).
Subsequent to the analyses described above, the duty schedule information stored via the electronic log-book application on the cell phone will be used as input to the FAST. Each selected crewmember’s data then will be processed through the FAST to determine (at a minimum) average day-to-day fatigue risk, amount of duty time below the established fatigue criterion level, and day-to-day effectiveness levels. These data will be analyzed via ANOVA as outlined for the cell phone and SleepBand data. These analyses will determine the impact of crew type and duty schedule on predicted performance and the correlation between predicted and measured performance. In addition, the SleepBand risk calculations (based on actual collected sleep data) will be compared to the FAST risk calculations (based on FAST-Autosleep predicted sleep data) to determine the extent to which future fatigue-mitigation work should entail the collection of actigraphy data in addition to FAST data.
Study Population:
Subject recruitment will be arranged in consultation with FAA officials as well as representatives from a number of US carriers. Potential subjects will receive an email (Attachment 2) from their employer or union representative notifying them that they may receive a paper-and-pencil survey (approved by CAMI IRB, June 13 2008) regarding their work as flight attendants. This email letter will also include a link to a website describing the research proposed herein (http://tinyurl.com/flightattendant). This description will also be included in the at-home survey. Volunteers may also be solicited through advertisements in periodicals and newsletters of particular interest to flight attendants. All electronic and print solicitations, whether private or public, will use the same text presented in Attachment 1. Flight attendants interested in participating in the study may register online, and will receive an automated message confirming receipt of their application and when to expect correspondence from a knowledgeable project researcher to determine their potential inclusion in the project.
A total of 210 flight attendants will be recruited for voluntary participation in the study for a period of no more than 30 consecutive days. All active flight attendants are eligible for participation. Although efforts will be made to recruit an equal number of female and male volunteers, the majority of the flight attendant population is female. Therefore, efforts will be made to recruit a sample of flight attendants that is proportional and consistant with current labor statistics. Prospective volunteers will not be systematically excluded based solely on age or ethnicity. Individuals selected for participation from the pool of applicants will be contacted and briefed in real-time by a member of the research staff, who will thoroughly explain the purpose of the project, the procedures involved, and the potential risks and benefits of participation. If the individual still wishes to participate, a member of the research staff will furnish a copy of the Informed Consent form (Attachment 3), explain it to the volunteer, and answer any additional questions before asking him/her to sign the form.
The final subject pool will represent a cross-section of the US flight attendant population, including members of participating Network, Low-Cost, and Regional carriers of senior, mid-level, and junior seniority status engaged in domestic and international flights:
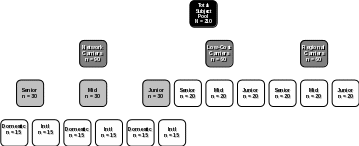
Subjects will receive no less than $400 US from IBR as a token of appreciation for completing the entire course of the project ($11.66 per day x 30 days + $50 study completion bonus). As described in the Consent From, all subjects are free to withdraw from the study at any time and for any reason. Individual subjects may also be removed from the study by the project research staff for safety reasons, compromised ability to perform regular work duties, changes in health status, changes in legal status, disruptive or inappropriate behavior, or non-compliance with the research protocol. Individuals who choose to withdraw or are removed from the study before completing the final duty cycle of their respective 30-day study period will receive no less than $11.66 US per day for each day of participation.
The proposed research will not include children. Since there are no serious physical risks associated with participation in this research, no medical evaluations other than those provided by the employers will be provided to the subjects initially, during the course of research, or at completion of the study.
Risks, Discomforts, and Inconveniences:
There are no serious physical risks associated with participation in this research. However, some individuals may experience slight discomfort from wearing the pedometer and actigraphy watch and/or carrying the PDA device, but no more so than from a standard wrist watch or cell phone. If redness or itching occurs due to a local allergic reaction to the metallic surfaces, corrective measures will recommended, such as applying moleskin tape to areas of the device(s) that contact the skin. Some individuals may experience mild discomfort in the form of eye or hand strain when filling logbooks and/or using the PDA device. Finally, some individuals may experience mild psychological discomfort in the form of annoyance, inconvenience, or boredom in fulfilling the repeated data collection requirements according to the agreed upon schedule(s) or in remembering to remove the pedometer and actigraph during exposure to water (e.g., routine hygiene or swimming). The least likely but most profound risk would be the unwarranted use of an individual’s data by airline management to make employment-related decisions regarding that individual. As detailed in the Informed Consent form (Attachment 3), this risk will be mitigated by data de-identification methods that shield the subjects from disclosure of their individual data to anyone outside the study team.
The Informed Consent Procedure:
Prospective subjects who have volunteered for participation and are selected by the research team will be contacted and briefed in real-time by a member of the research staff, who will thoroughly explain the purpose of the project, the procedures involved, and the potential risks and benefits of participation. If the individual still wishes to participate, a member of the research staff will furnish a copy of the Informed Consent form (Attachment 3), explain it to the volunteer, and answer any additional questions before asking him/her to sign the form.
References: None
Attachments:
Attachment 1: Explanation for Expedited Review Request
Attachment 2: Notification Email for Flight Attendant Survey with Subject Recruitment Solicitation
Attachment 3: Informed Consent Form
Attachment 1
Explanation for Expedited Review Request
The Institutes for Behavior Resources was recently awarded Cooperative Agreement # 08-G-006 from the Civil Aerospace Medical Institute (CAMI) to conduct the study entitled “Flight Attendant Work/Rest Patterns, Alertness, and Performance Assessment.” This study involves no more than minimal risk for the participants, all of whom will be 18 years of age or older, and qualifies for expedited review based on the CAMI-approved criteria described below.
Criterion:
Recording of data from subjects l8 years of age or older using noninvasive procedures routinely employed in clinical practice. This includes the use of physical sensors applied either to the surface of the body or at a distance and do not involve input of matter or significant amounts of energy into the subject or an invasion of the subject's privacy. It also includes such procedures as weighing, testing sensory activity, electrocardiography, electro/encephalography, thermography, detection of naturally-occurring radioactivity, diagnostic echography, and electroretinography. It does not include exposure to electromagnetic radiation outside the visible range (i.e. x-rays, microwaves, etc.).
Qualification:
Participants will be required to wear a commercially available pedometer (measures number of steps taken) around an ankle as well as a wristwatch-shaped actigraph (measures movement) on their non-dominant wrist throughout the study. By design, both of these devices monitor activity completely non-invasively and unobtrusively and are essentially weightless. Neither device emits or extracts any significant amount of energy. Although activity levels may be continuously monitored, neither device is capable of identifying the specific activities in which the subjects are engaged, and neither device contains any kind of individual tracking or positioning hardware or software.
Criterion:
Voice recording made for research purposes, such as investigations of speech defects.
Qualification:
As part of the assessment battery, the subjects will be required to recite a scripted statement into their cellphone/PDA devices for subsequent analysis of the voice recordings for physical signatures indicative of fatigue.
Criterion:
Research on individual or group behavior or characteristics of individuals, such as studies of perception, cognition, game theory, or test development, where the investigator does not manipulate the subject's behavior and the research will not involve stress to subjects.
Qualification:
The purpose of the project is to scientifically document naturally occurring work/rest patterns, alertness levels, and performance in flight attendants. As such, assignment to subgroups (carrier type, seniority level, flight lengths) will be based on inherent and/or otherwise pre-existing circumstances. No experimental manipulations per se, including physical or psychological stressors, will be systematically imposed on any of the participants.
Attachment 2
Notification of Flight Attendant Survey with Subject Recruitment Solicitation
Dear Flight Attendant/[union name] member,
Over the next few days, you may receive a survey from the Federal Aviation Administration (FAA). The U.S. Congress and the President have directed the FAA Civil Aerospace Medical Institute (CAMI) in Oklahoma City to conduct research concerning fatigue among U. S. flight attendants. The FAA is sending surveys to 30,000 randomly-selected U.S. flight attendants in October and will analyze and report participant responses to Congress by December 2009.
If you are selected, [airline name]/[union name] encourages you to complete the survey on-line or return the paper version mailed to you. Your honest and anonymous feedback will help government, industry, and employee representatives make the best possible decisions to ensure the safety and health of U. S. flight attendants and the traveling public.
In addition, CAMI is seeking 210 volunteers to participate in a field study in which flight attendants report and monitor duty periods, sleep, and activity over the course of a single month of flying using personal digital assistants (PDAs), wrist activity monitors, and pedometers. Volunteers will be paid $400.00 for their full participation. [airline name]/[union name] encourages you to consider volunteering by reviewing information posted at this weblink: http://tinyurl.com/flightattendant. The site explains how the study is conducted, requests information about the type of trips you typically fly and how to contact you, and provides a point of contact for further questions. All volunteers will receive a monthly email update of their status regarding selection. Study personnel will contact at least 50 flight volunteer attendants each month over four months to arrange participation. Research staff will then meet with selected volunteers at their crew base airport to provide all study equipment, and to thoroughly describe the study’s procedures as well the responsibilities and rights of everyone involved in the research.
In accordance with scientific sampling protocol, all information will be de-identified, and airline name]/[union name] will not know who is chosen to participate in the survey or the field study. Participation in both activities is strictly voluntary but is strongly encouraged.
Thank you for your support as we continue to enhance the safety of the flight attendant profession.
Sincerely,
[contact name]
Attachment 3
Informed Consent Form
CIVIL AEROSPACE MEDICAL INSTITUTE
Individual's Consent to Voluntary Participation in a Research Project
I, ______________________________, understand that this study, entitled " Flight Attendant Work/Rest Patterns, Alertness, and Performance Assessment," is sponsored by the Civil Aerospace Medical Institute (CAMI) of the Federal Aviation Administration (FAA) and is being directed by Steven R. Hursh, Ph.D. of the Institutes for Behavior Resources (IBR).
Nature and Purpose:
I have been recruited to volunteer as one of 210 subjects in the project named above. The purpose of the project is to systematically assess activity patterns, fatigue, and performance on- and off-duty in US-based flight attendants of various levels of seniority from network, low-cost, and regional carriers embarking on domestic and extended international flights. The time requirement is no more than 30 consecutive days.
Experimental Procedures:
As a volunteer participant in this research study, I will contribute to the scientific assessment of work/rest patterns, alertness, and performance in the US-based flight attendant population. The study requires my participation for 30 consecutive days. A wrist-watch shaped actigraph device will be worn on my non-dominant wrist continuously throughout the duration of the study to document my sleep/wake cycles and activity levels. A commercially available pedometer will also be used while on-duty to assess measure the number of steps I take to provide an estimate of workload levels.
I will be issued a cell phone or portable digital assistant (PDA) device appropriately programmed to deliver tasks routinely used in operational fatigue research, including the 5-minute Psychomotor Vigilance Task (PVT) for assessing attention/reaction time, the Profile of Mood States (POMS) for assessing mood, Visual Analog Scale (VAS) assessments of subjective sleepiness and alertness, the NASA Task Load Index (TLX) for assessing perceived workload, and voice recording software for detecting physical signatures of fatigue. Completion of the PVT, POMS, VAS, TLX, and voice recording assessments typically take no more than 8 minutes to complete.
I will be required to complete the PVT, POMS, VAS, and voice recording assessments will within 45 minutes after waking and immediately before bed every day throughout the 30-day study period. In addition, during duty days, I will be required to complete the PVT, POMS, VAS, and voice recording tasks one hour before each duty period and again along with the TLX within 15 min after each duty period. I understand that I should attempt to complete all assessments on the PDA-type device in a relatively isolated space with minimal distractions, but never while on-duty or while engaged in other activities where performing these tasks could compromise safety (such as driving).
I will also be required to use the PDA device to log my daily sleep/wake schedules throughout the entire study. On duty days, I must continue using the log to record sleep/wake cycles, but also record time spent commuting, time spent on-duty, time spent in-flight, time spent in on-duty breaks, and time spent in off-duty personal activities. I should log these data as soon as the information is available, but at least on the day they occur, and never during active duty or while engaged in other activities where diverted attention could compromise safety.
I may withdraw from the study at any time and for any reason. I also understand that the project research staff may remove me from the study at any time for safety reasons, compromised ability to perform regular work duties, changes in health status, changes in legal status, disruptive or inappropriate behavior, or non-compliance with the research protocol.
Discomfort and Risks:
There are no serious physical risks associated with participation in this research. However, I may experience slight discomfort from wearing the pedometer and actigraphy watch and/or carrying the PDA device. I may also experience mild discomfort in the form of eye or hand strain when filling logbooks and/or using the PDA device. Finally, I may experience mild psychological discomfort in the form of annoyance, inconvenience, or boredom in fulfilling the repeated data collection requirements according to the agreed upon schedule. The least likely but most profound risk would be the unwarranted use of my individual data by airline management to make employment-related decisions. However, to ensure anonymity, all data will be de-identified in all databases used for analysis and reporting. Only persons directly involved with the research will have access to my raw data, and under no circumstances will any individually identifiable data be published or made available to persons not directly associated with the research without my explicit, prior written approval. My employer will not have access to any data identified as mine.
As described above, since all data are collected either passively or during off-duty hours, my performance and safety during work shifts should not be impaired because of participation in this study. However, a member of the project research staff will inform me of any significant new findings that may affect my decision to remain in the study. Although this study is the first of its kind in flight attendants, similar assessments using the same techniques and procedures have been conducted with pilots as well as members of the railroad and commercial trucking industries.
Precautions for Female Subjects:
I understand that participation in this study poses no specific risks to female or pregnant flight attendants.
Benefits:
I will receive no less than $400 US as a token of appreciation for completing the entire course of the project. This total includes $11.66 per day of participation plus a $50 bonus for completing the study. If I withdraw or am otherwise removed from the study before completing the final duty cycle of my 30-day study period, I will be receive no less than $11.66 per day for each day of the study I completed up to the point of withdrawal.
In addition, potential benefits for me include enhanced knowledge regarding the effects of fatigue and the importance of sleep and activity management in relation to job performance. Moreover, my contributions may yield broader benefits to all US flight attendants, since the data collected for this project will be the first of its kind, and will serve as a foundation for decision-making by federal regulatory agencies, employee unions, and industry executives to improve safety, work conditions, and quality of life for commercial airline passengers and crew.
Subject Responsibilities:
I understand that by agreeing to participate in this study, I am responsible for completing all of the research tasks and assessments as described above in the “Experimental Procedures” section. Even if I agree to participate in this study, I understand that I am in no way exempt from any of the regulations, guidelines, and routine activities established by my employer, the Federal Aviation Administration, or any other applicable professional, regulatory, or legal authority.
Compensation and Injury:
I agree to immediately report any injury or suspected adverse effects to Dr. Steve Hursh at (410)-752-6080, ext 150 or [email protected], Dr. Melissa Mallis at 650-906-5994 or [email protected], or Dr. Pete Roma at 410-752-6080, ext 128 or [email protected]. I understand that accident insurance coverage is provided only through my already established health insurance and/or worker’s compensation benefits packages from my employer. Necessary medical care would be provided by local clinics and hospitals in accordance with my health insurance policy(s). I agree to provide CAMI, if requested, with copies of all insurance and medical records arising from any such care for injuries/medical problems incurred as a result of participation in this research project.
Subject's Assurances:
I understand that my participation in this study is completely voluntary. I choose to participate in this research study because I want to.
Dr. _______ has adequately answered any and all questions I have about this study, my participation, and the procedures involved. I understand that Drs. Hursh, Mallis, and Roma will be available to answer any questions concerning procedures throughout this study.
I understand that if new findings develop during the course of this research which may relate to my decision to continue participation, I will be informed.
I have not given up any of my legal rights nor released any individual or institution from liability for negligence.
I understand that my records and data from this study will be kept anonymous, and that I will not be individually identifiable by name or description in any reports or publications related to this study.
I understand that I may withdraw from this study at any time without penalty or loss of benefits to which I am otherwise entitled. I also understand that the research project team may terminate my participation in this study if they feel this to be in my best interest.
If I have questions about this study, or need to report any adverse effects from the research procedures, I may contact the following members of the research project staff:
Name |
Daytime Phone |
Evening/Weekend Phone |
|
Dr. Steven Hursh |
410-752-6080, ext 150 |
301-785-2341 |
|
Dr. Melissa Mallis |
650-906-5994 |
||
Dr. Pete Roma |
410-752-6080, ext 128 |
443-804-5750 |
|
Signature Lines:
I have read this consent document. I understand its contents, and I freely agree to participate in this study under the conditions described. I have received a copy of this consent form for my records.
Research Subject: __________________________________________________________________
Print Name Signature Date
Authorized Investigator: __________________________________________________________________
Print Name Signature Date
Witness: __________________________________________________________________
Print Name Signature Date
| File Type | application/msword |
| File Title | IBR CAMI IRB Draft |
| Author | IBR |
| Last Modified By | Taylor CTR Dahl |
| File Modified | 2009-01-08 |
| File Created | 2009-01-08 |
© 2026 OMB.report | Privacy Policy