Fluorosis Study Forms
National Health and Nutrition Examination Survey (NHANES)--2009-2010
Attachment B Thai Fluorosis 2010
NHANES Fluorosis Methodology Study
OMB: 0920-0237
Attachment B.
National Health and Nutrition Examination Survey (NHANES)
THAI Fluorosis Methodology Study
OMB no. 0920-0237
Expires: 12/31/2011
Assurance of confidentiality – All information which would permit identification of an individual, a practice, or an establishment will be held confidential, will be used for statistical purposes only by NCHS staff, contractors, and agents only when required and with necessary controls, and will not be disclosed or released to other persons without the consent of the individual or establishment in accordance with section 308(d) of the Public Health Service Act (42 USC 242m) and the Confidential Information Protection and Statistical Efficiency Act (PL-107-347).
Public reporting burden of this collection of information is estimated to average 15 minute per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road, MS D-74, Atlanta, GA 30333. ATTN: PRA (0920-0237).
APPENDIX C
SCREENING FORM AND
EXAMPLE OF REJECTION NOTIFICATION
INFORMATION
SCREENING FORM
Adhesive
label to contain the following information; Initials,
Class, School & ID
1. Is subject in good health?
(list medications, allergies, in comments section) Yes No
2. Does the subject have a complete record from the previous study? Yes No
3. Does subject have sufficient teeth (all four permanent molars) Yes No
available for examination?
4. Is the subject free from bonded orthodontic appliances? Yes No
5. Has parent/guardian signed an Informed Consent Form? Yes No
If any answer to the questions above is No the subject is ineligible for study and the subject should be given a rejection notification slip and Question 6 completed (No). If all answers are Yes the subject is eligible to take part in the study and Question 6 should be completed Yes.
6. IS SUBJECT ELIGIBLE FOR ENTRY INTO STUDY? Yes No
If no has “rejection notification” been sent to parent Yes No
7. Has the child agreed to take part in the study and have Yes No
they provided consent to do so?
COMMENTS

________________ ______________
Signature of screener Date
Adhesive
label to contain the following information; Initials,
Class, School & ID
Oral
Health Study II
Dear
parent,
Your
child had a dental examination today as part of the Oral Health
Study. Unfortunately we were unable to include them in the study for
the reason indicated below.
Thank
you for agreeing to help us with this study.
If
you require any further information please contact Assoc.
Prof. Patcharawan Srisilapanan
APPENDIX D
TF
SCORING INDEX
DDE INDEX
DEANS INDEX
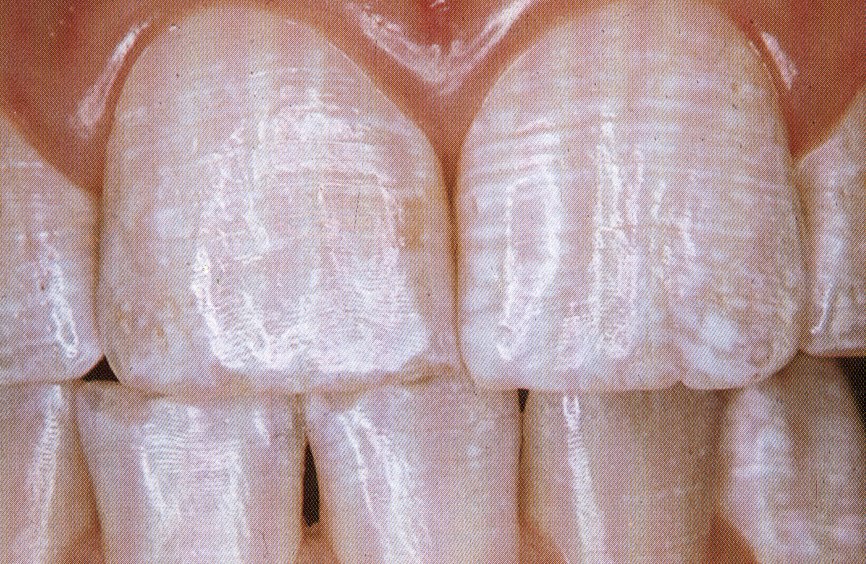
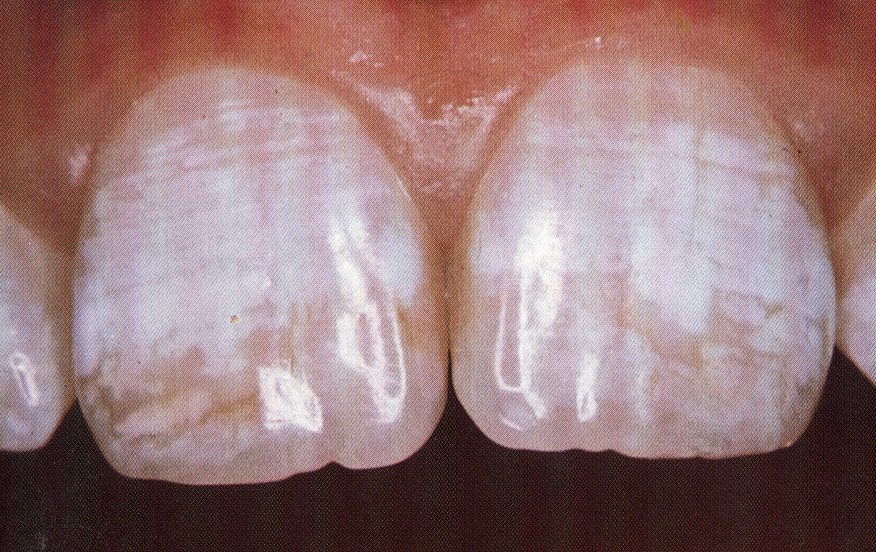
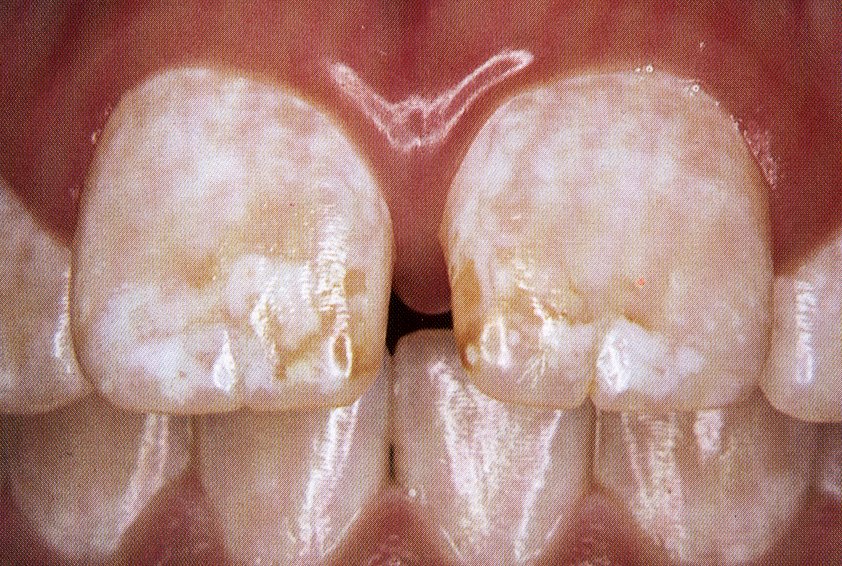
THE TF INDEX FOR FLUOROSIS
This is the diagrammatic representation of the TF index. Compare these to the clinical images which are included for comparison.
1


4
5
4
DDE INDEX
CODE
Normal 0
Demarcated opacities
White/cream 1
Yellow/brown 2
Diffuse opacities:
Diffuse – lines 3
Diffuse – patchy 4
Diffuse – confluent 5
Confluent/patchy + staining + loss of enamel 6
Hypoplasia:
Pits 7
Missing enamel 8
Any other defects 9
Combinations:
Demarcated and diffuse A
Demarcated and hypoplasia B
Diffuse and hypoplasia C
All three defects D
Extent of Defect:
Normal 0
Less than 1/3 1
At least 1/3 – 2/3 2
At least 2/3 3
DEANS INDEX
Score |
Criteria |
Normal |
The enamel represents the usual translucent semivitriform type of structure. The surface is smooth, glossy, and usually of a pale creamy white color. |
Questionable |
The enamel discloses slight aberrations from the translucency of normal enamel, ranging from a few white flecks to occasional white spots. This classification is utilized in those instances where a definite diagnosis of the mildest form of fluorosis is not warranted and a classification of "normal" is not justified. |
Small opaque, paper white areas scattered irregularly over the tooth but not involving as much as 25% of the tooth surface. Frequently included in this classification are teeth showing no more than about 1-2 mm of white opacity at the tip of the summit of the cusps of the bicuspids or second molars. |
|
The white opaque areas in the enamel of the teeth are more extensive but do not involve as much as 50% of the tooth. |
|
All enamel surfaces of the teeth are affected, and the surfaces subject to attrition show wear. Brown stain is frequently a disfiguring feature. |
|
Includes teeth formerly classified as "moderately severe and severe." All enamel surfaces are affected and hypoplasia is so marked that the general form of the tooth may be affected. The major diagnostic sign of this classification is discrete or confluent pitting. Brown stains are widespread and teeth often present a corroded-like appearance |
APPENDIX E
DATA COLLECTION CHARTS
![]()

SUBJECT HAPPY TO CONTINUE ON STUDY: YES NO (Circle)

Tooth
|
1.4 |
1.3 |
1.2 |
1.1 |
2.1 |
2.2 |
2.3 |
2.4 |
TF Score
|
|
|
|
|
|
|
|
|
DDE Score |
|
|
|
|
|
|
|
|
………………………………………… ……………………….. Signature
of clinician date
If
Examination is repeat please tick box
□
Top
copy to be sent to Manchester; Bottom Copy to be retained in Chiang
Mai
SUBJECT HAPPY TO CONTINUE ON STUDY: YES NO (Circle)

Tooth
|
1.4 |
1.3 |
1.2 |
1.1 |
2.1 |
2.2 |
2.3 |
2.4 |
DEANS SCORE
|
|
|
|
|
|
|
|
|
………………………………………… ……………………….. Signature
of clinician date
If
Examination is repeat please tick box
□
Top
copy to be sent to Manchester; Bottom Copy to be retained in Chiang
Mai
If
Examination is repeat please tick box
□
Top
copy to be sent to Manchester; Bottom Copy to be retained in Chiang
Mai
APPENDIX
F
QLF
AND PHOTOGRAPHIC
PROTOCOLS
QLF Protocol for fluorosis imaging.
Software Settings:
The following settings should be applied to the QLF software (QLF Patient) and also to the Jai Camera Software. Any variation in image size or appearance should first be investigated by confirming that these settings are correct. A copy of these settings should be placed on the appropriate computer for ease of use. The unit should be switched on 30 minutes before the first subject is imaged to ensure that the light source is stable.
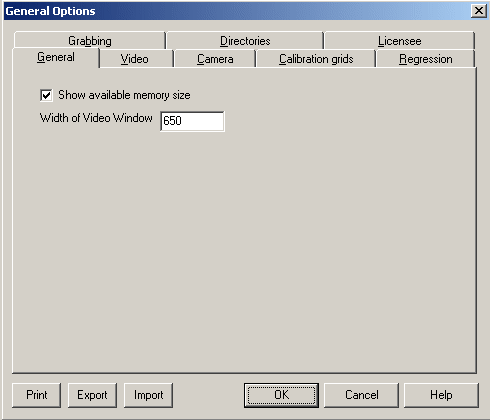
The
window size should be 650 pixels. This is a width measurement and
will permit the maximum image size to be acquired. Images of
differing sizes will require different blur levels when analysed and
therefore it is essential that this is maintained throughout the
study.
Image options. The gain, brightness, contrast and
saturation should be maintained at the levels shown below.
Variations in these will prevent analysis.
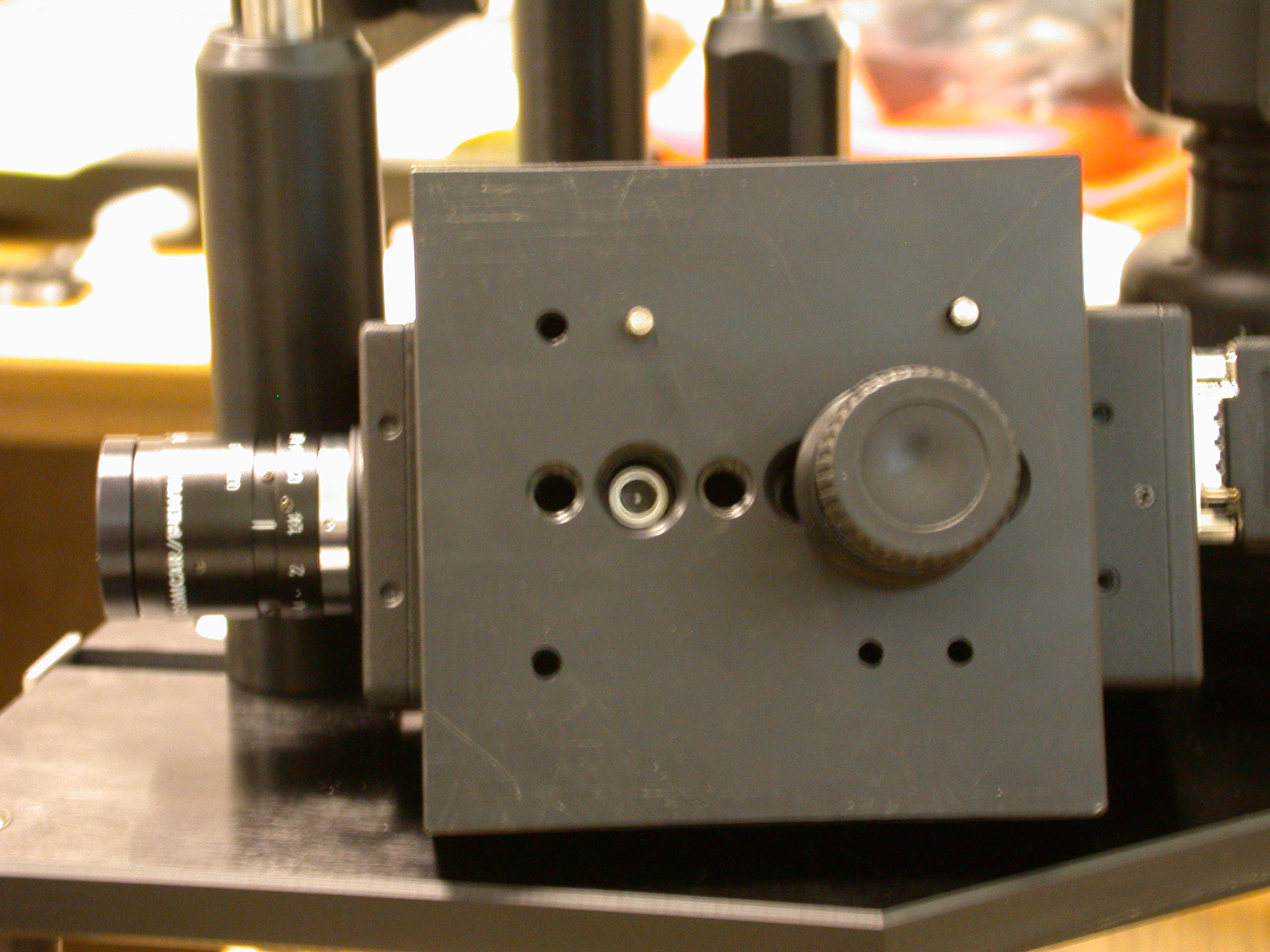
The final image demonstrates the mounting of the camera to the geometry device. The distance between the camera and the light source is crucial and therefore if the camera is dissembled the operator should check this before commencing imaging.
File storage and backup:
A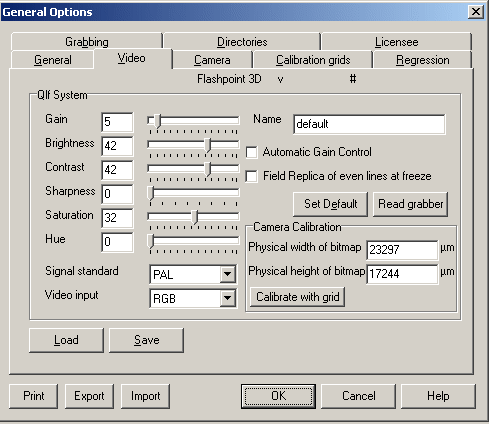 ll
images will be stored in a folder called “QLFData” this
will be on the route of the hard drive (i.e. c:/QLFData). At the end
of each session this folder should be backed up on to an external
hard drive which should be stored separately from the computer. The
photographic images will also be placed on the same drive, as will
the images from the intra-oral camera.
ll
images will be stored in a folder called “QLFData” this
will be on the route of the hard drive (i.e. c:/QLFData). At the end
of each session this folder should be backed up on to an external
hard drive which should be stored separately from the computer. The
photographic images will also be placed on the same drive, as will
the images from the intra-oral camera.
Imaging protocol:








APPENDIX G
TREATMENT
REQUIRED
FORM EXAMPLE
TREATMENT
REQUIRED FORM
EXAMPLE
Adhesive
label to contain the following information; Initials,
Class, School & ID
To be completed by the examining dentist
We have checked your child’s teeth as part of a clinical trial that you agreed to by signing a consent form.
When we examined your child’s teeth we thought that they would benefit from some additional treatment; we would therefore
recommend a dental inspection in a dental surgery as a matter of urgency
recommend a dental inspection in a dental surgery within the next few months
Comment
from dentist:
If your child does not have a dentist or you are having difficulty finding a dentist please contact our main dental clinic for advice:.
| File Type | application/msword |
| File Title | National Health and Nutrition Examination Survey (NHANES) |
| Author | bgl1 |
| Last Modified By | shari steinberg |
| File Modified | 2009-09-14 |
| File Created | 2009-09-14 |
© 2026 OMB.report | Privacy Policy
