Attach 2_IRB Protocol
Attach 2_IRB Protocol.03.16.08.docx
Follow-up of Kidney Cancer Patients from the Central European Multicenter Case-Control Study (CEERCC) (NCI)
Attach 2_IRB Protocol
OMB: 0925-0599
Attachment 2: IRB Protocol
3/16/2008
Response Special Studies Institutional Review Board 2/19/08
Title: Follow-up of Kidney Cancer Patients from the Central European Multicenter Case-Control Study
Investigators and their affiliations:
NCI: Drs. Lee Moore, Nat Rothman, Wong-Ho Chow, Ruth Pfeiffer
IARC PIs: Dr. Paul Brennan, Dr. Paolo Boffetta, Dr. Mia Hashibe, Dr. Rayjean Hung, Dr. Yuan-Chin Amy Lee, Ms. Valerie Gaborieau, Mr. Gilles Ferro, Mrs. Veronique Luzon
Study Centers: Dr. Dana Mates (Bucharest, Romania), Dr. Neonilia Szeszenia-Dabrowska (Lodz, Poland), Dr. David Zaridze (Moscow, Poland), Dr. Lenka Foretova (Brno, Czech Republic), Dr. Vladimir Janout (Olomouc, Czech Republic), Drs. Ivana Holcatova and Vladimir Bencko (Prague, Czech Republic)
Introduction
SURVIVAL AND MORTALITY
Kidney Cancer
Survival from kidney cancer is highly dependent upon stage at diagnosis. The
average 5-year survival rate for 1996-2002 from 17 SEER geographic areas is 60-65% (1). When the cancer is confined to the primary site, 5-year survival is very high (90.4%). However if regional and distant metastases are observed, 5-year survival is much lower, 61% and 9.5% respectively. Renal-cell carcinoma is among the most resistant tumors to therapy.
Generally, treatment for Stage I disease (T1, N0, M0) is surgical resection (1). Resection is either simple or radical. In patients who are not candidates for surgery, external beam radiation therapy (EBRT) can provide palliation. Radical surgical resection is also usually used for Stage II disease (T2, N0, M0). Stage III RCC (Any T3, any N1, M0) is also treated successfully with radical surgical resection. Depending upon the extent or aggressiveness of the disease, lymphadenectomy may be employed followed by dissection. EBRT may be given before or after surgery however the value of this treatment is not known. Patients with Stage IV and recurrent renal cancer (defined as any T4, any N2, any M1) are considered incurable and treatments vary. Some treatments that aid in the palliation of symptoms caused by the tumor include resection, cytokine therapy (such as interferon α), and newer anti-angiogenic therapies. RCC is considered resistant to chemotherapy, and is therefore not generally used.
In Central Europe, mortality rates for kidney cancers are higher (2-4), and 5-year survival rates (5) are lower than in the US and Western Europe overall. Survival has not improved substantially over the last decades except for Romania (Table1). Five-year survival rates in the Czech Republic, Poland, and Slovakia for kidney cancers are also lower relative to the survival rates in Europe overall (Table 2) (5). As shown in Table 1 and Table 2, women tend to have lower mortality rates and higher 5-year survival rates than men, for these cancers.
Table 1. Cancer mortality rates per 100,000 (2-4)

Table 2. Five-year survival rates from EUROCARE
for cancers diagnosed in 1990-1994
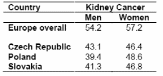
EUROCARE-3, a collaborative study on cancer patient survival in Europe, provides survival information for several Central and Eastern European countries, but not for Hungary, Russia and Romania (5). Additionally, survival data are available only for patients diagnosed up to 1994. Lack of information on histological types and treatment is also a limitation of the EUROCARE-3 project. While other studies have examined survival and prognostic factors for renal cancer in specific countries, a multicenter study of survival in Central and Eastern Europe has not been conducted to our knowledge.
Multiple Primary Tumors and RCC
The risk of second primary tumors is elevated in patients who have kidney cancer as a first primary (6-8). Similar to mortality and survival, there appears to be a sex difference in the proportion of cancer patients who develop second primary cancers; men develop second primaries at a higher proportion than women (Table 3). Potential risk factors for second primary cancers include treatment for the first primary, lifestyle factors, environmental factors, genetic variation, hormonal factors, and interactions among these factors (7).
Table 3. Proportion of cancer patients who developed second primary cancers
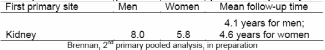
Data on secondary cancers obtained from the SEER registries have found that the observed to expected (O/E) ratio of subsequent primary cancers among renal cancer patients overall was 13% higher than the general population (8). The rate of a second primary was highest among those diagnosed with kidney cancer from ages 18-49 years (O/E=1.50 for all cancers; and O/E= 1.27 for all cancers excluding the kidney parenchyma). The cumulative incidence of developing any subsequent cancer following kidney cancer was 15.3% at 25 years (95% CI=14.7%-15.9%) including a 1.1% incidence of second kidney cancer. Other previous studies indicated elevated risk of subsequent cancers of the prostate, bladder, kidney, ureter, and thyroid as well as melanoma and overall deficits of cancers of the oral cavity and pharynx, larynx and ovary.
Objectives
The objectives of the feasibility assessment of our study are:
To evaluate the success rate or percentage of cases linked successfully to the data sources and to identify the optimal method of linkage in each center.
To determine whether the forms developed are practical for data abstraction and to compare their application to data abstraction from different sources.
To investigate the feasibility and success rates for contacting patients and/or the patients’ family and of conducting interviews with them.
To assess the accuracy of the information from interviews with patients or next of kin by cross-validating it with the information obtained from medical records or cancer registries.
Identify and compare methods used for clinical follow-up evaluation across centers.
To estimate the costs for each center for the complete follow-up component.
Kidney cancer cases for the feasibility follow-up (20% of total number of cases) will be selected by NCI, weighted by the total number of cases per center. After the patient list is prepared by the NCI, IARC will distribute the list to each center.
The objectives of the CEERCC Survival Study are:
(1) To assess the 5-year survival status of kidney cancer patients in the CEERCC study.
(2) To assess prevalence of recurrent disease and progression
(3) To investigate patient-, tumor- and genetic determinants of survival in cases. These include:
a. Patient-related factors
i. Age/Sex
ii. Tobacco, alcohol (before and after diagnosis)
iii. Socioeconomic status
iv. Genetic susceptibility
b. Tumor-Genetic factors
i. Anatomic site
ii. Histology
iii. Disease staging
iv. Tumor size
v. Extension of tumor
vi. VHL mutation/promoter hypermethylation
vii. other molecular markers
c. Treatment-related factors (these will be grouped and used as adjustment variables in the survival analysis).
i. Surgery
ii. Radiotherapy
iii. Chemotherapy
iv. Resection margin
STUDY DESIGN AND METHODS
Study Design
The Central and Eastern Europe multicenter case-control study,
completed in 2002, offers the opportunity to determinants that predict 5-year survival among kidney cancer patients. The number of cases and the subset of those subjects who have provided biological samples and have existing genetic data are shown in Table 4. To be eligible for this study, patients needed to have participated as a histologically confirmed renal cancer case in our previous case-control study entitled Central European Renal Cancer Case-Control Study.
Table 4. Number of kidney cancer cases in the CEERCC study
Center |
Total |
With DNA |
% |
ALL |
1097 |
954 |
87.0 |
Bucharest, Romania |
95 |
91 |
95.8 |
Lodz, Poland |
99 |
81 |
81.8 |
Moscow, Russia |
317 |
288 |
90.9 |
Brno, Czech Republic |
157 |
144 |
91.7 |
Olomouc, Czech Republic |
144 |
141 |
97.9 |
Prague, Czech Republic |
151 |
85 |
56.3 |
Ceske, Czech Republic |
134 |
124 |
92.5 |
The main method to obtain follow-up information is by linking the patient’s data obtained in the case-control study with vital statistics, cancer registry files, and medical records through active participation with patients’ physicians from the previous study.
The possible data sources for data abstraction for information on death, progression and recurrence are shown in Table 5. For example, we hope to be able to collect information on primary underlying cause of death from death records and possibly from cancer registry data or medical charts. The potential sources of data available in each country as preliminarily discussed in our meetings are shown in Table 6. If possible, medical charts will be collected from the hospital or physician’s office where the case subject was originally diagnosed and/or treated for their cancer. Before the interview, patients will learn about the study from their physicians during regular follow-up and participation will be requested by the physician. The physician will ask the subject whether they would prefer to be interviewed at home or at the hospital. The script that will be used when we contact the patient is included on page 1 of the attached questionnaire. Questionnaires will be used to assess tobacco and alcohol habits after diagnosis, through interview of the patient. The interview will also be used to confirm the accuracy of the information collected through passive sources, and also confirm survival status (alive/deceased), the most important outcome variable for this study. If we find that a patient is deceased we will try to obtain this information from the next-of-kin. It may be possible to assess tobacco use after cancer diagnosis through medical charts, but this is not expected to be consistent. The availability of tobacco use information will be assessed in a pilot study.
Table 5. Sources of information for the follow-up study

Table 6. Sources of follow-up data by country
-
Country
Treatment
Death
2nd Cancer
Tobacco/alcohol
Czech Republic
Possible from cancer registry
Death registry
Possible from cancer registry
Possible to contact
Poland
Cancer Registry
Death records not systematic
Cancer registry
Possible to contact
Russia
Medical records
Death records
Possible from medical records
Can contact patients/family
Romania
Medical records
Death records
Possible from medical records
Can contact patients/family
The precise linking procedure will depend on the input and specific situation at each center. Data linking will most likely be by the name, birth date, sex, and residency of the patients or possibly by medical ID if retained by investigators in the original study.
The patient will be considered lost to follow-up if the patients cannot be reached or tracked by any of the vital statistics records, cancer registry, medical chart/path report at the hospital, or available contact information. They will be considered lost to follow-up when all possible trails have failed to provide information on their status. We will also try to obtain information about the date last seen through the hospital or potential contacts or family members because this will tell us something about their cancer survivorship for a specific period of time, post treatment.
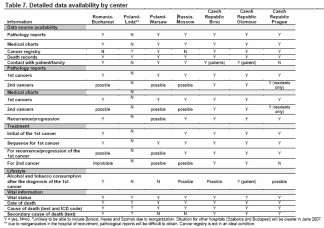
Study Location. Study locations and available data by center are listed in Table 7 below.
Subject Identification and Recruitment
To obtain data on survival status, treatment, disease, recurrence, progression and a limited list risk factors, forms have been created to abstract/record information from three potential sources (in appendix):
(1) Vital statistics
(2) Patient medical records
(3) Cancer registry
The data abstraction will be completed on the paper version of the forms. IARC will provide an ACCESS database for data entry from the paper forms. Some of the fields (specified in form) will require translation into English by the abstractor at the time of data entry.
Detailed instructions for the data abstraction have been provided in the appendix.
Case Definition and Disease Ascertainment/confirmation
Kidney cancer cases (20%-weighted by number of cases per center) that were included in the CEERCC study will be selected by the NCI and sent to IARC. IARC will distribute the list to each center. Children less than 18 are not eligible to participate in this study because there were no kidney cancer cases observed in the CEERCC study that were less than 18 years of age.
Data Collection (clinical evaluation, questionnaire, and/or abstracting methods)
INTERVIEWS OF PATIENTS/FAMILY MEMBERS
To capture the change in smoking habits after the initial diagnosis, we would like to interview the patients if possible. In addition, interviews of family members may be an alternative if the patients cannot be reached or are deceased. The interview questionnaire can be completed on the paper version or the ACCESS
database. IARC will provide the ACCESS database to enter the information from the paper forms if after the pilot, the study group decides to pursue subject interviews.
TRAINING OF ABSTRACTORS AND INTERVIEWERS
Training of abstractors and interviewers will facilitate the process of data collection and ensure data quality. Training will be conducted at each center since the linking procedures and records available in each center are expected to be different. The principal investigator of each center will first try to gather a data report from the vital statistic office, cancer registry, and a set of medical records for the first several patients, to demonstrate how to collect the information requested. For interviews, the interviewers will be trained with material available from the original case-control study. As in the case-control study, physicians and trained medical professionals will abstract information from clinical records.
Exposure Assessment
For tobacco use post-diagnosis, interview of the patient or family interviews is the most probable source of obtaining this information. It may be possible to assess tobacco use after cancer diagnosis through medical charts, but this is not expected to be consistent. The availability of tobacco information will be assessed in the pilot study.
Biological and/or Environmental Specimen Collection, Storage, and Assays NA
Data Management and Quality Control
One individual will act as the principal investigator at each center. This individual will oversee the follow-up of the cases and be responsible for training the abstractors and interviewers for data collection. In addition, one member of the expert group will act as coordinator of this group and be responsible for abstractor and interviewer training regarding the diagnosis, treatments and vital status. Drs. Moore, Chow, Hashibe, Boffetta, and Brennan will oversee the collection of survival and treatment data. IARC will be in charge of the overall coordination of the study. NCI will provide support for design, statistical analysis, and reports will be drafted collaboratively as in the case-control study.
A steering committee comprising the principle investigators in each center, along with Drs. Hashibe, Boffetta and Brennan (IARC) and Drs. Chow and Moore (NCI) will supervise the progress of the study. The success of the feasibility study and the quality and ability to confirm survival and treatment information collected will be evaluated at the next annual meeting at IARC, June 2008. NCI will be responsible for the decision to go forward and for incorporating necessary changes to the protocol and questionnaire for the main study.
MONITORING AND CRITERIA FOR WITHDRAWL FROM STUDY
In the consent for all subjects will be informed that their participation in this research is voluntary and that they may refuse to participate at any time without penalty or less of benefit to you or others.
ASSURANCE OF CONFIDENTIALITY
The information concerning participation of a subject in this study will be kept confidential and used only for scientific purposes, in accordance with applicable law of (Czech Republic, Poland, Romania, or Russia) and the United States state and federal law. No one except members of our research team will have access to questionnaire data and medical records. Questionnaires will never be labeled with a patients name, only the study identification code. Any information linking identifiers to a persons name will be kept locked at all times. Subject names or personally identifiers will not be used in any reports or released in any way.
ANALYSIS OF STUDY
Data will be received at IARC from each center and will be forwarded to the NCI. Statistical analysis will be performed, using SAS 9 software. Survival time (from diagnosis to death or to end of follow-up) will be calculated for each patient. Survival will be compared using the log-rank test to evaluate the Kaplan-Meier survival distributions. The Cox model will be used to analyze the impact of prognostic factors, including age, sex, tobacco smoking, genotype variation, treatment, tumor histology, and tumor marker variation. The Standardized Incidence Ratios (SIR) and 95% CI for second primary tumor (SPT) will be calculated using Eurocare rates for expected number of cancer computations.
The main outcome of this study will be used to compare the number of RCC deaths vs censoring (those alive at 5-years, those lost to follow-up, those that died of other causes). The four outcomes we intend to evaluate specifically include:
RCC death
Alive: disease recurrence (same clinical stage or disease independent of primary tumor)
Alive: disease progression (disease presents at higher clinical stage than primary diagnosis)
Censored (alive at 5-years, lost to follow-up, died of other causes)
As in the case-control study, physicians and experienced medical staff will be employed to abstract hospital records, pathology reports, and treatment information. After we distinguish the types of follow-up protocols used and procedures followed in each country, we will develop a definition of those cases confirmed to be disease-free (using “high-confidence methods, i.e. CT, PET, laboratory methods other), and patients for whom follow-up was not confirmed, incomplete, or undetermined (“low confidence confirmation”), so that we can stratify by this variable and conduct restricted analyses. We plan to collect information on methods used to evaluate disease status. Treatment variables will be grouped into broad categories. These new groups will be used as adjustment variables.
Lastly, we will initiate follow-up at date of diagnosis and collect survival at 5-years, controlling for treatment and perhaps with time dependent co-variates for treatment duration as needed. We will not discount any time during cancer treatment towards survival as this could make more advanced cases with longer treatment duration incorrectly appear to have a longer disease-free survival.
Statistical Power
Power calculations to determine the impact of VHL gene status, other etiologic and genetic risk factors on 5-year survival of RCC.
Table 8. Calculations for 80% power, two-sided test with alpha=0.05.
658 Living at 5-years; 439 Dead at 5-years |
Minimal HR |
VHL status: yes (93%)/no (7%)
|
2.2 |
Methylation (11%) vs no methylation |
1.8 |
Any VHL mutation (82%) vs no mutation |
1.6 |
Smoking (53% ever) |
1.5 |
BMI (29%: <25, 44%: 25-30, 27%: 30plus) |
1.3 (fitted with trend) |
Hypertension (yes 45%) |
1.5 |
Gender (female: 40%) |
1.45 |
Minor allele frequency=10% |
1.8 |
Minor allele frequency=20% |
1.6 |
Minor allele frequency=30% |
1.5 |
Human Subjects Protection (Address Inclusion of Children, Women, Minorities)
For the study, all subjects will be selected based on their participation in the previous case-control study. In the previous case-control study, each case included if histologically confirmed as an incident kidney cancer case.
Timeline
-
Month
1-6
7-12
13-18
19-24
25-30
31-36
Pilot study
Data collection
(Survival study)
Statistical analysis
Human Subjects Protections
(a) Subjects in this study will be selected based on having prior participation in a case-control study conducted in this area.
(b) Subjects will be contacted by phone and mail
(c) All subjects enrolled in the previous study will be included, there are no exclusions if subjects met the criteria for cases in the previous case-control study of RCC risk factors conducted it his area
(d) All discussion of risks and benefit to the subjects is described in the informed consent form presented below.
Informed Consent (proposed consent form to be used in study-see next page)
Consent form for follow-up Study of Cases in the Central and Eastern European RCC study
INTRODUCTION
You are being asked to participate in a study to follow-up cases that were previously enrolled in the European Kidney Cancer Case-Control Study. The purpose of this study is to investigate lifestyle factors, medical conditions, occupation and diet, and their effects on the outcome of kidney cancer patients in Europe. The study is being conducted by the Name of Local Collaborating Center and the International Agency for Research on Cancer located in Lyon, France EU and the National Cancer Institute, National Institutes of Health in Bethesda, MD, USA. Your participation in this study is voluntary. You may refuse to participate or withdraw in this study at any time without this affecting in any way, the treatment that you receive. Please read this consent form thoroughly, and ask the interviewer any questions that you may have about the study before signing this form.
EXPLAINATION OF PROCEDURES INVOLVED IN THE STUDY
If you agree to participate in this study, you will be asked to participate in an interview survey that will not take more than 60 minutes. In addition, we will ask your permission to collect relevant information from your hospital and cancer registry records. No penalties will result if you decide not to respond, either to the information collection as a whole, or to any particular question.
QUESTIONNAIRE
An interviewer will come to administer the questionnaire while you are in the hospital or at home. The interview takes approximately one hour and consists of questions related to your lifestyle, environment, and health. We will study your answers, your medical records, and the genetic and other information we found out from the earlier study. This will help us learn more about kidney cancer outcomes.
NOTIFICATION, COST, AND COMPENSATION
The research results are not suitable for use as clinical tests for your medical care. Therefore, the results of these studies will not be available to you. There will be no cost to you for participating in the study, other than your time, and there is no compensation or payment for completing the questionnaire.
POTENTIAL DISCOMFORT AND RISK
During the interviews, you may feel some discomfort when asked some questions. If you feel this way, you may refuse to answer them.
POTENTIAL BENEFITS
This is a research study and there will be no direct benefits to you other than the satisfaction of participating in this research for the possible benefit of future generations. Your participation is very important to the success of this scientific research.
ASSURANCE OF CONFIDENTIALITY
The information concerning your participation in the study will be kept confidential and used only for scientific purposes, in accordance with applicable law of (Czech Republic, Poland, Romania, or Russia) and the United States state and federal law. No one except members of the research team will have access to your answers and test results. Your employer will not be given any test results or information you provide us. Your questionnaire will not be labeled with your name. Your name will not be used in any report or released in any way. Any information that links your study identification number with your name will be kept under lock and key at all times.
RIGHTS AS A PARTICIPANT
Your participation in this medical research is voluntary and you may refuse to participate and/or withdraw your consent and discontinue participation at any time without penalty or less of benefits to which you are otherwise entitled. Your decision on this matter will not affect your medical care or employment. While the information collected as part of this research study continues to be linked to personally identifying information, it will be protected from access by anyone except those directly responsible for patient recruitment and contact. After this study is completed, all personal identifiers will be removed in place of study identifiers. Any documents containing linkage between identifiers with the study identifying codes will be kept under lock and key at the International Agency for Research on Cancer, and destroyed as soon as the study has been completed.
CERTIFICATION
I have read the explanation about this study and have been given the opportunity to discuss it and to ask questions. By agreeing to participate in this study, I do not waive any rights that I may have regarding access to and disclosure of my records. I hereby consent to take part in the study components marked “yes” and refuse to consent to participate in the components marked “no”. A copy of this consent form has been given to me.
YES NO Study Component
[ ] [ ] Interview
[ ] [ ] Access to hospital records
_____________________ _________ ________________ __________
Signature of Participant Date Signature of Witness Date
________________________________ ______________________________
Print Name Print Name
We appreciate your cooperation in this important research project. If you have further questions about this study, you may call Dr. (Name of Local Investigator) at (Local Phone Number) or you may write to him/her at the following address:
Name of Local Collaborating Center and Address
Consent form for follow-up Study of Next-of-Kin : Follow-up Study the Central and Eastern European RCC study
INTRODUCTION
You are being asked to participate in a study to follow-up cases that were previously enrolled in the European Kidney Cancer Case-Control Study. The purpose of this study is to investigate lifestyle factors, medical conditions, occupation and diet, and their effects on the outcome of kidney cancer patients in Europe. The study is being conducted by the Name of Local Collaborating Center and the International Agency for Research on Cancer located in Lyon, France EU and the National Cancer Institute, National Institutes of Health in Bethesda, MD, USA. His/her participation in this study was voluntary and you are being asked to participate in this follow-up study to, answer questions about your family member, as they are no longer able to do so. You may refuse to participate or withdraw in this study at any time. Please read this consent form thoroughly, and ask the interviewer any questions that you may have about the study before deciding to sign this consent form.
EXPLAINATION OF PROCEDURES INVOLVED IN THE STUDY
If you agree to participate in this study, you will be asked to participate in an interview survey about your family member that will not take more than 60 minutes. In addition, we will ask your permission to collect relevant information from your family member’s hospital and cancer registry records. No penalties will result if you decide not to respond, either to the information collection as a whole, or to any particular question.
QUESTIONNAIRE
An interviewer will come to administer the questionnaire while you are in the hospital or at home. The interview takes approximately one hour and consists of questions related to your family member’s lifestyle, environment, and health. We will study your answers given about your next-of-kin, their medical records, and the genetic and other information we found out about them from the earlier study. This will help us learn more about kidney cancer outcomes.
NOTIFICATION COST, AND COMPENSATION
The research results are not suitable for use as clinical tests for your medical care. Therefore, the results of these studies will not be available to you. There will be no cost to you for participating in the study, other than your time, and there is no compensation or payment for completing the questionnaire.
POTENTIAL DISCOMFORT AND RISK
During the interviews, you may feel some discomfort when asked some questions. If you feel this way, you may refuse to answer them.
POTENTIAL BENEFITS
This is a research study and there will be no direct benefits to you other than the satisfaction of participating in this research for the possible benefit of future generations. Your participation is very important to the success of this scientific research.
ASSURANCE OF CONFIDENTIALITY
The information concerning your participation in the study will be kept confidential and used only for scientific purposes, in accordance with applicable Lab of (Czech Republic, Poland, Romania, or Russia) and the United States state and federal law. No one except members of the research team will have access to your answers and test results. Your employer will not be given any test results or information you provide us. Your questionnaire will not be labeled with your name. Your name will not be used in any report or released in any way.
RIGHTS AS A PARTICIPANT
Your participation in this medical research is voluntary and you may refuse to participate and/or withdraw your consent and discontinue participation at any time without penalty or less of benefits to which you are otherwise entitled. Your decision on this matter will not affect your medical care or employment. We will keep all information we obtain from this interview secure and safe from access or use for any other intent but this research study. While the information collected as part of this research study continues to be linked to personally identifying information, it will be protected from access by anyone except those directly responsible for patient recruitment and contact. After this study is completed, all personal identifiers will be removed in place of study identifiers. Any documents containing linkage between identifiers with the study identifying codes will be kept under lock and key at the International Agency for Research on Cancer, and destroyed as soon as the study has been completed.
CERTIFICATION
I have read the explanation about this study and have been given the opportunity to discuss it and to ask questions. By agreeing to participate in this study, I do not waive any rights that I may have regarding access to and disclosure of my family member’s records. I hereby consent to take part in the study components marked “yes” and refuse to consent to participate in the components marked “no”. A copy of this consent form has been given to me.
YES NO Study Component
_________________________________
[ ] [ ] Interview
[ ] [ ] Access to hospital records
_____________________ _________ ________________ __________
Signature of Participant Date Signature of Witness Date
________________________________ ______________________________
Print Name Print Name
We appreciate your cooperation in this important research project concerning the health history of your family member. If you have further questions about this study, you may call Dr. (Name of Local Investigator) at (Local Phone Number) or you may write to him/her at the following address:
Name of Local Collaborating Center and Address
References
NCI SEER, website, 2007.
Levi, F., Lucchini, F., Negri, E., Boyle, P., and La Vecchia, C. Cancer mortality in Europe, 1995-1999, and an overview of trends since 1960. Int J Cancer., 110: 155-169, 2004.
Levi, F., Lucchini, F., Negri, E., Zatonski, W., Boyle, P., and La Vecchia, C. Trends in cancer mortality in the European Union and accession countries, 1980-2000. Ann Oncol., 15: 1425-1431, 2004.
Bray, F., Sankila, R., Ferlay, J., and Parkin, D. M. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer., 38: 99-166, 2002.
Sant, M., Aareleid, T., Berrino, F., Bielska, L. M., Carli, P. M., Faivre, J., Grosclaude, P., Hedelin, G., Matsuda, T., Moller, H., Moller, T., Verdecchia, A., Capocaccia, R., Gatta, G., Micheli, A., Santaquilani, M., Roazzi, P., and Lisi, D. EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol., 14 Suppl 5:v61-118.: v61-118, 2003.
Beisland, C., Talleraas, O., Bakke, A., and Norstein, J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int., 97: 698-702, 2006.
Travis, L. B., Rabkin, C. S., Brown, L. M., Allan, J. M., Alter, B. P., Ambrosone, C. B., Begg, C. B., Caporaso, N., Chanock, S., DeMichele, A., Figg, W. D., Gospodarowicz, M. K., Hall, E. J., Hisada, M., Inskip, P., Kleinerman, R., Little, J. B., Malkin, D., Ng, A. K., Offit, K., Pui, C. H., Robison, L. L., Rothman, N., Shields, P. G., Strong, L., Taniguchi, T., Tucker, M. A., and Greene, M. H. Cancer survivorship--genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst., 98: 15-25, 2006.
New Malignancies among Cancer Survivors SEER Cancer Registries, 1973-2000.National Cancer Institute, US Department of Health and Human Services, NIH . 2007.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Date of Review by Branch with Branch Chief signature |
| Author | Sara Karami |
| File Modified | 0000-00-00 |
| File Created | 2021-02-04 |
© 2026 OMB.report | Privacy Policy