0234-08-SS-a
0234-08-SS-a.doc
Notification of Intent to Use Schedule III, IV, or V Opioid Drugs for the Maintenance and Detoxification Treatment of Opiate Addiction
OMB: 0930-0234
Notification of Intent to Use Schedule III, IV, or V Opioid Drugs
for the Maintenance and Detoxification Treatment
of Opiate Addiction under 21 USC § 823(g)(2)
Supporting Statement
A. Justification
1. Circumstances of Information Collection
The Controlled Substances Act (CSA), as amended by the Drug Addiction Treatment Act of 2000 (DATA), establishes (21 USC § 823(g)(2)) conditions under which certain practitioners (physicians) may prescribe certain approved narcotic treatment medications for the maintenance or detoxification treatment of opioid addiction.
The legislation establishes criteria for waivers and a procedure for practitioners who are interested in waivers to obtain a unique Drug Enforcement Administration (DEA) identification number. To be eligible for a waiver the practitioner must: be a licensed physician; be registered by DEA; fulfill qualification requirements in the law for training and experience; and make written certifications relating to the capacity to provide ancillary services and maximum patient loads (currently no more than 30 patients).
Without the waivers provided under the new law, practitioners would have to seek and obtain the separate registration required under 21 USC 823(g)(1). Attachment A is a copy of 21 USC § 823(g)(2).
The Substance Abuse Mental Health Services Administration’s (SAMHSA), Center for Substance Abuse Treatment (CSAT) has the responsibility to receive, review, approve, or deny waiver requests. CSAT has entered into agreements with private contractors, and other Federal and State authorities to fulfill its responsibilities. SAMHSA seeks a revision for approval for this information collection (OMB No. 0930-0234), which expires on 03/31/2009).
Until August 7, 2005, the enactment date for the Drug Addiction Treatment Expansion Act, (P.L. 109-056) practitioners in group practices (defined under the Social Security Act) were limited to 30 patients per group. The new legislation eliminated the group practice limit, permitting physicians in group practices to treat up to 30 patients per physician. In December 2006, legislation modified the CSA to permit physicians to submit a second notification, after one year, to treat up to 100 patients. A minor modification to SAMHSA Form SMA-167 permitted physicians to submit the second notification in an efficient, uncomplicated manner. The online system was modified to accept second notifications as well.
Practitioners who meet the statutory requirements will be eligible to prescribe only those opioid treatment medications that are controlled in Schedules III, IV, or V, under the CSA, that are specifically approved by the Food and Drug Administration (FDA) for the treatment of opioid addiction, and are not the subject of an “adverse determination.” Only two medications Subutex® and Suboxone® fulfill these criteria.
The CSA establishes a set procedure for practitioners to obtain waivers. Interested practitioners are required to submit written notifications to the Secretary, HHS (authority delegated to the Administrator, Substance Abuse and Mental Health Services Administration (SAMHSA)). SAMHSA is required to determine whether the practitioner has met the criteria for a waiver within 45 days from the date of receipt of a notification. If SAMHSA determines the practitioner meets the legislative criteria, DEA is notified to assign a unique registration number to the practitioner. If SAMHSA does not respond to the practitioner within 45 days, DEA is required to release the unique identification number to the practitioner.
SAMHSA has implemented most of the waiver provisions of the new law “as written.” SAMHSA may develop regulations to establish a waiver notification renewal system to coincide with the physician’s DEA registration. In addition, possible new rules will exercise the authority provided to the Secretary to modify the individual physician patient limit. The information collection activity associated with these rulemaking initiatives will be the subject of a future PRA submission.
2. Purpose and Use of Information
As noted above, DATA amended the Controlled Substances Act (21 USC 823(g)(2)) to permit practitioners (physicians) to seek and obtain waivers to prescribe certain approved narcotic drugs for the treatment of opiate addiction. The legislation sets eligibility requirements and certification requirements as well as an interagency application process for physicians who seek waivers.
To facilitate the waiver review process, CSAT is seeking renewal of a “Notification of Intent to Use Schedule III, IV, or V Opioid Drugs for the Maintenance and Detoxification Treatment of Opiate Addiction under 21 USC 823(g)(2).” The form (SMA-167, Attachment B) may be completed and submitted on paper or on-line.
The information entered on the form will allow SAMHSA to determine whether practitioners are eligible for a waiver. The notification form is limited to two sets of items: certification of the qualifications for a waiver set forth in DATA; and only such additional information as is required to validate the statements made on the form regarding the notifying physician’s state medical licensure, and medical board certification and/or training/experience. SAMHSA has determined that the following information is necessary to process waiver notifications in accordance with the new law:
1. Practitioner name;
State medical license number;
DEA registration number;
Primary practice location information (address, telephone number, fax number);
E-mail address (optional);
Purpose of notification (new or immediate, or second notification to increase patient limit)
Certification that the practitioner will use only those schedule III, IV, or V drugs or combinations that have been approved by the FDA for use in maintenance or detoxification treatment and that have not been the subject of an adverse determination;
Certification of qualifying criteria for treatment and management of opiate-dependent patients;
9. Certification of capacity to refer patients for appropriate counseling and other appropriate ancillary services;
Certification of maximum patient load.
As discussed earlier, P.L 109-056, eliminated the group practice limit. However, due to an oversight, the recent legislation did not address a corresponding statutory provision. This corresponding provision establishes the terms for receipt of a waiver of registration by individual practitioners and prescribes the requirements of the notification to the Secretary [21 U.S.C. 823(g)(2)(D)(i)(I)-(III)]. The provision states that a waiver is not in effect unless “the practitioner is a member of a group practice, the notification states the names of the other practitioners in the practice and identifies the registrations issued for the other practitioners pursuant to subsection (f) of this section.”
SAMHSA believes that there is a reasonable argument that this requirement is no longer necessary and therefore does not intend to collect information regarding group practices. Initially, it was necessary to collect this information so that the 30 patient per group limit could be monitored and enforced. Since that limit has been eliminated by P.L. 109-05, SAMHSA believes that this information is no longer relevant. The burden on respondents to identify every physician in their group practice, together with their registration number, is substantial. Eliminating this unnecessary requirement will significantly reduced the reporting burden for physicians in group practices.
Practitioners will use the form for three types of notification: (a) new, (b) immediate, and (c) second notifications intent and need to treat up to 100 patients.. Under “new” notifications, practitioners will make their initial waiver requests to SAMHSA. “Immediate” notifications will inform SAMHSA and the Attorney General of a practitioner’s intent to prescribe immediately to facilitate the treatment of an individual patient under 21 USC 823(g)(2)(E)(ii). Second notifications will inform SAMHSA of a currently certified physician’s intent and need to treat up to 100 patients.
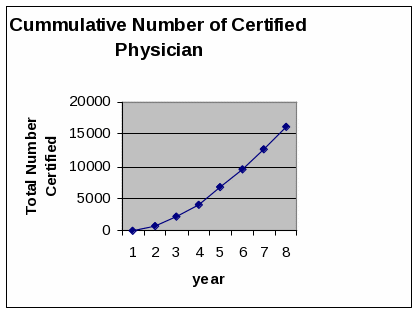
CSAT has developed a system for receiving, storing, reviewing, and verifying the information submitted with the notifications. CSAT has already received approximately 18,000 notifications using form SMA-167 (submitted by mail, by fax, or online) since the information activity was approved on May 15, 2002.
Practitioners will submit notifications to SAMHSA/CSAT's Division of Pharmacologic Therapies (DPT). DPT will then forward them to Synectics for Management Decisions, Inc. (Synectics, discussed below) which will, as an initial step, send an acknowledgment letter to the applicant physician (Attachment E1). Synectics will then verify physician credentials by contacting DEA (through DPT), State Medical Boards (through a subcontractor or directly as necessary), medical specialty societies (to confirm training and experience status) and other entities as appropriate. Synectics will forward reports on each applicant to CSAT/DPT where staff will review and approve or deny each waiver. If additional information is needed to determine qualifications, the applicant will receive an “incomplete” letter (Attachment E2). If it is determined that the physician meets the requirements under the law, the physician will receive a certification or conditional certification letter (Attachment E3). The letter will explain the status of their waiver request, provide information about medications that may be used under the waiver and provide contact information. SAMHSA has also developed letters that will notify physicians if their pending notifications will be deactivated (“on hold letters”) or if their certification has been suspended, withdrawn, or denied.
3. Use of Information Technology
In addition to submissions by mail, physicians may submit waiver notifications via an electronic version of the notification form, which is available at www.buprenorphine.samhsa.gov. The dedicated Web page containing the text of the notification form has all of the fields that are found on the paper version of the form. Many physicians have been able to access this website and submit notifications on line, using an email auto-response system for signature verification.
The Department of Health and Human Services, through the Office of the Assistant Secretary for Budget, Technology and Finance, is currently developing a department-wide strategy for compliance with the requirements of the Government Paperwork Elimination Act (GPEA). Operating Divisions of the Department (e.g., SAMHSA) are actively engaged in review of the following issues related to compliance with GPEA: digital signature software, PKI, and web site identification. As noted above, CSAT is accepting electronic notifications from approximately 20% of the respondents, and the Center will convert this system to the Department-wide standard once that is available.
4. Efforts to Identify Duplication
These notification requirements were enacted in October, 2000. The law requires physicians who wish to avail themselves of its waiver provisions to notify the Secretary of the Department of Health and Human Services. In an attempt to avoid unnecessary duplication of effort, SAMHSA/CSAT has arranged to serve as a single Federal point of contact and forward notifications, including “immediate” notifications, to DEA.
5. Involvement of Small Entities
Some applicants may be independent practitioners or members of small group practices that could be considered to be small businesses. The information being sought is the minimum needed to meet the requirements of DATA regardless of the size of the practice. This information collection will not have a significant impact on these businesses.
6. Consequences If Information Is Collected Less Frequently
Without providing this information, physicians will be unable to prescribe certain approved narcotic treatment drugs for the treatment of opiate addiction, as permitted under the Drug Addiction Treatment Act of 2000. All physicians who wish to prescribe the narcotic treatment drugs included under this statute must submit a notification.
7. Consistency With the Guidelines in 5 CFR 1320.5 (d)(2)
This information collection fully complies with 5 CFR 1320.5(d) (2).
8. Consultation Outside the Agency
The notice required under 5 CFR 1320.8(d) was published in the Federal Register on Tuesday, November 4, 2008, 73 FR 65612. No comments were received in response to that notice.
Other Consultations - In developing the waiver notification form SAMHSA and its contractor, Synectics for Management Decisions, Inc., consulted by telephone and through e-mail with staff of a number of organizations, and consulted the webpages of these organizations. Individuals were consulted regarding their views on the availability of data, data elements to be recorded, and clarity of instructions and record keeping. In particular, staff of organizations that review or certify physician credentials were consulted regarding the essential data required to perform such certifications.
The individuals consulted include:
Brad Bauer, National Sales Manager, ChoicePoint (770) 752-3828
Ryan Baldwin and Shannon Harris, technical staff, ChoicePoint—consulted as part of a conference call with Brad Bauer
Kelly Glenn, Med Advantage, (407) 282-5131
David R. Hooper, Manger of the Federation of State Medical Boards Physician Data Center, (817) 868-4000
Arrita Lawson, RN, Manager, National Provider Credentialing Service, Inc., (800) 327-5355
Armando F. Ramirez, Certification Division, Department of Education, American Osteopathic Association, (312) 202-8074
Sara Thran, Health Policy Staff, American Medical Association, (312) 464-4338
In addition, webpages and staff were consulted at the American Board of Medical Specialties, and the American Society of Addiction Medicine.
Finally, SAMHSA/CSAT established an interagency workgroup with representation from DEA and other Federal agencies (NIDA, ONDCP, FDA, HHS, DVA) in planning the implementation of DATA and this approach to information collection (Attachment C).
9. Payment to Respondents
Respondents will not receive any payment or gifts.
10. Assurance of Confidentiality
There are no study subject or patient protection concerns associated with this information collection activity. The waiver notification form includes the following statement of purpose and privacy:
This form is intended to facilitate the implementation of the provisions of 21 USC 823(g)(2). The Secretary of Health and Human Services will use the information provided to determine whether practitioners meet the qualifications for waivers from the separate registration requirements under the Controlled Substances Act (21 USC § 823(g)(1)). The Drug Enforcement Administration will assign an identification number to qualifying practitioners and the number will be included in the practitioner’s registration under 21 USC § 823(f).
SAMHSA has determined that the Opioid Treatment Waiver Notification System (OTWNS) constitutes a system of records under the Privacy Act. The Federal Register notice announcing establishment of OTWNS as a system of records was published on June 7, 2002 (Attachment D). SAMHSA has identified seven routine users/uses for the information from the system of records:
A. State medical licensing boards and medical specialty societies to verify practitioner qualifications.
B. Other federal law enforcement and regulatory agencies for law enforcement and regulatory purposes.
C. State and local law enforcement and regulatory agencies for law enforcement and regulatory purposes.
D. Persons registered under the Controlled Substance Act (21 U.S.C. § 823(f)) for the purpose of verifying the registration of customers and practitioners.
E. Members of Congress or congressional staff members to respond to a request for assistance from the Member by the individual of record.
The Department of Health and Human Services (HHS) may disclose information from this system of records to the Department of Justice, or to a court or other tribunal, when (a) HHS, or any component thereof; or (b) any HHS employee in his or her official capacity; or (c) any HHS employee in his or her individual capacity where the Department of Justice (or HHS, where it is authorized to do so) has agreed to represent the employee; or (d) the United States or any agency thereof where HHS determines that the litigation is likely to affect HHS or any of its components, is a party to litigation, and HHS determines that the use of such records by the Department of Justice, court or other tribunal is relevant and necessary to the litigation and would help in the effective representation of the governmental party, provided, however, that in each case HHS determines that such disclosure is compatible with the purpose for which the records were collected.
F. SAMHSA intends to disclose information from this system to an expert, consultant, or contractor (including employees of the contractor) of SAMHSA if necessary to further the implementation and operation of this program.
In addition, SAMHSA will release to the SAMHSA Buprenorphine Physician Locator only the physician name, address, and phone number, for those physicians who have explicitly consented to this disclosure. The Substance Abuse Treatment Facility Locator is available at no cost on the World Wide Web <http:findtreatment.samhsa.gov> and is widely used by the members of the treatment seeking public and referring professionals. It lists more than 11,000 facilities that offer specialized drug and alcohol abuse treatment programs and provides links to many other sources of information on substance abuse. While this disclosure may not be necessary for the implementation of DATA, SAMHSA believes that adding the information to the Locator will assist individuals seeking opioid treatment in finding approved providers, especially in rural settings. As such, this disclosure is consistent with the legislation’s goal of expanding the availability of substance abuse treatment.
Information provided on the Waiver Notification Form will be provided to third parties who specialize in verification of medical credentials for health care organizations. They will receive only the minimum information needed to identify the practitioner whose credentials are to be verified. The data will be provided only under standard privacy agreements with the verifying organizations. No other use of this information by a third party will be authorized. The complete information will be used only to review and certify waiver notifications.
Built in database authentication will allow access to practitioner information only by authorized CSAT or CSAT contractor personnel. This information will also be sent to the DEA by a secure channel. Information provided by practitioners may not be changed by them, CSAT staff, or the system contractor. A list of practitioners with valid waivers may be provided to pharmacists and the registered distributor, from time to time, containing information needed to verify the practitioners' authority to prescribe the drugs covered by the waiver. No other access will be permitted without the express permission of each practitioner.
11. Questions of a Sensitive Nature
There are no questions of a sensitive nature. The questions included on the application are basic items about the qualifications and licensing of practicing physicians.
12. Estimate of Annualized Hour Burden
The following table summarizes the estimated annual burden for the use of this form.
Purpose of Submission |
Number of respondents |
Responses/Respondent |
Burden/ Response (Hr.) |
Total Burden (Hrs.) |
Hourly Wage Cost ($) |
Total Wage Cost ($) |
Waiver Notification |
1,500 |
1 |
.083 |
125 |
$73.00 |
$9,125 |
Notification to Prescribe Immediately |
50 |
1 |
.083 |
4 |
$73.00 |
220 |
Notice to Treat up to 100 patients |
500 |
1 |
.040 |
20 |
$73.00 |
1460 |
Total |
2,050 |
-- |
-- |
149 |
-- |
$10,805 |
For the purpose of this information collection activity, SAMHSA/CSAT has assumed that the applicant practitioner (physician) will complete the form without assistance. As indicated in the chart above, SAMHSA estimates that completion of the form (for initial, immediate, and to increase patient limits) will require .083 hours or about five minutes. SAMHSA bases this estimate upon an informal test, wherein a group of physicians were asked to complete the form. The physicians reported a range of 4 to 6 minutes and an average of 5 minutes to complete the form. SAMHSA assumes an hourly wage rate of $73 for general practitioners and psychiatrists (See OES Code 32102(Physicians and Surgeons)) increased by 40 percent to include benefits.)
13. Estimates of Annualized Cost Burden to Respondents
Completing the waiver application should not require any additional costs for computer equipment or other record-keeping technology.
14. Estimates of Annualized Cost to the Government
CSAT has planned and allocated resources for the efficient and effective management and use of the information to be collected including the processing of the information in a manner which shall enhance, where appropriate, the utility of the information to the agencies and the public.
SAMHSA/CSAT estimates that it will require approximately 10 minutes to review and verify each notification processed by Synectics, and recommended for a waiver. This translates into approximately 200 hours per year. In addition, SAMHSA/CSAT estimates that it will require approximately 50 hours per year to review special cases that may require additional verification efforts. Together, the total estimated review time is approximately 250 hours. CSAT believes that this review will be conducted by a GS13-14 ($45/hour) level public health advisor within DPT. Accordingly, the total SAMHSA/CSAT annual cost to review these forms is approximately $11,250.
As discussed above, a waiver processing and verification system has been developed and will be maintained by a contractor, Synectics, under a task-order contract that includes provision for other activities to support implementation of DATA. The estimated cost of the waiver processing and verification system for the five-year life of the contract is $2,075,000 or an annualized cost of $415,000 per year over the potential five-year term of the contract if all option years are exercised. This includes general management and reporting costs that can be attributed to the waiver processing and verification activity.
Thus, the total annual cost is estimated to be approximately $426,250.
15. Changes in Burden
Currently, there are 135 total burden hours in the OMB inventory. CSAT requests 149 total burden hours. The increase of 14 hours is due to the fact that CSAT has processed over 18,000 notifications under the previous PRA approval. Although SAMHSA certified more physicians in 2008 than in any previous year, SAMHSA expects the number of notifications submitted to decrease over the next few years as fewer physicians are remaining for certification. The addition 14 hours of burden are estimated to address the number of second notifications that physicians will submit to increase their patient limit to up to 100. There are approximately 7,000 physicians eligible to submit a second notification.
16. Time Schedule, Publication and Analysis Plan
SAMHSA/CSAT has accepted approximately 12,000 notifications under the previous approval. The Center estimates receiving approximately 2000 notifications per year. There is no statutory expiration date in P.L. 106-310. SAMHSA is seeking approval of the required data collection for three years and will seek a renewal at the end of that period.
Under DATA (21 USC 823(g)(2)(J)(ii)(I)), the Secretary (as well as DEA) “may make a determination of whether treatments provided under subpart (A) have been effective forms of maintenance treatment and detoxification treatment in clinical settings; may make a determination of whether such waivers have significantly increased (relative to the beginning of such period) the availability of maintenance treatment and detoxification treatment; and may make a determination of whether such waivers have adverse consequences for the public health.” SAMHSA/CSAT completed and forwarded an evaluation to Congress that concluded that the waiver system has expanding treatment, has provided for effective treatment, and has not produced negative public health problems.
Display of Expiration Date
The expiration date for OMB approval will be displayed.
Exceptions to Certification Statement
This collection of information involves no exceptions to the Certification for Paperwork Reduction Act Submissions.
Attachments
A. Drug Addiction Treatment Act of 2000
B. SMA-167 - Notification of intent to Use Schedule III, IV, or V Opioid Drugs for the Maintenance and Detoxification Treatment of Opiate Addiction under 21 USC 823(g)(2)
Interagency Workgroup on the Implementation of Drug Addiction Treatment Act of 2000
D. Privacy Act Documents (Federal Register Notice)
E. Physician Response Letters
1. Acknowledgment
2. Incomplete
3. Approval/Conditional Approval
| File Type | application/msword |
| File Title | Notification of Intent to Use Schedule III, IV, or V Opioid Drugs |
| Author | djohnson |
| Last Modified By | NREUTER |
| File Modified | 2009-01-29 |
| File Created | 2009-01-29 |
© 2026 OMB.report | Privacy Policy