Attachment C Consent Form
Voluntary Partner Surveys to Implement Executive Order 12862 in the Health Resources and Services Administration
Attachment C NHSC Brand_Materials Testing FGs Consent Form FINAL 6 28 10
National Health Service Corps (NHSC) Brand and Materials Feedback
OMB: 0915-0212
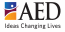
OMB No. 0915-0212
Exp. Date: XX/XX/20XX
Attachment C: Focus Group Consent Form
Project: NHSC Brand and Materials Testing Focus Groups with Potential Members
Client: Health Resources and Services Administration, National Health Service Corps
OMB Public Burden Statement: An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The OMB control number for this project is 0915-0212. Public reporting burden for this collection of information is estimated to average 5 minutes per response, including the time for reviewing instructions, searching existing data sources, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to: HRSA Reports Clearance Officer, 5600 Fishers Lane, Room 10-33, Rockville, MD 20857
Informed Consent Form
Identification of Project |
HRSA National Health Service Corps (NHSC) Brand and Materials Testing Focus Groups with Potential Members
|
Statement of Age of Subject |
I state that I am at least 18 years of age, in good physical health, and wish to participate in a focus group being conducted by Lori Roche, HRSA Division of Site and Clinician Recruitment, 5600 Fishers Lane, 8A-55, Rockville, MD 20857.
|
Purpose |
The purpose of this focus group is to help HRSA obtain feedback from current and future healthcare providers on materials being developed for a program that advance the provision of healthcare in medically underserved areas. The results will help refine and improve the materials, which will be used to promote the program and recruit new clinicians and program sites.
|
Procedures
|
Respondents will participate in a focus group discussion with up to 9 participants and led by a trained facilitator. The total time involved, including instructions, will be no more than 90 minutes.
|
Privacy |
All information collected is private. I understand that the information I provide will be grouped with data others provide for the purpose of reporting and presentation and that my name will not be used. I understand that the discussion will be audio taped, but my voice will not be played to others besides the research team without my written permission.
|
Risks |
I understand that the risks of my participation are expected to be minimal in nature.
|
Benefits, Freedom to Withdraw, & Ability to Ask Questions |
I understand that this focus group is not designed to help me personally but to help the investigators learn about preferences for program and communications materials. I am free to ask questions or withdraw from participation at any time and without penalty.
|
Contact Information for Sponsoring Agency |
Name: Lori Roche Position: Program Director Telephone: 301-443-0652 Email: [email protected]
|
Printed Name of Focus Group Participant _____________________________
Signature of Focus Group Participant ________________________________
Date______________________
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | anschwartz |
| File Modified | 0000-00-00 |
| File Created | 2021-02-03 |
© 2026 OMB.report | Privacy Policy