Standard Form Instructions
Att_INSTRUCTIONS FOR STANDARD FORMS.docx
US-Brazil Higher Education Consortia Program Application Guidelines
Standard Form Instructions
OMB: 1840-0761
INSTRUCTIONS FOR STANDARD FORMS
● Application for Federal Assistance (SF 424)
● Department of Education Supplemental Form for the SF 424
● Department of Education Budget Summary Form (ED 524)
● Disclosure of Lobbying Activities (SF-LLL)
● Survey Instructions on Ensuring Equal Opportunity for Applicants
INSTRUCTIONS FOR THE SF-424
INSTRUCTIONS FOR DEPARTMENT OF EDUCATION SUPPLEMENTAL INFORMATION FOR SF 424
1. Project Director. Name, address, telephone and fax numbers, and e-mail address of the person to be contacted on matters involving this application.
2. Novice Applicant. Check “Yes” or “No” only if assistance is being requested under a program that gives special consideration to novice applicants. Otherwise, leave blank.
Check “Yes” if you meet the requirements for novice applicants specified in the regulations in 34 CFR 75.225 and included on the attached page entitled “Definitions for Department of Education Supplemental Information for SF 424.” By checking “Yes” the applicant certifies that it meets these novice applicant requirements. Check “No” if you do not meet the requirements for novice applicants.
3. Human Subjects Research. (See I. A. “Definitions” in attached page entitled “Definitions for Department of Education Supplemental Information For SF 424.”)
If Not Human Subjects Research. Check “No” if research activities involving human subjects are not planned at any time during the proposed project period. The remaining parts of Item 3 are then not applicable.
If Human Subjects Research. Check “Yes” if research activities involving human subjects are planned at any time during the proposed project period, either at the applicant organization or at any other performance site or collaborating institution. Check “Yes” even if the research is exempt from the regulations for the protection of human subjects. (See I. B. “Exemptions” in attached page entitled “Definitions for Department of Education Supplemental Information For SF 424.”)
If Human Subjects Research is Exempt from the Human Subjects Regulations. Check “Yes” if all the research activities proposed are designated to be exempt from the regulations. Insert the exemption number(s) corresponding to one or more of the six exemption categories listed in I. B. “Exemptions.” In addition, follow the instructions in II. A. “Exempt Research Narrative” in the attached page entitled “Definitions for Department of Education Supplemental Information For SF 424.”
If Human Subjects Research is Not Exempt from Human Subjects Regulations. Check “No” if some or all of the planned research activities are covered (not exempt). In addition, follow the instructions in II. B. “Nonexempt Research Narrative” in the page entitled “Definitions for Department of Education Supplemental Information For SF 424
Human Subjects Assurance Number. If the applicant has an approved Federal Wide (FWA) on file with the Office for Human Research Protections (OHRP), U.S. Department of Health and Human Services, that covers the specific activity, insert the number in the space provided. If the applicant does not have an approved assurance on file with OHRP, enter “None.” In this case, the applicant, by signature on the SF-424, is declaring that it will comply with 34 CFR 97 and proceed to obtain the human subjects assurance upon request by the designated ED official. If the application is recommended/selected for funding, the designated ED official will request that the applicant obtain the assurance within 30 days after the specific formal request.
Note about Institutional Review Board Approval. ED does not require certification of Institutional Review Board approval with the application. However, if an application that involves non-exempt human subjects research is recommended/selected for funding, the designated ED official will request that the applicant obtain and send the certification to ED within 30 days after the formal request.
DEFINITIONS FOR DEPARTMENT OF EDUCATION SUPPLEMENTAL INFORMATION FOR SF 424
(Attachment to Instructions for Supplemental Information for SF 424)
Definitions:
Novice Applicant (See 34 CFR 75.225). For discretionary grant programs under which the Secretary gives special consideration to novice applications, a novice applicant means any applicant for a grant from ED that—
Has never received a grant or subgrant under the program from which it seeks funding;
Has never been a member of a group application, submitted in accordance with 34 CFR 75.127-75.129, that received a grant under the program from which it seeks funding; and
Has not had an active discretionary grant from the Federal government in the five years before the deadline date for applications under the program. For the purposes of this requirement, a grant is active until the end of the grant’s project or funding period, including any extensions of those periods that extend the grantee’s authority to obligate funds.
In the case of a group application submitted in accordance with 34 CFR 75.127-75.129, a group includes only parties that meet the requirements listed above.
PROTECTION OF HUMAN SUBJECTS IN RESEARCH
I. Definitions and Exemptions
A. Definitions.
A research activity involves human subjects if the activity is research, as defined in the Department’s regulations, and the research activity will involve use of human subjects, as defined in the regulations.
—Research
The ED Regulations for the Protection of Human Subjects, Title 34, Code of Federal Regulations, Part 97, define research as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” If an activity follows a deliberate plan whose purpose is to develop or contribute to generalizable knowledge it is research. Activities which meet this definition constitute research whether or not they are conducted or supported under a program that is considered research for other purposes. For example, some demonstration and service programs may include research activities.
—Human Subject
The regulations define human subject as “a living individual about whom an investigator (whether professional or student) conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information.” (1) If an activity involves obtaining information about a living person by manipulating that person or that person’s environment, as might occur when a new instructional technique is tested, or by communicating or interacting with the individual, as occurs with surveys and interviews, the definition of human subject is met. (2) If an activity involves obtaining private information about a living person in such a way that the information can be linked to that individual (the identity of the subject is or may be readily determined by the investigator or associated with the information), the definition of human subject is met. [Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a school health record).]
B. Exemptions.
Research activities in which the only involvement of human subjects will be in one or more of the following six categories of exemptions are not covered by the regulations:
(1) Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (a) research on regular and special education instructional strategies, or (b) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods.
(2) Research involving the use of educational tests cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (a) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (b) any disclosure of the human subjects’ responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation. If the subjects are children, exemption 2 applies only to research involving educational tests and observations of research involving educational tests and observations of public behavior when the investigator(s) do not participate in the activities being observed. Exemption 2 does not apply if children are surveyed or interviewed or if the research involves observation of public behavior and the investigator(s) participate in the activities being observed. [Children are defined as persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law or jurisdiction in which the research will be conducted.]
(3) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior that is not exempt under section (2) above, if the human subjects are elected or appointed public officials or candidates for public office; or federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter.
(4) Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
(5) Research and demonstration projects which are conducted by or subject to the approval of department or agency heads, and which are designed to study, evaluate, or otherwise examine: (a) public benefit or service programs; (b) procedures for obtaining benefits or services under those programs; (c) possible changes in or alternatives to those programs or procedures; or (d) possible changes in methods or levels of payment for benefits or services under those programs.
(6) Taste and food quality evaluation and consumer acceptance studies, (a) if wholesome foods without additives are consumed or (b) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture. II. Instructions for Exempt and Nonexempt Human Subjects Research Narratives If the applicant marked “Yes” for Item 3 of Department of Education Supplemental Information for SF 424, the applicant must provide a human subjects “exempt research” or “nonexempt research” narrative. Insert the narrative(s) in the space provided. If you have multiple projects and need to provide more than one narrative, be sure to label each set of responses as to the project they address.
A. Exempt Research Narrative.
If you marked “Yes” for item 3 a. and designated exemption numbers(s), provide the “exempt research” narrative. The narrative must contain sufficient information about the involvement of human subjects in the proposed research to allow a determination by ED that the designated exemption(s) are appropriate. The narrative must be succinct.
B. Nonexempt Research Narrative.
If you marked “No” for item 3 a. you must provide the “nonexempt research” narrative. The narrative must address the following seven points. Although no specific page limitation applies to this section of the application, be succinct.
(1) Human Subjects Involvement and Characteristics: Provide a detailed description of the proposed involvement of human subjects. Describe the characteristics of the subject population, including their anticipated number, age range, and health status. Identify the criteria for inclusion or exclusion of any subpopulation. Explain the rationale for the involvement of special classes of subjects, such as children, children with disabilities, adults with disabilities, persons with mental disabilities, pregnant women, prisoners, institutionalized individuals, or others who are likely to be vulnerable.
(2) Sources of Materials: Identify the sources of research material obtained from individually identifiable living human subjects in the form of specimens, records, or data. Indicate whether the material or data will be obtained specifically for research purposes or whether use will be made of existing specimens, records, or data.
(3) Recruitment and Informed Consent: Describe plans for the recruitment of subjects and the consent procedures to be followed. Include the circumstances under which consent will be sought and obtained, who will seek it, the nature of the information to be provided to prospective subjects, and the method of documenting consent. State if the Institutional Review Board (IRB) has authorized a modification or waiver of the elements of consent or the requirement for documentation of consent.
(4) Potential Risks: Describe potential risks (physical, psychological, social, legal, or other) and assess their likelihood and seriousness. Where appropriate, describe alternative treatments and procedures that might be advantageous to the subjects.
(5) Protection Against Risk: Describe the procedures for protecting against or minimizing potential risks, including risks to confidentiality, and assess their likely effectiveness. Where appropriate, discuss provisions for ensuring necessary medical or professional intervention in the event of adverse effects to the subjects. Also, where appropriate, describe the provisions for monitoring the data collected to ensure the safety of the subjects.
(6) Importance of the Knowledge to be Gained: Discuss the importance of the knowledge gained or to be gained as a result of the proposed research. Discuss why the risks to subjects are reasonable in relation to the anticipated benefits to subjects and in relation to the importance of the knowledge that may reasonably be expected to result.
(7) Collaborating Site(s): If research involving human subjects will take place at collaborating site(s) or other performance site(s), name the sites and briefly describe their involvement or role in the research.
Copies of the Department of Education’s Regulations for the Protection of Human Subjects, 34 CFR Part 97 and other pertinent materials on the protection of human subjects in research are available from the Grants Policy and Oversight Staff, Office of the Chief Financial Officer, U.S. Department of Education, Washington, DC 20202-4250, telephone: (202) 245-6120, and on the U.S. Department of Education’s Protection of Human Subjects in Research Web Site: http://www.ed.gov/about/offices/list/OCFO/humansub.html
NOTE: The State Applicant Identifier on the SF 424 is for State Use only. Please complete it on the OMB Standard 424 in the upper right corner of the form (if applicable).
INSTRUCTIONS FOR ED 524
General Instructions
This
form is used to apply to individual U.S. Department of Education (ED)
discretionary grant programs. Unless directed otherwise, provide the
same budget information for each year of the multi-year funding
request. Pay attention to applicable program specific instructions,
if attached. Please consult with your Business Office prior to
submitting this form.
Section A - Budget Summary
U.S. Department of Education Funds
All applicants must complete Section A and provide a breakdown by the applicable budget categories shown in lines 1-11.
Lines 1-11, columns (a)-(e): For each project year for which funding is requested, show the total amount requested for each applicable budget category.
Lines 1-11, column (f): Show the multi-year total for each budget category. If funding is requested for only one project year, leave this column blank.
Line 12, columns (a)-(e): Show the total budget request for each project year for which funding is requested.
Line 12, column (f): Show the total amount requested for all project years. If funding is requested for only one year, leave this space blank.
Indirect Cost Information:
If you are requesting reimbursement for indirect costs on line 10, this information is to be completed by your Business Office. (1): Indicate whether or not your organization has an Indirect Cost Rate Agreement that was approved by the Federal government. (2): If you checked “yes” in (1), indicate in (2) the beginning and ending dates covered by the Indirect Cost Rate Agreement. In addition, indicate whether ED or another Federal agency (Other) issued the approved agreement. If you check “Other,” specify the name of the Federal agency that issued the approved agreement. (3): If you are applying for a grant under a Restricted Rate Program (34 CFR 75.563 or 76.563), indicate whether you are using a restricted indirect cost rate that is included on your approved Indirect Cost Rate Agreement or whether you are using a restricted indirect cost rate that complies with 34 CFR 76.564(c)(2). Note: State or Local government agencies may not use the provision for a restricted indirect cost rate specified in 34 CFR 76.564(c)(2). Check only one response. Leave blank, if this item is not applicable.
Section B - Budget Summary
Non-Federal Funds
If you are required to provide or volunteer to provide matching funds or other non-Federal resources to the project, these should be shown for each applicable budget category on lines 1‑11 of Section B.
Lines 1-11, columns (a)-(e): For each project year, for which matching funds or other contributions are provided, show the total contribution for each applicable budget category.
Lines 1-11, column (f): Show the multi-year total for each budget category. If non-Federal contributions are provided for only one year, leave this column blank.
Line 12, columns (a)-(e): Show the total matching or other contribution for each project year.
Line 12, column (f): Show the total amount to be contributed for all years of the multi-year project. If non-Federal contributions are provided for only one year, leave this space blank.
Section C - Budget Narrative [Attach separate sheet(s)]
Pay
attention to applicable program specific instructions,
if
attached.
Provide an itemized budget breakdown, and justification by project year, for each budget category listed in Sections A and B. For grant projects that will be divided into two or more separately budgeted major activities or sub-projects, show for each budget category of a project year the breakdown of the specific expenses attributable to each sub-project or activity.
If applicable to this program, provide the rate and base on which fringe benefits are calculated.
If you are requesting reimbursement for indirect costs on line 10, this information is to be completed by your Business Office. Specify the estimated amount of the base to which the indirect cost rate is applied and the total indirect expense. Depending on the grant program to which you are applying and/or your approved Indirect Cost Rate Agreement, some direct cost budget categories in your grant application budget may not be included in the base and multiplied by your indirect cost rate. For example, you must multiply the indirect cost rates of “Training grants" (34 CFR 75.562) and grants under programs with “Supplement not Supplant” requirements ("Restricted Rate" programs) by a “modified total direct cost” (MTDC) base (34 CFR 75.563 or 76.563). Please indicate which costs are included and which costs are excluded from the base to which the indirect cost rate is applied.
When calculating indirect costs (line 10) for "Training grants" or grants under "Restricted Rate" programs, you must refer to the information and examples on ED’s website at: http://www.ed.gov/fund/grant/apply/appforms/appforms.html.
You may also contact (202) 377-3838 for additional information regarding calculating indirect cost rates or general indirect cost rate information.
Provide other explanations or comments you deem necessary.
Please note—your Budget Narrative will not be counted as part of the 20-page application
Instructions for Standard Forms
Instructions for the SF-424
Instructions for the Department of Education Supplemental Form for the SF 424
Definitions for the Department of Education Supplemental Form for the SF 424
Instructions for Completion of Disclosure of Lobbying Activities (SF-LLL)
Survey Instructions on Ensuring Equal Opportunity for Applicants
Instructions for the SF-424
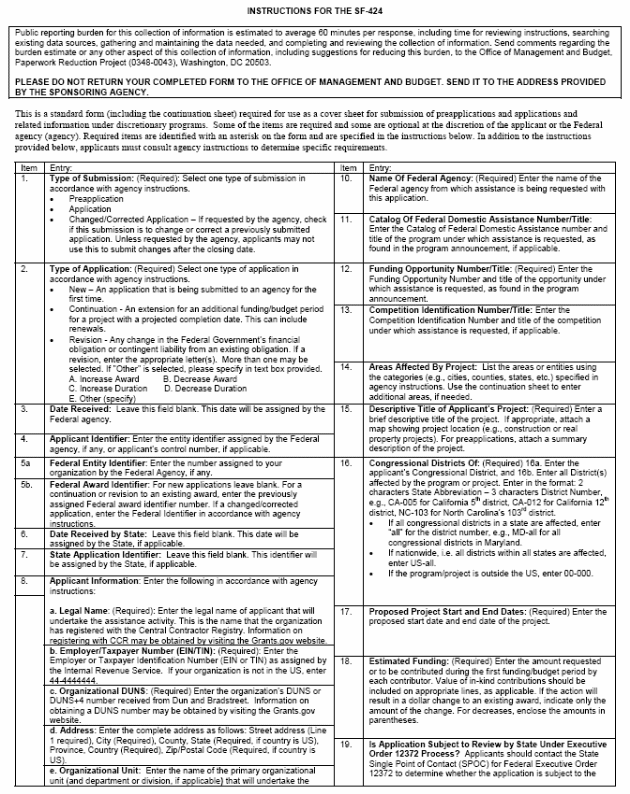
Public reporting burden for this collection of information is estimated to average 60 minutes per response, including time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding the burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to the Office of Management and Budget, Paperwork Reduction Project (0348-0043), Washington, DC 20503.
PLEASE DO NOT RETURN YOUR COMPLETED FORM TO THE OFFICE OF MANAGEMENT AND
BUDGET. SEND IT TO THE ADDRESS PROVIDED BY THE SPONSORING AGENCY.
This is a standard form (including the continuation sheet) required for use as a cover sheet for submission of pre-applications and applications and related information under discretionary programs. Some of the items are required and some are optional at the discretion of the applicant or the Federal agency (agency). Required items are identified with an asterisk on the form and are specified in the instructions below. In addition to the instructions provided below, applicants must consult agency instructions to determine specific requirements.
Item |
Entry: |
Item |
Entry: |
|
1. |
Type of Submission: (Required): Select one type of submission in accordance with agency instructions.
|
10. |
Name Of Federal Agency: (Required) Enter the name of the Federal agency from which assistance is being requested with this application. |
|
11. |
Catalog Of Federal Domestic Assistance Number/Title: Enter the Catalog of Federal Domestic Assistance number and title of the program under which assistance is requested, as found in the program announcement, if applicable.
|
|||
2. |
Type of Application: (Required) Select one type of application in accordance with agency instructions.
A. Increase Award B. Decrease Award C. Increase Duration D. Decrease Duration E. Other (specify) |
12. |
Funding Opportunity Number/Title: (Required) Enter the Funding Opportunity Number and title of the opportunity under which assistance is requested, as found in the program announcement. |
|
13. |
Competition Identification Number/Title: Enter the Competition Identification Number and title of the competition under which assistance is requested, if applicable. |
|||
14. |
Areas Affected By Project: List the areas or entities using the categories (e.g., cities, counties, states, etc.) specified in agency instructions. Use the continuation sheet to enter additional areas, if needed. |
|||
3. |
Date Received: Leave this field blank. This date will be assigned by the Federal agency.
|
15. |
Descriptive Title of Applicant’s Project: (Required) Enter a brief descriptive title of the project. If appropriate, attach a map showing project location (e.g., construction or real property projects). For pre-applications, attach a summary description of the project. |
|
4. |
Applicant Identifier: Enter the entity identifier assigned by the Federal agency, if any, or applicant’s control number, if applicable. |
|||
5a |
Federal Entity Identifier: Enter the number assigned to your organization by the Federal Agency, if any. |
16. |
Congressional Districts Of: (Required) 16a. Enter the applicant’s Congressional District, and 16b. Enter all District(s) affected by the program or project. Enter in the format: 2 characters State Abbreviation – 3 characters District Number, e.g., CA-005 for California 5thth district, CA-012 for California 12th district, NC-103 for North Carolina’s 103rd district.
|
|
5b. |
Federal Award Identifier: For new applications leave blank. For a continuation or revision to an existing award, enter the previously assigned Federal award identifier number. If a changed/corrected application, enter the Federal Identifier in accordance with agency instructions. |
|||
6. |
Date Received by State: Leave this field blank. This date will be assigned by the State, if applicable. |
|||
7. |
State Application Identifier: Leave this field blank. This identifier will be assigned by the State, if applicable. |
|||
8. |
Applicant Information: Enter the following in accordance with agency instructions:
a. Legal Name: (Required): Enter the legal name of applicant that will undertake the assistance activity. This is the name that the organization has registered with the Central Contractor Registry. Information on registering with CCR may be obtained by visiting the Grants.gov website. |
|||
|
||||
17. |
Proposed Project Start and End Dates: (Required) Enter the proposed start date and end date of the project. |
|||
b. Employer/Taxpayer Number (EIN/TIN): (Required): Enter the Employer or Taxpayer Identification Number (EIN or TIN) as assigned by the Internal Revenue Service. If your organization is not in the US, enter 44-4444444. |
||||
18. |
Estimated Funding: (Required) Enter the amount requested or to be contributed during the first funding/budget period by each contributor. Value of in-kind contributions should be included on appropriate lines, as applicable. If the action will result in a dollar change to an existing award, indicate only the amount of the change. For decreases, enclose the amounts in parentheses. |
|||
c. Organizational DUNS: (Required) Enter the organization’s DUNS or DUNS+4 number received from Dun and Bradstreet. Information on obtaining a DUNS number may be obtained by visiting the Grants.gov website. |
||||
d. Address: Enter the complete address as follows: Street address (Line 1 required), City (Required), County, State (Required, if country is US), Province, Country (Required), Zip/Postal Code (Required, if country is US). |
||||
19. |
Is Application Subject to Review by State Under Executive Order 12372 Process? Applicants should contact the State Single Point of Contact (SPOC) for Federal Executive Order 12372 to determine whether the application is subject to the State intergovernmental review process. Select the appropriate box. If “a.” is selected, enter the date the application was submitted to the State |
|||
e. Organizational Unit: Enter the name of the primary organizational unit (and department or division, if applicable) that will undertake the assistance activity, if applicable. |
||||
f. Name and contact information of person to be contacted on matters involving this application: Enter the name (First and last name required), organizational affiliation (if affiliated with an organization other than the applicant organization), telephone number (Required), fax number, and email address (Required) of the person to contact on matters related to this application. |
||||
20. |
Is the Applicant Delinquent on any Federal Debt? (Required) Select the appropriate box. This question applies to the applicant organization, not the person who signs as the authorized representative. Categories of debt include delinquent audit disallowances, loans and taxes.
If yes, include an explanation on the continuation sheet. |
|||
9. |
Type of Applicant: (Required) Select up to three applicant type(s) in accordance with agency instructions. |
21. |
Authorized Representative: (Required) To be signed and dated by the authorized representative of the applicant organization. Enter the name (First and last name required) title (Required), telephone number (Required), fax number, and email address (Required) of the person authorized to sign for the applicant. A copy of the governing body’s authorization for you to sign this application as the official representative must be on file in the applicant’s office. (Certain Federal agencies may require that this authorization be submitted as part of the application.)
|
|
|
|
|||
|
|
|||
Instructions for the Department of Education Supplemental Information for SF 424
Project Director. Name, address, telephone and fax numbers, and e-mail address of the person to be contacted on matters involving this application.
2. Novice Applicant. Check “Yes” or “No” only if assistance is being requested under a program that gives special consideration to novice applicants. Otherwise, leave blank.
Check “Yes” if you meet the requirements for novice applicants specified in the regulations in 34 CFR 75.225 and included on the attached page entitled “Definitions for Department of Education Supplemental Information for SF 424.” By checking “Yes” the applicant certifies that it meets these novice applicant requirements. Check “No” if you do not meet the requirements for novice applicants.
3. Human Subjects Research. (See I. A. “Definitions” in attached page entitled “Definitions for Department of Education Supplemental Information For SF 424.”)
If Not Human Subjects Research. Check “No” if research activities involving human subjects are not planned at any time during the proposed project period. The remaining parts of Item 3 are then not applicable.
If Human Subjects Research. Check “Yes” if research activities involving human subjects are planned at any time during the proposed project period, either at the applicant organization or at any other performance site or collaborating institution. Check “Yes” even if the research is exempt from the regulations for the protection of human subjects. (See I. B. “Exemptions” in attached page entitled “Definitions for Department of Education Supplemental Information For SF 424.”)
3a. If Human Subjects Research is Exempt from the Human Subjects Regulations. Check “Yes” if all the research activities proposed are designated to be exempt from the regulations. Insert the exemption number(s) corresponding to one or more of the six exemption categories listed in I. B. “Exemptions.” In addition, follow the instructions in II. A. “Exempt Research Narrative” in the attached page entitled “Definitions for Department of Education Supplemental Information For SF 424.”
3a. If Human Subjects Research is Not Exempt from Human Subjects Regulations. Check “No” if some or all of the planned research activities are covered (not exempt). In
addition, follow the instructions in II. B. “Nonexempt Research Narrative” in the page entitled “Definitions for Department of Education Supplemental Information For SF 424
3a. Human Subjects Assurance Number. If the applicant has an approved Federal Wide (FWA) on file with the Office for Human Research Protections (OHRP), U.S. Department of Health and Human Services, that covers the specific activity, insert the number in the space provided. If the applicant does not have an approved assurance on file with OHRP, enter “None.” In this case, the applicant, by signature on the SF-424, is declaring that it will comply with 34 CFR 97 and proceed to obtain the human subjects assurance upon request by the designated ED official. If the application is recommended/selected for funding, the designated ED official will request that the applicant obtain the assurance within 30 days after the specific formal request.
Note about Institutional Review Board Approval. ED does not require certification of Institutional Review Board approval with the application. However, if an application that involves non-exempt human subjects research is recommended/selected for funding, the designated ED official will request that the applicant obtain and send the certification to ED within 30 days after the formal request.
Paperwork Burden Statement. According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless such collection displays a valid OMB control number. The valid OMB control number for this information collection is 1890-0017. The time required to complete this information collection is estimated to average between 15 and 45 minutes per response, including the time to review instructions, search existing data resources, gather the data needed, and complete and review the information collection. If you have any comments concerning the accuracy of the estimate(s) or suggestions for improving this form, please write to: U.S. Department of Education, Washington, DC 20202-4700. If you have comments or concerns regarding the status of your individual submission of this form write directly to: Joyce I. Mays, Application Control Center, U.S. Department of Education, Potomac Center Plaza, 550 12th Street, S.W. Room 7076, Washington, DC 20202-4260.
Definitions for Department of Education Supplemental Information for SF 424
(Attachment to Instructions for Supplemental Information for SF 424)
Definitions:
Novice Applicant (See 34 CFR 75.225). For discretionary grant programs under which the Secretary gives special consideration to novice applications, a novice applicant means any applicant for a grant from ED that—
Has never received a grant or sub-grant under the program from which it seeks funding;
Has never been a member of a group application, submitted in accordance with 34 CFR 75.127-75.129, that received a grant under the program from which it seeks funding; and
Has not had an active discretionary grant from the Federal government in the five years before the deadline date for applications under the program. For the purposes of this requirement, a grant is active until the end of the grant’s project or funding period, including any extensions of those periods that extend the grantee’s authority to obligate funds.
In the case of a group application submitted in accordance with 34 CFR 75.127-75.129, a group includes only parties that meet the requirements listed above.
PROTECTION OF HUMAN SUBJECTS IN RESEARCH
I. Definitions and Exemptions
A. Definitions.
A research activity involves human subjects if the activity is research, as defined in the Department’s regulations, and the research activity will involve use of human subjects, as defined in the regulations.
—Research
The ED Regulations for the Protection of Human Subjects, Title 34, Code of Federal Regulations, Part 97, define research as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.” If an activity follows a deliberate plan whose purpose is to develop or contribute to generalizable knowledge it is research. Activities, which meet this definition constitute research whether or not they are conducted or supported under a program that is considered research for other purposes. For example, some demonstration and service programs may include research activities.
—Human Subject
The regulations define human subject as “a living individual about whom an investigator (whether professional or student) conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information.” (1) If an activity involves obtaining information about a living person by manipulating that person or that person’s environment, as might occur when a new instructional technique is tested, or by communicating or interacting with the individual, as occurs with surveys and interviews, the definition of human subject is met. (2) If an activity involves obtaining private information about a living person in such a way that the information can be linked to that individual (the identity of the subject is or may be readily determined by the investigator or associated with the information), the definition of human subject is met. [Private information includes information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a school health record).]
Research activities in which the only involvement of human subjects will be in one or more of the following six categories of exemptions are not covered by the regulations:
(1) Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (a) research on regular and special education instructional strategies, or (b) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods.
(2) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (a) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (b) any disclosure of the human subjects’ responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation. If the subjects are children, exemption 2 applies only to research involving educational tests and observations of public behavior when the investigator(s) do not participate in the activities being observed. Exemption 2 does not apply if children are surveyed or interviewed or if the research involves observation of public behavior and the investigator(s) participate in the activities being observed. [Children are defined as persons who have not attained the legal age for consent to treatments or procedures involved in the research, under the applicable law or jurisdiction in which the research will be conducted.]
(3) Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior that is not exempt under section (2) above, if the human subjects are elected or appointed public officials or candidates for public office; or federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter.
(4) Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
(5) Research and demonstration projects which are conducted by or subject to the approval of department or agency heads, and which are designed to study, evaluate, or otherwise examine: (a) public benefit or service programs; (b) procedures for obtaining benefits or services under those programs; (c) possible changes in or alternatives to those programs or procedures; or (d) possible changes in methods or levels of payment for benefits or services under those programs.
(6) Taste and food quality evaluation and consumer acceptance studies, (a) if wholesome foods without additives are consumed or (b) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture.
II. Instructions for Exempt and Nonexempt Human Subjects Research Narratives
If the applicant marked “Yes” for Item 3 of Department of Education Supplemental Information for SF 424, the applicant must provide a human subjects “exempt research” or “nonexempt research” narrative. Insert the narrative(s) in the space provided. If you have multiple projects and need to provide more than one narrative, be sure to label each set of responses as to the project they address.
A. Exempt Research Narrative.
If you marked “Yes” for item 3 a. and designated exemption numbers(s), provide the “exempt research” narrative. The narrative must contain sufficient information about the involvement of human subjects in the proposed research to
allow a determination by ED that the designated exemption(s) are appropriate. The narrative must be succinct.
B. Nonexempt Research Narrative.
If you marked “No” for item 3 a. you must provide the “nonexempt research” narrative. The narrative must address the following seven points. Although no specific page limitation applies to this section of the application, be succinct.
(1) Human Subjects Involvement and Characteristics: Provide a detailed description of the proposed involvement of human subjects. Describe the characteristics of the subject population, including their anticipated number, age range, and health status. Identify the criteria for inclusion or exclusion of any subpopulation. Explain the rationale for the involvement of special classes of subjects, such as children, children with disabilities, adults with disabilities, persons with mental disabilities, pregnant women, prisoners, institutionalized individuals, or others who are likely to be vulnerable
(2) Sources of Materials: Identify the sources of research material obtained from individually identifiable living human subjects in the form of specimens, records, or data. Indicate whether the material or data will be obtained specifically for research purposes or whether use will be made of existing specimens, records, or data.
(3) Recruitment and Informed Consent: Describe plans for the recruitment of subjects and the consent procedures to be followed. Include the circumstances under which consent will be sought and obtained, who will seek it, the nature of the information to be provided to prospective subjects, and the method of documenting consent. State if the Institutional Review Board (IRB) has authorized a modification or waiver of the elements of consent or the requirement for documentation of consent.
(4) Potential Risks: Describe potential risks (physical, psychological, social, legal, or other) and assess their likelihood and seriousness. Where appropriate, describe alternative treatments and procedures that might be advantageous to the subjects.
(5) Protection Against Risk: Describe the procedures for protecting against or minimizing potential risks, including risks to confidentiality, and assess their likely effectiveness. Where appropriate, discuss provisions for ensuring necessary medical or professional intervention in the event of adverse effects to the subjects. Also, where appropriate, describe the provisions for monitoring the data collected to ensure the safety of the subjects.
(6) Importance of the Knowledge to be Gained: Discuss the importance of the knowledge gained or to be gained as a result of the proposed research. Discuss why the risks to subjects are reasonable in relation to the anticipated benefits to subjects and in relation to the importance of the knowledge that may reasonably be expected to result.
(7) Collaborating Site(s): If research involving human subjects will take place at collaborating site(s) or other performance site(s), name the sites and briefly describe their involvement or role in the research.
Copies of the Department of Education’s Regulations for the Protection of Human Subjects, 34 CFR Part 97 and other pertinent materials on the protection of human subjects in research are available from the Grants Policy and Oversight Staff, Office of the Chief Financial Officer, U.S. Department of Education, Washington, DC 20202-4250, telephone: (202) 245-6120, and on the U.S. Department of Education’s Protection of Human Subjects in Research Web Site: http://www.ed.gov/about/offices/list/OCFO/humansub.html
NOTE: The State Applicant Identifier on the SF 424 is for State Use only. Please complete it on the OMB Standard 424 in the upper right corner of the form (if applicable).
Instructions for Completion of SF-LLL, Disclosure of Lobbying Activities
This disclosure form shall be completed by the reporting entity, whether sub-awardee or prime Federal recipient, at the initiation or receipt of a covered Federal action, or a material change to a previous filing, pursuant to title 31 U.S.C. section 1352. The filing of a form is required for each payment or agreement to make payment to any lobbying entity for influencing or attempting to influence an officer or employee of any agency, a Member of Congress, an officer or employee of Congress, or an employee of a Member of Congress in connection with a covered Federal action. Complete all items that apply for both the initial filing and material change report. Refer to the implementing guidance published by the Office of Management and Budget for additional information.
1. Identify the type of covered Federal action for which lobbying activity is and/or has been secured to influence the outcome of a covered Federal action.
2. Identify the status of the covered Federal action.
3. Identify the appropriate classification of this report. If this is a follow-up report caused by a material change to the information previously reported, enter the year and quarter in which the change occurred. Enter the date of the last previously submitted report by this reporting entity for this covered Federal action.
4. Enter the full name, address, city, State and zip code of the reporting entity. Include Congressional District, if known. Check the appropriate classification of the reporting entity that designates if it is, or expects to be, a prime or sub-award recipient. Identify the tier of the sub-awardee, e.g., the first sub-awardee of the prime is the 1st tier. Sub-awards include but are not limited to subcontracts, sub-grants and contract awards under grants.
5. If the organization filing the report in item 4 checks “Sub-awardee,” then enter the full name, address, city, State and zip code of the prime Federal recipient. Include Congressional District, if known.
6. Enter the name of the federal agency making the award or loan commitment. Include at least one organizational level below agency name, if known. For example, Department of Transportation, United States Coast Guard.
7. Enter the Federal program name or description for the covered Federal action (item 1). If known, enter the full Catalog of Federal Domestic Assistance (CFDA) number for grants, cooperative agreements, loans, and loan commitments.
8. Enter the most appropriate Federal identifying number available for the Federal action identified in item 1 (e.g., Request for Proposal (RFP) number; Invitations for Bid (IFB) number; grant announcement number; the contract, grant, or loan award number; the application/proposal control number assigned by the Federal agency). Included prefixes, e.g., “RFP-DE-90-001.”
9. For a covered Federal action where there has been an award or loan commitment by the Federal agency, enter the Federal amount of the award/loan commitment for the prime entity identified in item 4 or 5.
10. (a) Enter the full name, address, city, State and zip code of the lobbying registrant under the Lobbying Disclosure Act of 1995 engaged by the reporting entity identified in item 4 to influence the covered Federal action.
(b) Enter the full names of the individual(s) performing services, and include full address if different from 10(a). Enter Last Name, First Name, and Middle Initial (MI).
11. The certifying official shall sign and date the form, print his/her name, title, and telephone number.
According to the Paperwork Reduction Act, as amended, no persons are required to respond to a collection of information unless it displays a valid OMB control Number. The valid OMB control number for this information collection is OMB No. 0348-0046. Public reporting burden for this collection of information is estimated to average 10 minutes per response, including time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding the burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to the Office of Management and Budget, Paperwork Reduction Project (0348-0046), Washington, DC 20503
Survey Instructions on Ensuring Equal Opportunity for Applicants
Provide the applicant’s (organization) name and DUNS number and the grant name and CFDA number.
Self-explanatory.
Self-identify.
Self-identify.
4. 501(c)(3) status is a legal designation provided on application to the Internal Revenue Service by eligible organizations. Some grant programs may require nonprofit applicants to have 501(c)(3) status. Other grant programs do not.
5. Self-explanatory.
6. For example, two part-time employees who each work half-time equal one full-time equivalent employee. If the applicant is a local affiliate of a national organization, the responses to survey questions 2 and 3 should reflect the staff and budget size of the local affiliate.
7. Annual budget means the amount of money your organization spends each year on all of its activities.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Authorised User |
| File Modified | 0000-00-00 |
| File Created | 2021-02-03 |
© 2026 OMB.report | Privacy Policy