eSTAMP Parts 1-2 Protocol IRB Clean 12
eSTAMP Parts 1-2 Protocol IRB Clean 12.11.2012.docx
Formative Research and Tool Development
eSTAMP Parts 1-2 Protocol IRB Clean 12
OMB: 0920-0840
Title: Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
Parts 1 and 2: Preliminary studies of qualitative and cognitive assessment of materials, packaging and instructions and user proficiency
Investigators: A.D. McNaghten, PhD, MHSA
Patrick Sullivan, DVM, PhD
Brian Mustanski, PhD
Michael Newcomb, PhD
Alexandra Ricca, MPH
Akshay Sharma, MBBS, MPH
Craig Sineath, MPH
Sponsor: US Centers for Disease Control and Prevention
Date: December 11, 2012
Parts 1 and 2: Preliminary studies of qualitative and cognitive assessment of materials, packaging and instructions and user proficiency
Background
To evaluate the acceptability, use and effectiveness of HIV self-test kits among men who have sex with men (MSM) in cities with high HIV prevalence, the study “Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities”, known as eSTAMP, is being conducted in several US cities. The study will be conducted in four parts; each part will be independent and will provide information to develop and implement the next part of the study. Part 1 will assess willingness to participate in the study and the acceptability of home testing, and will evaluate the materials, packaging and instructions for conducting self-test activities through focus groups and individual in-depth interviews to obtain feedback and reactions from participants about materials proposed for Parts 2, 3 and 4. Part 2 will evaluate the use of the self-test materials and dried blood spot (DBS) collection by participants under controlled conditions, assessing the extent to which untrained users can proficiently conduct testing procedures with the use of provided printed and video instructions. Participant testing procedures will be observed by trained HIV counselors who will also verify participants’ results. For quality control purposes, Part 2 will include MSM known to be HIV-positive. Part 3 will be an evaluation of the performance of the self-test kits by the proposed study population in real world settings by sending participants a package containing test kits and a DBS specimen collection kit with packaging for specimen transport, then comparing the user-administered and interpreted rapid HIV self-test results to a standard of a laboratory-administered enzyme immunoassay (EIA). Part 4 will evaluate the use and effectiveness of self-test kits as a public health strategy for increasing testing among MSM by determining whether the distribution of user-administered and interpreted HIV self-tests to HIV-negative or HIV status unknown MSM results in a higher frequency of MSM HIV testing at least 3 times in a 12 month period compared to a standard of referring MSM to testing locations. A secondary aim of Part 4 is to evaluate the extent to which MSM (both HIV-negative and HIV-positive) distribute HIV self-test kits to their social and sexual networks.
This protocol outlines the procedures for Parts 1 and 2, which will provide information on whether users of self-administered HIV rapid test kits understand the instructions for conducting the rapid tests proposed for use in later stages of the study, and if users can correctly administer and interpret the HIV self-tests. Parts 1 and 2 also seek to obtain feedback for improving the rapid test instructions, and to determine how to modify the packaging of materials to increase the likelihood that test kit recipients will use the tests they receive and distribute test kits within their social and sexual networks.
Research Team
The research team is composed of five researchers from the Rollins School of Public Health, Emory University, Atlanta (Sullivan, McNaghten, Ricca, Sharma and Sineath), and two researchers from Northwestern University (Mustanski and Newcomb). Sullivan (PhD, DVM) and McNaghten (PhD) are Faculty with the Rollins School of Public Health, Emory University, and have considerable experience in HIV research and qualitative methodologies. Mustanski (PhD) and Newcomb (PhD) are faculty at Northwestern University, and are trained clinical psychologists. Tregear (PhD) is a Project Director at MANILA Consulting, with extensive experience in scientific management, and in meta-analytic and review methodology. Sullivan and McNaghten will be responsible for the design of the studies; Ricca, Sharma and Sineath will conduct the focus group discussions and in-depth interviews in Atlanta and Chicago, and Mustanski and Newcomb will recruit participants and conduct focus group discussions in Chicago, and participate in analysis of data.
The CDC Project Officers (Robin MacGowan and Pollyanna Chavez) will provide technical assistance, as needed, including consultation about study design, monitoring study progress, data management, and supervising data transfer through the Secure Data Network. The CDC Project officers and staff will also participate in conducting qualitative and quantitative data analyses, preparing manuscripts, and presenting at scientific meetings. CDC Project Coordinator (Arin Freeman) will assist the CDC project officers to coordinate project-related activities.
The CDC Project Officers or other CDC staff will not collect data from or interact with research participants and are therefore not engaged in the research activities. The study site will transfer all study data to CDC via the Secure Data Network during the conduct of the study. While individual identifiers will be linked to the data in the local database, no individually identifiable private information will be shared with CDC.
Preliminary studies in the area that support this research
Dr. Sullivan has considerable experience recruiting MSM online, and recruiting MSM into online research studies with an emphasis on HIV prevention. In the past 2 years, Sullivan’s research team has collected over 15,000 internet-based surveys of MSM in the United States and Africa. In Sullivan’s Barriers to Online HIV Prevention (BOPR) Study (funded by Emory Center for AIDS Research), a 29-day period of advertising on MySpace.com in March and April 2009 resulted in 30,559 click-throughs. Of the men who clicked on the advertisement, 9,005 MSM were eligible and consented to participate in a non-incentivized 40-minute online survey, which queried participants on sexual risk behavior and HIV testing history. In May 2010, a five-day period of advertising on Facebook resulted in the recruitment of 1,923 MSM (who self-reported being in a relationship for a minimum of three months) for an online survey of couples HIV counseling and testing. Additionally, a national online HIV prevention survey of MSM, for which banner advertisements were displayed on both Facebook and Black Gay Chat, enrolled 3,428 MSM in November 2010 in a period of 14 days. Both of the aforementioned studies were not incentivized. Sullivan’s research team has also had success in recruiting MSM into longitudinal research. For the Checking In. Study (RC1MD004370), 3,524 MSM consented to participate in a 12-month prospective study of HIV behavioral risks, for which the eligibility criteria was restrictive on race/ethnicity, mobile phone ownership, and willingness to receive an at-home test HIV test kit. The number of consenting participants, who represent a geographically diverse sample of MSM, are the result of 14 weeks of banner advertisements displayed on MySpace.com, Facebook.com, and Black Gay Chat.
Further, data from Sullivan’s BOPR study has informed Sullivan’s research team on the development of online recruitment methods to increase recruitment of the target population. An analysis of click-through data from the BOPR study identified that black men are more likely to click on a banner advertisement displaying a black model compared to an advertisement displaying a white model (aOR=1.83; CI: 1.72-1.98), while white men are less likely to click on an advertisement displaying a black model compared to a white model advertisement (aOR=0.74; CI: 0.70-0.79). These data suggest that studies which aim to recruit specific populations may see increased click-through by displaying advertisements depicting models of concordant race. This strategy was implemented in Sullivan’s Checking In. study, which allowed for recruitment specifically of black and Hispanic MSM, resulting in a consenting population consisting of the following: 42% white MSM, 38% black MSM, and 20% Hispanic MSM.
Training
All research personnel will have completed Collaborative Institutional Training Initiative (CITI) training before the research begins. In addition, all study personnel who will have access to study data will sign a confidentiality agreement before the study begins. Any other staff who request to analyze data after the study is completed will be required to complete CITI training, be added to the IRB protocol, and sign a confidentiality agreement before being allowed access to the data.
Part 1
Part 1 will include both focus group discussions and in-depth interviews. The focus group discussions will be conducted to identify issues related to recruitment, barriers to participation, perceptions of the accuracy and acceptability of HIV self-tests, and willingness to conduct self-tests and to provide test kits to others in their sexual and social networks. In-depth interviews will be conducted primarily to gather qualitative data that will be used to make the rapid testing and DBS specimen collection instructions clearer, but will also gather information regarding how to improve the packaging and marketing of test kits for later stages of the study. Information gained from Part 1 will inform Parts 2, 3, and 4 of the study.
Specific aims of Part 1
To examine attitudes towards and perceptions among focus group participants regarding participation in a study of HIV self-testing among MSM
To seek feedback and reactions from participants about self-testing materials and packaging proposed for Parts 2, 3 and 4
To understand what can be modified about the packaging of study materials to increase likelihood of kit use and distribution to network members
To determine recruitment methods to be used in Parts 3 and 4
To examine attitudes toward recruitment, participation, and retention in online studies, including establishing levels of comfort and trust of MSM to participate in an online survey in Parts 3 and 4 which will ask questions about high-risk sexual behavior
To determine if instructions proposed for HIV self-testing are understood by typical users of rapid tests to be targeted in Parts 3 and 4
Design
Sample
Population: The population for the focus groups will be men over the age of 18 years who self-report that they have had anal intercourse with a man in the past year. Participants do not have to self-identify as gay, bisexual or transgender, although such terms will be used in recruitment scripts to attempt to recruit eligible men. Participants in the Atlanta focus groups must be current residents of Atlanta, and they must be able to participate in the focus group in English. An effort will be made to include an equal number of black and white participants in Atlanta. Four focus groups will be conducted in Atlanta, one with HIV-positive participants. Participants in the Chicago focus group must be current residents of Chicago, and they must be able to participate in the focus group in English. An effort will be made to include mostly Hispanic participants in Chicago. One focus group will be conducted in Chicago. We will recruit a total of 60 participants for the focus groups; 48 (predominately black and white) in Atlanta and 12(predominately Hispanic) in Chicago.
The population for the in-depth interviews will be men over the age of 18 years who self-report that they have had anal intercourse with a man in the past year. Participants do not have to self-identify as gay, bisexual or transgender, although such terms will be used in recruitment scripts to attempt to recruit eligible men. Participants must be current residents of Atlanta, and they must be able to participate in the interview in English. We will recruit up to 20 men for the in-depth interviews.
Vulnerable populations: No vulnerable populations will be included in the focus groups or in-depth interviews.
Inclusion criteria: The inclusion criteria for Part 1 focus groups are: (1) male sex at birth; (2) currently identify as male; (3) at least 18 years of age; (4) resident of Atlanta or Chicago; (5) self-reported anal intercourse with a man in the past 12 months; (6) able to provide informed consent; and (7) English speaking.
The inclusion criteria for Part 1 in-depth interviews are: (1) male sex at birth; (2) currently identify as male; (3) at least 18 years of age; (4) resident of Atlanta; (5) self-reported anal intercourse with a man in the past 12 months; (6) able to provide informed consent; and (7) English speaking.
Exclusion criteria: The exclusion criteria for Part 1 focus groups are: (1) not male sex at birth; (2) do not currently identify as male; (3) under 18 years of age; (4) not a resident of Atlanta or Chicago; (5) no history of anal intercourse with a man in the past 12 months; (6) not able to provide informed consent; and (7) not able to speak English.
The exclusion criteria for Part 1 in-depth interviews are: (1) not male sex at birth; (2) do not currently identify as male; (3) under 18 years of age; (4) not a resident of Atlanta; (5) no history of anal intercourse with a man in the past 12 months; (6) not able to provide informed consent; and (7) not able to speak English.
Setting and Recruitment
Online recruitment: Online recruitment will be our main source of recruitment for Part 1 focus groups and in-depth interviews. The goal is to recruit participants who would be typical users of HIV self-test kits in Parts 3 and 4. Therefore, recruitment will be conducted through banner advertisements (Appendix A [example]) displayed on social networking sites such as Facebook. Recruitment for focus groups will be targeted only towards men who indicate in their Facebook profile that they are interested in men, whose profile age is at least 18 years and who report their city of residence as Atlanta or Chicago. Recruitment for in-depth interview participants will be targeted only towards men who indicate in their Facebook profile that they are interested in men, whose profile age is at least 18 and who report their city of residence as Atlanta.
Men who click through the banner advertisement will be provided with a brief explanation of the study, and those who consent (Appendix B) will then be taken to a short eligibility screener (Appendix C), which will confirm that they meet eligibility criteria to participate in a focus group or in-depth interview. Men who are determined by the screener to meet eligibility criteria will be asked to provide a nickname or name of choice, email address and telephone number so that they can be contacted by staff from Emory University or Northwestern University for this activity.
Emory study staff will contact men who are residents of Atlanta and Northwestern study staff will contact men who are residents of Chicago for participation in the focus groups at Emory University or Northwestern University. Staff in both cities will provide basic information about the study procedures, and schedule men for a time to attend focus group discussions. Emory study staff will contact men for participation in an in-depth interview, provide basic information about the study procedures, and schedule men for a time to attend an individual interview at Emory University.
Recruitment from current and past research studies: If the desired sample size is not reached through online recruitment, we will recruit men through current or past research studies. Currently, Sullivan’s Involve[MEN]t (IRB00042405) and MAN Project (IRB00047855) studies together have enrolled approximately 850 black and white MSM in Atlanta. The majority of these participants have agreed to be contacted for future research. Mustanski and Newcomb’s studies (STU00046626/STU00046626 and STU00047881/STU00047881) together currently have approximately 450 MSM enrolled and the majority of these participants have agreed to be contacted for future research. Recruitment for focus groups will occur from the Atlanta-based studies and Chicago-based studies while recruitment for in-depth interviews will only occur from the Atlanta-based studies. A recruitment script for the focus groups and in-depth interviews is in Appendix D. Men who are interested in participating will be given a link to provide consent (Appendix B), and those who consent will complete a short eligibility screener (Appendix C), which will confirm that they meet eligibility criteria to participate in a focus group or in-depth interview. Men who are determined by the screener to meet eligibility criteria will be asked to provide a nickname or name of choice, email address and telephone number so they can be contacted to be scheduled for the focus group or in-depth interview.
Procedures
Study design: The study design for Part 1 is a qualitative research study with focus groups and in-depth interviews.
Focus groups are small groups of between 6-12 participants. Participants are selected based on the above inclusion criteria. The aim is to create diverse groups of participants who do not know each other, and to promote free flowing discussion among group members. Each focus group is led by a moderator (Sullivan, McNaghten, Newcomb, Mustanski, Ricca, Sharma or Sineath) with a note-taker (Sullivan, Ricca, Sharma or Sineath) present to record key themes as they arise.
In-depth interviews are one-one-one interviews between a participant and interviewer. Participants are selected based on the above inclusion criteria. Each interview is led by an interviewer (Sullivan, McNaghten, Ricca, Sharma or Sineath).
Interaction with participants: Men who agree to participate in a focus group will be registered upon arrival and assigned a unique identifying number. Those selected to participate will be consented prior to the focus group (Appendix E) and informed that the focus group discussion will be audio recorded. Participants will be called by their number into the room where the focus group will be conducted. Thirteen chairs will be arranged in a circle (up to 12 participants and the moderator), and the note taker will sit at the side of the room. The moderator will begin by explaining the purpose of the focus group, and will ask permission from the participants to tape record the discussion. It will be made clear that no names will be used in the discussion and that tapes will be destroyed after their content has been transcribed. The facilitator will then outline some guidelines for the discussion: no names to be used, one person talking at a time, all opinions are valid. The discussion will then follow the focus group discussion guide (Appendix F).
The focus groups will seek to assess:
The operations, feasibility and acceptability of rapid HIV self-tests
Attitudes and perceptions towards participation in an HIV study of self-testing among MSM
Attitudes toward recruitment, participation, and retention in the online study portion of Parts 3 and 4, including establishing levels of comfort and trust of MSM to participate
Attitudes toward giving test kits to others within their sexual and social networks
Focus group participants will examine the test kit packaging to be provided to participants in Parts 2, 3 and 4. The test kit package for Parts 2 and 3 will include two FDA-approved rapid tests (OraQuick ®In-Home HIV Test [OraQuick] and SURE CHECK® HIV 1/ 2 Assay [Sure Check]) and one dried blood spot (DBS) specimen collection kit. The Part 4 test kit package will include: 2 oral fluid tests (OraQuick) and 2 finger-stick blood tests (Sure Check). Participants will be asked to provide their opinions regarding issues relevant to the research study including: recruitment and willingness to provide contact information, barriers to participation, receiving test kits in the mail, their perception of the accuracy of the test kits, and their willingness to use the test kits and give them to others in their sexual and social networks. Participants will be asked to talk about their ideas about how the kits could be packaged or provisioned to increase the likelihood that the participant would be comfortable both using and distributing the kits.
Men who arrive for an in-depth interview will be registered upon arrival and assigned a unique identifying number. Those selected to participate will be consented prior to the beginning of the interview (Appendix G). The interviewer will begin by explaining the purpose of the interview, and will ask permission from the participant to video and audio record the interview. It will be made clear that no names will be used in the interview and that tapes will be destroyed after their content has been transcribed. Each participant will be administered an individual qualitative interview, according to the interview guide included in Appendix H.
The in-depth interview will seek to assess:
The participant’s understanding of what is expected of him after opening the packaged study materials
The participant’s understanding of the instructional materials (written, video) that explain how to administer and interpret the Sure Check test and how to collect and package the DBS specimen
The participant’s understanding of the educational brochure (written, video) that explain concepts such as window period for each test
The participant’s opinions about how willing he would be to use the tests with the type of packaging and instructions demonstrated during the interview
Men will be shown the test kit packaging to be provided to participants for use in Parts 2 and 3. The test kit package will contain: 1 oral fluid test (OraQuick), 1 finger-stick blood test (Sure Check), and one DBS specimen collection kit. Participants will be asked to interact with the packages and provide feedback about their understanding of the sequence of conducting the individual tests in the package from the package layout and instructions. They will also be asked to examine the written and video instructions for the Sure Check test kit and DBS specimen collection kit, including “placemats” developed by the study team that will guide participants through the procedures for that specific kit. They will be asked to describe to the interviewer their understanding of how to administer the Sure Check test, and how to collect, package and ship the DBS specimen. They will also be observed as they interact with the package to visually confirm if package instructions are followed. The interview will be conducted to elicit men’s understanding of and reactions to the packaging that will be used in Parts 2 and 3, and the instructional materials for the Sure Check tests and DBS specimen collection kits that will be used in Parts 2, 3 and 4. Participants will be asked to share their ideas about how the instructions could be clearer and how the packaging could be changed to increase the likelihood that the participant would be comfortable using the test kits.
Token of Appreciation: Focus group and in-depth interview participants in Part 1 will receive $50 as a token of appreciation. Participants will also receive light refreshments during the focus group discussion. No compensation is available for injury resulting from participation in the research; the investigators see no more than minimal risk of study participation.
Respondent burden: The estimated burden for focus group participants is approximately 120 minutes (registration, participant selection, study overview, and consent process is approximately 30 minutes; focus group is approximately 90 minutes). The estimated burden for in-depth interview participants is approximately 75 minutes (study overview and consent process is approximately 15 minutes; interview is approximately 60 minutes).
Protection of human subjects: The following procedures will be used to ensure protection of human subjects during Part 1:
During the focus group and in-depth interview screening processes nicknames or name of choice, email addresses and phone numbers are collected to provide contact information to confirm participation, but names are not used during the focus group discussion or interview. Contact information used to confirm participation will be held in a password-protected database on a MANILA secure server, accessible only by study staff. The database will be backed-up to a hard drive housed in another location, also accessible only by study staff. The contact information (name, email address and phone number) in the database will be destroyed after the study is over, and will never be associated with the study data collected. Emory study staff will notify MANILA when to destroy the information in the database; MANILA will use Norton CleanSweep software to delete the database file from the secure server and the back-up file(s) from the separate hard drive. No identifying information will be collected, except for the focus group audio recordings and the in-depth interview audio and video recordings. The audio and video files will be stored on a password protected network accessible only by study staff. Audio and video recordings will be destroyed within 4 weeks after transcription, which will take place within 4 weeks of the data collection. The recruitment materials for both the focus groups and in-depth interviews will include an email and telephone number from which potential participants can receive information on participation; this process does not require participants to give any personal information.
The consent procedures for both the focus groups and the in-depth interviews outline the voluntary nature of the research and explain the potential benefits and disadvantages of the research.
The participant will be given a copy of the consent form that includes contact information for the research team members and the IRB.
Costs of participation: There will be no costs to participants for participation in the focus group or in-depth interview. All participants will be compensated for their time.
Benefits of participation: There is no direct benefit to the participant of participation in the focus group or in-depth interview; the main benefit of the research comes from the new information that will be applied to Parts 2, 3 and 4. This is explained to focus group and in-depth interview participants in the consent form.
Handling of adverse reactions: There is only a minimal risk of discomfort for focus group and in-depth interview participants. Also, it will be made clear that the research team will be available after the focus group and in-depth interview should any participant wish to ask confidential questions or receive more information on local HIV/AIDS services.
Data collection and analysis
Purpose: The purpose of the Part 1 focus groups is to determine men’s willingness to participate in the study and barriers to participation, recruitment methods and willingness to provide contact information, the acceptability of receiving test kits in the mail, their perception of the accuracy of the test kits, and their willingness to use the test kits and give them to others in their sexual and social networks. As such, we propose to analyze the focus group data through a review of the transcripts of the focus groups by study staff. Major themes will be identified, but formal coding procedures will not be used in this analysis. The investigators will develop lists of specific issues or concerns regarding the use of self-administered HIV tests and participation in the research study, as well as general suggestions to improve recruitment, participation, and the test kits’ usability or acceptability.
The purpose of the Part 1 qualitative individual in-depth interview is to confirm the clarity of test kit instructions including sequence of testing, and of instructions for the Sure Check test kit and the DBS specimen collection kit prior to the Part 2 user proficiency study. As such, we propose to analyze the individual in-depth interview data through a review of the transcripts of the interviews by study staff. Major themes will be identified, but formal coding procedures will not be used in this analysis. The investigators will develop lists of specific suggestions for modification of instructions or materials, as well as general suggestions to improve usability or acceptability. These items will be used to revise the instructions and packaging before Part 2.
Transcription: All recorded data will be transcribed within 4 weeks of the focus group discussions and within 4 weeks of verifying all individual interviews. Following transcription, all of the original voice and video recordings will be destroyed.
Rationale for proposed number of subjects: The aim is to have five focus groups consisting of approximately 8 participants each, for a total of approximately 40 participants. We will over-recruit for each focus group by 50% to allow for participants who do not report to their scheduled focus group. Thus, we will recruit 12 participants per focus group for a total of 60 participants, and expect 40 participants to consent to participate.
We will conduct in-depth interviews with up to 15 participants, recruiting up to 20 participants, if necessary. If saturation is reached prior to interviewing 15 men we will stop recruitment and not conduct any more interviews.
Data handling: Only the research team members will have access to the transcripts and recordings of the Part 1 focus group discussions and in-depth interviews. Ricca and Sharma will be responsible for verbatim transcriptions of each of the tapes and videos and general themes for the focus group discussions and in-depth interviews. After transcription, all tapes and videos will be destroyed. The resultant text files will be loaded into NVIVO or similar qualitative analysis software.
Data security: All focus group and in-depth interview participants will be assigned a unique identification number for the study. Consent forms with names or nicknames will be separated from interviews, and a master list linking the identifiers and names will be developed. All confidential data (i.e., phone numbers and email addresses) collected regarding study participants will be maintained in locked filing cabinets, separated from all other study data. Electronic audio and video files will be stored on password protected computers accessed only by study staff transcribing the data. Access to confidential files is managed by the Principal Investigator and is limited to study staff directly involved in this research on a need-to-know basis. No confidential data will be permitted off site, except when data are in transit from the focus group and interview sites to the research office.
Confidentiality
During the focus group and in-depth interview screening processes nicknames or name of choice, email addresses and phone numbers are collected to provide contact information to confirm participation, but names are not used during the focus group discussions or in-depth interviews. Contact information used to confirm participation will be held in a password-protected database accessible only by study staff. This contact information will be held separately from focus group and in-depth interview notes. Information in the database will be destroyed at the end of the study, and will never be associated with the study data collected.
Informed consent
Verbal informed consent will be obtained for the focus groups (Appendix E) and in-depth interviews (Appendix G). Men who report for participation in either a focus group or an in-depth interview will be given basic information about the purpose of the study, and provided with a written informed consent form for their records. For Part 1, we request a waiver of written documentation of informed consent. This is because the written consent document would be the only identifying document once voice and video recordings have been destroyed. Therefore, relying on verbal consent will reduce risk of loss of privacy for the participants. Upon arriving at the research site, potential participants will be taken through the consent process by one of the research team members. The consent process will take approximately 10 minutes per individual and will take place in a room at the research site that provides visual and audio privacy. The consent procedure will begin with welcoming remarks from the team member, who will then establish that the potential participants are aware of why they are at the research site. The team member will then explain the consent process to the potential participant, explaining (1) what is meant by consent; (2) why we need to take consent; and (3) the purpose of the consent form. The participant will then be asked if they feel comfortable reading the consent form; if not, the researcher will read the consent form to the participant, asking questions along the way to ensure they understand the content. The consent forms are written in English for the focus groups and for the in-depth interviews. After reading the consent form, the participants will be asked if they understand the content, and will be asked if they understand that their participation is voluntary. The participant will be given a copy of the consent form for him to keep. Each consent form has a Flesch-Kinkaid Reading Level of <8.0. Study staff will answer any questions that participants have and will document verbal consent.
Part 2
Part 2 will consist of a user proficiency assessment to determine if potential users of rapid HIV test kits can successfully conduct the self-tests, accurately interpret the results, and prepare the DBS specimen. Results of the user proficiency assessment will be used to make the printed and video testing instructions clearer prior to Parts 3 and 4. Part 2 will be conducted using the same packaging and test kits to be used in Part 3: 1 oral fluid test (OraQuick), 1 DBS specimen collection kit, and 1 finger-stick blood test (Sure Check)..
Specific aims of Part 2
To determine the extent to which untrained users can proficiently conduct rapid HIV self-testing procedures and interpret the results, and conduct DBS specimen collection procedures with the use of provided print and video instructions
To demonstrate the operations, feasibility and acceptability of rapid HIV self-tests
To compare the results of participants’ self-administered and interpreted rapid HIV self-tests compared to laboratory-administered EIA
Design
Sample
Population: The target population of MSM for the research will be men over the age of 18 years who self-report that they have had anal intercourse with men in the past year. Participants do not have to self-identify as gay, bisexual or transgender, although such terms will be used in recruitment scripts to attempt to recruit eligible men. Participants must be current residents of Atlanta. We will recruit a total of approximately 30 men who assume they are HIV-negative or are unaware of their HIV status, and 10 known HIV-positive men to participate in the proficiency study.
Vulnerable populations: No vulnerable populations will be included.
Inclusion criteria: The inclusion criteria for Part 2 proficiency assessment are: (1) male sex at birth; (2) currently identify as male; (3) at least 18 years of age; (4) resident of Atlanta; (5) self-reported anal intercourse with a man in the past 12 months; (6) able to provide informed consent; (7) never diagnosed with a bleeding disorder; (8) not part of an HIV vaccine trial; (8) not taking antiretroviral medication for HIV; and (10) English speaking. We will include 40 participants.
Exclusion criteria: The exclusion criteria for Part 2 user proficiency assessment are: (1) not male sex at birth; (2) do not currently identify as male; (3) under 18 years of age; (4) not a resident of Atlanta; (5) no history of anal intercourse with a man in the past 12 months; (6) not able to provide informed consent; (7) ever diagnosed with a bleeding disorder; (8) part of an HIV vaccine trial; (9) taking antiretroviral medication for HIV; and (10) not able to speak English.
Setting and Recruitment
Current and previous research studies will be our sole source of recruitment for Part 2 user proficiency assessment. Currently, Sullivan’s Involve[MEN]t (IRB00042405) and MAN Project (IRB00047855) studies have together enrolled approximately 850 black and white MSM in Atlanta. The majority of these participants have agreed to be contacted for future research. A recruitment script for the proficiency assessment research is in Appendix I. Men who are interested in participating will be given a link to consent, and those who consent will be taken to a short eligibility screener (Appendix C), which will confirm that they meet eligibility criteria to participate in the proficiency assessment. Men who are determined by the screener to meet eligibility criteria will be asked to provide a nickname or name of choice, email address and telephone number so that they can be contacted.
Emory study staff will contact men who have indicated their interest in participating, provide basic information about the study procedures, and schedule men for a time to attend a study session for Part 2 at Emory University. We will recruit a total of 60 participants for the proficiency assessment; 45 known HIV-negative men and 15 known HIV-positive men. We anticipate that 20 men will not arrive for this activity; therefore, we expect this will yield a sample size of 40 men.
Procedures
Study design: The study design for Part 2 is a qualitative research study with observed proficiency assessment.
Data collection: After screening and participant selection, participants will be called into the room in which the proficiency assessment will be conducted. Participants will be instructed to document results for each test in the electronic application designed to guide the testing processes. Participants will complete a survey regarding the self-testing process. Finally, each participant will use an electronic application to review a panel of test result images for that particular test and will select responses to indicate if they interpret the test as positive, negative, or indeterminate.
Interaction with participant: Men who meet the eligibility criteria will be consented to having their hands video recorded and their voice audio recorded (Appendix J). All men will be informed that a trained HIV counselor on the Emory study team will be available following each proficiency assessment session to provide HIV testing to men who want to learn or confirm their status, and to provide counseling. Emory staff will use a finger-stick Clearview or Insti rapid test. Test results and counseling will be conducted in a private, secure area. Men in the HIV negative/unaware group will be informed that if any of their tests are reactive, they will have the option of having the Emory staff confirm their status using a finger-stick Clearview or Insti rapid test, to have their blood drawn by the trained counselor for confirmatory supplemental EIA testing, and be provided with counseling which includes referrals to local HIV care services. Information will be collected by study staff for HIV reporting purposes (name, mailing address and phone number) and if they are confirmed HIV-positive, this information will be reported by telephone to the Georgia Department of Public Health. If the confirmatory supplemental EIA testing is negative, this information will be destroyed.
All men participating in the proficiency assessment will be assigned a participant number that will be associated with their test results, but not linked to their contact information. The proficiency assessment sessions will be conducted to determine men’s ability to understand the sequence of use of the testing package components and the instructional materials for the Sure Check test kit and the DBS specimen collection kit included in the package.
The proficiency assessment will seek to assess:
The participant’s understanding of the materials (written, video) that explain how to administer and interpret the Sure Check test and how to collect and package the DBS specimen
The participant’s ability to properly conduct the self-tests and self DBS collection and accurately interpret the results (rapid tests only)
The participant’s opinions about how willing he would be to use and distribute either of the tests within his social and sexual networks
Each participant will be given the same testing kit package as will be distributed to home testing participants in Part 3 containing: 1 oral fluid test (OraQuick), 1 DBS specimen collection kit, and 1 finger-stick blood test (Sure Check). Participants will open the test kit package and follow the package instructions to begin the process of first conducting the oral fluid test, second collecting a DBS specimen, and third conducting the finger-stick blood test. Participants will use the written instructions included with each individual test and will also have access to an electronic application that will provide video instructions and timers specific to each test. Participants will conduct the testing process following the kit instructions, and will have the option to document test times and results for both OraQuick and Sure Check in the electronic application. Participants also have the option of documenting the Sure Check test time and result on an individual test kit “placemat” (instructional sheets designed to guide the placement of materials and the steps for administering and interpreting the Sure Check test and conducting the DBS specimen collection). Following each rapid test administered and interpreted by the participant, a study staff member trained in HIV testing will interpret the participant’s test results. If any of the tests among the HIV-negative/unaware participants is preliminary positive, they will have the option of having the Emory staff confirm their status using a finger-stick Clearview or Insti rapid test, to have their blood drawn by the trained counselor for confirmatory supplemental EIA testing, and be provided with counseling which includes referrals to local HIV care services. This is a voluntary service that the participant can accept or not. Researchers will observe the men’s ability to conduct the tests and interpret the results of each self-test, and to collect the DBS specimen and properly package it for transport, and will document their observations (Appendix K). Men will package a prefabricated DBS specimen for transport. Participants’ DBS specimen cards will be collected by Emory study staff, anonymized so the specimen card cannot be linked back to the participant, and transported to CDC for laboratory testing. Considerations for further educational materials or provisions to packaging will be assessed. Following the self-testing component, participants will complete a survey about the self-testing process (Appendix L). Finally, each participant will use an electronic application to review a panel of test result images for OraQuick and Sure Check (Appendix M). At the end of their session, participants will have the option to receive counselor provided HIV screening from trained Emory study staff and to speak to the trained HIV counselor or other study staff.
Token of Appreciation: Proficiency assessment participants in Part 2 will receive $75 as a token of appreciation. No compensation is available for injury resulting from participation in the research; the investigators see no more than minimal risk of study participation.
Respondent burden: The estimated burden for participants in Part 2 can take approximately 80 minutes. The breakdown of time is as follows: the consent process will take approximately 10 minutes. The specimen collection for the DBS will take approximately 5 minutes, and packaging the specimen will take approximately 2 minutes. The OraQuick test will take approximately 3 minutes to perform and 20 minutes for the results. The Sure Check test will take approximately 5 minutes for the finger prick and 15 minutes for results. All self-test results will be read by a trained tester. The self-test survey will take approximately 10 minutes, and the review of test result images will take approximately 10 minutes. Participants are welcome to receive counselor provided HIV screening and to speak to the trained HIV counselor for as long as he likes.
Protection of human subjects: The following procedures will be used to ensure protection of human subjects:
For HIV negative men, no identifying information will be collected, except for the voice and video recording. The audio and video files will be stored on a password protected network accessible only by study staff. Audio and video recordings will be destroyed within 4 weeks after transcription, which will take place within 4 weeks of the data collection. The recruitment material will include an email and telephone number from which potential participants can receive information on participation; this process does not require participants to give any personal information. During the screening process nicknames or name of choice, email addresses and phone numbers are collected to provide contact information to confirm participation, but names are not used during the proficiency assessment session. Contact information used to confirm participation will be held in a password-protected database on a MANILA secure server, accessible only by study staff. The database will be backed-up to a hard drive housed in another location, also accessible only by study staff. The contact information (name, email address and phone number) in the database will be destroyed at the end of the study, and will never be associated with the study data collected. Emory study staff will notify MANILA when to destroy the information in the database; MANILA will use Norton CleanSweep software to delete the database file from the secure server and the back-up file(s) from the separate hard drive. HIV test results data will contain only the participants’ study identification number and cannot be linked to their contact information. If any of the tests among the HIV-negative/unaware participants is preliminary positive, they will have the option of having the Emory staff confirm their status using a finger-stick Clearview or Insti rapid test, to have their blood drawn by the trained counselor for confirmatory supplemental EIA testing, and be provided with counseling which includes referrals to local HIV care services. This is a voluntary service that the participant can accept or not. Information will be collected by the Emory staff for HIV reporting purposes (name, mailing address and phone number) and if they are confirmed HIV-positive, this information will be reported by telephone to the Georgia Department of Public Health. Their information remains confidential when it is reported to the state health department. If the confirmatory testing is negative, this information will be destroyed. This information will be kept in a locked filing cabinet accessible only by Emory staff and kept only until the confirmatory results are received.
The consent procedure outlines the voluntary nature of the research and explains the potential benefits and disadvantages of the research.
The participant will be given a copy of the consent form that includes contact information for the research team members and the IRB.
Costs of participation: There will be no costs to participants for participation in the research. Participants will be provided a token of appreciation for their time.
Benefits of participation: Subjects will learn their current HIV status based upon a self-test or from a trained counselor if they accept the additional testing service. There are no additional direct benefits to the participant of participation in the research; the main benefit of the research comes from the new information that will be applied to Parts 3 and 4. This is explained to the participant in the consent form.
Handling of adverse reactions: There is only a minimal risk of discomfort for participants in the research. Trained HIV counselors will be present to assist persons who test HIV positive during the pre-test. Also, it will be made clear that the research team will be available after the proficiency assessment session in Part 2 has ended should any participant wish to ask confidential questions or receive more information on local HIV/AIDS services.
Data collection and analysis
Purpose: The purpose of the Part 2 proficiency assessment is to assess the ability of participants to follow package and test kit instructions and accurately conduct self-testing and DBS specimen collection, and to accurately interpret their test results. Further improvements will be made from participants’ feedback regarding the packaging and instructions for Sure Check and DBS collection prior to Parts 3 and 4.
The interpretation of the self-administered oral fluid and finger-stick blood test results interpreted by the participant will be compared with the interpretation of the results by the trained tester. We will calculate percent agreement between the participants’ and the tester’s interpretation of the test results.
Participants’ interpretation of the panel of test result images presented on the electronic application will be assessed to determine if they accurately identified positive, negative, or invalid results.
Results of proficiency assessment participants’ self-administered and interpreted rapid HIV self-tests will be compared to laboratory-administered EIA conducted on each participant’s DBS specimen at a CDC laboratory. We will calculate percent agreement between the oral fluid test result and the EIA and the finger-stick blood test result and the EIA.
Transcription: All recorded data will be transcribed within 4 weeks of verifying the content of all of the recordings. Following transcription, all of the original voice and video recordings will be destroyed.
Rationale for proposed number of subjects: The aim is to conduct proficiency assessment with 30 known HIV-negative men and 10 known HIV-positive men for a total of approximately 40 participants. We will over-recruit by 50% to allow for participants who do not report to their proficiency assessment session. Thus, we will recruit 45 known HIV-negative men and 15 known HIV-positive men and expect 30 and 10, respectively, to consent to participate.
Data handling: Only the research team members will have access to the transcripts and voice and video recordings of the Part 2 proficiency tests. Ricca and Sharma will be responsible for verbatim transcriptions of each of the tapes and general themes of each video. After transcription, all tapes and videos will be destroyed. The resultant text files will be loaded into NVIVO or similar qualitative analysis software. Similarly, only the research team members will have access to the participants’ and study staffs’ interpretations of the user-administered rapid tests, participants’ responses to the HIV test results panel interpretations, and participants’ oral fluid test, finger-stick blood test and anonymized laboratory-tested EIA results.
Data security: All proficiency assessment participants will be assigned a unique identification number for the study. Consent forms will be separated from interviews, and a master list linking the identifiers and names of choice will be developed. All confidential data collected regarding study participants will be maintained in locked filing cabinets, separated from all other study data. Access to confidential files is managed by the Principal Investigator and is limited to study staff directly involved in this research on a need-to-know basis. No confidential data will be permitted off site, except when data are in transit from the proficiency assessment site to the research office.
Confidentiality
During the proficiency assessment screening process nicknames or names of choice, email addresses and phone numbers are collected to provide contact information to confirm participation, but names are not used during the proficiency assessment session. Contact information used to confirm participation will be held in a password-protected database accessible only by study staff. Information in this database will be destroyed at the end of the study, and will never be associated with the study data collected. HIV test results data will contain only participants’ study identification number and cannot be linked to their contact information.
If any test result is preliminarily positive, they will have the option of their blood drawn by the trained counselor for confirmatory supplemental EIA testing, and will be provided with counseling which includes referrals to local HIV care services. This is a voluntary service that the participant can accept or not. Information will be collected by the Emory staff for HIV reporting purposes (name, mailing address and phone number) and if they are confirmed HIV-positive, this information will be reported by telephone to the Georgia Department of Public Health. Their information remains confidential when it is reported to the state health department. If the confirmatory testing is negative, this information will be destroyed. This information will be kept in a locked filing cabinet accessible only by Emory staff and kept only until the confirmatory results are received.
Informed consent
Men who report for participation in Part 2 will be given basic information about the purpose of the study, and provided with a written informed consent form (Appendix J). Upon arriving at the research site, potential participants will be taken through the consent process by one of the research team members. The consent process will take approximately 10 minutes per individual and will take place in a room at the research site that provides visual and audio privacy. The consent procedure will begin with welcoming remarks from the team member, who will then establish that the potential participants are aware of why they are at the research site. The team member will then explain the consent process to the potential participant, explaining (1) what is meant by consent; (2) why we need to take consent; and (3) the purpose of the consent form. The participant will then be asked if they feel comfortable reading the consent form; if not, the researcher will read the consent form to the participant, asking questions along the way to ensure they understand the content. Participants will be told that their voice will be recorded and their hands will be video recorded as part of the study procedures, and study staff will confirm their understanding of consent to be recorded. They will also be informed that if they are found to be preliminarily HIV positive they will have the option of having their blood drawn by the trained counselor for confirmatory supplemental EIA testing, and will be provided with counseling which includes referrals to local HIV care services. This is a voluntary service that the participant can accept or not. Information will be collected by the Emory staff for HIV reporting purposes (name, mailing address and phone number) and if they are confirmed HIV-positive, this information will be reported by telephone to the Georgia Department of Public Health. Their information remains confidential when it is reported to the state health department. If the confirmatory testing is negative, this information will be destroyed. This information will be kept in a locked filing cabinet accessible only by Emory staff and kept only until the confirmatory results are received. Emory study staff will provide them with referral information for additional supplemental testing and care. They will have the option to request a written document (in person, or via mail or email) with their preliminary positive test results that they can take to their own healthcare provider (Appendix N). The consent form is written in plain English. After reading the consent form, the participants will be asked if they understood the content, and will be asked if they understand that there will be a proficiency assessment involving 2 HIV test kits and a DBS specimen collection and that their participation is voluntary. Participants who consent will sign the consent form and will be given a copy of the consent form to keep. The consent form has a Flesch-Kinkaid Reading Level of <8.0.
APPENDIX A
Banner Advertisements from Prior Research Studies

Examples of banner advertisement images:




APPENDIX B
Eligibility Screener Consent Form
Emory University, Rollins School of Public Health
Consent to be a Research Subject
Flesch-Kincaid Reading Level: 7.9
Title: Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
Principal Investigator: Patrick Sullivan, DVM PhD
Funding Sources: Emory University and MANILA Consulting Group, Inc are conducting this study which is sponsored by the Centers for Disease Control and Prevention (CDC).
Purpose: The
Emory University Rollins School of Public Health is doing a research
study of men who use the Internet. The purpose of this study is
to learn about behaviors that put people at risk for getting
diseases transmitted by having sex (like HIV). The information we
learn from this study will help create better HIV prevention
programs for people in our community.
Procedures:
If you would like to be in the study, you will take a 5 minute
Internet survey to see if you are eligible. All of your answers
to the survey questions will be confidential.
In the survey you will be asked questions about the following topics:
a. Your age, race/ethnicity and gender
b. Your sex behavior
c. If you have ever tested for HIV or been in an HIV vaccine trial
d. Your contact information
You
will take this survey on a computer. The web site where the survey is
located is secure and any answers you give us will be safely
stored on a password-protected computer. Researchers will not be
able to link your responses to you or your Facebook page. You can
refuse to answer a question at any time. If you don’t
answer a question, or if you want to end the survey, there will be
no penalty to you.
Based
on your responses to this survey, you may be asked to participate in
a research study. If you are asked and agree to participate in the
study, we will ask that you provide additional information.
Risks
and Discomforts: All of
your answers will remain private. Some of the questions in the
survey are about sex and may make you feel uncomfortable.
Your participation is completely voluntary and you can refuse to
answer a question at any time.
Benefits: There
are no direct benefits by taking this survey. The information from
the Internet survey may be used to determine eligibility for a
research study.
Compensation: If you agree to take this survey, you will not receive any compensation (money or otherwise) for taking this survey.
Privacy: Certain
offices and people other than the researchers may look at your
medical charts and study records. Government agencies and Emory
employees overseeing proper study conduct may look at your study
records. These offices may include the Emory Institutional Review
Board, the Emory Office of Research Compliance, Food and Drug
Administration, the Office for Human Research Protections and/or the
U.S. Centers for Disease Control and Prevention. The contact
information you give us will be kept in a secure location and will
not identify you. If you are eligible to participate this information
will be used to contact you to make an appointment for the research
study, then it will be destroyed. The survey answers you give us
will be grouped with survey answers from other persons.
Researchers will not be able to link your responses to you or
your Facebook page. If you are asked and agree to participate in
the research study, you will be asked to provide additional
information. You do not have to participate in the study and you
can still participate in this survey even if you do not want to
provide additional information for the research study.
Voluntary Participation and Withdrawal
Being in this research is voluntary and you have the right to refuse to answer all questions in the survey. You can stop at any time after giving your consent.
Contact Persons
If you have any questions about this study or feel you have been harmed in this study please contact a member of the research team:
Patrick Sullivan, DVM PhD
Emory University
Rollins School of Public Health
1518 Clifton Road NE
Room 464
Atlanta, GA 30322
(404) 727-2038
If you have any questions about your rights as a participant in this study or feel you have been harmed by being in this study you can contact the institutional review board at Emory University.
For Emory University contact:
Emory IRB
1599 Clifton Road
5th Floor East
Atlanta, GA 30322 USA
Tel: 404 712 0720
Toll free: 877 503 9797
Email:[email protected]
You may print a copy of this form for your records if you like.
If you agree to the above and would like to participate in this study, please click “Agree” below.
Appendix C
Eligibility Screener
Eligibility Screener
You are invited to be in a research study being done by Emory University’s Rollins School of Public Health and Manila Consulting Group, Inc, and is sponsored by the Centers for Disease Control and Prevention.
Thank
you for your interest in our study. First, we have a few questions to
determine if you’re eligible. Please take note of the following
information:
1.
Your answers are anonymous: we don't have any information about who
you are beyond the questions you answer.
2.
Some questions are about sensitive topics; you can choose not to
answer any question that you are not comfortable with.
3.
If you have any questions or comments, you may contact the Principal
Investigator, Dr. Patrick Sullivan of Emory University, at (404)
727-2038.
_____________________________________________________
Thank you for your interest in our survey. Please take note of the following information:
Your answers are confidential: the information you provide us will be kept private and known only to study staff.
This survey includes some personal questions about sexual behaviors, HIV status, HIV testing practices. You can choose to not answer any questions that make you feel uncomfortable.
AUTO1. Date of Interview: __ __/ __ __ / __ __ __ __
(M M / D D / Y Y Y Y )
AUTO2. Time Began Eligibility Screener __ __:__ __ :__ __ [24 Hour time HH:MM:SS]
QS1. How old are you? _ _ _
If ES1 <18 skip to End1
QS2. Do you consider yourself to be Hispanic or Latino?
No
Yes
I prefer not to answer
Don't know
QS3. Which racial group or groups do you consider yourself to be in? Check all that apply:
American Indian or Alaska Native
Asian
Black or African American
Native Hawaiian or Other Pacific Islander
White
I prefer not to answer
Does not apply
Don’t know
Q4. What zipcode do you live in?
__ __ __ __ __
If QS4≠ one of the study cities, skip to End 1
Gender Assessment and Identity
QS5. What was your sex at birth?
[Check only one]
Male
Female
Intersex/Ambiguous
I prefer not to answer
Don't know
QS6. Do you consider yourself to be male, female, or transgender?
[Check only one]
Male
Female
Transgender
I prefer not to answer
Don't know
If QS5 and QS6 =Male, go to QS7
If QS5 and QS6 ≠ MALE, skip to End 1
Sex risk assessment
The next question is about having sex with other men. For this question, "anal sex" means you put your penis in his anus (butt) or he put his penis in your anus (butt).
QS7. Have you had sex with a man in the past 12 months?
No
Yes
I prefer not to answer
Don't know
If QS7 ≠Yes, skip to End1
Bleeding Disorder
QS8. Have you ever been diagnosed with a bleeding disorder?
No
Yes
I don't know
I prefer not to answer
If QS8 = “Yes”, “I don’t know” or “I prefer not to answer”, skip to End 1
HIV Testing
QS-9. Have you ever been tested for HIV? An HIV test checks whether someone has the virus that causes AIDS.
No
Yes
I prefer not to answer
If QS9= Yes, go to QS10
If QS9= No, go to QS11
If QS9= I prefer not to answer, skip to End 1
QS10. Have you ever tested positive for HIV?
No
Yes
I don't know
I prefer not to answer
If QS10 = I prefer not to answer, skip to End 1
If QS10 = “Yes”, then classify participant into “Positive Arm”
QS11. Are you taking antiretroviral medications to prevent HIV?
No
Yes
I don't know
I prefer not to answer
If QS11 = “Yes”, “I don’t know” or “I prefer not to answer”, skip to End 1
QS12. Have you ever been part of an HIV vaccine trial?
No
Yes
I don't know
I prefer not to answer
If QS12 = “Yes”, “I don’t know” or “I prefer not to answer”, skip to End 1
If QS12 = “No” then classify participant into “Negative Arm” and proceed to collect contact information
Contact Information
We will communicate with you via email, phone or text message to schedule a time for you to participate in a research session. We will also contact you to remind you about the date, time and place of the research session. This information will not be shared or used for any other research purposes.
*required
QS13a. Email address*: ____________________
QS13b. Telephone number to receive calls*: ___________________
QS13c. Telephone number to receive text messages: ___________________
If QS13 is not answered go to End 1
QS14. We will not ask your name as part of the participation in the study. Please provide us with an alias or name of your choice that we can use throughout the study to communicate with you.
Nickname or name of choice _______________
QS15. Your login will be the email address you provided. Please create a password that you will use to access the study website.
End 1. If the participant does not qualify:
Thank you for your interest in this study. Unfortunately, you are not eligible to participate any further. Thank you for your time.
End survey.
End 2. If the participant qualifies:
Congratulations! You qualify to participate in this health study.
AUTO3. Time Ended Eligibility Screener: __ __:__ __ : __ __ [24 Hour time HH:MM:SS]
APPENDIX D
Focus Group and In-Depth Interview Recruitment Script
Focus Group and In-Depth Interview Recruitment Script
Hello [name of participant], my name is [name] and I am calling from the eSTAMP study at [Emory University or Northwestern University]. You clicked on our banner ad online and indicated that you are willing to participate in research and I would like to discuss an opportunity you may be interested in. A token of appreciation will be provided if you are eligible and you decide to participate in the study. Do you have a minute to go over this?
If no: Okay, no problem at all. Thank you for your time and have a great day.
If yes and calling for Focus Group: Okay, thank you! We are conducting focus groups to help us understand how men who are not trained in testing can test themselves at home. We want to talk about the test kits we will use as part of the study and also determine how men who have sex with men feel about participating in an online study and home testing for HIV. A token of appreciation will be provided if you are eligible and you decide to participate in the study. If you are interested, we would ask you to come in to [Emory University or Northwestern University] for about 2 hours to talk to us about what you think. Is this something that you might be interested in?
If no: Okay, no problem at all. Thank you for your time and have a great day.
If yes: Great! How about we set up a time for you to come in? [Review times, confirm contact information, set up meeting.]
If yes and calling for In-Depth Interview: Okay, thank you! We are conducting in-depth interviews to help us evaluate instructional materials and packaging for HIV self-tests. A token of appreciation will be provided if you are eligible and you decide to participate in the study. If you are interested, we would ask you to come in to Emory University for about 1.5 hours to talk to us about how you think we can make our product better. Is this something that you might be interested in?
If no: Okay, no problem at all. Thank you for your time and have a great day.
If yes: Great! How about we set up a time for you to come in? [Review times, confirm contact information, set up meeting.]
APPENDIX E
Focus Group Consent Form
Emory University, Rollins School of Public Health
Consent to be a Research Subject
Flesch-Kincaid Reading Level: 7.9
Title: Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
Principal Investigator: Patrick Sullivan, DVM PhD
Funding Sources: Emory University and MANILA Consulting Group, Inc are conducting this study which is sponsored by the Centers for Disease Control and Prevention (CDC).
Introduction
You are invited to be in a research study being done by Emory University’s Rollins School of Public Health. You are being asked because you have told us that you are a man who has sex with men who is over 18 years of age. We expect to have 10 men taking part in the focus group. If you decide to take part, the things we learn from you today will help improve a men’s health study.
Purpose
To plan for a study on HIV home testing among men who have sex with men (MSM), we want to know how we can make the best packages for HIV home test kits and how we can make the online study easy for men to participate. This study will find out what men think about being in an online HIV study and what they think about testing for HIV at home.
The focus group is to get your thoughts on how willing MSM are to use at-home HIV test kits, kind of like tests that women use to check if they are pregnant. We are not asking you to be in the actual study at this time; we just want you to share your ideas that will help us best create test kit packages that will be used at a later time.
Procedures
When you came here you were given a number. If we pick you to be in the focus group, you will be called by your number and invited into a secure room where the focus group will take place. Before the focus group starts, the leader will discuss the consent. You will then have the chance to ask any questions and make sure everyone is comfortable with the process. We will also give you a toll-free number for any concerns that you may have with the focus group or the study staff.
The focus group will take about 1.5 hours and will be audio recorded and recorded by a note-taker. The tapes and written notes will be destroyed after transcription of the focus group. The study team will ask how you would feel about being asked to be in an online HIV study and how you would feel about taking part. You will also be shown packages of HIV home test kits that may be sent to participants in an online study. You will be asked what you like and don’t like about them. You will also be asked about the types of tests that may be used in the study, if you would be likely to use them, and who, if anyone, you would likely give these test kits to.
Risks and Discomforts
We do not expect there to be any risks or discomforts in this study. However, this is a group discussion, and we cannot be certain that others in the group will not discuss what was said. You will be asked about taking part in an online HIV study and receiving HIV test kits for use at home. You may feel uneasy talking about these issues. You can choose not to answer any of the questions asked.
Benefits
Taking part in this study may not benefit you personally, but researchers will learn new things that will help them better design the online HIV study and improve the health of MSM.
Token of Appreciation
If you decide to take part in the focus group you will be given $50.
Privacy
Certain offices and people other than the researchers may look at your medical charts and study records. Government agencies and Emory employees overseeing proper study conduct may look at your study records. These offices may include the Emory Institutional Review Board, the Emory Office of Research Compliance, Food and Drug Administration, the Office for Human Research Protections and/or the U.S. Centers for Disease Control and Prevention.
We will not collect any information that will identify you. We got your contact information to get in touch with you for the focus group; after contacting you this information was destroyed. No names will be used in the focus group. We will record the focus group to allow the researchers to listen to them later and to identify the main issues. The tapes will be stored in a locked cabinet where only the study staff will have access to them. We will transcribe the tapes within 10 days and then destroy the tapes and the written notes. If a name or any other personal information is said by accident, we will not include it in the transcription. Agencies and Emory departments and committees that make rules and policy about how research is done have the right to review these records. So do companies and agencies that pay for the study which includes MANILA Consulting Group, Inc. and CDC. We will keep all records that we produce private to the extent we are required to do so by law.
Voluntary Participation and Withdrawal
Being in this research is voluntary and you have the right to refuse to be in this focus group. You can stop at any time after giving your consent without losing benefits that you are otherwise entitled to. The researcher may stop you from taking part in this study at any time if he/she decides it is in your best interest, if you do not follow study instructions, or if others’ behavior becomes disruptive.
Contact Persons
If you have any questions about this study or feel you have been harmed in this study please contact a member of the research team:
Patrick Sullivan, DVM PhD
Emory University
Rollins School of Public Health
1518 Clifton Road NE
Room 464
Atlanta, GA 30322
(404) 727-2038
If you have any questions about your rights as a participant in this study or feel you have been harmed by being in this study you can contact the institutional review board at Emory University.
For Emory University contact:
Emory IRB
1599 Clifton Road
5th Floor East
Atlanta, GA 30322 USA
Tel: 404 712 0720
Toll free: 877 503 9797
Email:[email protected]
You may keep a copy of this form for your records if you like.
If you agree to the above and would like to participate in this study, please give your verbal consent and the study staff will sign below.
Participant Number |
|
|
|
|
|
Printed name of Person Obtaining Consent |
|
|
|
|
|
Signature of Person Obtaining Consent |
Date |
Time |
Appendix F
Focus Group Discussion Guide
Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
Focus Group Discussion Guide
Introduction:
Hi, my name is (YOUR NAME) and I want to thank you for joining us today. We are planning a study on HIV home testing for local men who have sex with men. We want to know your thoughts about home HIV test kits. During the focus group, I’m going to show you examples of HIV test kit packages that will be for guys to test themselves for HIV at home and read their test results, and to collect a blood specimen that they’ll prepare and mail to the researchers. This is like a home test women do for pregnancy, or a test people do to check their blood sugar at home. As of July 2012, people can buy an at-home test kit like this in a drug store, and in the future, it may be possible to get one from the health department or a community-based organization. In the next year, we will also be doing a research study of at-home testing, in which we will mail out home test kits to men around the United States.
Before we begin, I want you to know that there are no right or wrong answers in our discussion. We will simply be discussing your views, opinions and experiences on the use of rapid HIV tests, so please feel comfortable to say what you honestly feel. I would like to tape record the whole session. Please do not be concerned about this: all measures will be taken by the researchers to maintain your privacy. Please also remember to respect each other’s privacy and do not repeat the conversation outside of this room. Information you tell us will ONLY be used for this research project. As we are tape recording the interview, we ask that you refrain from using names or identifying yourself, others, or your partners. Any time during the focus group you can ask for a break, refuse to answer any question, and you are always free to leave. Do you have any questions before we start? Great. Let’s begin.
Question Guide:
Questions for package to be used in Part 2 and Part 3.
First show group entire package, pass around, allow time for each participant to look and feel.
What are your first thoughts on the HIV test kit package?
1a. Do you like the size? What about how heavy or light it is?
1b. Do you think it is discrete? i.e., is it not obvious that this package contains HIV test kits?
1c. Do you like the design?
1d. What, if anything, would you change about the packaging? The labeling of the box?
1e. On a scale of 1 to 10 with 1 being the least appealing and 10 being the most appealing, how would you rate the package?
Ask why they rated the package the way they did.
Facilitator: Open package, pull out instructions, educational brochure and frequently asked questions, placemat and kits.
Facilitator: Pass around instructions and allow participants to read through them.
Please take time to read the instructions insert. What are your first thoughts on the instructions?
2a. Do they make sense? Can you explain them to the group in your own words?
2b. What, if anything, would you change?
2c. Please pay close attention to the wording and images. On a scale of 1 to 10 with 1 being hard to understand and 10 being easy to understand, how would you rate the written instructions? Think about wording and the images. Ask why they rated the written instructions the way they did.
Facilitator: Pass around educational brochure and frequently asked questions
Please take time to read the educational brochure and frequently asked questions. What are your first thoughts on the brochure and frequently asked questions?
3a. Do they make sense? Please tell me the main points in your own words.
3b. What, if anything, would you change?
3c. On a scale of 1 to 10 with 1 being hard to understand and 10 being easy to understand, how would you rate the educational brochure? How would you rate the frequently asked questions? Think about wording and the images. Ask why they rated the educational brochure and frequently asked questions the way they did.
Facilitator: Pass around test kits and DBS collection materials
Please take time to look at the Sure Check test kit and package. What are your first thoughts on the packages?
4a. Are they clearly marked?
4b. Do you understand the differences between each test kit? Can you explain to me what the differences are?
4c. On a scale of 1 to 10 with 1 hard to understand the differences in the kits and 10 being easy to understand the differences between the kits, how would you rate the kit packaging? Ask why they rated the package the way they did.
Facilitator: Open the Sure Check test kit and the DBS specimen collection kit. Pass around the placemat and instructions for Sure Check first, followed by the placemat and instructions for the DBS kit
Please take time to look at the Sure Check placemat and instructions. What are your first thoughts on the placemat and instructions?
5a. Would you use the placemat? Please tell me the main points of the placemat in your own words.
5b. Do the instructions and the placemat make sense when used together?
5c. Would you use the instructions and placemat together if you were testing yourself at home?
5d. What, if anything, would you change?
5e. On a scale of 1 to 10 with 1 being hard to use and 10 being easy to use, how would you rate the placemat? Ask why they rated the placemat and instructions the way they did.
Please take time to look at the DBS specimen collection placemat and instructions. What are your first thoughts on the placemat?
6a. Would you use the placemat? Please tell me the main points of the placemat in your own words.
6b. Do the instructions and the placemat make sense when used together?
6c. Would you use the instructions and placemat together if you were testing yourself at home?
6d. What, if anything, would you change?
6e. On a scale of 1 to 10 with 1 being hard to use and 10 being easy to use, how would you rate the placemat? How would you rate the instructions? Ask why they rated the placemat and instructions the way they did.
Thinking of all the pieces of the package together, what, if anything, do you think is missing?
7a. Are they clearly marked?
7b. Do you understand the differences between each test kit and what order they are to be used in?
7c.
Facilitator: Begin video for Sure Check instructions Allow for participants to watch the video.
What are your first thoughts on the video instructions?
8a. Do they make sense? Can you explain them to the group in your own words?
8b. What, if anything, would you change?
8c. On a scale of 1 to 10 with 1 being hard to understand and 10 being easy to understand, how would you rate the video instructions? Ask why they rated the video instructions the way they did.
Facilitator: Begin video for DBS specimen collection. Allow for participants to watch the video.
What are your first thoughts on the video instructions?
9a. Do they make sense? Can you explain them to the group in your own words?
9b. What, if anything, would you change?
9c. On a scale of 1 to 10 with 1 being hard to understand and 10 being easy to understand, how would you rate the video instructions? Ask why they rated the video instructions the way they did.
Imagine you are part of an online research study. You know that you will receive a package in the mail with test kits and instructions. What are your thoughts on the package we just showed you being delivered to your house? Keep in mind, being part of the research study is completely voluntary.
10a. Would you want to open the package right away?
10b. How likely would you be to test yourself as soon as the package arrived?
10c. What, if anything, would you suggest we change about the packaging to increase the chances that you would test yourself with the kits within 2 days of the package arriving?
10d. What, if anything, would keep you from testing yourself as soon as the package arrived?
10e. What other barriers can you think of to testing yourself and mailing a specimen back in the prepaid mailer?10f. Would you have concerns about privacy based on what the outside package looked like?
10g. On a scale of 1 to 10 with 1 being very unlikely and 10 being very likely, how likely would you be to use these test kits if they were sent to you as part of a study? Why?
Questions for package to be used in Part 4 for Intervention Arm.
First show group entire package, pass around, allow time for each participant to look and feel.
What are your first thoughts on the HIV test kit package?
11a. Do you like the size? What about how heavy or light it is?
11b. Do you think it is discreet? i.e., it is not obvious that this package contains HIV test kits.
11c. Do you like the design?
11d. What, if anything, would you change about the packaging? The color of the box? Labeling of the box?
11e. On a scale of 1 to 10 with 1 being the least appealing and 10 being the most appealing, how would you rate the package?
Ask why they rated the package the way they did.
Facilitator: Open package, pull out package instructions, educational brochure, frequently asked questions, and kits.
Again, imagine you are part of an online research study. You know that you will receive a package in the mail with test kits and instructions. What are your thoughts on the package we just showed you being delivered to your house? Keep in mind, being part of a research study is completely voluntary.
12a. Would you be interested in opening the package and getting started right away?
12b. How likely would you be to test yourself as soon as the package arrived?
12c. What, if anything, would keep you from testing yourself as soon as the package arrived?
What, if anything, would you suggests we change about the packaging to increase the chances that you would test yourself with the kits within 2 days of the package arriving?
12d. What, if anything, would keep you from testing yourself as soon as the package arrived?
12e. What other barriers can you think of to testing yourself and mailing a specimen back in the prepaid mailer?
12f. Would you have concerns about privacy based on what the outside package looked like?
12g. On a scale of 1 to 10 with 1 being very unlikely and 10 being very likely, how likely would you be to use these test kits if they were sent to you as part of a study? Why?
12h. On a scale of 1 to 10 with 1 being very unlikely and 10 being very likely, how likely would you be to give these test kits away if they were sent to you as part of a study? Why?
Questions for package to be used in Part 4 for HIV Positive Group.
First show group entire package, pass around, allow time for each participant to look and feel.
What are your first thoughts on the HIV test kit package?
13a. Do you like the size? What about how heavy or light it is?
13b. Do you think it is discreet? i.e., it is not obvious that this package contains HIV test kits.
13c. Do you like the design?
13d. What, if anything, would you change about the packaging? The color of the box? Labeling of the box?
13e. On a scale of 1 to 10 with 1 being the least appealing and 10 being the most appealing, how would you rate the package?
Ask why they rated the package the way they did.
Imagine you are in the research study and were asked to distribute kits to your friends or sexual partners. You may give these kits to anyone you wish. The packaging in the kit will look similar to what you have just looked at and will have information on how to report test results. You do not have to tell your friend or sexual partner where you got the kit unless you would like to. Would you do this?
14a. Please give us a reason why you would NOT distribute kits to your friends or sexual partners.
14b. Please give us a reason why you WOULD distribute kits to your friends or sexual partners.
Is there anything else you want to add to help us develop the perfect packaging of these HIV self-test kits for use by for gay men or guys who have sex with other guys in a nationwide online study?
END OF FOCUS GROUP
Thank the participants for their time. Ask participants if they have any questions. Give token of appreciation.
APPENDIX G
In-Depth Interview Consent Form
E mory
University, Rollins School of Public Health
mory
University, Rollins School of Public Health
Consent to be a Research Subject
Flesch-Kincaid Reading Level: 7.9
Title: Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
Principal Investigator: Patrick Sullivan, DVM PhD
Funding Sources: Emory University and MANILA Consulting Group, Inc are conducting this study which is sponsored by the Centers for Disease Control and Prevention (CDC).
Introduction
You are invited to be in a research study being done by Emory University’s Rollins School of Public Health. You are being asked because you have told us that you are a man who has sex with men who is over 18 years of age. We expect to have up to 15 men taking part in these interviews. If you decide to take part, the things we learn from you today will help improve a men’s health study.
Purpose
To plan for a study on HIV home testing among men who have sex with men (MSM), we want to know how we can make the instructions for using HIV home test kits easy for men to follow. This study will find out if men understand how to use the HIV home test kits we want to use in the study. We are not asking you to be in the actual study at this time; the things you tell us will help us make home test kit packages that will be used at a later time.
Procedures
When you came here you were given a number. If we pick you to be interviewed, you will be called by your number to go into a secure room where the interview will take place. Before the interview starts, the interviewer will discuss the consent. You will then have the chance to ask any questions and make sure you are comfortable with the process. We will give you a toll-free number for any concerns that you may have with the study or study staff.
The interview will take about 1.5 hours and will be audio and video recorded so the researchers can view and listen to the interview at a later date. The tapes will be destroyed after transcription of the interview. Participants will be asked to interact with the test kit package and each test and describe their understanding of how to do each test. They won’t actually test themselves; they will just talk through what they understand study participants will do. They will be asked to share ideas about how the instructions could be more clear and how the packaging could be changed so that men will be comfortable using the test kits.
Risks and Discomforts
We do not expect there to be any risks or discomforts in this study. You will be asked questions about taking part in an online HIV study and receiving HIV test kits for use at home. You may feel uncomfortable talking about these issues. You can choose not to answer any of the questions asked.
Benefits
Taking part in this study may not benefit you personally, but researchers will learn new things that will help them better design the online HIV study and improve the health of MSM.
Token of Appreciation
If you decide to take part you will be given $50.
Privacy
Certain offices and people other than the researchers may look at your medical charts and study records. Government agencies and Emory employees overseeing proper study conduct may look at your study records. These offices may include the Emory Institutional Review Board, the Emory Office of Research Compliance, Food and Drug Administration, the Office for Human Research Protections and/or the U.S. Centers for Disease Control and Prevention.
We will not collect any information that will identify you personally. We got your contact information to get in touch with you for the interview; after contacting you this information was destroyed. No names will be used in the interview. We will record the interview to allow the researchers to watch and listen to later and to identify the main issues. The tapes will be stored in a locked cabinet where only the study staff will have access to them. We will transcribe the tapes within 10 days and then destroy the tapes. If a name or any other personal information is said by accident, we will not include it in the transcription. Agencies and Emory departments and committees that make rules and policy about how research is done have the right to review these records. So do companies and agencies that pay for the study which includes MANILA Consulting Group, Inc. and CDC. We will keep all records that we produce private to the extent we are required to do so by law.
Voluntary Participation and Withdrawal
Being in this research is voluntary and you have the right to refuse to participate. You can stop at any time after giving your consent without losing benefits that you are otherwise entitled to. The researcher may stop you from taking part in this study at any time if he/she decides it is in your best interest or if you do not follow study instructions.
Contact Persons
If you have any questions about this study or feel you have been harmed in this study please contact a member of the research team:
Patrick Sullivan, DVM PhD
Emory University
Rollins School of Public Health
1518 Clifton Road NE
Room 464
Atlanta, GA 30322
(404) 727-2038
If you have any questions about your rights as a participant in this research study or feel you have been harmed by being in this study you can contact the institutional review board at Emory University.
For Emory University contact:
Emory IRB
1599 Clifton Road
5th Floor East
Atlanta, GA 30322 USA
Tel: 404 712 0720
Toll free: 877 503 9797
Email:[email protected]
You may keep a copy of this form for your records if you like.
If you agree to the above information and would like to be in this study, please give your verbal agreement and the study staff will sign below.
Participant Number |
|
|
|
|
|
Printed name of Person Obtaining Consent |
|
|
|
|
|
Signature of Person Obtaining Consent |
Date |
Time |
APPENDIX H
In-Depth Interview Guide
Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
In-Depth Interview Guide
Introduction: Hello, may name is [name] and I am a researcher at Emory University. We appreciate your willingness to help us as we develop a new kind of HIV testing product for men in our community.
During the next hour, I’m going to show you an example of a product that will be for guys to test themselves for HIV at home and read their test results. This is like a home test women do for pregnancy, or a test people do to check their blood sugar at home. As of July 2012, people can buy an at-home test kit like this in a drug store, and in the future, it may be possible to get one from the health department or a community-based organization. In the next year, we will also be doing a research study of at-home testing, in which we will mail out home test kits to men around the United States.
Today, we’re most interested in your ideas about how the test kits are packaged and if you understand the procedures for conducting self-testing. Imagine that you were participating in an Emory research study, and that the box I’m going to show you came in the mail for your use in the study. We’re going to have you look at the instruction materials (paper and a video) that we have prepared for guys who will use the tests, and we’ll ask you to talk through those instructions and tell us your understanding of how you would test yourself with the different test kits. You won’t actually test yourself. I am going to listen and I may ask you questions as you interact with the test kits and talk through what you would do with the test kits.
Here is the box that would come in the mail. Please open it as you would if you received it at home. As you see each of the contents in the box, please talk out loud about what you are seeing, what you understand you are supposed to do with the contents, and in what order. As we mentioned in the consent, we’ll also be videotaping your hands and the work area; this will help us understand better what you are seeing and doing as you describe how you would use the kits.
At this point, please do not actually open the individually packaged tests; please just tell me about what you understand about what you need to do with the materials in the box. We’ll actually open the individual tests a little later.
[The participant is given the box. Interviewer will record the participant’s comments on the materials. If the participant is not verbalizing comments on the materials, the interviewer will prompt with open-ended prompts such as: “Please tell me which part of the kit you are looking at, and what you are supposed to do with it.” “Please remember to talk out loud about what you are seeing, and what you are supposed to do with what you see in your own words.” “Please tell me if there’s anything that is unclear about the kit, or what you are supposed to do with it.”
After the participant has removed all of the kit contents and talked about what he understands he is supposed to do with them, ask:
“Are there any of the steps in the instructions that you think could be made clearer?” Use open-ended probes to elicit information about the nature of what was not clear, and how the participant thinks it could be made clearer. ]
APPENDIX I
Proficiency Assessment Recruitment Script
Proficiency Assessment Recruitment Script
Hello [name of participant], my name is [name] and I am calling from the [Involvement, MAN Project, IMPACT] study at Emory University. You have indicated that you are willing to participate in future research and I would like to discuss an opportunity you may be interested in. A token of appreciation will be provided if you are eligible and you decide to participate in the study. Do you have a minute to go over this?
If no: Okay, no problem at all. Thank you for your time and have a great day.
If yes: Okay, thank you! We are conducting a study to help us understand how men who are not trained in testing can test themselves for HIV at home. We want to have men use the test kits we are planning to use for a future study to see if they can follow the test kit instructions, accurately conduct self-testing, and interpret the results. A token of appreciation will be provided if you are eligible and you decide to participate in the study. If you are interested, we would ask you to come in to Emory University for about 1.5 hours to participate in a self-testing session. Is this something that you might be interested in?
If no: Okay, no problem at all. Thank you for your time and have a great day.
If yes: Great! How about we set up a time for you to come in? [Review times, confirm contact information, set up meeting.]
APPENDIX J
Proficiency Assessment Consent Form
Emory University, Rollins School of Public Health
Consent to be a Research Subject
Flesch-Kincaid Reading Level: 7.9
Title: Evaluation of Rapid HIV Self-Testing among MSM in High Prevalence Cities
Principal Investigator: Patrick Sullivan, DVM PhD
Funding Sources: Emory University and MANILA Consulting Group, Inc are conducting this study which is sponsored by the Centers for Disease Control and Prevention (CDC).
Introduction
You are invited to be in a research study being done by Emory University’s Rollins School of Public Health. You are being asked because you have told us that you are a man who has sex with men who is over 18 years of age. We expect to have up to 40 men taking part in this research. If you decide to take part, the things we learn from you today will help improve a men’s health study.
Purpose
To plan for a study on HIV home testing among men who have sex with men (MSM), we want to know if men can easily use the HIV home test kits we want to use in the study. We are not asking you to be in the actual study at this time; the things you tell us will help us improve the home test kit instructions that will be used at a later time.
Procedures
Participants will be given a testing kit package that they will open and conduct the testing process following the kit instructions. Written instructions are included with each individual test and there will also be access to an electronic application that will provide video instructions and timers for each test. After each rapid test done and interpreted by the participant, a study staff member trained in HIV testing will interpret the test results. Participants will also collect and prepare a dried blood spot specimen for shipping. After the self-testing, participants will complete a survey about the self-testing process, and each participant will use an electronic application to review a panel of test result images. At the end of their session, participants will have the option to receive counselor provided HIV screening from trained Emory staff and to speak to the trained HIV counselor or other study staff.
The testing will take about 1.5 hours and will be audio and video recorded. The tapes will be destroyed after any transcription of the interview. Participants will be asked to do 2 self-tests and collect a few drops of blood. They will record their test results on paper and in an electronic application. A trained tester will also read the results. They will be asked to share ideas about how the instructions could be more clear. After each test they will view test results in an electronic application and record the result of each test.
Those who test positive on any of the self-tests or the post-test during the study will be offered HIV counseling and testing by trained Emory staff. This is a voluntary service that the participant can accept or not. Information will be collected by the Emory staff for HIV reporting purposes (name, mailing address and phone number) and if they are confirmed HIV-positive, this information will be reported by telephone to the Georgia Department of Public Health. This information remains confidential when it is reported to the state health department. If the confirmatory testing is negative, this information will be destroyed. We will also give you a toll-free number for any concerns that you may have with the study or study staff. If we pick you to take part, you will go into a secure room where the session will take place. Before the testing starts, the study staff will discuss the consent. You will then have the chance to ask any questions and make sure you are comfortable with the process.
Risks and Discomforts
We do not expect there to be any risks. There may be minor discomfort from the finger-stick HIV tests and bruising may occur. You may feel uneasy talking about HIV. You can choose not to answer any of the questions asked.
Benefits
Subjects will learn their current HIV status based upon a self-test or from a trained counselor if they accept the additional testing service. There are no additional direct benefits to the participant of participation in the research, but researchers will learn new things that will help them better design the online HIV study and improve the health of MSM.
Token of Appreciation
If you decide to take part you will be given $75 for completing the self-testing activities for this study. You will not receive compensation for the voluntary HIV testing provided by Emory University.
Confidentiality
Certain offices and people other than the researchers may look at your medical charts and study records. Government agencies and Emory employees overseeing proper study conduct may look at your study records. These offices may include the Emory Institutional Review Board, the Emory Office of Research Compliance, Food and Drug Administration, the Office for Human Research Protections and/or the U.S. Centers for Disease Control and Prevention.
We will not collect any information that will identify you personally. No names will be used in the session. We will record the session to allow the researchers to watch and listen to later and to identify the main issues. The tapes will be stored in a locked cabinet where only the study staff will have access to them. We will transcribe the tapes within 10 days and then destroy the tapes. If a name or any other personal information is said by mistake, we will not include it. Agencies and Emory departments and committees that make rules and policy about how research is done have the right to review these records. So do companies and agencies that pay for the study which includes MANILA Consulting Group, Inc. and CDC. We will keep all records that we produce private to the extent we are required to do so by law.
If any test result is preliminarily positive, they will be offered HIV counseling and testing by trained Emory staff. This is a voluntary service that the participant can accept or not. Information will be collected by the Emory staff for HIV reporting purposes (name, mailing address and phone number) and if they are confirmed HIV-positive, this information will be reported by telephone to the Georgia Department of Public Health. This information remains confidential when it is reported to the state health department. If the confirmatory testing is negative, this information will be destroyed.
Voluntary Participation and Withdrawal
Being in this research is voluntary and you have the right to refuse to take part. You can stop at any time after giving your consent without losing benefits that you are otherwise entitled to. Study staff may stop you from taking part in this study at any time if they decide it is in your best interest or if you do not follow study instructions.
Contact Persons
If you have any questions about this study or feel you have been harmed in this study please contact a member of the research team:
Patrick Sullivan, DVM PhD
Emory University
Rollins School of Public Health
1518 Clifton Road NE
Room 464
Atlanta, GA 30322
(404) 727-2038
If you have any questions about your rights as a participant in this study or feel you have been harmed by being in this study you can contact the institutional review board at Emory University.
For Emory University contact:
Emory IRB
1599 Clifton Road
5th Floor East
Atlanta, GA 30322 USA
Tel: 404 712 0720
Toll free: 877 503 9797
Email:[email protected]
You may keep a copy of this form for your records if you like.
If you agree to the above information and would like to be in this study, please give your written agreement below.
Print Volunteer’s Name |
|
|
|
|
|
Signature of Volunteer |
Date |
Time |
|
|
|
Printed name of Person Obtaining Consent |
|
|
|
|
|
Signature of Person Obtaining Consent |
Date |
Time |
APPENDIX K
Proficiency Assessment Observation Forms
Untrained user observation form 1a
To be completed by Study Staff
Date: ____/____/____
Self-Tests Conducted by Participant ID # _______________
Observations by: ____________________________
Ambient Temperature of room where observed self-test is being conducted: _____
Storage Temperature of room or fridge where test kits are being stored: _____
Have controls been run? ___________
Result of controls: __________________
What time device is the study staff using during observation?
What time device is the participant using during self-testing?
Form 1a. Evaluation of effectiveness and safety of sample collection by untrained potential users
Monitor the ability of study participants to properly collect a test specimen. Note down any deviations from the procedure described in the test kit, along with any impact on the test result (no impact, invalid test result, incorrect test result) (see Form 3 below).
OraQuick
Lot Number:______
Expiration Date: _______
Reads package instructions
Watches video instructions
Gathers materials needed
Identifies an appropriate workspace (fully lighted area, not under A/C air, flat surface)
Allows the test kit to come to operating temperature (59-99F)
Examines test kit pouch (makes sure it is unopened, makes sure there is an absorbent/desiccant packet)
Covers workspace with a clean, disposable, absorbent workspace cover
Sets an Oraquick reusable test stand on workspace
Successfully opens the pouch
Leaves the test device in the pouch, to prevent contamination, until ready to use
Positions vial in stand (no spillage, vial to bottom of stand)
Removes test device from pouch
Does not touch the flat pad
Inserts the flat pad above the teeth against the outer gum, between upper gum and upper lip and swabs top gum, then bottom gum once, as shown in picture
Does not swab the inside of the cheek, tongue, or roof of mouth
Successfully completes all collection steps (if not, note what was missing/incorrect below):
_________________________________________________________
_________________________________________________________
Untrained user observation form 1b
To be completed by Study Staff
Date: ____/____/____
DBS collection conducted by Participant ID # _______________
Observations by: ____________________________
Ambient Temperature of room where observed DBS collection is being conducted: _____
Form 1b. Evaluation of effectiveness and safety of DBS specimen collection by untrained potential users
Monitor the ability of study participants to properly collect a DBS specimen. Note down any deviations from the procedure described in the collection kit.
Dried blood spot collection kit
Lot Number:______
Expiration Date: _______
Writes Collection Date
Reads package instructions
Watches video instructions
Washes hands with soap and warm (not hot) tap water
Dries hands thoroughly
Examines package contents
Lays out package contents
Writes date and time on the DBS card
Lays DBS card flat on table with circles facing up
Chooses a puncture site off center of the fingertip, preferably on the middle or ring finger.
Cleans Puncture Site with Alcohol Pad
Stimulates Blood Flow to the Fingers
Positions Hand on a table or countertop with the palm up
Presses the lancet firmly against his finger. Does not remove the device from finger until he hears a click.
Wipes away the first drop with sterile gauze
Turns hand over and lets large drops of blood collect at the puncture site.
Participant has steady blood flow
Participant has low blood flow
To increase blood flow, gently applies pressure at base of finger.
To increase blood flow, the participant stands up and keeps hand below waistline.
To increase blood flow, the participant uses the gauze to wipe the opening with a solid swipe to get the blood flowing again
Once a large drop of blood has formed, drops or touches the blood drop gently inside the circles printed on the DBS Card.
Does not touch card with finger
Applies blood drops inside the circle until it is completely filled.
After he fills one circle, moves to the next circle; fills the remaining circles one at a time.
Once he leaves a circle he doesn’t go back.
Fills less than 3 circles
Fills 3 - 4 circles
Fills 5 circles
Applies bandage
Places Used Safety Lancet(s) in Lancet Disposal Container
SURE CHECK® HIV 1/ 2 Assay (Sure Check)
Lot Number:______
Expiration Date: _______
Reads package instructions
Watches video instructions
Gathers materials needed
Identifies an appropriate workspace (fully lighted area, not under A/C air, flat surface)
Allows the test kit to come to operating temperature (64-86F)
Examines test kit pouch (makes sure it is unopened, makes sure there is an absorbent/desiccant packet)
Covers workspace with a clean, disposable, absorbent workspace cover
Opens pouch and arranges materials provided (sampler with test strip, buffer vial, safety lancet, bandage, desiccant, disposable rack)
Removes buffer vial from top of sampler
Places vial in disposable rack
Cleans finger with antiseptic wipe
Allows the finger to dry thoroughly or wipe dry with sterile gauze pad
Pricks finger using lancet
Punctures skin just off the center of the finger
Wipes away the first drop with sterile gauze
Does not squeeze fingertip
Collects sample from second drop
Touches blood drop with capillary tip of sampler
Sampler tip is full
Successfully completes all collection steps (if not, note what was missing/incorrect below):
_________________________________________________________
_________________________________________________________
Untrained user observation form 2
To be completed by Study Staff
Date: ____/____/____
Tests Conducted by Participant ID # _______________
Observations by: ____________________________
Form 2. Evaluation of the ability of untrained potential users to perform the test properly
Monitor study participants for their ability to follow the instructional materials on the running of the test after the specimen is collected, including application of the specimen to the device, the addition of reagents, and proper timing of the test. Note any deviation from the instructional materials.
OraQuick
Reads package instructions
Watches video instructions
Successfully inserts flat pad of the test device into the vial (no spillage, result window forward, flat pad touching bottom of vial)
Did NOT touch flat pad when inserting test kit
Accurately records start time and temperature on provided slip
Accurately records end time on provided slip
Does not remove the device from vial while test is running
Reads results between 20-40 minutes
Successfully completes all steps (if not, note what was missing/incorrect below):
_________________________________________________________
_________________________________________________________
SURE CHECK®
Reads package instructions
Watches video instructions
Pushes capillary tip of sampler completely into buffer vial through foil cover
Pushes to the bottom of the vial until sampler and buffer vial snap together tightly (3 snaps)
Does not jab into foil cover
Accurately records start time and temperature on provided slip
Accurately records end time on provided slip
Keeps the buffer vial upright in the disposable rack
Reads result between 15-20 minutes
Does not read results after 20 minutes
Successfully completes all steps (if not, note what was missing/incorrect below):
Untrained user observation form 3
To be completed by Study Staff
Date: ____/____/____
Tests Conducted by Participant ID # _______________
Observations by: ____________________________
Form 3. Evaluation of the ability of untrained potential users to read and interpret self- testing results (see page 5)
Monitor study participants for their ability to correctly interpret their own test results (negative, preliminary positive, invalid) and identify any follow-up actions that should be taken consistent with the informational materials provided with the test kit (e.g., additional counseling, follow-up testing).
Compare the results of testing by untrained potential users to the results of testing by trained personnel using appropriate statistical methods, including percent positive agreement and percent negative agreement, each with 95% two-sided confidence interval lower bounds of at least 95%.
Rapid Self- Test 1: OraQuick
Observe and write down:
Start time
End time
Number of minutes from starting timer to reading test result
Once the participant finishes self-testing with OraQuick enter room to check device and interpret result within the optimal period (20-40 minutes)
Result (Please check one of the answers):
____ Preliminary Positive
_____Negative
_____Invalid
Select the image that most looks like your device:
Strong positive
Weak positive
Negative
Invalid
Blank
None
Ask the participant the following questions:
If you found any difficulty conducting the test, please indicate what was difficult.
Do you have any comment or question about the directions/material provided?
Do you have any comment or question about the test device?
Rapid Self- Test 2: SURE CHECK® HIV 1/ 2 Assay (Sure Check)
Observe and write down:
Start time
End time
Number of minutes from starting timer to reading test result
Once the participant finishes self-testing with Sure Check enter room to check device and interpret result within the optimal period (15-20 minutes)
Result (Please check one of the answers):
____ Preliminary Positive
_____Negative
_____Invalid
Select the image that most looks like your device:
Strong positive
Weak positive
Negative
Invalid
Blank
Ask the participant the following questions:
If you found any difficulty conducting the test, please indicate what was difficult.
Do you have any comment or question about the directions/material provided?
Do you have any comment or question about the test device?
Did participant accept rapid testing from Emory staff to confirm results?
Yes
No
Test used and results
___ Clearview
___ Insti
____ Preliminary Positive
_____Negative
_____Invalid
APPENDIX L
Proficiency Assessment Survey
Self-Test Results
To be completed by Participant
Date: ____/____/____
Tests Conducted by Participant ID # _______________
Instructions: After performing each self-test, please check the result option. Write down any comment you have in the comment boxes.
Rapid Self- Test 1: OraQuick
Sample Type: Oral Fluid
Result (Please check one of the answers):
____ Preliminary Positive
_____Negative
_____Invalid
Rapid Self- Test 2: SURE CHECK®
Sample Type: Whole blood finger stick
Result (Please check one of the answers):
____ Preliminary Positive
_____Negative
_____Invalid
Proficiency Assessment Survey
Date: ____/____/____
Participant ID # _______________
What is the highest level of education you have completed?
___ Less than high school
___ Some high school
___ High school diploma or GED
___ Some college, Associate’s Degree, or Technical Degree
___ College, post graduate or professional school
What is your age?
______
Do you consider yourself to be Hispanic or Latino?
___ No
___ Yes
Which racial group or groups do you consider yourself to be in? Check all that apply:
___ American Indian or Alaska Native
___ Asian
___ Black or African American
___ Native Hawaiian or Other Pacific Islander
___ White
Have you ever had a job where you conduct laboratory tests or experiments?
___ Yes
___ No
Have you ever used “The Home Access® HIV-1 Test System”, where you collect your own blood sample and ship it to the Home Access laboratory?
___ Yes
___ No
Have you ever used any other kind of over-the-counter self-test before? Examples of these tests include cholesterol.
___ Yes. Which test? ___________________________
___ No
Post-test Evaluation
Self-testing
What type of additional training or information, if any, would you have liked to have had before performing the tests? (Check all that apply)
___ No additional training/information was needed besides the instructions provided
___ Additional video demonstration
___ Demonstration in person by laboratory worker
___ More detailed written instructions
___ Other: ________________________________________________
After you completed the OraQuick (oral fluid test), how confident were you that you could perform the test according to the instructions?
___ Very Confident
___ Somewhat confident
___ Not very confident
___ Not confident at all
After you completed the Sure Check test (finger stick test), how confident were you that you could perform the test according to the instructions?
___ Very Confident
___ Somewhat confident
___ Not very confident
___ Not confident at all
What questions, if any do you still have about how to perform the test?
DBS specimen collection and packaging
What type of additional training, if any, would you have liked to have had before the dried blood spot sample? (Check all that apply)
___ No additional training was needed besides the instructions provided
___ Additional video demonstration
___ Demonstration in person by laboratory worker
___ More detailed written instructions
___ Other: ________________________________________________
After you collected the blood sample on the DBS card, how confident were you that you could collect your own blood sample according to the instructions?
___ Very Confident
___ Somewhat confident
___ Not very confident
___ Not confident at all
After you finished packaging the DBS card, how confident were you that you could package the DBS card according to directions?
___ Very Confident
___ Somewhat confident
___ Not very confident
___ Not confident at all
What questions, if any do you still have about how to collect and package a DBS sample?
APPENDIX M
Proficiency Assessment Testing Panel Images
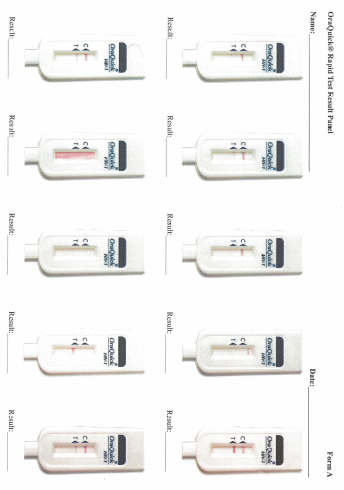
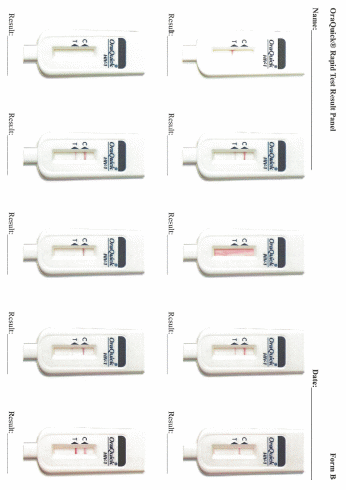
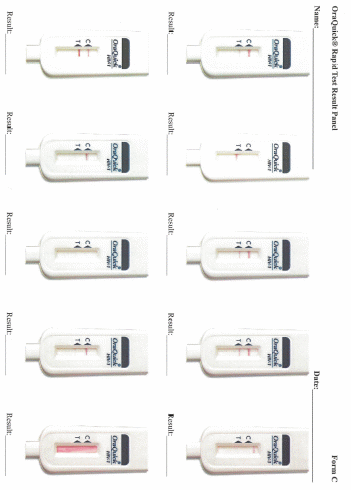 S
S ure
Check Results Panel Form D
ure
Check Results Panel Form D
Forms A-D. Evaluation of the ability of untrained potential users to read and interpret examples of test results
Study participants whose HIV status was not known prior to testing will be evaluated for their ability to correctly interpret a set of test results obtained with the investigational test (i.e., a random mix of devices with non-reactive, weakly reactive, strongly reactive, and invalid test results). Each study participant will be asked to interpret approximately 10 test results of each type. Results will be evaluated using appropriate statistical methods, including calculations of percent agreement for each type of result, each with 95% two-sided confidence interval lower bounds of at least 98% for non-reactive, strong reactive and invalid results and at least 95% for weak reactive results.
APPENDIX N
eSTAMP Participant Self-Test Referral Sheet
eSTAMP Participant Self-Test Referral Sheet
Completed by eSTAMP Study Staff
Dear eSTAMP Study Participant:
Thank you for your participation in the study and for using one or both of the following HIV rapid test kits we provided:
OraQuick® In-Home HIV Test, an oral fluid HIV test you conducted, interpreted, then entered your test results on our study website; and/or
Sure Check® HIV 1/2 Assay, a blood finger-stick HIV test you conducted, interpreted, then entered your test results on our study website; and/or
Early detection and treatment of HIV is extremely important. Because you had a preliminary positive test result on one or more of the tests you used as part of the study, we recommend you take follow-up precautions and seek further testing for HIV. To assist you and your health care provider, we ask that you take this form with you to your first medical visit, so that your provider is aware of the type of test(s) you have completed and the results. This information can help you and your provider identify the next steps that are right for you.
Below are the results based on your interpretation and the counselor’s interpretation of the rapid HIV tests that you conducted on yourself. You can take this letter to your health care provider to help explain why you are seeking follow-up HIV testing.
Participant assessment of self-testing results
Preliminary
Positive Negative Invalid Test Date
OraQuick® In-Home HIV Test ______ _______ _____ ___/___/__
Sure Check® HIV 1/2 Assay ______ _______ _____ ___/___/__
Trained counselor’s assessment of participant’s self-testing results
Preliminary
Positive Negative Invalid Test Date
OraQuick® In-Home HIV Test ______ _______ _____ ___/___/__
Sure Check® HIV 1/2 Assay ______ _______ _____ ___/___/__
Trained counselor’s HIV rapid test results
Preliminary
Positive Negative Invalid Test Date
Clearview® Complete HIV 1/2 ______ _______ _____ ___/___/__
INSTITM HIV-1 Antibody Test ______ _______ _____ ___/___/__
Dear Provider:
If you have questions regarding the HIV rapid tests used in this study, please contact:
Colleen Kelly, MD
Emory University
Atlanta, GA
404-616-9724
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | aricca |
| File Modified | 0000-00-00 |
| File Created | 2021-02-03 |
© 2026 OMB.report | Privacy Policy