CMS-10237 Part-C Medicare Advantage Application
Medicare Advantage Applications - Part C and regulations under 42 CFR 422 subpart K
2011PartC_MA_AppV15_09142009_CLEAN_Draft
Medicare Advantage Applications - Part C (CY 2011)
OMB: 0938-0935
PART C -MEDICARE ADVANTAGE
APPLICATION
For all new applicants and existing Medicare Advantage contractors seeking to expand a service area -- CCP, PFFS, MSA, RPPO, SNPs, and EGWPs
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Medicare & Medicaid Services (CMS)
Center for Drug and Health Plan Beneficiary Choice (CPC)
Medicare Drug and Health Plan Contract Administration Group (MCAG)
Medicare Advantage Coordinated Care Plans (CCPs) must offer Part D prescription drug benefits under at least one Medicare Advantage plan in each county of its service area, and therefore must timely submit a Medicare Advantage-Prescription Drug(MA-PD) application to offer Part D prescription drug benefits as a condition of approval this application.
PUBLIC REPORTING BURDEN: According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-0935. The time required to complete this information collection is estimated to average 33 hours per response, including the time to review instructions, search existing data resources, and gather the data needed, and complete and review the information collection. If you have any comments, concerning the accuracy of the time estimate(s) or suggestions for improving this form, please write to CMS, Attn: Reports Clearance Officer, 7500 Security Boulevard, Baltimore, Maryland 21244-1850.
1.5 Health Plan Management System (HPMS) 7
1.8 Due date for MA Application 9
1.9 Withdrawing a Pending Initial and Service Area Application Requests 10
1.10 Application Determination Appeal Rights 11
2. INSTRUCTIONS 12
2.2 Applicant Seeking to Offer New Employer/Union-Only Group Waiver Plans (EGWPs) 12
2.3 Applicants Seeking to Offer Employer/Union Direct Contract Private Fee-For Service (PFFS) MAO 13
2.4 Applicants Seeking to Offer Special Needs Plans (SNP) 13
2.6 Chart of Required Attestations by Type of Applicant 14
2.7 Health Service Delivery Tables Instructions 16
2.8 Document (Upload) Submission Instructions 17
2.9 MA Part D (MA-PD) Prescription Drug Benefit Instructions 17
3. ATTESTATIONS 18
3.1 Experience & Organization History 18
3.2 Administrative Management 18
3.9 CMS Provider Participation Contracts & Agreements 30
3.10 Contracts for Administrative & Management Services 32
3.11 Health Services Management & Delivery 35
3.12 Quality Improvement Program 37
3.14 Working Aged Membership 45
3.17 Communications between Medicare Advantage Organization and CMS 47
3.20 Health Insurance Portability and Accountability Act of 1996 (HIPAA) 51
3.22 Medicare Advantage Certification 53
3.24 RPPO Essential Hospital 56
3.28 General Administration/Management 62
3.29 MSA Demonstration Addendum 63
4. Document Upload Templates 65
4.1 Experience & Organization History 65
4.2 CMS Provider Participation Contracts and/or Agreements Matrix 67
4.3 Contracts for Administrative & Management Services Matrix 71
4.4 CMS Insurance Coverage Table for Medicare Advantage Organizations 74
4.6 Regional Preferred Provider Organization (RPPO) Essential Hospital Designation Table 80
4.7 Regional Preferred Provider Organization (RPPO) Essential Hospital Attestation 81
4.8 Quality Improvement Program Template 82
5. APPENDIX I-Solicitations for Special Needs Plan Proposal 83
6. APPENDIX II- Employer/Union-Only Group Waiver Plans (EGWPs) MAO “800 Series” 119
6.3 Request for Additional Waiver/Modification of Requirements (Optional) 120
7. APPENDIX III : Employer/Union Direct Contract Private Fee-For Service (PFFS) MAO Application 128
7.3 Request for Additional Waiver/Modification of Requirements (Optional) 129
1. GENERAL INFORMATION
The Medicare Prescription Drug, Improvement, and Modernization Act (MMA) significantly revised the Medicare + Choice managed care program, now called the Medicare Advantage (MA) program, and added outpatient prescription drugs to Medicare (offered by either stand-alone prescription drug plan sponsors or MA organizations). The MMA changes make managed care more accessible, efficient, and attractive to beneficiaries seeking options to meet their needs. The MA program offers several kinds of plans and health care choices, such as regional preferred provider organization plans (RPPOs), private fee-for-service plans (PFFS), Special Needs Plans (SNPs), and Medical Savings Account plans (MSAs).
The Medicare outpatient prescription drug benefit is a landmark addition to the Medicare program. More people have prescription drug coverage and are saving money on prescription drugs than ever before. Costs to the government for the program are lower than expected, as are premiums for prescription drug plans.
People with Medicare not only have more quality health care choices than in the past but also more information about those choices. The Centers for Medicare & Medicaid Services (CMS) welcome organizations that can add value to these programs, make them more accessible to Medicare beneficiaries, and meet all the contracting requirements.
The MA program is comprised of a variety of product types including:
Health Maintenance Organizations (HMOs) with/without a Point of Service (POS) benefit
Local Preferred Provider Organizations (LPPOs)
Regional Preferred Provider Organizations (RPPOs)
Special Needs Plans (SNPs)
Private Fee-for-Service (PFFS) plans
Medical Savings Account plans (including Medical Savings Account Demonstration plans)
Employer Group Waiver plans
Note: For facts sheets on each of these types of product offerings go to http://www.cms.hhs.gov/home/medicare.asp
Qualifying organizations may contract with CMS to offer any of these types of products. To offer one or more of these products an application must be submitted according to the instructions in this application.
Note: The Medicare Modernization Act requires that CCPs offer at least one MA plan that includes a Part D prescription drug benefit (an MA-PD) in each county of its service area. To meet this requirement, the applicant must timely complete and submit a separate Medicare Advantage Group Prescription Drug Plan application (MA-PD application) in connection with this Part C Application.
PFFS plans have the option to offer the Part D drug benefit. MSA plans cannot offer the Part D drug benefit.
The following are key references about the MA program:
Social Security Act -- 42 USC 1395 et seq. http://www.ssa.gov/OP_Home/ssact/title18/1800.htm
Medicare Regulations--42 CFR 422
Medicare Managed Care Manual-- http://www.cms.hhs.gov/Manuals/Medicare Marketing Guidelines –http://www.cms.hhs.gov/ManagedCareMarketing/
CMS Central and Regional Office staffs are available to provide technical support to all Applicants during the application process. While preparing the application, applicants may call Letticia Ramsey in the CMS Central Office at (410) 786-5262 or by email [email protected]. Applicants should contact a Regional Office to request assistance for specific issues in response to deficiency letters. Below is a list of CMS Regional Office contacts.
This list is also available at http://www.cms.hhs.gov/HealthPlansGenInfo/Downloads/cmsregional.pdf
RO I CMS – BOSTON REGIONAL OFFICE
John F. Kennedy Federal Building, Room 2375, Boston, MA 02203
Telephone: 617-565-1267
States: Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont
RO II CMS – NEW YORK REGIONAL OFFICE
26 Federal Plaza, Room 3811, New York, NY 10278
Telephone: 212-616-2353
States: New Jersey, New York, Puerto Rico, and Virgin Islands
RO III CMS – PHILADELPHIA REGIONAL OFFICE
Public Ledger Building, Suite 216, 150 S. Independence Mall West, Philadelphia, PA 19106-3499
Telephone: 215-861-4224
States: Delaware, District Of Columbia, Maryland, Pennsylvania, Virginia, and West Virginia
RO IV CMS – ATLANTA REGIONAL OFFICE
Atlanta Federal Center, 61 Forsyth Street, SW, Suite 4T20, Atlanta, GA 30303-8909
Telephone: 404-562-7362
States: Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, and Tennessee
RO V CMS – CHICAGO REGIONAL OFFICE
233 North Michigan Avenue, Suite 600, Chicago, IL 60601-5519
Telephone: 312-353-3620
States: Illinois, Indiana, Michigan, Minnesota, Ohio, and Wisconsin
RO VI CMS – DALLAS REGIONAL OFFICE
1301 Young Street, Room 833, Dallas, TX 75202
Telephone: 214-767-4471
States: Arkansas, Louisiana, Oklahoma, New Mexico, and Texas
RO VII CMS – KANSAS CITY REGIONAL OFFICE
Richard Bolling Federal Office Building, 601 East 12th Street., Room 235, Kansas City, MO, 64106
Telephone: 816-426-5783
States: Iowa, Kansas, Missouri, and Nebraska
RO VIII CMS -- DENVER REGIONAL OFFICE
1600 Broadway, Suite 700, Denver, CO 80202
Telephone: 303-844-2111
States: Colorado, Montana, North Dakota, South Dakota, Utah, and Wyoming
RO IX CMS – SAN FRANCISCO REGIONAL OFFICE
Division of Medicare Health Plans Operations
90 7th Street, Suite 5-300 (5w), San Francisco, CA 94103-6707
Telephone: 415-744-3617
States: Arizona, California, Guam, Hawaii, Nevada, American Samoa, and The Commonwealth Of Northern Mariana Islands
RO X CMS -- SEATTLE REGIONAL OFFICE
Medicare Managed Care Branch
2201 6th Avenue, Rx-47, Room 739, Seattle, WA 98121-2500
Telephone: 206-615-2351
States: Alaska, Idaho, Oregon, and Washington
For general information about this application, please send an email to the following email address: [email protected].
CMS conducts special training sessions and user group calls for new Applicants and existing contractors. All Applicants are strongly encouraged to participate in these sessions, which are announced via the Health Plan Management System (see below) and/or CMS main website.
The HPMS is the primary information collection vehicle through which MA organizations will communicate with CMS in support of the application process, bid submission process, ongoing operations of the MA program, and reporting and oversight activities.
Applicants are required to enter contact and other information collected in the HPMS in order to facilitate the application review process. Applicants must promptly enter organizational data into the HPMS and keep the system accurate. This ensures that CMS has timely information and is able to provide guidance to the appropriate contacts within the organization. In the event that an applicant is awarded a contract, this information will also be used for frequent communications during implementation. Therefore, it is important that this information be accurate at all times.
The HPMS is also the vehicle used to disseminate CMS guidance to MA organizations. The information is then incorporated in the appropriate manuals. It is imperative for MA organizations to independently check the HPMS notices and incorporate the guidance as indicated in the notices.
Organizations interested in offering a new Medicare Advantage product or expanding the service area of an existing product or submitting a PFFS network transition application must complete a Notice of Intent to Apply by November 17, 2009. Upon submitting the completed form to CMS, the organization will be assigned a pending contract number (H number) to use throughout the application and subsequent operational processes.
Once the new contract number is assigned, the applicant should request a CMS User ID. An application for Access to CMS Computer Systems (for HPMS access) is required and can be found at: https://applications.cms.hhs.gov. Upon approval of the CMS User ID request, the applicant will receive a CMS User ID(s) and password(s) for HPMS access. Existing MAO’s requesting service area expansions do not need to apply for a new MAO contract number.
On or before the first Monday of June of every year, MA organizations must submit a bid, comprised of the proper benefits and pricing for each MA plan for the upcoming year based on its determination of expected revenue needs. Each bid will have 3 components: original Medicare benefits (A/B); prescription drugs under Part D (if offered under the plan); and supplemental benefits. Bids must also reflect the amount of enrollee cost sharing. CMS will review bids and request additional information if needed. MA Organizations must submit the benefit plan or plans it intends to offer under the bids submitted. No bid submission is needed at the time the application is submitted. Further instructions and time frames for bid submissions are provided at http://www.cms.hhs.gov/MedicareAdvantageApps.
In order to prepare plan bids, Applicants will use the HPMS to define its plan structures, associated plan service areas, and then download the Plan Benefit Package (PBP) and Bid Pricing Tool (BPT) software. For each plan being offered, Applicants will use the PBP software to describe the detailed structure of its MA benefit and the BPT software to define its bid pricing information.
Once the PBP and BPT software have been completed for each plan being offered, Applicants will upload their bids to the HPMS. Applicants will be able to submit bid uploads to the HPMS on its PBP or BPT one or more times between May and the CY bid deadline, which is the first Monday in June each year CMS will use the last successful upload received for a plan as the official bid submission.
CMS will provide technical instructions and guidance upon release of the HPMS bid functionality as well as the PBP and BPT software. In addition, systems training will be available at the Bid Training in Spring 2010.
All MA organizations must submit information about its membership to CMS electronically and have the capability to download files or receive electronic information directly. Prior to the approval of a contract, MA organizations must contact the MMA Help Desk at 1-800-927-8069 for specific guidance on establishing connectivity and the electronic submission of files. Instructions are also on the MMA Help Desk web page, http://www.cms.hhs.gov/mmahelp, in the Plan Reference Guide for CMS Part C/D systems link. The MMA Help Desk is the primary contact for all issues related to the physical submission of transaction files to CMS.
Applicants may seek to protect its information from disclosure under the Freedom of Information Act (FOIA) by claiming that FOIA Exemption 4 applies. The Applicant is required to label the information in question “confidential” or “proprietary” and explain the applicability of the FOIA exemption it is claiming. When there is a request for information that is designated by the Applicant as confidential or that could reasonably be considered exempt under FOIA Exemption 4, CMS is required by its FOIA regulation at 45 CFR §5.65(d) and by Executive Order 12,600 to give the submitter notice before the information is disclosed. To decide whether the Applicant’s information is protected by Exemption 4, CMS must determine whether the Applicant has shown that: (1) disclosure of the information might impair the government's ability to obtain necessary information in the future; (2) disclosure of the information would cause substantial harm to the competitive position of the submitter; (3) disclosure would impair other government interests, such as program effectiveness and compliance; or (4) disclosure would impair other private interests, such as an interest in controlling availability of intrinsically valuable records, which are sold in the market. Consistent with our approach under other Medicare programs, CMS would not release information that would be considered proprietary in nature if applicant has shown it meets requirements for FOIA Exemption 4.
Payment Information Form
Please complete the Payment Information form that is located at:
http://www.cms.hhs.gov/MedicareAdvantageApps/Downloads/pmtform.pdf
The document contains financial institution information and Medicare contractor data.
If the applicant has questions about this form, please contact Yvonne Rice at (410) 786-7626. The completed form needs to be faxed to Yvonne Rice at (410) 786-0322.
Applications must be submitted by 11:59 P.M. EST, February 25, 2010. CMS will not review applications received after this date and time. Applicants’ access to application fields within the HPMS will be blocked after this date and time.
Below is a tentative timeline for the Part C (MA program) application review process:
APPLICATION REVIEW PROCESS * |
|
Date |
Milestone |
November 17, 2009
|
1. Submit notice of intent to apply to CMS 2. Request HPMS Access (Includes User ID and Password Request) 3. Request CMS Connectivity |
January 5, 2010 |
Final Applications Posted by CMS |
February 25, 2010 |
Completed Applications due to CMS
|
June 7, 2010 |
All bids due to CMS |
September 2010 |
CMS completes review and approval of bid data. CMS executes MA, MA-PD contracts with organizations whose bids are approved and who otherwise meet CMS requirements. |
November 2010 |
2010 Annual Coordinated Election Period begins for January 1, 2011 effective date for 2011 plans. |
* Note: all dates listed above are subject to change.
Applicant organizations seeking to withdraw an entire pending application or seeking to withdraw counties from a pending application’s service area must submit a written request to such effect on the organization’s letterhead and signed by an authorized corporate official by May 21, 2010 (tentative date). Zip code withdrawal requests must likewise be requested through a written request by an authorized official, though must be submitted to CMS by April 5, 2010 (tentative due date for an organization’s response to the application deficiency email). Additionally, any applicant seeking to withdraw zip codes (rendering their application a “partial-county” request) must also submit through HPMS a partial county justification as explained in the application instructions.
To submit via email, send the request in PDF format as an attachment to the email message to [email protected].
Mail requests should be addressed to:
MCAG/DMAO/CAT
Attn: Contracting and Applications Team
Mail Stop: C4-22-04
7500 Security Blvd.
Baltimore, MD 21244
Send faxed requests to the attention of the Contracting and Applications Team Lead at (410) 786-8933.
The following information must be included in the letter:
Applicant Organization’s Legal Entity Name
Full and Correct Address and Point of Contact information for follow-up, if necessary
Contract Number (H#)
Exact Description of the Nature of the Withdrawal:
Withdrawal from individual Medicare market counties (keeping Medicare employer group counties, e.g., 800 series plan(s)
Withdrawal from employer group counties (keeping the individual Medicare market)
Withdrawal of the entire application.
Withdrawal of specifically named counties from both individual Medicare and employer group markets
If CMS determines that the applicant is not qualified to enter into a contract with CMS under Part C of Title XVIII of the Social Security Act and denies this application, the applicant has the right to appeal this determination through a hearing before a CMS Hearing Officer. Administrative appeals of MA-PD application denials are governed by 42 CFR 422, Subpart N. The request for a hearing must be in writing, signed by an authorized official of applicant organization and received by CMS within 15 calendar days from the date CMS notifies the MAO organization of its determination (See 42 CFR 422.662). If the 15th day falls on a weekend or federal holiday, you have until the next regular business day to submit your request.
The appealing organization must receive a favorable determination resulting from the hearing or review as specified under Part 422, Subpart N prior to July 15, 2010 in order to qualify for a Medicare contract to begin January 1, 2011.
Applicants must complete the 2011 MA application using the HPMS as instructed. CMS will only accept submissions using this current 2011version of the MA application. All documentation must contain the appropriate CMS-issued contract number.
In preparing a response to the prompts throughout this application, the Applicant must mark “Yes” or “No” in sections organized with that format. By responding “Yes”, the applicant is responding that it will be compliant as of the date of the contract, unless it is stated in the attestation or application that it requires an earlier compliance date.
CMS may verify an Applicant’s readiness and compliance with Medicare requirements through on-site visits at the Applicant’s facilities as well as through other program monitoring techniques throughout the application process, as well as at any time both prior to and after the start of the contract year. Failure to meet the requirements represented in this application and to operate MA plans consistent with the applicable statutes, regulations, and the MA contract, and other CMS guidance could result in the suspension of plan marketing and enrollment. If these issues are not corrected in a timely manner, the Applicant will be disqualified from participation in the MA program.
Throughout this application, applicants are asked to provide various documents and/or tables in the HPMS. There is a summary of all required documents to be submitted at the end of each attestation section.
CMS strongly recommends and encourages Medicare Advantage applicants to refer to the 42 CFR 422 regulations to clearly understand the nature of the requirement in order to provide an appropriate response. Nothing in this application is intended to supersede the regulations at 42 CFR 422. Failure to reference a regulatory requirement in this application does not affect the applicability of such requirement, and Applicants are required to comply with all applicable requirements of the regulations in Part 422 of 42 CFR. Applicants must read the HPMS notices and visit the CMS web site periodically to stay informed about new or revised guidance documents.
Applicants that wish to offer MA, MA-PD products under Employer/Union-Only Group Waivers must complete and timely submit a separate EGWP application. Please see APPENDIX II- Employer/Union-Only Group Waiver Plans (EGWPs) MAO “800 Series” of this application for details about the EGWP.
All Applicants will be able to enter their EGWP service areas directly into the HPMS during the application process (refer to the HPMS User Guide). Applicants may provide coverage to employer group members wherever they reside (i.e., nationwide). However, in order to provide coverage to retirees wherever they reside, Applicants must set their service area to include all areas where retirees reside during the plan year (i.e., set national service areas).
Applicants that wish to offer Employer/Union Direct Contract Private Fee-For Service (PFFS) MAO must complete and timely submit a separate EGWP application. Please see APPENDIX III : Employer/Union Direct Contract Private Fee-For Service (PFFS) MAO Application of this application for details about the Direct Contract PFFS MAO.
In general, MAOs can cover beneficiaries only in the service areas in which they are licensed and approved by CMS to offer benefits. CMS has waived these requirements for Direct Contract MAOs. Direct Contract PFFS MAO applicants can extend coverage to all of their Medicare-eligible actives/retirees regardless of whether they reside in one or more MAO regions in the nation. In order to provide coverage to retirees wherever they reside, Direct Contract PFFS MAO applicants must set their service area to include all areas where retirees may reside during the plan year (no mid-year service area expansions will be permitted).
Direct Contract PFFS MAOs that offer Part D coverage (i.e., MA-PDs) will be required to submit pharmacy access information for the entire defined service area during the application process and demonstrate sufficient access in these areas in accordance with employer group waiver pharmacy access policy.
All 2011 Applicants seeking to offer a new or expand the service area of an existing Special Needs Plan (SNP) must complete and timely submit a separate SNP application. Please refer to APPENDIX I-Solicitations for Special Needs Plan Proposal for application instructions and details.
Initial Applications are for:
Applicants that are seeking a MA contract to offer a MA product for the first time, or to offer a MA product they do not already offer.
Existing MA contractors that are seeking a MA contract to offer a type of MA product they do not current offer.
Existing PFFS contractors required to transition some or all of their service area to network based product.
NOTE: A RPPO applicant may apply as a signal entity or as a joint enterprise. Joint Enterprise applicants must provide as part of their application a copy of the agreement executed by the State-licensed entities describing their rights and responsibilities to each other and to CMS in the operation of a Medicare Part D benefit plan. Such an agreement must address at least the following issues:
Termination of participation in the joint enterprise by one or more of the member organizations; and
Allocation of CMS payments among the member organizations.
Service Area Expansion Applications are for:
Existing MAO contractors that are seeking to expand the service area of an existing contract number.
This chart (Chart 1) describes the required attestations that must be completed for each type of application and applicant. The purpose of this chart is to provide the Applicant with a summary of the attestation topics. First, the applicant must determine if the application will be an initial or service area explanation type. Then the applicant must select type of MA product it will provide. The corresponding location of each attestation is provided under the column labeled “section #”, which corresponds to this application package.
Chart 1 - Required Attestations by Type of Application
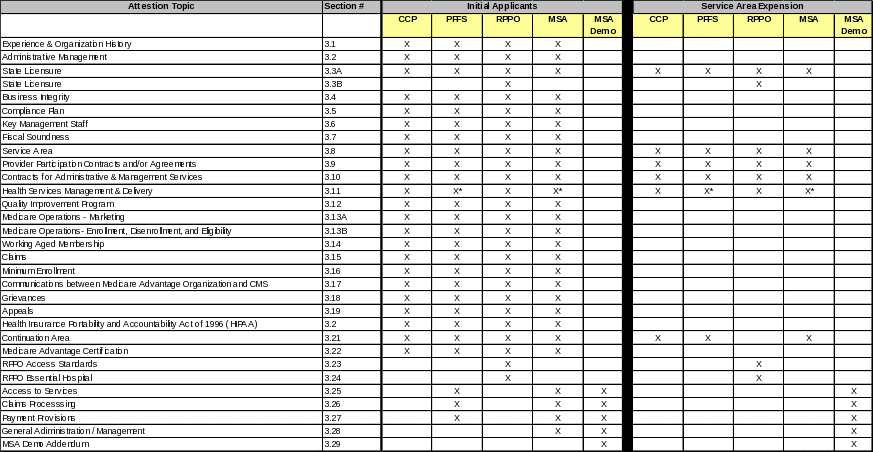
*Indicates Applicant with a network
Applicants will be demonstrating network adequacy through an automated review process and revised Health Service Delivery Tables (HSD). Detailed instructions on how to complete each of the required HSD Tables are available in a separate file along with the HSD Table templates. Detailed HSD instructions and table templates are available in the MA Download file in the HPMS.
As part of the application module in the Health Plan Management System (HPMS), CMS will be providing applicants with an automated tool for submitting network information via revised and automated HSD tables. The revised tables will then be reviewed automatically against default adequacy measures for each required provider type in each county. This new process will permit applicants to determine if they have achieved network adequacy before completing the submission of their application. Further, CMS will make these default values known prior to the opening of the application module. As such, applicants will see the values (providers and facilities of each required type in each county) that CMS requires before the application module opens. Applicants that believe that CMS default values for a given provider type in a given county are not in line with local patterns of care may seek an exception, in which case the applicant will submit required information to support the exceptions request and HSD review will occur manually by a CMS reviewer as it has in the past. Applicants that submit HSD tables that 'clear' CMS's default values will still be required to submit signed contracts and other documents that demonstrate the accuracy of the HSD tables submission. Applicants may still be determined to have network deficiencies even if they 'pass' the automated review.
CMS will be providing training to applicants on the new automated system, the new HSD tables, and the default values for determining network adequacy before the application module opens, and expects to annually post the default values for determining network adequacy in November of each year, prior to the last date for submitting the Notice of Intent to Apply.
Application forms and tables associated with the applications are available in separate Microsoft Word or Excel files that are available at http://www.cms.hhs.gov/MedicareAdvantageApps/. Microsoft Word files located on the CMS web site are posted in a .zip format and can also be found in the MA Download file in the HPMS.
Applicants must generally submit separate completed copies of each table template for each area/region or county that the Applicant is requesting. Specific instructions on how to complete and submit each table will be outlined in the 2011 HPMS User Guide for the Part C Application.
Applicants must include their assigned H number in the file name of all submitted these documents. Applicants are encouraged to be descriptive in naming all files. If the Applicant is required to provide multiple versions of the same document, the Applicant should insert a number, letter, or even the state name at the end of each file name for easy identification.
The Part D Application for MA-PD Applicants is an abbreviated version of the application used by stand-alone Prescription Drug Plan (PDPs), as the regulation allows CMS to waive provisions that are duplicative of MA requirements or where a waiver would facilitate the coordination of Part C and Part D benefits. Further, the Part D Application for MA-PD Applicants includes a mechanism for Applicants to request CMS approval of waivers for specific Part D requirements under the authority of 42 CFR 423.458 (b)(2). The Part D Application for MA-PD Applicants can be found at: http://www.cms.hhs.gov/PrescriptionDrugCovContra/04_RxContracting_ApplicationGuidance.asp#TopOfPage. Specific instructions to guide MA-PD Applicants in applying to offer a Part D benefit during 2011 are provided in the Part D Application for MA-PD Applicants and must be followed.
Note: Failure to file the required Part D Application for MA-PD Applicants will render the MA-PD Application incomplete and could result in the denial of this application.
Failure to submit supporting documentation consistent with these instructions may delay the review by CMS and may result in the Applicant receiving a Notice of Intent to Deny or Denial.
The purpose of this section is to allow applicants to submit information describing their experience and organizational history. A description of the Medicare Advantage organization’s structure of ownership, subsidiaries, and business affiliations will enable CMS to more fully understand additional factors that contribute to the management and operation of Medicare Advantage plans. The following attestations were developed to implement the regulations of 42 CFR 422.503(b).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: EXPERIENCE & ORGANIZATIONAL HISTORY |
YES |
NO |
|
|
|
|
|
|
In the HPMS, upload the following:
History/Structure/Organizational Charts -- (This is a brief summary of the applicant’s history, structure and ownership. Include organizational charts to show the structure of ownership, subsidiaries, and business affiliations.)
The purpose of the administrative management attestations is to ensure that Medicare Advantage organizations have the appropriate resources and structures available to effectively and efficiently manage administrative issues associated with Medicare beneficiaries. CMS requires that Medicare Advantage plans have sufficient personnel and systems to organize, implement, control and evaluate financial and marketing activities, oversee quality assurance, and manage the administrative aspects of the organization. The following attestations were developed to implement the regulations of 42 CFR 422.503(b) (4) (ii).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: ADMINISTRATIVE MANAGEMENT |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In the HPMS, upload the following:
CMS Insurance Coverage Table (as applicable)
In efforts to ensure that all Medicare Advantage organizations operate in compliance with state and federal regulations, CMS requires Medicare Advantage organizations to be licensed under state law. This will ensure that Medicare Advantage organizations adhere to state regulations aimed at protecting Medicare beneficiaries. The following attestations were developed based on the Code of Federal Regulations 422.400.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: STATE LICENSURE |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
In the HPMS, upload the following:
State Licensing Certificate (executed copy)
CMS State Certification Form (signed and dated by appropriate State officials)
State Corrective Plans / State Monitoring Explanation (as applicable)
Note: Federal Preemption Authority – The Medicare Modernization Act amended section 1856(b) (3) of the Social Security Act and significantly broadened the scope of Federal preemption of State law. The revised MA regulations at Sec. 422.402 state that MA standards supersede State law or regulation with respect to MA plans other than licensing laws and laws relating to plan solvency.
In efforts to ensure that all Medicare Advantage organizations operate in compliance with state and federal regulations, CMS requires Medicare Advantage organizations to be licensed under state law. This will ensure that Medicare Advantage organizations adhere to state regulations aimed at protecting Medicare beneficiaries. The following attestations were developed to implement the regulations of 42 CFR 422.400.
In the HPMS, complete the 3.3.A and the table below 3.3.B.
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: STATE LICENSURE |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
In the HPMS, upload the following:
Provide in the HPMS “State Licensure”
Provide in the HPMS “State Corrective Plans/State Monitoring Explanation” (as applicable)Provide in the HPMS “State Approval of d/b/a”
Provide in the HPMS, a complete “CMS State Licensing Status for MA Regional PPO Table” for each MA Region.
Provide in the HPMS, a signed “CMS State Licensure Attestation for MA Regional PPOs”
Note: Federal Preemption Authority – The Medicare Modernization Act amended section 1856(b) (3) of the Social Security Act and significantly broadened the scope of Federal preemption of State law. The revised MA regulations at Sec. 422.402 state that MA standards supersede State law or regulation with respect to MA plans other than licensing laws and laws relating to plan solvency.
Note: For states or territories such as Puerto Rico whose licenses renew after the first Monday in June, Applicant is required to submit the new license in order to operate as an MA or MA-PD.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: BUSINESS INTEGRITY |
YES |
NO |
|
|
|
|
|
|
B. If Applicant answered “No” to either question above; upload in the HPMS a Business Integrity Disclosure, which contains a brief explanation of each action, including the following:
1.
Legal names of the parties.
2. Circumstances.
3. Status
(pending or closed).
4. If closed, provide the details concerning resolution and any monetary payments, or settlement agreements or corporate integrity agreement.
The purpose of a compliance plan is to ensure that the Medicare Advantage organization, including but not limited to compliance officers, organization employees, contractors, managers and directors, abide by all Federal and State regulations, standards, and guidelines. To accomplish this objective, the plan should include the following components: training/education, communication plan, disciplinary standards, internal monitoring/auditing procedures, etc. The following attestations were developed to implement the regulations of42 CFR 422.503(b) (4) (vi).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: COMPLIANCE PLAN |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: Applicant will upload the timeline document into application section: Uploads (Other). |
|
|
|
|
|
In the HPMS, upload the following:
Template Timeline re: development and staffing of compliance program
The purpose of this section is to ensure that qualified staffs are available to support Medicare Advantage organizations. Position descriptions for the key management staff and an organizational chart showing the relationships of the various departments will demonstrate that Medicare Advantage organizations meet this requirement. The following attestations were developed to implement the regulations of 42 CFR 422.503(b) (4) (ii).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: KEY STAFF MANAGEMENT |
YES |
NO |
|
|
|
|
|
|
In the HPMS, upload the Contact Management/Information/Data page following:
Contact |
Name/Title |
Mailing Address |
Phone/Fax Numbers |
Email Address |
Corporate Mailing |
|
|
|
|
CEO – Sr. Official for Contracting |
|
|
|
|
Chief Financial Officer |
|
|
|
|
Medicare Compliance Officer |
|
|
|
|
Enrollment Contact |
|
|
|
|
Medicare Coordinator |
|
|
|
|
System Contact |
|
|
|
|
Customer Service Operations Contact |
|
|
|
|
General Contact |
|
|
|
|
User Access Contact |
|
|
|
|
Backup User Access Contact |
|
|
|
|
Marketing Contact |
|
|
|
|
Medical Director |
|
|
|
|
Bid Primary Contact |
|
|
|
|
Payment Contact |
|
|
|
|
HIPAA Security Officer |
|
|
|
|
HIPAA Privacy Officer |
|
|
|
|
CEO- CMS Administrator Contact |
|
|
|
|
Quality Director |
|
|
|
|
Provide in the HPMS, position descriptions for the key management staff and an organizational chart showing the relationships of the various departments.
In the HPMS, complete the table below:
YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: FISCAL SOUNDNESS |
YES |
NO |
|
|
|
2. Applicant maintains a fiscally sound organization. Specifically, a fiscally sound organization must: 1) have sufficient cash flow and adequate liquidity to meet obligations as they become due, 2) a recent balance sheet demonstrating a reserve level that meets the State regulatory reserve minimum, and 3) net income (NOTE: a net loss is acceptable if the organization’s net worth is at least two times greater than the reported net loss for the accounting period).
|
|
|
3. Applicant agrees to immediately notify CMS if it becomes fiscally unsound during the contract period. Additionally, applicant will immediately notify CMS if the State identifies any financial concerns that will impact the applicant’s ability to operate its Medicare Advantage contract.
|
|
|
4. Applicant is in compliance with all State requirements and is not under any type of supervision, corrective action plan, or special monitoring by the State regulator.
|
|
|
In the HPMS, upload the following:
Financial Plan
Financial Disclosure
The purpose of the service area attestations is to clearly define what areas will be served by the Medicare Advantage organization. A service area for local Medicare Advantage plans is defined as a geographic area composed of a county or multiple counties, while a service area for Medicare Advantage regional plans is a region approved by CMS. The Medicare Advantage information requested below are intended to provide CMS reviewers with a visual understanding of boundaries (e.g., traffic arteries), physical attributes (e.g., rivers, lakes, mountains) and location of facilities to ensure that Medicare Advantage organizations have adequate network of resources relative to geographic factors. The following attestations were developed to implement the regulations of 42 CFR 422.2.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: SERVICE AREA |
YES |
NO |
|
|
|
|
|
|
In the HPMS, on the Contract Management/Contract Service Area/Service Area Data page, enter the state and county information for the area the Applicant proposes to serve.
Note: The service area for the MSA demonstration plan can only be offered at the entire state or territory level.
This section contains attestations that address the requirements of 42 CFR 422.504 -- that MA organizations have oversight for contractors, subcontractors, and other entities. The intent of the regulations is to ensure services provided by these parties meet contractual obligations, laws, regulations and CMS instructions. The MA organization is held responsible for compliance of its providers and subcontractors with all contractual, legal, regulatory, and operational obligations. Beneficiaries shall be protected from payment or fees that are the obligation of the MA organization. Further guidance is provided in Chapter 11, Medicare Managed Care manual.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: PROVIDER CONTRACTS AND AGREEMENTS |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provide in the HPMS a template copy of each primary provider contract(s) and agreement(s) between the Applicant and its health care contractors (i.e., direct contract with physicians, medical group, IPA, PHO, hospitals, skilled nursing facilities, etc.).
Provide in the HPMS, a template copy of each downstream subcontract that may exist between a Medical group(s), IPA(s), PHO, etc. and its downstream providers (e.g., individual physicians). (For example: If the Applicant contracts with an IPA, which contracts with individual physicians, the Applicant must provide in HPMS a sample copy of the contract/agreement between the IPA and physicians in addition to the contract between it and the IPA referenced above in section B).
Provide in HPMS, a completed “CMS Provider Participation Contracts and/or Agreements Matrix”, which is a crosswalk to show where in each provider contract/agreement the referenced CMS regulations are included. Applicant should complete a matrix for each applicable primary contracted provider and subcontracted provider.
Note: As part of the application process, Applicants will need to provide signature pages for provider contracts that the CMS reviewers select. Reviewers will provide specific instructions during the application review.
This section describes the requirements the Applicant must demonstrate to ensure any contracts for administrative/management services comply with the requirements of all Medicare laws, regulations, and CMS instructions in accordance with 42 CFR 422.504(i) (4) (v). Further guidance is provided in Chapter 11, Medicare Managed Care manual.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: CONTRACTS FOR ADMINISTRATIVE MANAGEMENT SERVICES |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. In the HPMS, complete the Delegated Business Function Table, shown in Exhibit 1 below:
Exhibit 1: Delegated Business Function Table
Note: If you plan to delegate a specific function but cannot at this time name the entity with which you will contract, enter "Not Yet Determined" so that CMS is aware of your plans to delegate that function even if you are still in contract negotiations. If you delegate a particular function to a number of different entities (e.g., claims processing to multiple medical groups), then list the five most significant entities for each delegated business function identified and in the list for the sixth, enter "Multiple Additional Entities".
C. Provide in the HPMS, a completed “CMS Administrative/Management Delegated Contracting or Arrangement Matrix”.
The purpose of the Health Service Management and Delivery attestations is to ensure that all applicants deliver timely and accessible health services for Medicare beneficiaries. CMS recognizes the importance of ensuring continuity of care and developing policies for medical necessity determinations. In efforts to accomplish this, Medicare Advantage organizations will be required to select, evaluate, and credential providers that meet CMS’ standards, in addition, to ensuring the availability of a range of providers necessary to meet the health care needs of Medicare beneficiaries. The following attestations were developed to implement the regulations of 42 CFR 422.112, 422.114.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: HEALTH SERVICES MANAGEMENT AND DELIVERY |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. Provide in the HPMS, completed HSD tables 1 through 5. Applicants offering multiple benefit plans must submit separate tables for each county and each plan. Only one HSD table is needed for different plans that have the same network and service area. NOTE: Applicants offering provider specific plans must submit separate HSD Tables.
The purpose of the section is to ensure that all applicants have a quality improvement program. A quality improvement program will ensure that Medicare Advantage organizations have the infrastructure available to increase quality, performance, and efficiency of the program on an on-going basis; and to identify actual or potential triggers or activities for the purpose of mitigating risk and enhancing patient safety. This process will provide the Medicare Advantage organizations an opportunity to resolve identified areas of concern. The following attestations were developed to implement the regulations of 42 CFR 422.152 and Medicare Manual Chapter 5.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: QUALITY IMPROVEMENT PROGRAM |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In the HPMS, upload the following:
The purpose of the Medicare Operations Marketing attestations is to ensure that all applicants comply with all CMS regulations, and guidance including but not limited to the managed care manual, user guides, the annual call letter, and communications through the HPMS. Medicare Advantage organizations are required to provide comprehensive information in written form and via a call center to ensure that Medicare beneficiaries understand the features of the Medicare Advantage plans. The following attestations were developed to implement the regulations of 42 CFR 422.2260 to 422.2276.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: MEDICARE OPERATIONS – MARKETING |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This section identifies attestations that meet the intent of 42 CFR 422.50 to 422.74, which address the eligibility requirements to enroll, continue enrollment, and disenroll in a Medicare Advantage plan. The intent of these regulations is to ensure that all Medicare Advantage organizations fully comply with the requirements set forth to ensure services adhere to standard processes and meet contractual obligations, laws, regulations and CMS instructions.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: MEDICARE OPERATIONS – ENROLLMENT, DISENROLLMENT, AND ELIGIBILITY |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The purpose of these attestations is to ensure that applicants report all working aged members to CMS, as well as to identify amounts payable, coordinate benefits to enrollees, identify primary Medicare patients. The following attestations were developed to implement the regulations of CFR 42 CFR 422.108.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: WORKING AGED MEMBERSHIP |
YES |
NO |
|
|
|
The purpose of these attestations is to ensure that the applicant properly dates and processes all claims, per CMS instructions listed herein, as well as providing applicant with instructions of how to appropriately notify beneficiary of claim decisions. The following attestations were developed to implement the regulations of 42 CFR 422.504(c) and 42 CFR 422.520(a).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: CLAIMS |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The purpose of these attestations is to ensure that the applicant, whether from a rural or urban area, has the minimum number of individuals enrolled. Additionally, if the applicant cannot satisfy the minimum enrollment number, it provides guidance for what the applicant needs to do to comply with CMS guidelines. The following attestations were developed to implement the regulations of 42 CFR 422.514.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: MINIMUM ENROLLMENT |
YES |
NO |
|
|
|
|
|
|
|
|
|
CMS is committed to ensuring clear communications with Medicare Advantage organizations. The purpose of this section is to ensure that all applicants have effective and timely communications. This will help improve and support administrative coordination between CMS and Medicare Advantage organizations. The following attestations were developed to implement the regulations of 42 CFR 422.504(b).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: COMMUNICATIONS |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CMS is committed to the guaranteed rights of Medicare beneficiaries to have access to, education on, decision making authority for, and receive quality health care. To ensure that beneficiaries have the ability to express their concerns and those concerns are promptly acted on, Medicare Advantage organizations must have a grievance program structured in compliance with CMS regulations and guidelines. In this capacity, grievances are defined as any complaint or dispute, other than one that constitutes an organization determination, expressing dissatisfaction with any aspect of an organization's or provider's operations, activities, or behavior, regardless of whether remedial action is requested. The following attestations were developed to implement the regulations of 42 CFR 422.561, 422.564.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: GRIEVANCES |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
Note: A grievance is any complaint or dispute, other than one that involving an organization determination, expressing dissatisfaction with the manner in which a Medicare health plan or delegated entity provides health care services, regardless of whether any remedial action can be taken. An enrollee or their representative may make the complaint or dispute, either orally or in writing, to a Medicare health plan, provider or facility. An expedited grievance may also include a complaint that a Medicare health plan refused to expedite an organization determination or reconsideration, or invoked an extension to an organization determination or reconsideration period. In addition, grievances may include complaints regarding the timeliness, appropriateness, access to, and/or setting of a provided health service, procedure, or item. Grievance issues may also include complaints that a covered health service procedure or item during a course of treatment did not meet accepted standards for delivery of health care.
CMS recognizes the importance of the appeals process for both the Medicare Advantage organizations and Medicare beneficiary. The purpose of this section is to ensure that beneficiaries have the opportunity to submit an appeal. Correspondingly, Medicare Advantage organizations must have an appeals process structured in compliance with CMS regulations and guidelines. An appeal is defined as any of the procedures that deal with the review of adverse organization determinations on the health care services the enrollee believes he or she is entitled to receive, including delay in providing, arranging for, or approving the health care services (such that a delay would adversely affect the health of the enrollee), or on any amounts the enrollee must pay for a service, as defined under §422.566(b). These procedures include reconsiderations by the Medicare Advantage organization, and if necessary, an independent review entity, hearings before ALJs, review by the Medicare Appeals Council (MAC), and judicial review. The following attestations were developed to implement the regulations of 42 CFR 422.561.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: APPEALS |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and CMS issued guidance 07/23/2007 and 8/28/2007; 2008 Call Letter
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT OF 1996 (HIPAA) |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. Provide in the HPMS a complete “Data Use Attestation”
The purpose of a continuation area is to ensure continuity of care for enrollees that no longer reside in the service area of a plan and permanently move into the geographic area designated by the Medicare Advantage organization as a continuation area. A continuation area is defined as an additional area (outside the service area) within which the Medicare Advantage organization offering a local plan furnishes or arranges to furnish services to its continuation-of-enrollment enrollees. Enrollees must reside in a continuation area on a permanent basis and provide documentation that establishes residency, such as a driver’s license or voter registration card. A continuation area does not expand the service area of any Medicare Advantage local plan. The following attestations were developed to implement the regulations of 42 CFR 422.54.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: CONTINUATION AREA |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: MEDICARE ADVANTAGE CERTIFICATION |
YES |
NO |
|
|
|
|
|
|
|
|
|
NOTE: Once the Part C application is complete, applicants seeking to offer a Part D plan must complete the Part D application in the HPMS. PFFS organizations have the option to offer Part D plans. MSAs are not allowed to offer Part D plans.
The purpose of this section is to allow applicants an opportunity to clearly define access standards. Attestations in this section will allow CMS to evaluate access standards based on the organization’s explanation of urban and rural areas, discussions of the patterns of care, descriptions of how the geo-access standards were developed, and the percentage of beneficiaries that will fall within the standards for each type of provider. The following attestations were developed to implement the regulations of 42 CFR 422.112.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: ACCESS STANDARDS |
YES |
NO |
|
|
|
|
|
|
|
|
|
Provide in the HPMS, access standards for the following specified provider types, including the percentage of beneficiaries that will fall within the standards and stated in terms of distance and time (___% of beneficiaries fall within xx miles/xx minutes of 2 Primary Care Providers):
Contracted Hospitals with Full Emergency Facilities
Contracted Primary Care Providers
Contracted Skilled Nursing Facilities
Contracted Home Health Agencies
Contracted Ambulatory Clinics
Contracted Providers of End Stage Renal Disease Services
Contracted Outpatient Laboratory and Diagnostic Services
Contracted Specialists in the following areas:
General Surgery
Otology/Laryngology/Rhinology
Anesthesiology
Cardiology
Dermatology
Gastroenterology
Neurology
Obstetrics and Gynecology
Ophthalmology
Orthopedic Surgery
Psychiatry/Mental Health
Pulmonary Disease
Urology
Chiropractic
Optometry
Podiatry
Provide in the HPMS, a chart listing all counties (or other units of analysis as relied upon by applicant in establishing standards) and indicate whether each county meets or does not meet each contracted access standard for a contracted provider type.
Provide in the HPMS, an access plan describing the applicants proposed mechanism for ensuring beneficiary access to the identified type(s) of provider(s) for each area in which the applicant does not meet its access standards through it contracted network. Access plans may include requests for essential hospital designations, facilitating enrollee access to non-contracted providers at preferred cost sharing levels, or other proposed mechanisms as approved by CMS.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: ESSENTIAL HOSPITAL |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
Provide in the HPMS, a completed “CMS Essential Hospital Designation Table”.
Provide in the HPMS, a completed “CMS Attestation Regarding Designation of Essential Hospitals”.
Private Fee for Service (PFFS) APPLICANTS ONLY
The purpose of these attestations is to provide the applicant with information regarding the offering of the various PFFS models, including a network, partial network, or non-network PFFS models to its members, as applicable. Additionally, these attestations will instruct the applicant of the documents and/or information that will need to be uploaded into the HPMS. The following attestations were developed to implement the regulations of 42 CFR 422.114(a) (2) (iii).
Please note that, effective for contract year 2011, Section 1862(d) of the SSA, as amended by Section 162(a)(1) of MIPPA, requires those PFFS plans operating in “network areas” to meet the access standards described in section 1852(d)(4)(B) of the Act through contracts with providers. The list of those areas considered “network areas” for purposes of the 2011 application and contracting requirements can be found at http://www.cms.hhs.gov/PrivateFeeforServicePlans/. CMS will not accept a non-network or partial network application that includes any of the areas identified as “network areas” in the referenced document. Furthermore, Applicants wishing to offer both network PFFS products and non or partial network PFFS products must do so under separate contracts.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: ACCESS TO SERVICES (PFFS AND/OR MSA) |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
UPLOAD: Applicants proposing to furnish certain categories of service through a contracted network are required to submit a narrative description of the proposed network through an upload in the HPMS. Please ensure that the categories are clearly defined in the narrative description. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provide in the HPMS, Description of Proposed Services for combination networks (as applicable).
Provide in the HPMS, completed HSD tables 1 through 5 for network model PFFS plans. Applicants offering multiple benefit plans must submit separate tables for each county and each plan. Only one HSD table is needed for different plans that have the same network and service area.
Provide in the HPMS, a description on how the applicant will follow CMS’s national coverage decisions and written decision of carriers and intermediaries (LMRP) throughout the United States. [Refer to 42 CFR 422.101 (b)].
Provide in the HPMS, a description on how applicant’s policies will ensure that health services are provided in culturally competent manner to enrollees of different backgrounds.
Private Fee for Service (PFFS) APPLICANTS ONLY
The purpose of these attestations is to verify that applicant uses a validated claims system, properly implements the Reimbursement Grid and pays all providers according to the PFFS plan's terms and conditions of payment. Additionally upon request, the applicant will submit to CSM its complete and thorough Provider Dispute Resolution Policies and Procedures (P&Ps), bi-weekly report detailing complaints, and/or bi-weekly report detailing appeals and/or claims.
The following attestations were developed to implement the regulations of 42 CFR 422.216
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: CLAIMS PROCESSING |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This section may be applicable to PFFS, MSA & MSA Demo Plans
The purpose of these attestations is to ensure that the applicant has an appropriate system in place to properly pay providers and to ensure that members are not being overcharged. Additionally, it instructs applicants to upload a Reimbursement Grid in the HPMS. The following attestations were developed to implement the regulations of 42 CFR 422.216 (c).
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: PAYMENT PROVISIONS |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provide in the HPMS, a completed Payment Reimbursement grid. NOTE: Organization may use any format for the Payment Reimbursement grid that best outlines the organization’s rates.
This section is applicable to MSA and MSA Demo Plans
The purpose of these attestations is to ensure that the applicant is offering Medical Savings Accounts (MSA) plans that follow requirements set forth in laws, regulations and CMS instructions. The applicant may establish a relationship with a banking partner and have a system in place to receive Medicare deposits to MSA plan enrollee accounts. The following sections of Code of Federal Regulations 42 CFR 422 contain provisions that are specific to Medical Savings Accounts : 422.2, 422.4(a) and (c), 422.56, 422.62(d), 422.100(b)(2), 422.102(b), 422.103, 422.104, 422.111(a), 422.152, 422.252, 422.254(e), 422.256(e), 422.262(b)(2), 422.270(a)(1), 422.304(c)(2), and lastly, 422.314.
In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: MEDICAL SAVINGS ACCOUNTS (MSA) & MSA DEMO |
YES |
NO |
|
|
|
|
|
|
|
|
|
NOTE: MSA Applicant must upload the banking contract for review by CMS and applicant to ensure that ALL CMS direct and/or any delegated contracting requirements are included in the contract. |
|
|
|
|
|
|
|
|
This section is applicable to MSA Demo Plans
The purpose of these attestations is to request information on demonstration design. The Medicare MSA demonstration allows for design parameters that are not in current regulation.
A. In the HPMS, complete the table below:
RESPOND ‘YES’ OR ‘NO’ TO EACH OF THE FOLLOWING STATEMENTS: MSA DEMONSTRATION ADDENDUM |
YES |
NO |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B. Provide in the HPMS, the following:
Description of any differential in cost sharing for supplemental benefits from the standard Medicare A/B benefits and for in-network and out-of-network services.
Description of the preventive services that will have full or partial coverage before the deductible is met.
Figures on projected enrollment and the characteristics of beneficiaries who are most likely to enroll in the applicant’s plans (for example, what type of Medicare coverage do they currently have?).
Description of non-Medicare covered preventive services and whether or not any cost sharing for these services will apply to the plan deductible.
Description of the frequency of periodic deposits and how the applicant will address cases where the enrollee incurs high health costs early in the year.
Description on how the applicant will track enrollee usage of information provided on the cost and quality of providers. Be sure to include how you intend to track use of health services between those enrollees who utilize transparency information with those who do not.
Description of how the applicant will recover current-year deposit amounts for members who are disenrolled from the plan before the end of the calendar year.
Note: CMS REQUEST THAT YOU LIMIT THIS DOCUMENT TO EIGHT (8) PAGES.
Please Check:
_____New to the Medicare Advantage program
SECTION 1: All Applicants (new and existing) must complete this section.
Please give a brief summary of applicant’s history
Structure:
Ownership:
Attach a diagram of applicant’s structure of ownership.
Attach CVs of all key personnel.
Attach a diagram of applicant relation to subsidiaries, and business affiliations.
SECTION II: Applicants that are new to the Medicare Advantage Program must complete this section.
What were the results of that audit?
Briefly describe the financial status of the applicant’s company.
Briefly explain the applicant’s your marketing philosophy.
Who in the applicant’s organization can appoint and remove the executive manager?
Please submit a brief description and/or a flow chart of the applicant’s claim process.
Please submit a brief description and/or flow chart of the applicant’s grievances process.
Please provide a brief description and flow chart of the applicant’s appeals process.
If applicable, please provide the name of the claims systems that applicant tested to demonstrate the systems’ ability to pay Medicare FFS payments.
Instructions for CMS Provider Participation Contracts and/or Agreements Matrix
This matrix must be completed by MA applicants and should be use to reflect the applicants first tier, downstream and related entity contracts and/or agreements.
Instructions:
Provide in HPMS using a PDF format, a separate matrix for each county or partial county.
Enter name of the provider(s)/group(s) or entity that the MA organization contracts with to provide services to Medicare enrollees. Each matrix will need to be filled out for all first tiers, downstream and related entity providers.
Designate if provider is first tier contracted provider with a "(1)" next to the name of that provider(s)/group(s) or other entity.
Designate downstream contracted provider(s), group, or other entity with a "(DS)".
Under each column, list the page number where the provision that meets the regulatory requirement can be found in each of the contracts and/or agreements templates for that particular provider(s), group(s) and other contracted entities.
Note: This matrix contains a brief description of MA regulatory requirements; please refer to full regulatory citations for an appropriate response.
CMS PROVIDER PARTICIPATION CONTRACTS AND/OR AGREEMENTS MATRIX- 1
COUNTY:______________________
IPA/Group/Provider Name First Tier, Downstream and Related Entities Contracts and/or Agreements |
|
|
|
|
|
|
CMS REGULATIONS – 42 CFR 422
|
Section/Page
|
Section/Page
|
Section/Page
|
Section/Page
|
Section/Page |
|
All Provider Contracts |
||||||
Record Retention. HHS, the Comptroller General or their designees have the right to audit, evaluate and inspect audit any pertinent information including books, contracts, records, including medical records, and documentation related to CMS’ contract with the MA organization for a period of 10 years from the final date of the contract period or the completion of any audit, whichever is later. 422.504(i)(2)(i) and (ii); |
|
|
|
|
|
|
Privacy and Accuracy of Records. Providers and suppliers agree to safeguard beneficiary privacy and confidentiality and assure the accuracy of beneficiary health records. 422.504 (a)13. 422.504(a)13 |
|
|
|
|
|
|
Hold Harmless - Providers may not hold beneficiary liable for payment of fees that are the legal obligation of the MAO. 422.504(g)(1)(i); 422.504(i)(3)(i) |
|
|
|
|
|
|
Delegated Activities: Compliance with MAO’s contractual obligations. A provision requiring that any services performed will be inconsistent and comply with the MA organization’s contractual obligations. 422.504(i)(3)(iii) |
|
|
|
|
|
|
Prompt Payment The agreement specifies a prompt payment requirement, the terms and conditions of which are developed and agreed to by the MAO and contracted providers and suppliers. 422.520(b) |
|
|
|
|
|
|
Delegated Activities: Selection of Providers. If the MAO delegates selection of providers, written arrangements must state the MAO retains the right to approve, suspend, or terminate such arrangement. 422.504(i)(5) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Delegated Activities – List of Delegated Activities and Reporting Responsibilities The contract must clearly state the delegated activities and reporting responsibilities. 422.504(i)(4)(i) |
|
|
|
|
|
|
Delegated Activities – Revocation. Agreement provides for the revocation of the delegated activities and reporting requirements or specifies other remedies in instances when CMS or the MA organization determines that such parties have not performed satisfactorily. 422.504(i)(4)(ii) |
|
|
|
|
|
|
Delegated Activities – Monitoring Agreement provides that the performance of the parties is monitored by the MA organization on an ongoing basis 422.504(i)(4)(iii) |
|
|
|
|
|
|
Delegated Activities - Credentialing The credentials of medical professionals affiliated with the party or parties will either be reviewed by the MA organization OR the credentialing process will be reviewed and approved by the MA organization and the MA organization must audit the credentialing process on an ongoing basis 422.504(i)(4)(iv) |
|
|
|
|
|
|
Compliance with applicable Medicare laws and Regulations Must comply with all applicable Medicare laws, regulations, and CMS instructions 422.504(i)(4)(v) |
|
|
|
|
|
|
Date and Signature Line
|
|
|
|
|
|
|
Administrative Contracting Requirements for Management/Delegation of Contracts and/or Agreements
For contracts and/or agreements that directly relate to MA Organization’s core functions under its contract with CMS
NAME OF CONTRACTOR (FIRST TIER, DOWNSTREAM and RELATED ENTITY) |
|
|
|
|
|
|
CMS REGULATIONS – 42 CFR 422
|
Section/Page
|
Section/Page
|
Section/Page
|
Section/Page
|
Section/Page |
|
Record Retention. HHS, the Comptroller General or their designees have the right to audit, evaluate and inspect audit any pertinent information including books, contracts, records, including medical records, and documentation related to CMS’ contract with the MA organization for a period of 10 years from the final date of the contract period or the completion of any audit, whichever is later. 422.504(i)(2)(i) and (ii); |
|
|
|
|
|
|
Privacy and Accuracy of Records. Providers and suppliers agree to safeguard beneficiary privacy and confidentiality and assure the accuracy of beneficiary health records.
|
|
|
|
|
|
|
Hold Harmless - Providers may not hold beneficiary liable for payment of fees that are the legal obligation of the MAO. 422.504(g)(1)(i); 422.504(i)(3)(i) |
|
|
|
|
|
|
Delegated Activities: Compliance with MAO’s contractual obligations. A provision requiring that any services performed will be in consistent and comply with the MA organization’s contractual obligations 422.504(i)(3)(iii) |
|
|
|
|
|
|
Delegated Activities: Selection of Providers. If the MAO delegates the selection of providers, written arrangements must state the MAO retains the right to approve, suspend, or terminate such arrangement. 422.504(i)(5) |
|
|
|
|
|
|
Delegated Activities: List of Delegated Activities and Reporting Responsibilities The contract must clearly state the delegated activities and reporting responsibilities. 422.504(i)(4)(i) |
|
|
|
|
|
|
Delegated Activities: Revocation. Agreement provides for the revocation of the delegated activities and reporting requirements or specifies other remedies in instances when CMS or the MA organization determines that such parties have not performed satisfactorily. 422.504(i)(3)(ii); 422.504(i)(4)(ii) |
|
|
|
|
|
|
Delegated Activities: Monitoring Agreement provides that the performance of the parties is monitored by the MA organization on an ongoing basis 422.504(i)(3)(ii); 422.504(i)(4)(iii) |
|
|
|
|
|
|
Delegated Activities: Credentialing The credentials of medical professionals affiliated with the party or parties will either be reviewed by the MA organization OR the credentialing process will be reviewed and approved by the MA organization; and the MA organization must audit the credentialing process on an ongoing basis 422.504(i)(4)(iv)(A)(B) |
|
|
|
|
|
|
Compliance with applicable Medicare laws and Regulations Must comply with all applicable Medicare laws, regulations, and CMS instructions 422.504(i)(4)(v) |
|
|
|
|
|
|
Dated and Signed
|
|
|
|
|
|
|
Type |
Carrier |
Entity Covered |
Description: Deductibles, Co-insurance, Minimum & Maximum Benefits |
Premiums |
Period Policies are in Effect |
Other Arrangements to Cover These Risks |
Reinsurance
|
|
|
|
|
|
|
Risk of insolvency
|
|
|
|
|
|
|
Out-of-area emergency |
|
|
|
|
|
|
Malpractice 1. Plan |
|
|
|
|
|
|
2. Affiliated Providers |
|
|
|
|
|
|
General Liability |
|
|
|
|
|
|
Casualty
|
|
|
|
|
|
|
Fire
|
|
|
|
|
|
|
Theft
|
|
|
|
|
|
|
Fidelity bond
|
|
|
|
|
|
|
INSTRUCTIONS
(MA State Certification Form)
General:
This form is required to be submitted with all Medicare Advantage (MA) applications. The MA applicant organization is required to complete the items above the line (items 1 - 3), then forward the document to the appropriate State Agency Official who should complete those items below the line (items 4-7). After completion, the State Agency Official should return this document to the applicant organization for submission to CMS as part of its application for a MA contract. Applicants should place this document in the Organizational and Contractual section of the application in the Legal Entity subsection.
The questions provided must be fully completed. If additional space is needed to respond to the questions, please add pages as necessary. Provide additional information whenever you believe further explanation will clarify the question.
The MA State Certification Form demonstrates to CMS that the MA contract being sought by the applicant organization is within the scope of the license granted by the appropriate State regulatory agency, that the organization meets state solvency requirements and that it is authorized to bear risk. A determination on the organization’s MA application will be based upon the organization’s entire application was submitted to CMS, including documentation of appropriate licensure.
Items 1 - 3 (to be completed by the Applicant):
List the name, d/b/a (if applicable) and complete address of the organization that is seeking to enter into the MA contract with CMS.
Indicate the type of license (if any) applicant organization currently holds in the State where applicant organization is applying to offer an MA contract.
Specify the type of MA contract applicant organization is seeking to enter into with CMS.
New Federal Preemption Authority – The Medicare Modernization Act amended section 1856(b)(3) of the Social Security Act to significantly broaden the scope of Federal preemption of State laws governing plans serving Medicare beneficiaries. Current law provides that the provisions of Title XVIII of the Social Security Act supersede State laws or regulations with respect to MA plans other than laws relating to licensure or plan solvency.
Items 4 - 7 (to be completed by State Official):
List the reviewer’s pertinent information in case CMS needs to communicate with the individual conducting the review at the State level.
List the requested information regarding other State departments/agencies required to review requests for licensure.
A. Circle where appropriate to indicate whether the applicant meets State financial solvency requirements.
B. Indicate State Agency or Division, including contact name and complete address, which is responsible for assessing whether the applicant meets State financial solvency requirements.
A. Circle where appropriate to indicate whether the applicant meets State licensure requirements.
B. Indicate State Agency or Division, including contact name and complete address, which is responsible for assessing whether the applicant meets State licensing requirements.
MEDICARE ADVANTAGE (MA)
MA applicant should complete items 1-3.
MA Applicant Information (Organization that has applied for MA contract(s)):
Name_____________________________________________________
D/B/A (if applicable) _________________________________________
Address___________________________________________________
City/State/Zip_______________________________________________
Type of State license or Certificate of Authority currently held by referenced applicant: (Circle more than one if entity holds multiple licenses)
● HMO ● PSO ● PPO ● Indemnity ● Other________
Comments:
Type of MA application referenced applicant has filed with the Centers for Medicare & Medicaid Services (CMS): (Circle all that are appropriate)
● HMO ● PPO ● MSA ● PFFS ●Religious Fraternal
Requested Service Area: _______________________________________________
I certify that ____________________’s application to CMS is for the type of MA plan(s) and the service area(s) indicated above in questions 1-3.
____________________________________
MA Organization
__________________ ____________________________________
Date CEO/CFO Signature
____________________________________
Title
(An appropriate State official must complete items 4-7.)
Please note that under section 1856(b)(3) of the Social Security Act and 42 CFR 422.402, other than laws related to State licensure or solvency requirements, the provisions of title XVIII of the Social Security Act preempt State laws with respect to MA plans.
State official reviewing MA State Certification Request:
Reviewer’s Name_______________________________________
State Oversight/Compliance Officer _________________________
Agency Name__________________________________________
Address______________________________________________
City/State_____________________________________________
Telephone_____________________________________________
E-Mail Address _________________________________________
Name of other State agencies (if any) whose approval is required for licensure:
Agency______________________________________________
Contact Person________________________________________
Address______________________________________________
City/State____________________________________________
Telephone____________________________________________
E-Mail Address _______________________________________
Financial Solvency:
Does the applicant organization named in item 1 above meet State financial solvency requirements? (Please circle the correct response)
● Yes ● No
Please indicate which State Agency or Division is responsible for assessing whether the named applicant organization meets State financial solvency requirements.
_______________________________________________________________________
State Licensure:
Does the applicant organization named in item 1 above meet State Licensure requirements? (Please circle the correct response)
● Yes ● No
Please indicate which State Agency or Division is responsible for assessing whether this organization meets State licensure requirements.
_____________________________________________________________________
I hereby certify to the Centers for Medicare & Medicaid Services (CMS) that the above organization (doing business as(d/b/a)_________________________) is:
(check one)
________ licensed in the State of ___________ as a risk bearing entity, or
________ authorized to operate as a risk bearing entity in the State of ________________
and
(check one)
________ is in compliance with state solvency requirements, or
________ state solvency requirement not applicable [please explain below].
By signing the certification, the State of __________ is certifying that the organization is licensed and/or that the organization is authorized to bear the risk associated with the MA product circled in item 3 above. The State is not being asked to verify plan eligibility for the Medicare managed care products(s) or CMS contract type(s) requested by the organization, but merely to certify to the requested information based on the representation by the organization named above.
____________________________________
Agency
__________________ ____________________________________
Date Signature
____________________________________
Title
ESSENTIAL HOSPITAL DESIGNATION TABLE
Please complete this form with the indicated information about each hospital that applicant seeks to have designated as essential. Please note that, under Section 1858(h) of the Social Security Act (the Act) and 42 CFR 422.112(c)(3), applicant organization must have made a good faith effort to contract with each hospital that it seeks to have designated as essential. A “good faith” effort is defined as having offered the hospital a contract providing for payment rates in amounts no less than the amount the hospital would have received had payment been made under section 1886(d) of the Act. The attestation on the following page must be completed and submitted with the completed chart.
Hospital name and address (including county) |
Contact person and phone |
Hospital Type/Provider Number |
Method by which offer was communicated |
Date(s) offer refused/how refused |
Why hospital is needed to meet RPPO’s previously submitted access standards, including distance from named hospital to next closest Medicare participating contracted hospital |
Happy Care Medical Center 211 Green St., Foxdale, Delaware County, PA 21135
|
Any Body, CFO (215) 345-1121 |
Acute Care/ 210076 |
2 Letter Offers followed by 2 phone calls
|
Letter dated 8/02/05. Confirmed by phone call with CFO |
Nearest Medicare participating inpatient facility with which applicant contracts is in downtown Philadelphia, PA – 35 or more miles away from beneficiaries in Delaware County. Applicant’s hospital access standard is 98% of beneficiaries in Delaware County and northern half of Chester County have access to inpatient facility within 30 miles drive. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Regional Preferred Provider Organization (RPPO) Attestation Regarding Designation of “Essential” Hospitals
Applicant Organization named below (the Organization) attests that it made a good faith effort consistent with Section 1858(h) of the Social Security Act (the Act) and 42 CFR 422.112(c)(3), to contract with each hospital identified by the Organization in the attached chart at rates no less than current Medicare inpatient fee-for-service amounts and that, in each case, the hospital refused to enter into a contract with the Organization.
CMS is authorized to inspect any and all books or records necessary to substantiate the information in this attestation and the corresponding designation requests.
The Organization agrees to notify CMS immediately upon becoming aware of any occurrence or circumstance that would make this attestation inaccurate with respect to any of the designated hospitals. I possess the requisite authority to execute this attestation on behalf of the Organization.
Name of Organization: ______________________________________________
Printed Name of CEO: ______________________________________________
Signature:_________________________________________________________
Medicare Advantage RPPO Application/Contract Number(s):
R#_____ ______ ______ ______ ______ ______ ______ ______ ______ ______
NOTE: This attestation form must be signed by any organization that seeks to designate one or more hospitals as “essential.”
Provide a projected timeline with specific dates supporting your attestation to develop and implement your organization’s Quality Improvement Program. The timeline must show how you will meet the CMS requirements to establish the QI program in full no later than CMS contract signature date (estimated to be in September) and implement it no later than CMS contract effective date (i.e., January 1). Specifically, CMS requires that you develop detailed policies and procedures, flowcharts, and system reports by the contract signature date and implement them fully no later than the contract effective date.
The timeline and supporting narrative (if you need elaborate upon your timeline) must include specific dates by which your organization will develop the following key program components:
A Quality Improvement Program that is formally evaluated at least annually.
A policymaking body that exercises oversight and accountability of the Quality Improvement Program.
A mechanism for assuring formal ongoing communication and collaboration among the policy making body that oversees the Quality Improvement Program and the other functional areas (e.g., health services, management, appeals & grievances and management).
A Chronic Care Improvement Program.
An adequate health information system that collects, integrates, analyzes, and reports data necessary to implement its Quality Improvement Program.
A capability to conduct quality improvement projects.
A process for identifying and correcting significant systemic problems that comes to its attention through internal surveillance, complaints, or other mechanisms.
Hiring and assigning appropriate medical and administrative staff to each program component
QUALITY IMPROVEMENT PROGRAM TIMELINE
Deadline for Completion |
Key Quality Improvement Program Component |
(e.g., 09/01/2009 |
Quality Improvement Program, evaluated semi-annually) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solicitations for Special Needs Plan Proposal
Specific Requirements for Dual-eligible SNPs: All 2011 applicants seeking to offer a new or expand the service area of an existing dual-eligible SNP must have a contract with the State Medicaid agency overlapping the CMS MA contracting period. Under the State Medicaid agency contract, the MA organization must meet all of the contractual terms listed in the State Medicaid Agency Contract Upload Document which is a separate document included in the application packet. Specifically, the MA organization must retain responsibility for providing, or contracting for benefits to be provided, for individuals entitled to receive medical assistance under Title XIX. Please note that State Medicaid agencies are not required to enter into contracts with MA organizations for dual-eligible SNPs.
Specific Requirements for Institutional SNPs: All 2011 applicants seeking to offer a new or expand the service area of an existing institutional SNP must specify whether the plan will target only institutionalized individuals, only institutional equivalent individuals living in the community but requiring an institutional level of care, or both subtypes of individuals. Institutional SNPs targeting institutional equivalent individuals are required to use the respective State level of care assessment tool to determine the need for institutional level of care for prospective enrollees. The eligibility assessment must be performed by an entity other than the MA organization offering the SNP.
Specific Requirements for Severe or Disabling Chronic Condition SNPs: All 2011 applicants seeking to offer a new or expand the service area of an existing severe or disabling chronic condition SNP must exclusively serve an individual confirmed to have one of the CMS-approved chronic conditions. For the sole purpose of determining eligibility for a chronic condition SNP, CMS has identified several severe or disabling chronic conditions that meet the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA) definition: Has one or more co-morbid and medically complex chronic conditions that are substantially disabling or life-threatening, has a high risk of hospitalization or other significant adverse health outcomes, and requires specialized delivery systems across domains of care. The list of CMS-approved chronic conditions is found in the C-SNP Proposal section of this application.
Requirements for All (both new and existing) SNPs:
Enrollment Requirements: Both existing and new SNPs can only enroll individuals who meet the statutory definition of special needs individual for the specific SNP. Applicants should refer to the definition section below to assure that their proposal will comply with enrolling only those beneficiaries who meet the statutory definition of special needs individual for their specific type SNP.
Care Management Requirements: All SNPs are required to implement an evidence-based model of care having explicit components. These components include: 1) measurable goals specific to the target special needs individuals; 2) an adequate staff structure having care management roles; 3) an interdisciplinary care team for each beneficiary; 4) a provider network having specialized expertise pertinent to the target special needs individuals; 5) training on the model of care for plan personnel and contractors; 6) comprehensive health risk assessment for each beneficiary; 7) an individualized plan of care having goals and measurable outcomes for each beneficiary; 8) a communication network that facilitates coordination of care; and 9) evaluation of the effectiveness of the model of care. The MA organization must design its model of care to accommodate the needs of the most vulnerable members of its target population, i.e., the frail, the disabled, those near the end-of-life, those having multiple or medically complex chronic conditions, and those who develop end-stage renal disease after enrollment.
Quality Reporting Requirements: All MA organizations are required to collect, analyze, report, and act on data through a systematic and continuous quality improvement program. As an MA plan, each SNP must implement a quality improvement program that focuses on measuring indices of quality, beneficiary health outcomes, and evaluating the effectiveness of its model of care in meeting the needs of its targeted special needs individuals.
For each SNP, MA organizations must coordinate the systematic collection of data using indicators that are objective, clearly defined, and preferably based on valid and reliable measures. Indicators should be selected from a variety of quality and outcome measurement domains such as functional status, care transitioning, disease management, behavioral health, medication management, personal and environmental safety, beneficiary involvement and satisfaction, and family and caregiver support. SNPs must document all aspects of the quality improvement program including data collection and analysis, actions taken to improve the performance of the model of care, and the participation of the interdisciplinary team members and network providers in quality improvement activities. The MA organization should document quality improvement activities and maintain the information for CMS review upon request and during audits.
MA organizations are required to report HEDIS measures (if enrollment threshold is met), Structure & Process measures, HOS survey (if enrollment threshold is met), CAHPS survey (if enrollment threshold is met), Part C Reporting Data, and Medication Therapy Management measures for each SNP.
Definitions:
Full Benefit Dual Eligible (FBDE aka Medicaid only): An individual who does not meet the income or resource criteria for QMB or SLMB, but is eligible for Medicaid either categorically or through optional coverage groups such as medically needy, or special income levels for institutionalized, or home and community-based waivers.
Qualified Medicare Beneficiary (QMB): An individual entitled to Medicare Part A, has income at the 100% Federal Poverty Level (FPL) or less, and resources that do not exceed twice the SSI limit. This individual is eligible for Medicaid payment of Medicare Part B premium, deductibles, co-insurance and co-pays, (except for Part D).
Qualified Medicare Beneficiary Plus (QMB+): An individual who meets standards for QMB eligibility and also meets criteria for full Medicaid benefits in the State. These individuals often qualify for full Medicaid benefits by meeting Medically Needy standards, or through spending down excess income to the Medically Needy level.
Specified Low-Income Medicare Beneficiary (SLMB): An individual entitled to Medicare Part A, has income that exceeds 100% FPL but less than 120% FPL, and resources do not exceed twice the SSI limit. A SLMB is eligible for Medicaid payment of the Medicare Part B premium.
Specified Low-Income Medicare Beneficiary Plus (SLMB+): An individual who meets the standards for SLMB eligibility, but who also meets the criteria for full State Medicaid benefits. Such individuals are entitled to payment of the Medicare Part B premium, as well as full State Medicaid benefits. These individuals often qualify for Medicaid by meeting the Medically Needy standards, or through spending down excess income to the Medically Needy level.
Qualified Disabled and Working Individual (QDWI): An individual who has lost Medicare Part A benefits due to a return to work, but is eligible to enroll in and purchase Medicare Part A. The individual’s income may not exceed 200% FPL and resources may not exceed twice the SSI limit. The individual may not be otherwise eligible for Medicaid. QDWIs are eligible only for Medicaid payment of the Part A premium.
Qualifying Individual (QI): An individual entitled to Medicare Part A, has income at least 120% FPL but less than 135% FPL, and resources that do not exceed twice the SSI limit, and not otherwise eligible for Medicaid benefits. This individual is eligible for Medicaid payment of Medicare Part B premium.
All duals: A SNP that has a State Medicaid agency contract to enroll all categories of Medicaid eligible individuals, who are also Medicare entitled, e.g., FBDE, QMB, QMB+, SLMB, SLMB+, QI and QDWI.
Full duals: A SNP that has a State Medicaid agency contract to enroll Medicaid eligible individuals, who are also Medicare entitled, in the following categories: 1) FBDE, 2) QMB+ and 3) SLMB+.
Zero cost share: A SNP that has a State Medicaid agency contract to enroll Medicaid eligible individuals, who are also Medicare entitled, in the following categories: 1) QMB, 2) QMB+ and 3) any other dual eligible beneficiaries for which the State holds harmless for Part A and Part B cost sharing except Part D.
Medicaid Subset: A SNP that meets the following three criteria.
Is a Dual-eligible SNP;
Has a contract with the State Medicaid agency; and
Has a State-determined targeted subpopulation.
Institutional SNP: A SNP that enrolls eligible individuals who continuously reside or are expected to continuously reside for 90 days or longer in a long-term care (LTC) facility. These LTC facilities may include a skilled nursing facility (SNF); nursing facility (NF); (SNF/NF); an intermediate care facility for the mentally retarded (ICF/MR); and/or an inpatient psychiatric facility. An institutional SNP to serve Medicare residents of LTC facilities must have a contractual arrangement with (or own and operate) the specific LTC facility(ies).
Institutional equivalent SNP: An institutional SNP that enrolls eligible individuals living in the community but requiring an institutional level of care based on the State assessment. The assessment must be performed using the respective State level of care assessment tool and by administered by an entity other than the organization offering the SNP. This type of SNP may restrict enrollment to individuals that reside in a contracted assisted living facility (ALF) if necessary to ensure uniform delivery of specialized care.
SNP Proposal Applications Instructions
Initial (new) SNP
An applicant, including an existing MA contractor, offering a new SNP must submit their SNP proposal by completing the HPMS SNP Proposal Application template and submitting all completed upload documents per HPMS User Guide instructions. A SNP proposal application must be completed for each SNP type to be offered by the MA. The service area of the proposed SNP cannot exceed the existing or pending service area for the MA contract.
All applicants requesting to offer a dual-eligible SNP must have a State Medicaid Agency contract or be in negotiation with the State Medicaid Agency toward that goal. A dual-eligible SNP must have a State Medicaid Agency contract in place prior to the beginning of the 2011 contract year and the contract must overlap the entire CMS MA contract year.
In general, CMS recommends and encourages MA applicants to refer to 42 CFR 422 regulations to clearly understand the nature of the requirement. Nothing in this solicitation is intended to supersede the regulations at 42 CFR 422. Failure to reference a regulatory requirement does not affect the applicability of such requirement. Other associated MA and Part D applications must also be completed and submitted. Applicants must read HPMS notices and visit the CMS web site periodically to stay informed about new or revised guidance documents.
SNP Service Area Expansion (SAE)
An MA organization having an existing contract who wants to expand the service area of the SNP must adhere to the same requirements for submission of an initial SNP proposal application. The service area of the proposed SNP cannot exceed the existing or pending service area for the MA contract.
1. C-SNP Proposal Application
Attestation |
Response |
SNP Proposal Applications |
|
1. Applicant is applying to offer a new severe or disabling chronic condition SNP. |
Yes/No |
2. How many new severe or disabling chronic condition SNPs? |
Insert number. |
3. Applicant is applying to expand an existing severe or disabling chronic condition SNP. |
Yes/No |
2. C-SNP Service Area
Attestation |
Response |
SNP Service Area |
|
1. Provide a separate service area listing (State and County name and code) for each different type of dual-eligible SNP being offered.
NOTE: SNP service area must be equal to or less than the approved or pending MA service. |
Service Area Upload Document |
2. Applicant’s service area is equal to or less than the approved or pending MA service area. |
Yes/No |
3. Applicant’s service area covers more than one State. If yes, respond to question #4 in this section. If no, proceed to the next section. |
Yes/No |
4. Provide the names of the States. |
Insert text. |
3. C-SNP Attestations
Attestation |
Response |
Severe or Disabling Chronic Conditions |
|
1. Applicant will offer a chronic condition SNP (C-SNP) covering one or more of the following severe or disabling chronic conditions |
Yes/No |
2. C-SNP covering only chronic alcohol and other drug abuse |
Yes/No |
3. C-SNP covering only autoimmune disorders |
Yes/No |
4. C-SNP covering only cancer |
Yes/No |
5. C-SNP covering only cardiovascular disorders |
Yes/No |
6. C-SNP covering only chronic heart failure |
Yes/No |
7. C-SNP covering only dementia |
Yes/No |
8. C-SNP covering only diabetes mellitus |
Yes/No |
9. C-SNP covering only end-stage liver disease |
Yes/No |
10. C-SNP covering only end-stage renal disease requiring dialysis |
Yes/No |
11. C-SNP covering only severe hematologic disorders |
Yes/No |
12. C-SNP covering only HIV\AIDS |
Yes/No |
13. C-SNP covering only chronic lung disorders |
Yes/No |
14. C-SNP covering only chronic disabling mental health conditions |
Yes/No |
15. C-SNP covering only neurologic disorders |
Yes/No |
16. C-SNP covering only stroke |
Yes/No |
17. Single C-SNP covering both cardiovascular disorders and chronic heart failure (NOTE: Enrollees must have at least one of the chronic conditions in the group) |
Yes/No |
18. Single C-SNP covering both cardiovascular disorders and diabetes (NOTE: Enrollees must have at least one of the chronic conditions in the group) |
Yes/No |
19. Single C-SNP covering both chronic heart failure and diabetes (NOTE: Enrollees must have at least one of the chronic conditions in the group) |
Yes/No |
20. Single C-SNP covering three conditions - cardiovascular disorders, chronic heart failure, and diabetes (NOTE: Enrollees must have at least one of the chronic conditions in the group) |
Yes/No |
21. Single C-SNP covering both cardiovascular disorders and stroke (NOTE: Enrollees must have at least one of the chronic conditions in the group) |
Yes/No |
22. Customized grouping of CMS-approved chronic conditions (NOTE: Enrollees must have all chronic conditions in the customized group) |
Yes/No |
4. D-SNP Proposal Application
Attestation |
Response |
SNP Proposal Applications |
|
1. Applicant is applying to offer a new dual-eligible SNP. |
Yes/No |
2. How many new dual-eligible SNPs? |
Insert number. |
3. Applicant is applying to expand an existing dual-eligible SNP. |
Yes/No |
5. D-SNP Service Area
Attestation |
Response |
SNP Service Area |
|
1. Provide a separate service area listing (State and County name and code) for each different type of dual-eligible SNP being offered.
NOTE: Applicant’s proposed service area must be equal to or less than the counties included in the approved or pending State Medicaid Agency(ies) contract(s). |
D-SNP State Medicaid Agency Contract Upload Document |
2. Applicant’s service area is equal to or less than the approved or pending MA service area. |
Yes/No |
3. Applicant’s service area is equal to or less than the counties approved in the State Medicaid Agency(ies) contract. |
Yes/No |
4. Applicant’s service area covers more than one State. If yes, respond to question #5 in this section. If no, proceed to the next section. |
Yes/No |
5. Provide the names of the States. |
Insert text. |
6. D-SNP State Medicaid Agency(ies) Contract(s)
Attestation |
Response |
State Medicaid Agency Contracts |
|
1. Applicant has a contract with the State Medicaid Agency (ies) that covers the MA application year. If yes, go to question #2. If no, go to question #3. |
Yes/No |
2. Provide copy of ALL signed State Medicaid Agency (ies) contract(s) and, for EACH contract, a corresponding Contract Matrix that references where to locate the MIPPA required provisions. |
Upload executed contracts and corresponding Contract Matrix for each State Medicaid Agency contract. |
3.
Applicant
has contacted the State Medicaid Agency (ies), initiated contract
negotiation, and will have a signed State Medicaid Agency (ies)
contract for the MA application year. |
Yes/No |
4. Download the “State Medicaid Agency Contract Upload Document” and provide a narrative description of the status of your negotiation with the State Medicaid Agency (ies). |
Upload the completed D-SNP State Medicaid Agency Contract Upload Document. |
5. Provide the State Medicaid contract begin date. |
For each of multiple contracts, reject if < 01/01/2010 |
6. Provide the State Medicaid contract end date. |
For each of multiple contracts, reject if > 12/31/2010 |
Attestation |
Response |
State Medicaid Agency(ies) contract enrolled population |
|
1. Applicant will have an executed State Medicaid Agency (ies) contract to cover one or more of the enrollment categories listed below. Select all enrollment categories that apply.
NOTE: The selected enrollment categories must match those listed in the executed State Medicaid Agency (ies) contract. |
Drop-down Menu |
a. Qualified Medicare Beneficiary Plus(QMB+) dual-eligible |
|
b. Specified Low-income Medicare Beneficiary Plus (SLMB+) dual-eligible |
|
c. Other full benefit dual-eligible also known as "Medicaid only” |
|
d. Qualified Medicare Beneficiary (QMB) dual-eligible |
|
e. Specified Low-income Medicare Beneficiary (SLMB) dual-eligible |
|
f. Qualifying Individual (QI) dual-eligible |
|
g. Qualified Disabled and Working Individual (QDWI) dual-eligible |
|
h. Dual-eligible who are institutionalized |
|
i. Dual-eligible who are institutional equivalent |
|
j. Medicaid Subset enrollment category other than those listed above |
|
2. Provide a description of 1.j., the Medicaid Subset for an enrollment population other than what is listed above in 1.a.-1.i. |
Insert text. |
Attestation |
Response |
State Medicaid Agency(ies) contact |
|
1. Provide the name of the contact individual at the State Medicaid Agency (ies). |
Insert Text |
2. Provide the address of the State Medicaid Agency contact person. |
Insert Text |
3. Provide the phone number of the State Medicaid Agency contact person. |
Insert number |
4. Provide the e-mail address of the State Medicaid Agency contact person. |
Insert text |
7. I-SNP Proposal Application
Attestation |
Response |
SNP Proposal Applications |
|
1. Applicant is applying to offer a new institutional SNP. |
Yes/No |
2. How many new institutional SNPs? |
Insert number. |
3. Applicant is applying to expand an existing institutional SNP. |
Yes/No |
8. I-SNP Service Area
Attestation |
Response |
SNP Service Area |
|
1. Provide a separate service area listing (State and County name and code) for each different type of dual-eligible SNP being offered.
NOTE: SNP service area must be equal to or less than the approved or pending MA service. |
Service Area Upload Document |
2. Applicant’s service area is equal to or less than the approved or pending MA service area. |
Yes/No |
3. Applicant’s service area covers more than one State. If yes, respond to question #4 in this section. If no, proceed to the next section. |
Yes/No |
4. Provide the names of the States. |
Insert text. |
9. I-SNP Attestations
Attestation |
Response |
SNPs enrolling individuals residing in institutions |
|
1. Applicant will enroll individuals residing in a long term care facility under contract with or owned by the organization offering the SNP. |
Yes/No |
2. Provide a list of contracted long-term care facilities. |
Upload I-SNP Upload Document. |
3. Provide attestation for Special Needs Plans (SNP) serving institutionalized beneficiaries. |
Upload I-SNP Attestation Document. |
Attestation |
Response |
|
SNPs enrolling individuals eligible as institutional equivalent |
||
1. Applicant will enroll individuals who are institutional equivalents residing in the community. |
Yes/No |
|
2. Provide a list of assisted-living facilities (respond only if applicant is contracting with ALFs). |
Upload document. |
|
3. Applicant owns or has an executed contract(s) with each of the ALFs on the list (if applicable)? |
Yes/No/NA |
|
4. Applicant uses the respective State level of care (LOC) assessment tool to determine eligibility for each institutional equivalent beneficiary.
NOTE: The applicant must use the respective State (LOC) assessment tool to determine eligibility for institutional equivalent individuals living in the community. |
Yes/No |
|
5. Provide a copy of the State LOC assessment tool. |
Upload document. |
|
6. Provide the URL for the State LOC assessment tool if accessible on the State website. |
http://xxxxxxxxxxxx.xxx |
|
7. Applicant uses an unrelated third party entity to perform the LOC assessment.
NOTE: The applicant must use an unrelated third party entity to perform the LOC assessment. |
Yes/No |
|
8. Provide the name of the entity (ies) performing the LOC assessment. |
Insert text. |
|
9. Provide the address of the entity (ies) performing the LOC assessment. |
Insert text. |
|
10. Provide the relevant credential (e.g., RN for registered nurse, LSW for licensed social worker, etc.) of the staff from the entity (ies) performing the LOC assessment. |
Insert text. |
|
10. ESRD Waiver Request
Attestation |
Response |
ESRD Waiver Requests |
|
1. Applicant requests an ESRD waiver. If yes, respond to questions 2 through 6 below. If no, proceed to the next section. |
Yes/No |
2. Provide a description of how it intends to serve the unique needs of the ESRD enrollees in the ESRD Waiver Request Upload Document. |
Upload ESRD Upload Document. |
3. Provide a list of the contracted dialysis facility (ies). |
Upload ESRD Upload Document. |
4. Provide a list of the contracted transplant facility (ies). |
Upload ESRD Upload Document. |
5. Provide a description of any additional service(s) provided to members with ESRD. |
Upload ESRD Upload Document. |
6. Provide a description of the interdisciplinary care team coordinator role in the assessment and delivery of services needed by members with ESRD. |
Upload ESRD Upload Document. |
11. Model of Care Attestations
Attestation |
Response |
Written Care Management Plan |
|
1. Applicant has a written care management plan that describes its model of care. |
Yes/No |
2. Complete and upload the Model of Care Matrix Upload Document. |
Upload |
3. Upload a copy of the written care management plan. |
Upload |
Attestation |
Response |
Model of Care Goals |
|
1. Applicant has the goal to improve access to medical, mental health, and social services for its enrolled special needs individuals. |
Yes/No |
2. Applicant has the goal to improve access to care for its enrolled special needs individuals. |
Yes/No |
3. Applicant has the goal to improve coordination of care through an identified point of contact for its enrolled special needs individuals. |
Yes/No |
4. Applicant has the goal to provide seamless transitions across healthcare settings, care providers, and health services for its enrolled special needs individuals. |
Yes/No |
5. Applicant has the goal to improve access to preventive health services for its enrolled special needs individuals. |
Yes/No |
6. Applicant has the goal to assure appropriate utilization of services by its enrolled special needs individuals. |
Yes/No |
7. Applicant has the goal to assure cost-effective health services delivery for its enrolled special needs individuals. |
Yes/No |
8. Applicant has the goal to improve beneficiary health outcomes through reducing hospitalization and nursing facility placement for its enrolled special needs individuals. |
Yes/No |
9. Applicant has the goal to improve beneficiary health outcomes through improved independence and self-management for its enrolled special needs individuals. |
Yes/No |
10. Applicant has the goal to improve beneficiary health outcomes through improved mobility and functional status for its enrolled special needs individuals. |
Yes/No |
11. Applicant has the goal to improve beneficiary health outcomes through improved pain management for its enrolled special needs individuals. |
Yes/No |
12. Applicant has the goal to improve beneficiary health outcomes through improved quality of life as perceived by its enrolled special needs individuals. |
Yes/No |
13. Applicant has the goal to improve beneficiary health outcomes through improved satisfaction with health status and healthcare services for its enrolled special needs individuals. |
Yes/No |
14. Applicant’s model of care goals are written as measurable outcomes. |
Yes/No |
15. Applicant’s care management plan specifies how it will determine that model of care goals are met. |
Yes/No |
16. Applicant’s care management plan specifies what action it will take if goals are not met. |
Yes/No |
Attestation |
Response |
Staff Structure and Care Management Roles |
|
1. Applicant has appropriate staff (employed or contracted) to perform administrative functions. Specific functions include: |
Yes/No |
2. Processes enrollment |
Yes/No |
3. Verifies eligibility of enrollees |
Yes/No |
4. Processes claims |
Yes/No |
5. Processes and facilitates resolution of consumer or provider complaints |
Yes/No |
6. Communicates telephonically and disseminates written plan information to beneficiaries, network providers, |
Yes/No |
7. Applicant has appropriate staff (employed or contracted) to collect, analyze, report, and act on performance and health outcome data. Specific tasks include: |
Yes/No |
8. Conducts a quality improvement program |
Yes/No |
9. Reviews and analyzes utilization data |
Yes/No |
10. Survey beneficiaries, plan personnel and network providers, oversight agencies, and the public. |
Yes/No |
11. Applicant has appropriate staff (employed or contracted) to coordinate care for beneficiaries across care settings and providers. Specific functions include: |
Yes/No |
12. Authorizes and/or facilitates access to specialists and therapies |
Yes/No |
13. Advocates, informs, educates beneficiaries on services and benefits |
Yes/No |
14. Identifies and facilitates access to community resources and social services |
Yes/No |
15. Triages beneficiaries care needs |
Yes/No |
16. Conducts risk assessment |
Yes/No |
17. Facilitates the implementation of the individualized care plan for each beneficiary |
Yes/No |
18. Schedules or facilitates scheduling appointments and follow-up services. |
Yes/No |
19. Facilitates transportation services |
Yes/No |
20. Requests consultation and diagnostic reports from network specialists |
Yes/No |
21. Facilitates translation services |
Yes/No |
22. Applicant has appropriate staff (employed or contracted) to deliver medical, mental health, and social services to beneficiaries. |
Yes/No |
23. Applicant has appropriate staff (employed or contracted) to manage healthcare information related to medical, mental health, and social services delivered to beneficiaries. Specific functions include: |
Yes/No |
24. Assures maintenance and sharing of healthcare records |
Yes/No |
25. Assures HIPAA compliance |
Yes/No |
26. Applicant has appropriate staff (employed or contracted) to perform administrative oversight duties. Specific administrative oversight duties include: |
Yes/No |
27. Oversees plan operations and develops policies |
Yes/No |
28. Authorizes and/or facilitates access to specialists and therapies |
Yes/No |
29. Assures current licensure and competency of providers |
Yes/No |
30. Monitors contractual services to assure contractor compliance |
Yes/No |
31. Assures statutory and regulatory compliance |
Yes/No |
32. Evaluate the effectiveness of the model of care |
Yes/No |
33. Applicant has appropriate staff (employed or contracted) to perform clinical oversight duties. Specific clinical oversight duties include: |
Yes/No |
34. Conducts and/or observes interdisciplinary team meetings randomly |
Yes/No |
35. Assures care and pharmacotherapy are delivered as planned by the interdisciplinary team |
Yes/No |
36. Coordinates care across settings and providers |
Yes/No |
37. Assures providers adhere to nationally-recognized clinical practice guidelines in clinical care |
Yes/No |
38. Assures clinical services are appropriate and timely |
Yes/No |
39. Monitors provision of services and benefits to assure follow-up |
Yes/No |
40. Monitors provision of services to assure care is seamlessly transitioned across settings and providers |
Yes/No |
41. Conducts targeted medical chart reviews |
Yes/No |
42. Conducts medication reviews |
Yes/No |
Attestation |
Response |
Interdisciplinary Care Team |
|
1. Applicant assigns each beneficiary to an interdisciplinary care team composed of primary, ancillary, and specialty care providers. Members of the interdisciplinary care team include some or all of the following: |
Yes/No |
2. Primary care physician |
Yes/No |
3. Nurse practitioner, physician’s assistant, mid-level provider |
Yes/No |
4. Social worker, community resources specialist |
Yes/No |
5. Registered nurse |
Yes/No |
6. Restorative health specialist (physical, occupational, speech, recreation) |
Yes/No |
7. Behavioral and/or mental health specialist (psychiatrist, psychologist, drug or alcohol therapist) |
Yes/No |
8. Board-certified physician |
Yes/No |
9. Dietitian, nutritionist |
Yes/No |
10. Pharmacist, clinical pharmacist |
Yes/No |
11. Disease management specialist |
Yes/No |
12. Nurse educator |
Yes/No |
13. Pastoral specialists |
Yes/No |
14. Caregiver/family member whenever feasible |
Yes/No |
15. Preventive health/health promotion specialist |
Yes/No |
16. Applicant facilitates the participation of the beneficiary on the Interdisciplinary Care Team whenever feasible. |
Yes/No |
17. Applicant assures that the interdisciplinary care team works together to manage beneficiary care by performing care management functions. Care management functions include: |
Yes/No |
18. Develop and implement an individualized care plan with the beneficiary/caregiver |
Yes/No |
19. Conduct care coordination meetings annually |
Yes/No |
20. Conduct care coordination meetings on a regular schedule |
Yes/No |
21. Conduct face-to-face meetings |
Yes/No |
22. Maintain a web-based meeting interface |
Yes/No |
23. Maintain web-based electronic health information |
Yes/No |
24. Conduct case rounds on a regular schedule |
Yes/No |
25. Maintain a call line or other mechanism for beneficiary inquiries and input |
Yes/No |
26. Conduct conference calls among plan, providers, and beneficiaries |
Yes/No |
27. Develop and disseminate newsletters or bulletins |
Yes/No |
28. Maintain a mechanism for beneficiary complaints and grievances |
Yes/No |
29. Use e-mail, fax, and written correspondence to communicate |
Yes/No |
Attestation |
Response |
Provider Network and Use of Clinical Practice Guidelines |
|
1. Applicant has a network of providers and facilities having specialized clinical expertise pertinent to the targeted special needs population. The provider network includes: |
Yes/No |
2. Acute care facility, hospital, medical center |
Yes/No |
3. Laboratory |
Yes/No |
4. Long-term care facility, skilled nursing facility |
Yes/No |
5. Pharmacy |
Yes/No |
6. Radiography facility |
Yes/No |
7. Rehabilitative facility |
Yes/No |
8. Primary care providers |
Yes/No |
9. Nursing professionals |
Yes/No |
10. Mid-level practitioners |
Yes/No |
11. Rehabilitation/restorative therapy specialists |
Yes/No |
12. Social worker/social services specialists |
Yes/No |
13. Mental health specialists |
Yes/No |
14. Medical specialists pertinent to targeted chronic conditions and identified co-morbid conditions |
Yes/No |
15. Pharmacists and/or clinical pharmacists |
Yes/No |
16. Oral health specialists |
Yes/No |
17. Applicant gives priority to having board-certified specialists in the provider network. |
Yes/No |
18. Applicant assures that the provider and facility network having specialized clinical expertise pertinent to the targeted special needs population delivers services. Specific services include: |
Yes/No |
19. Assess, diagnose, and treat in collaboration with the interdisciplinary care team |
Yes/No |
20. Provide 24-hour access to a clinical consultant |
Yes/No |
21. Conduct conference calls with the interdisciplinary care team as needed |
Yes/No |
22. Assist with developing and updating individualized care plans |
Yes/No |
23. Assist with conducting disease management programs |
Yes/No |
24. Provide wound management services |
Yes/No |
25. Provide pharmacotherapy consultation and/or medication management clinics |
Yes/No |
26. Conduct home visits for clinical assessment or treatment |
Yes/No |
27. Conduct home safety assessments |
Yes/No |
28. Provide home health services |
Yes/No |
29. Provide home-based end-of-life care |
Yes/No |
30. Conduct risk prevention programs such as fall prevention or wellness promotion |
Yes/No |
31. Provide telemonitoring services |
Yes/No |
32. Provide telemedicine services |
Yes/No |
33. Provide in-patient acute care services |
Yes/No |
34. Provide hospital-based or urgent care facility-based emergency services |
Yes/No |
35. Provide long-term facility care |
Yes/No |
36. Applicant has a process to assure that its network facilities and providers have current licensure and/or certification to perform services that meet the specialized needs of special needs individuals. |
Yes/No |
37. Applicant has a credentialing review every three years to assure that its network providers are credentialed and competent to perform services that meet the specialized needs of special needs individuals. |
Yes/No |
38. Applicant has a process to coordinate the delivery of services through a provider and facility network having clinical expertise pertinent to the targeted special needs population. The process includes some or all of the following: |
Yes/No |
39. Applicant contracts with providers having the clinical expertise to meet the specialized needs of the targeted SNP population |
Yes/No |
40. Applicant contracts with facilities that provide diagnostic and treatment services to meet the specialized needs of the targeted SNP population |
Yes/No |
41. Applicant’s contract directs how the network providers and facilities will deliver services to beneficiaries |
Yes/No |
42. Applicant has employed or contracted administrative staff that approve all referrals to network or out-of network providers prior to the delivery of services and notifies the interdisciplinary care team |
Yes/No |
43. Applicant has the beneficiary’s interdisciplinary care team approve all referrals to the network or out-of-network providers prior to the delivery of services and notifies the plan’s administrative staff |
Yes/No |
44. Applicant has the beneficiary directly contact network or out-of-network providers to schedule necessary services |
Yes/No |
45. Applicant has the beneficiary notify the plan and/or interdisciplinary team regarding necessary services |
|
46. Applicant tracks and analyzes services utilization to assure appropriate use of services |
Yes/No |
47. Applicant contacts beneficiaries to remind them about upcoming appointments |
Yes/No |
48. Applicant contacts beneficiaries to follow-up on missed appointments |
Yes/No |
49. Applicant has a process to coordinate the seamless transition of care across healthcare settings and providers. The process includes: |
Yes/No |
50. Applicant has written procedures that direct how the network providers and facilities will deliver services to beneficiaries including transition of care from setting-to-setting, provider-to-provider, and provider-to-facility |
Yes/No |
51. Applicant monitors the transition of care from setting-to-setting, provider-to-provider, provider-to-facility, and notification to the interdisciplinary care team |
Yes/No |
52. Applicant has written procedures that require notification to the interdisciplinary care team and respective providers when transitions of care occur |
Yes/No |
53. Applicant tracks and analyzes transitions of care to assure timeliness and appropriateness of services |
Yes/No |
54. Applicant disseminates the results of the transition of care analysis to the interdisciplinary care team |
Yes/No |
55. Applicant contacts beneficiaries to monitor their status after a transition of care from provider-to-provider, facility-to-facility, or provider-to-facility |
Yes/No |
56. Applicant has a process to monitor its providers and assure they deliver evidence-based services in accordance with nationally recognized clinical protocols and guidelines when available (see the Agency for Healthcare Research and Quality’s National Guideline Clearinghouse, http://www.guideline.gov/). The process includes some or all of the following: |
Yes/No |
57. Applicant has written procedures to assure that employed providers deliver services in accordance with nationally recognized clinical protocols and guidelines when available |
Yes/No |
58. Applicant’s contract with providers stipulates that contracted providers deliver services in accordance with nationally recognized clinical protocols and guidelines when available |
Yes/No |
59. Applicant conducts periodic surveillance of employed and contracted providers to assure that nationally recognized clinical protocols and guidelines are used when available and maintains monitoring data for review during CMS monitoring visits |
Yes/No |
Attestation |
Response |
Model of Care Training for Personnel and Providers |
|
1. Applicant trains employed and contracted personnel on the model of care to coordinate and/or deliver all services. Applicant conducts training using one or more of the following methods: |
Yes/No |
2. Face-to-face training |
Yes/No |
3. Web-based interactive training |
Yes/No |
4. Self-study program (electronic media, print materials) |
Yes/No |
5. Applicant maintains documentation that model of care training was completed by employed and contracted personnel. |
Yes/No |
6. Applicant designated personnel responsible to oversee training implementation and maintain training records for review upon CMS request. |
Yes/No |
7. Applicant has a process for taking action when the required model of care training has not been completed by employed or contracted personnel. |
Yes/No |
Attestation |
Response |
Health Risk Assessment |
|
1. Applicant conducts a comprehensive initial health risk assessment of the medical, functional, cognitive, and psychosocial status as well as annual health risk reassessments for each beneficiary. The process for health risk assessment includes some or all of the following: |
Yes/No |
2. Initial comprehensive health risk assessment is conducted within 90 days of enrollment and results are used to develop the individualized care plan |
Yes/No |
3. Annual comprehensive health risk assessment is conducted and results are used to update the individualized care plan |
Yes/No |
4. Comprehensive initial and annual health risk assessment examines medical, psychosocial, cognitive, and functional status |
Yes/No |
5. Comprehensive health risk assessment is conducted face-to-face by the applicant |
Yes/No |
6. Comprehensive health risk assessment is conducted telephonically by the applicant |
Yes/No |
7. Comprehensive health risk assessment is conducted by the beneficiary completing an electronic or paper-based questionnaire |
Yes/No |
8. Applicant develops or selects and utilizes a comprehensive risk assessment tool that will be reviewed during oversight activities. The tool consists of: |
Yes/No |
9. An existing validated health risk assessment tool |
Yes/No |
10.A plan-developed health risk assessment tool |
Yes/No |
11. An electronic health risk assessment tool |
Yes/No |
12.A paper health risk assessment tool |
Yes/No |
13. Applicant standardized the use of the health risk assessment tool for all beneficiaries |
Yes/No |
14. Applicant periodically reviews the effectiveness of the health risk assessment tool |
Yes/No |
15. Provide a copy of the comprehensive health risk assessment tool. |
Yes/No |
16. Applicant has a process to analyze identified health risks and stratify them to develop an individualized care plan that mitigates health risks. The process includes some or all of the following: |
Yes/No |
17. Comprehensive health risk analysis is conducted by a credentialed healthcare professional |
Yes/No |
18. Applicant notifies the Interdisciplinary Care Team, respective providers, and beneficiary about the results of the health risk analysis |
Yes/No |
19. Applicant uses predictive modeling software to stratify beneficiary health risks for the development of an individualized care plan |
Yes/No |
20. Applicant manually analyzes health risk data to stratify beneficiary health risks for the development of an individualized care plan |
Yes/No |
21. Applicant tracks and trends population health risk data to inform the development of specialized benefits and services |
|
Attestation |
Response |
Individualized Care Plan |
|
1. Applicant has written procedures that direct how the interdisciplinary care team develops and implements a comprehensive individualized plan of care for each beneficiary. The system includes some or all of the following: |
Yes/No |
2. Each beneficiary is assigned an interdisciplinary care team that develops the individualized care plan with beneficiary involvement when feasible |
Yes/No |
3. Results from the initial health risk assessment are used to develop the individualized care plan |
Yes/No |
4. Beneficiary’s medical history is used to develop the individualized care plan |
Yes/No |
5. Beneficiary’s healthcare preferences are incorporated in the individualized care plan |
Yes/No |
6. Interdisciplinary care team members update the individualized care plan as beneficiary health status changes |
Yes/No |
7. Interdisciplinary care team notifies respective providers and beneficiaries when they update care plans that result from health status changes |
Yes/No |
8. Applicant has a written process to facilitate beneficiary/caregiver participation in care planning when feasible. The process includes some or all of the following: |
Yes/No |
9. Beneficiaries and/or caregivers participate face-to-face in care planning |
Yes/No |
10. Beneficiaries and/or caregivers participate telephonically in care planning |
Yes/No |
11. Beneficiaries and/or caregivers participate in care planning through an exchange of written correspondence with their interdisciplinary team |
Yes/No |
12. Beneficiaries and/or caregivers participate in care planning through a web-based electronic interface or virtual correspondence |
Yes/No |
13. Applicant has a written procedure for maintaining the documented care plan for each beneficiary that complies with HIPAA. |
Yes/No |
14. Applicant facilitates access to the documented care plan for the Interdisciplinary Care Team, respective providers, and beneficiaries |
Yes/No |
Attestation |
Response |
Health Risk Assessment |
|
1. Applicant has written procedures to coordinate the delivery of services and benefits through communication systems connecting plan personnel, providers, and beneficiaries. These systems include some or all of the following: |
Yes/No |
2. Call-line for beneficiary inquiries |
Yes/No |
3. Call-line for provider network inquiries |
Yes/No |
4. Care coordination meetings |
Yes/No |
5. Case rounds |
Yes/No |
6. Complaints and grievances documentation and system for resolution |
Yes/No |
7. Committees (standing and ad hoc) |
Yes/No |
8. Conference calls |
Yes/No |
9. E-mails, faxes, written correspondence |
Yes/No |
10. Electronic network for meetings, training, information, communication |
Yes/No |
11. Electronic records (administrative data and/or clinical care) |
Yes/No |
12. Newsletter, bulletin |
Yes/No |
13. Person-to-person direct interface |
Yes/No |
14. Applicant has written procedures to coordinate communication among the interdisciplinary care team members and the beneficiary. The system includes some or all of the following: |
Yes/No |
15. Regularly scheduled face-to-face team meetings |
Yes/No |
16. Regularly scheduled team conference calls |
Yes/No |
17. Regularly scheduled web-based team networking |
Yes/No |
18. Team access to shared electronic health information |
Yes/No |
19. Randomly scheduled team meetings conducted when needed |
Yes/No |
20. Applicant has written procedures describing how communication among stakeholders is documented and maintained as part of the administrative and clinical care records. Documentation includes some or all of the following: |
Yes/No |
21. Written minutes |
Yes/No |
22. Recordings |
Yes/No |
23. Transcripts from recordings |
Yes/No |
24. Newsletters, bulletins |
Yes/No |
25. Web-based database |
Yes/No |
26. Applicant’s written plan identifies the personnel having oversight responsibility for its communication network. |
Yes/No |
Attestation |
Response |
Care Management for the Most Vulnerable Subpopulations |
|
1. Applicant has written procedures to identify the most vulnerable beneficiaries enrolled in the SNP. |
Yes/No |
2. Applicant delineated additional services it will provide for its most vulnerable beneficiaries. These add-on services address the specialized needs of the following vulnerable special needs individuals within each target population: |
Yes/No |
3. Frail |
Yes/No |
4. Disabled |
Yes/No |
5. Beneficiaries developing end-stage renal disease after enrollment |
Yes/No |
6. Beneficiaries near the end-of-life |
Yes/No |
7. Beneficiaries having multiple and complex chronic conditions |
Yes/No |
Attestation |
Response |
Performance and Health Outcome Measurement |
|
1. Applicant collects, analyzes, reports, and acts on data to annually evaluate the effectiveness of its model of care. This evaluation process includes examining the effectiveness of some or all of the following model of care elements by demonstrating: |
Yes/No |
2. Improved access to medical, mental health, and social services |
Yes/No |
3. Improved access to affordable care |
Yes/No |
4. Improved coordination of care through a single point of care management |
Yes/No |
5. Improved transition of care across settings and providers |
Yes/No |
6. Improved access to preventive health services |
Yes/No |
7. Improved beneficiary health outcomes |
Yes/No |
8. Quality and/or improved service delivery and oversight of services through appropriate staffing and implementation of roles |
Yes/No |
9. Quality and/or improved coordination of care through implementation of the interdisciplinary care team |
Yes/No |
10. Quality and/or improved service delivery through a competent provider network having specialized expertise and implementing evidence-based practice guidelines |
Yes/No |
11. Quality and/or improved coordination of care through identification and stratification of health risks |
Yes/No |
12. Quality and/or improved coordination of care through implementation of a dynamic individualized care plan addressing identified health risks |
Yes/No |
13. Quality and/or improved coordination of care through effective communication networks and documentation of care |
Yes/No |
14. Quality and/or improved coordination of care for vulnerable beneficiaries through implementation of the model of care |
Yes/No |
15. Applicant has written procedures to collect, analyze, report, and act on data using a variety of strategies. Strategies include some or all of the following: |
Yes/No |
16. Internal quality assurance specialists implementing and evaluating a performance improvement program |
Yes/No |
17. External quality assurance consultants implementing and evaluating a performance improvement program |
Yes/No |
18. Participation by plan, provider network, and beneficiaries/caregivers |
Yes/No |
19. Data collection and analysis via electronic software |
Yes/No |
20. Data collection and analysis via manual techniques |
Yes/No |
21. Applicant takes actions to improve the model of care. Actions include some or all of the following: |
Yes/No |
22. Changes in policies or procedures |
Yes/No |
23. Changes in staffing patterns or personnel |
Yes/No |
24. Changes in provider or facility network |
Yes/No |
25. Changes in systems of operation |
Yes/No |
26. Communication of results internally and externally |
Yes/No |
27. Applicant documents its evaluation of the effectiveness of its model of care and assures access to the documentation for all stakeholders. |
Yes/No |
28. Applicant designates personnel having oversight responsibility for the evaluation of the model of care effectiveness. |
Yes/No |
29. Applicant communicates the results of its model of care evaluation to all stakeholders. |
Yes/No |
12. Quality Improvement Program Requirements
Attestation |
Response |
SNP Quality Improvement Program Requirements |
|
1. Applicant has a written plan including policies, procedures, and a systematic methodology to conduct an overall quality improvement program that is specific to its targeted special needs individuals. |
Yes/No |
2. Applicant has a health information system to collect, analyze, and integrate valid and reliable data to conduct its overall quality improvement program. |
Yes/No |
3. Applicant has a system to maintain health information for CMS review as requested. |
Yes/No |
4. Applicant has a system to ensure that data collected, analyzed, and reported are accurate and complete. |
Yes/No |
5. Applicant conducts an annual review of the effectiveness of its quality improvement program. |
Yes/No |
6. Applicant takes action to correct problems identified through its quality improvement activities as well as complaints from beneficiaries and providers. |
Yes/No |
7. Applicant conducts one or more chronic care improvement programs to improve health outcomes for beneficiaries having chronic conditions. |
Yes/No |
8. Applicant identifies beneficiaries with multiple or severe chronic conditions that would benefit from participation in a chronic care improvement program. |
Yes/No |
9. Applicant has a mechanism to monitor beneficiaries that participate in a chronic care improvement program. |
Yes/No |
10. Applicant conducts one or more quality improvement projects on clinical or non-clinical areas. |
Yes/No |
11. For each quality improvement project, applicant measures performance, applies interventions to improve performance, evaluates performance, and conducts periodic follow-up to ensure the effectiveness of the intervention. |
Yes/No |
12. For each quality improvement project, applicant evaluates performance using quality indicators that are objective, clearly defined, and correspond to measurable outcomes such as changes in health status, functional status, and beneficiary satisfaction. |
Yes/No |
13. For each quality improvement project, applicant collects, analyzes, reports, and acts on valid and reliable data, and achieves demonstrable improvement from interventions. |
Yes/No |
14. For each special needs plan, applicant collects, analyzes, and reports data that measure health outcomes and indices of quality pertaining to the management of care for its targeted special needs population (i.e., dual-eligible, institutionalized, or chronic condition) at the plan level. |
Yes/No |
15. For each special needs plan, applicant collects, analyzes, and reports data that measure access to care (e.g., service and benefit utilization rates, or timeliness of referrals or treatment). |
Yes/No |
16. For each special needs plan, applicant collects, analyzes, and reports data that measure improvement in beneficiary health status (e.g., quality of life indicators, depression scales, or chronic disease outcomes). |
Yes/No |
17. For each special needs plan, applicant collects, analyzes, and reports data that measure staff implementation of the SNP model of care (e.g., National Committee for Quality Assurance accreditation measures or medication reconciliation associated with care setting transitions indicators). |
Yes/No |
18. For each special needs plan, applicant collects, analyzes, and reports data that measure comprehensive health risk assessment (e.g., accuracy of acuity stratification, safety indicators, or timeliness of initial assessments or annual reassessments). |
Yes/No |
19. For each special needs plan, applicant collects, analyzes, and reports data that measure implementation of an individualized plan of care (e.g., rate of participation by IDT members and beneficiaries in care planning). |
Yes/No |
20. For each special needs plan, applicant collects, analyzes, and reports data that measure use and adequacy of a provider network having targeted clinical expertise (e.g., service claims, pharmacy claims, diagnostic reports, etc.) |
Yes/No |
21. For each special needs plan, applicant collects, analyzes, and reports data that measure delivery of add-on services and benefits that meet the specialized needs of the most vulnerable beneficiaries (frail, disabled, near the end-of-life, etc.). |
Yes/No |
22. For each special needs plan, applicant collects, analyzes, and reports data that measure provider use of evidence-based practices and/or nationally recognized clinical protocols. |
Yes/No |
23. For each special needs plan, applicant collects, analyzes, and reports data that measure the effectiveness of communication (e.g., call center utilization rates, rates of beneficiary involvement in care plan development, analysis of beneficiary or provider complaints, etc.). |
Yes/No |
24. For each special needs plan, applicant collects, analyzes, and reports data that measure CMS-required data on quality and outcomes measures that will enable beneficiaries to compare health coverage options. These data include HEDIS, HOS, and/or CAHPS data. |
Yes/No |
25. For each special needs plan, applicant collects, analyzes, and reports data that measure CMS-required Part C Reporting Data Elements that will enable CMS to monitor plan performance. |
Yes/No |
26. For each special needs plan, applicant collects, analyzes, and reports CMS-required Medication Therapy Management measures that will enable CMS to monitor plan performance. |
Yes/No |
27. Provide a copy of the MAO’s written plan that describes its overall quality improvement program. |
Upload |
28. Provide a completed copy of the Quality Improvement Program Matrix Upload Document. |
Upload Quality Improvement Program Matrix Upload Document. |
13. C-SNP Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
||
2011 Severe or Disabling Chronic Condition SNP Upload Document |
||
Applicant's Contract Name (as provided in HPMS): |
||
Enter contract name here. |
||
CMS Contract Number: |
||
Enter CMS contract number here. |
||
Provide the service area (County, State name and code) for the severe or disabling chronic condition SNP being offered. |
||
County Name |
State Name |
State & County Code |
Enter County name here. |
Enter State 2 letter abbreviation here. |
Enter State & County Code here. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14. D-SNP Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
||
2011 D-SNP State Medicaid Agency Contract Upload Document |
||
Applicant's Contract Name (as provided in HPMS): |
||
Enter contract name here.
|
||
CMS Contract Number: |
||
Enter CMS contract number here.
|
||
1. Provide the service area (County, State name and code) for the dual-eligible SNP being offered. |
||
County Name |
State Name |
State & County Code |
Enter County name here. |
Enter State 2 letter abbreviation here. |
Enter State & County Code here. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2. Provide the State Medicaid Agency (ies) contract approved service area.
NOTE: The service area specified in the State Medicaid contract must at a minimum cover the same service area as the MA SNP. |
||
County Name |
State Name |
State & County Code |
Enter County name here. |
Enter State 2 letter abbreviation here. |
Enter State & County Code here. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3. Provide a description of your progress toward negotiating the MA organization’s responsibilities, including financial obligations, to provide or arrange for Medicaid benefits covered in the State Medicaid contract.
NOTE: The contract must specify the following items:
|
||
Enter your response to #3 here.
|
||
4. Provide a full description of your progress toward negotiating the category(ies) of eligibility for dual-eligible beneficiaries enrolled under the SNP as describe in the Statute at sections 1902(a), 1902(f), 1902(p), and 1905.
NOTE: A “Medicaid subset” SNP is defined as:
|
||
Enter your response to #4 here.
|
||
5. Provide a full description of your progress toward negotiating the Medicaid benefits covered in the State Medicaid contract.
NOTE: These are the Medicaid medical services that the organization is obligated to provide under its State contract, not the non-Medicare mandatory Part C services covered under the MA contract. |
||
Enter your response to #5 here.
|
||
6. Provide a full description of your progress toward negotiating the cost-sharing protections covered in the State Medicaid contract.
NOTE: Specifically indicate that the Medicaid entity will not bill or hold the member responsible in any way for charges or deductibles for Medically Necessary Covered Services. |
||
Enter your response to #6 here.
|
||
7. Provide a full description of your progress toward negotiating the identification and sharing of information on Medicaid provider participation covered in the State Medicaid contract.
NOTE: The description must contain language indicating that the MA SNP has written procedures for ensuring Medicaid network adequacy including access standards. |
||
Enter your response to #7 here.
|
||
8. Provide a full description of your progress toward negotiating the process to verify Medicaid eligibility of individuals through the State.
NOTE: The targeted group(s) must be specified in the State Medicaid Agency contract. |
||
Enter your response to #8 here.
|
||
9. Provide a full description of your progress toward negotiating the process to coordinate Medicare and Medicaid services for dual-eligible enrollees. |
||
Enter your response to #9 here.
|
||
15. D-SNP State Medicaid Agency Contract Matrix
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
||||
STATE CONTRACT/SUB CONTRACT REQUIREMENTS
<organization name, plan name/area, PBP number, and date of preparation> |
||||
CMS Regulations – 42 CFR 422.107 (c) |
Page Number(s) |
Section Number |
Comments |
Reviewer Findings (For CMS Reviewer Use Only) |
MA organizations responsibility, including financial obligations, to provide or arrange for Medicaid benefits
The contract must specify the following items:
|
|
|
|
|
Category(ies) of eligibility for dual-eligible beneficiaries enrolled under the SNP, as described under the Statute at sections 1902(a), 1902(f), 1902(p), and 1905.
A “Medicaid subset” SNP is defined as:
NOTE: If applicable, please use State aid codes to identify category of duals being enrolled. Additional guidance can be found at the following link: http://www.cms.hhs.gov/DualEligible/02_DualEligibleCategories.asp#TopOfPage |
|
|
|
|
Medicaid benefits covered under the SNP
These are the Medicaid medical services that the organization is obligated to provide under its State contract, not the non-Medicare mandatory Part C services covered under the MA contract.
|
|
|
|
|
Cost-sharing protections covered under the SNP
Specifically indicate that the Medicaid entity will not bill or hold the member responsible in any way for charges or deductibles for Medically Necessary Covered Services. NOTE: Covered services under Medicaid may also, depending on State law and policy, include a provider network and prior authorization component, except for emergency services. |
|
|
|
|
Identification and sharing of information on Medicare provider participation
Must contain language indicating that the MA SNP has written procedures for ensuring Medicaid network adequacy, including access standards.
|
|
|
|
|
Verification of enrollee’s eligibility for both Medicare and Medicaid
The targeted group(s) must be specified in the State Medicaid agency contract.
|
|
|
|
|
Service area covered by the SNP
The service area specified in the State Medicaid contract must at a minimum, cover the same service area as the MA SNP.
|
|
|
|
|
The contract period for the SNP
The contracting period between the State Medicaid agency and the DE SNP must specify that it will continue through the contract year. (January 1-December 31 of the year following the due date, October 1, 2009) If not, the plan may indicate the evergreen clause within the contract and provide an explanation of when the State issues updated rates.
|
|
|
|
|
16. I-SNP Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
|
Applicant's Contract Name (as provided in HPMS): |
|
Enter contract name here. |
|
CMS Contract Number: |
|
Enter CMS contract number here. |
|
Specific I-SNP Population: Place an “X” to the left. (Mark only one.) |
|
|
A. Applicant will enroll ONLY individuals living in an institution. |
|
B. Applicant will enroll ONLY individuals who are institutional equivalent and living in the community. |
|
C. Applicant will enroll BOTH institutionalized and institutional equivalent individuals. |
Applicant Enrolling ONLY Institutionalized |
||
1. Provide the service area (County, State name and code) for the institutional SNP being offered. |
||
County Name |
State Name |
State & County Code |
Enter County name here. |
Enter State 2 letter abbreviation here. |
Enter State & County Code here. |
|
|
|
|
|
|
|
|
|
2. Provide a list of contracted long-term care facilities. |
||
Name of Contracted Long-term Care Facilities |
Medicaid Provider # |
Facilities Address |
Enter name of long-term care facilities here. |
Enter Medicaid provider # here. |
Enter facilities address here. |
|
|
|
|
|
|
|
|
|
Applicant Enrolling ONLY Institutional Equivalent Individuals |
||
1. Provide the service area (County, State name and code) for the institutional SNP being offered. |
||
County Name |
State Name |
State & County Code |
Enter County name here. |
Enter State 2 letter abbreviation here. |
Enter State & County Code here. |
|
|
|
|
|
|
|
|
|
2. Provide the name of the entity (ies) performing the level of care (LOC) assessment for enrolling individuals living in the community. |
||
Enter name of the entity (ies) performing the LOC assessment here. |
||
3. Provide the address of the entity (ies) performing the LOC assessment. |
||
Enter the address of the entity (ies) performing the LOC assessment here. |
||
4. Provide the relevant credential (e.g., RN for registered nurse, LSW for licensed social worker, etc.) of the staff from the entity (ies) performing the LOC assessment. |
||
Enter the relevant credential from the staff of the entity (ies) performing the LOC assessment here. |
||
5. Provide a list of assisted-living facilities (if applicant is contracting with ALFs) |
||
Name of Assisted-living Facilities |
Medicaid Provider # |
Facilities Address |
Enter Name of assisted-living facilities here. |
Enter Medicaid provider # here. |
Enter facilities address here. |
|
|
|
|
|
|
|
|
|
Applicant Enrolling BOTH Institutionalized and Institutional Equivalent |
||
1. Provide the service area (County, State name and code) for the institutional SNP being offered. |
||
County Name |
State Name |
State & County Code |
Enter County name here. |
Enter State 2 letter abbreviation here. |
Enter State & County Code here. |
|
|
|
|
|
|
|
|
|
2. Provide a list of contracted long-term care facilities. |
||
Name of Contracted Long-term Care Facilities |
Medicare Provider # |
Facilities Address |
Enter name of long-term care facilities here. |
Enter Medicaid provider # here. |
Enter facilities address here. |
|
|
|
|
|
|
|
|
|
3. Provide the name of the entity (ies) performing the level of care (LOC) assessment for enrolling individuals living in the community. |
||
Enter name of the entity (ies) performing the LOC assessment here. |
||
4. Provide the address of the entity (ies) performing the LOC assessment. |
||
Enter the address of the entity (ies) performing the LOC assessment here. |
||
5. Provide the relevant credential (e.g., RN for registered nurse, LSW for licensed social worker, etc.) of the staff from the entity (ies) performing the LOC assessment. |
||
Enter the relevant credential from the staff of the entity (ies) performing the LOC assessment here. |
||
6. Provide a list of assisted-living facilities (if applicant is contracting with ALFs) |
||
Name of Assisted-living Facilities |
Medicaid Provider # |
Facilities Address |
Enter Name of assisted-living facilities here. |
Enter Medicaid provider # here. |
Enter facilities address here. |
|
|
|
|
|
|
|
|
|
17. I-SNP Attestation Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
2011 Institutional SNP Attestation Upload Document |
Applicant's Contract Name (as provided in HPMS): |
Enter contract name here. |
CMS Contract Number: |
Enter contract number here. |
Provide attestation for Special Needs Plans (SNP) Serving institutionalized beneficiaries. |
Attestation
for Special Needs Plans (SNP) Serving Institutionalized
Beneficiaries
I attest that in the event the above referenced organization has a CMS approved institutional SNP to provide services to community dwelling beneficiaries who otherwise meet the institutional status as determined by the State, the SNP will assure that the necessary arrangements with community resources are in place to ensure beneficiaries will be assessed and receive services as specified by the SNP Model of Care.
I attest that if a SNP enrollee changes residence, the SNP will have appropriate documentation that it is prepared to implement the SNP Model of Care at the beneficiary’s new residence, or disenroll the beneficiary according to CMS enrollment/disenrollment policies and procedures. Appropriate documentation includes the executed MAO contract with the LTC facility to provide the SNP Model of Care, and written documentation of the necessary arrangements in the community setting to ensure beneficiaries will be assessed and receive services as required under the SNP Model of Care.
_________________________________________
____________________________
|
18. ESRD Waiver Request Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
||
2011 ESRD Upload Document |
||
Applicant's Contract Name (as provided in HPMS): |
||
Enter contract name here. |
||
Applicant’s CMS Contract Number: |
||
Enter contract number here. |
||
1. Provide a description of how applicant intends to serve the unique needs of the ESRD enrollees. |
||
Enter your response to #1 here. |
||
2. Provide a description of any additional service(s) provided to members with ESRD. |
||
Enter your response to #2 here. |
||
3. Provide a description of the interdisciplinary care team coordinator role in the assessment and delivery of services needed by members with ESRD. |
||
Enter your response to #3 here. |
||
4. Provide a list of the contracted dialysis facility (ies). |
||
Name of Contracted Dialysis Facility(ies) |
Medicare Provider # |
Facilities Address |
Enter name of contracted dialysis facility (ies) here. |
Enter Medicare provider # here. |
Enter facilities address here. |
|
|
|
|
|
|
|
|
|
5. Provide a list of the contracted transplant facility (ies). |
||
Name of Contracted Transplant Facility(ies) |
Medicare Provider # |
Facilities Address |
Enter Name of contracted transplant facility (ies) here. |
Enter Medicare provider # here. |
Enter facilities address here. |
|
|
|
|
|
|
|
|
|
19. Model of Care Matrix Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
|
Applicant's Contract Name (as provided in HPMS) |
|
Enter contract name here. |
|
Applicant’s CMS Contract Number |
|
Enter contract number here. |
|
Care Management Plan Outlining the Model of Care |
|
In the following table, list the document, page number, and section of the corresponding description in your care management plan for each model of care element. |
|
Model of Care Elements |
Corresponding Document Page Number/Section |
1. Description of the SNP-specific Target Population (e.g., Medicaid subset D-SNP, institutional equivalent individuals enrolled in I-SNP, diabetes C-SNP, or chronic heart failure/cardiovascular C-SNP) |
|
2. Measurable Goals a. Describe the specific goals including:
b. Describe the goals as measurable outcomes and indicate how MAO will know when goals are met c. Discuss actions MAO will take if goals are not met in the expected time frame |
|
3. Staff Structure and Care Management Roles a. Identify the specific employed or contracted staff to perform administrative functions (e.g., process enrollments, verify eligibility, process claims, etc.) b. Identify the specific employed or contracted staff to perform clinical functions (e.g., coordinate care management, provide clinical care, educate beneficiaries on self-management techniques, consult on pharmacy issues, counsel on drug dependence rehab strategies, etc.) c. Identify the specific employed or contracted staff to perform administrative and clinical oversight functions (e.g., verifies licensing and competency, reviews encounter data for appropriateness and timeliness of services, reviews pharmacy claims and utilization data for appropriateness, assures provider use of clinical practice guidelines, etc.) |
|
4. Interdisciplinary Care Team (ICT) a. Describe the composition of the ICT and how the MAO determined the membership b. Describe how the MAO will facilitate the participation of the beneficiary whenever feasible c. Describe how the ICT will operate and communicate (e.g., frequency of meetings, documentation of proceedings and retention of records, notification about ICT meetings, dissemination of ICT reports to all stakeholders, etc.) |
|
5. Provider Network having Specialized Expertise and Use of Clinical Practice Guidelines and Protocols a. Describe the specialized expertise in the MAO’s provider network that corresponds to the target population including facilities and providers (e.g., medical specialists, mental health specialists, dialysis facilities, specialty outpatient clinics, etc.) b. Describe how the MAO determined that its network facilities and providers were actively licensed and competent c. Describe who determines which services beneficiaries will receive (e.g., is there a gatekeeper, and if not, how is the beneficiary connected to the appropriate service provider, etc.) d. Describe how the provider network coordinates with the ICT and the beneficiary to deliver specialized services (e.g., how care needs are communicated to all stakeholders, which personnel assures follow-up is scheduled and performed, how it assures that specialized services are delivered to the beneficiary in a timely and quality way, how reports on services delivered are shared with the plan and ICT for maintenance of a complete beneficiary record and incorporation into the care plan, how services are delivered across care settings and providers, etc.) e. Describe how the MAO assures that providers use evidence-based clinical practice guidelines and nationally recognized protocols (e.g., review of medical records, pharmacy records, medical specialist reports, audio/video-conferencing to discuss protocols and clinical guidelines, written protocols providers send to MAO Medical Director for review, etc.) |
|
6. Model of Care Training for Personnel and Provider Network a. Describe how the MAO conducted initial and annual model of care training including training strategies and content (e.g., printed instructional materials, face-to-face training, web-based instruction, audio/video-conferencing, etc.) b. Describe how the MAO assures and documents completion of training by the employed and contracted personnel (e.g., attendee lists, results of testing, web-based attendance confirmation, electronic training record, etc.) c. Describe who the MAO identified as personnel responsible for oversight of the model of care training d. Describe what actions the MAO will take when the required model of care training has not been completed (e.g., contract evaluation mechanism, follow-up communication to personnel/providers, incentives for training completion, etc.) |
|
7. Health Risk Assessment a. Describe the health risk assessment tool the MAO uses to identify the specialized needs of its beneficiaries (e.g., identifies medical, psychosocial, functional, and cognitive needs, medical and mental health history, etc.) b. Describe when and how the initial health risk assessment and annual reassessment is conducted for each beneficiary (e.g., initial assessment within 90 days of enrollment, annual reassessment within one year of last assessment; conducted by phone interview, face-to-face, written form completed by beneficiary, etc.) c. Describe the personnel who review, analyze, and stratify health care needs (e.g., professionally knowledgeable and credentialed such as physicians, nurses, restorative therapist, pharmacist, psychologist, etc.) d. Describe the communication mechanism the MAO institutes to notify the ICT, provider network, beneficiaries, etc. about the health risk assessment and stratification results (e.g., written notification, secure electronic record, etc.) |
|
8. Individualized Care Plan a. Describe which personnel develops the individualized plan of care and how the beneficiary is involved in its development as feasible b. Describe the essential elements incorporated in the plan of care (e.g., results of health risk assessment, goals/objectives, specific services and benefits, outcome measures, preferences for care, add-on benefits and services for vulnerable beneficiaries such as disabled or those near the end-of-life, etc) c. Describe the personnel who review the care plan and how frequently the plan of care is reviewed and revised (e.g., developed by the interdisciplinary care team (ICT), beneficiary whenever feasible, and other pertinent specialists required by the beneficiary’s health needs; reviewed and revised annually and as a change in health status is identified, etc.) d. Describe how the plan of care is documented and where the documentation is maintained (e.g., accessible to interdisciplinary team, provider network, and beneficiary either in original form or copies; maintained in accordance with industry practices such as preserved from destruction, secured for privacy and confidentiality, etc.) e. Describe how the plan of care and any care plan revisions are communicated to the beneficiary, ICT, MAO, and pertinent network providers |
|
9. Communication Network a. Describe the MAO’s structure for a communication network (e.g., web-based network, audio-conferencing, face-to-face meetings, etc.) b. Describe how the communication network connects the plan, providers, beneficiaries, public, and regulatory agencies c. Describe how the MAO preserves aspects of communication as evidence of care (e.g., recordings, written minutes, newsletters, interactive web sites, etc.) d. Describe the personnel having oversight responsibility for monitoring and evaluating communication effectiveness |
|
10. Care Management for the Most Vulnerable Subpopulations a. Describe how the MAO identifies its most vulnerable beneficiaries b. Describe the add-on services and benefits the MAO delivers to its most vulnerable beneficiaries |
|
11. Performance and Health Outcome Measurement a. Describe how the MAO will collect, analyze, report, and act on to evaluate the model of care (e.g., specific data sources, specific performance and outcome measures, etc.) b. Describe who will collect, analyze, report, and act on data to evaluate the model of care (e.g., internal quality specialists, contracted consultants, etc.) c. Describe how the MAO will use the analyzed results of the performance measures to improve the model of care (e.g., internal committee, other structured mechanism, etc.) d. Describe how the evaluation of the model of care will be documented and preserved as evidence of the effectiveness of the model of care (e.g., electronic or print copies of its evaluation process, etc.) e. Describe the personnel having oversight responsibility for monitoring and evaluating the model of care effectiveness (e.g., quality assurance specialist, consultant with quality expertise, etc.) f. Describe how the MAO will communicate improvements in the model of care to all stakeholders (e.g., a webpage for announcements, printed newsletters, bulletins, announcements, etc.) |
|
20. Quality Improvement Program Matrix Upload Document
Please complete and upload this document into HPMS per the HPMS MA Application User Guide instructions. |
|
Applicant's Contract Name (as provided in HPMS) |
|
Enter contract name here. |
|
Applicant’s CMS Contract Number |
|
Enter contract number here. |
|
Quality Improvement Program Plan |
|
In the following table, list the document, page number, and section of the corresponding description of your quality improvement program components in your written plan. |
|
Quality Improvement Program Components |
Corresponding Document Page Number/Section |
1. Description of the SNP-specific Target Population a. Identify the SNP-specific target population (e.g., Medicaid subset D-SNP, institutional equivalent individuals enrolled in I-SNP, diabetes C-SNP, or chronic heart failure/cardiovascular C-SNP) b. Describe the purpose of the quality improvement program in relation to the target population c. Describe how the MAO identifies and monitors the most vulnerable members of the population (i.e., frail, disabled, near the end-of-life, multiple or complex chronic conditions, or developing ESRD after enrollment) and the quality improvement activities designed for these individuals. d. Outline the components of the overall quality improvement program including the MAO’s internal activities and the following CMS required activities:
|
|
2. Health Information System a. Describe the health information system and how the system enables the MAO to:
b. Describe how the MAO manages the health information system to comply with HIPAA and privacy laws, and professional standards of health information management |
|
3. MAO-determined Internal Quality Improvement Activities a. Describe the quality improvement activities the MAO has designed that address the target population and are not specifically required by CMS. b. Describe how the MAO maintains documentation on internal quality improvement activities and makes it available to CMS if requested. |
|
4. Chronic Care Improvement Program (CCIP) a. Describe the chronic care improvement program(s) and how CCIP(s) relate to the SNP target population b. Describe how the MAO identifies SNP beneficiaries who would benefit from participation in the CCIP(s) c. Describe how the MAO monitors the beneficiaries who participate in the CCIP(s), and how it evaluates the health outcomes, quality indices, and/or improved operational systems post-intervention. |
|
5. Quality Improvement Projects (QIP) a. Describe the quality improvement project(s) and how QIP(s) relate to the SNP target population including:
b. Describe how the MAO identifies SNP beneficiaries who would benefit from participation in the QIP(s) c. Describe how the MAO monitors the beneficiaries who participate in the QIP(s) d. Describe how it evaluates the health outcomes, quality indices, and/or improved operational systems post-intervention, and achieves demonstrable improvement e. Describe how the MAO conducts systematic and periodic follow-up to assure improvements are sustained |
|
6. SNP-specific Care Management Measurement a. Describe how the MAO will evaluate the effectiveness of its model of care including:
b. Describe how the MAO maintains documentation on model of care evaluation and makes it available to CMS as requested and during onsite audits. c. Describe how the MAO determines what actions to take based on the results of its model of care evaluation. |
|
7. HEDIS and Structure & Process Measures (NCQA) a. Describe how the MAO collects and reports the required HEDIS measures and Structure & Process measures to NCQA (Note: SNPs having 30 or more enrolled members are required to report these measures) b. Describe how the MAO assures accuracy of HEDIS and Structure & Process measures. c. Describe how the MAO determines what actions to take based on the results of HEDIS data and Structure & Process measurement. |
|
8. Health Outcomes Survey - HOS (NCQA) a. Describe how the MAO participates in reporting HOS (Note: MAOs having 500 or more enrolled members are required to report HOS information) b. Describe how the MAO determines what actions to take based on the HOS survey results. |
|
9. Consumer Assessment of Healthcare Providers and Systems – CAHPS Survey (Wilkerson & Associates) a. Describe how the MAO participates in reporting CAHPS (Note: MAOs having 600 or more enrolled members are required to report CAPHS information) b. Describe how the MAO determines what actions to take based on the CAHPS survey results. |
|
10. Part C Reporting Elements a. Describe how the MAO collects, analyzes, and reports Part C reporting data elements to CMS. b. Describe how the MAO assures accuracy of Part C reporting data elements. c. Describe how the MAO determines what actions to take based on the results of Part C reporting data elements. |
|
11. Part D Medication Therapy Management Reporting a. Describe how the MAO collects, analyzes, and reports Medication Therapy Management measures to CMS. b. Describe how the MAO assures accuracy of Medication Therapy Management measures. c. Describe how the MAO determines what actions to take based on the results of Medication Therapy Management measurement. |
|
12. Communication on Quality Improvement Program with Stakeholders a. Describe how the MAO will facilitate the participation of providers, the interdisciplinary care team, and beneficiaries/caregivers in its overall quality improvement program. b. Describe how the MAO will communicate improvements in care management resulting from its overall quality improvement program to all stakeholders (e.g., a webpage for announcements, printed newsletters, bulletins, announcements, etc.) c. Describe how the MAO maintains documentation on its overall quality improvement program and makes it available to CMS as requested and during onsite audits. |
|
The Medicare Modernization Act (MMA) provides employers and unions with a number of options for providing coverage to their Medicare-eligible members. Under the MMA, these options include purchasing benefits from sponsors of prescription drug-only plans (PDPs), making special arrangements with Medicare Advantage Organizations (MAOs) and Section 1876 Cost Plans to purchase customized benefits, including drug benefits, for their members; and directly contracting with CMS to become Part D or MAO plan sponsors themselves. Each of these approaches involves the use of CMS waivers authorized under Sections 1857(i) or 1860D-22(b) of the Social Security Act (SSA). Under this authority, CMS may waive or modify requirements that “hinder the design of, the offering of, or the enrollment in” employer-sponsored group plans. CMS may exercise its waiver authority for PDPs, MAOs and Cost Plan Sponsors that offer employer/union-only group waiver plans (EGWPs). EGWPs are also known as “800 series” plans because of the way they are enumerated in CMS systems.
Which Applicants Should Complete this Appendix?
This appendix is to be used by MAOs seeking to offer the following new “800 series” EGWPs: Private Fee-For-Service (PFFS) Plans, Local Coordinated Care Plans (CCPs), Regional Preferred Provider Organization Plans (RPPOs), Regular Medical Savings Accounts (MSAs) and Demonstration MSAs. CMS issues separate contract numbers for each type of offering and thus a separate application is required for each corresponding contract. However, MAO applicants may submit one application to be eligible to offer new MA-only and new MA-PD EGWPs under the same contract number. All applications are required to be submitted electronically in the Health Plan Management System (HPMS). Please follow the application instructions below and submit the required material in support of your application to offer new “800 series” EGWPs.
For waiver guidance and rules on Part C and Part D Employer contracts, see Chapter 9 of the Medicare Managed Care Manual and Chapter 12 of the Prescription Drug Benefit Manual.
New MAO applicants seeking to offer new “800 series” EGWPs. New MAOs include applicants that have not previously applied to offer plans to individual beneficiaries or “800 series” EGWPs.
Note: All new MAOs intending to offer Part D EGWPs (i.e., MA-PDs) must also complete the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors. The 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors must also be submitted electronically through HPMS. These requirements are also applicable to new MAOs applying to offer “800 series” Regular MSA or Demonstration MSA plans that do not intend to offer plans to individual beneficiaries in 2011. Together these documents will comprise a completed application for new MAOs. Failure to complete, if applicable, the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors, may result in a denial of the EGWP application.
Existing MAOs that currently offer plans to individual beneficiaries under an existing contract but that have not previously applied to offer EGWPs (MA-only or MA-PD) under this same contract.
Note: Existing MAOs are only required to complete this application.
Separate Applications Required For Each Contract Number
A separate application must be submitted for each contract number under which the MAO applicant is applying to offer new “800 series” EGWPs.
As a part of the application process, applicants may submit individual waiver/modification requests to CMS. The applicant should submit this additional waiver/modification as an upload via HPMS to the Attestation Waiver Request in the appropriate MA or Part D supplemental upload pages.
These requests must be identified as requests for additional waivers/modifications and must fully address the following items:
Specific provisions of existing statutory, regulatory, and/or CMS policy requirement(s) the entity is requesting to be waived/modified (please identify the specific requirement (e.g., “42 CFR 422.66,” or “Section 40.4 of Chapter 2 of the Medicare Managed Care Manual (MMCM)”) and whether you are requesting a waiver or a modification of these requirements);
How the particular requirements hinder the design of, the offering of, or the enrollment in, the employer-sponsored group plan;
Detailed description of the waiver/modification requested including how the waiver/modification will remedy the impediment (i.e., hindrance) to the design of, the offering of, or the enrollment in, the employer-sponsored group plan;
Other details specific to the particular waiver/modification that would assist CMS in the evaluation of the request; and
Contact information (contract number, name, position, phone, fax and email address) of the person who is available to answer inquiries about the waiver/modification request.
Designation of Application as “800 SERIES” EGWP ONLY (NO INDIVIDUAL PLANS WILL BE OFFERED)
Checking the box below is optional. Check the box below if you are applying to offer only “800 series” plans under this contract (i.e. no plans will be offered to individual beneficiaries). Do not check the box below if you intend to offer plans to individual beneficiaries and also “800 series” plans under this contract number.
I am hereby designating this MSA application as one that will offer only “800 series” plans. No plans will be offered to individual Medicare beneficiaries under this contract number.
{Entity MUST complete if it is applying to offer only MSA “800 series” EGWPs. No plans will be offered to individual Medicare beneficiaries under this contract number).}
EGWP Attestation for Contract _________
1. EGWP Service Area
MSA or Demonstration MSA Applicants:
If applicant is seeking to offer “800 series” EGWP may designate national service areas and provide coverage to employer group members wherever they reside (i.e., nationwide).
If applicant is seeking to offer individual plans in addition to “800 series” EGWPs must have a national service area (i.e., 50 states and Washington, DC) designated in the Health Plan Management System (HPMS). These MAO applicants will not be initially required to have networks in place to cover members nationally. Applicants offering both individual and “800 series” plans will not initially be required to have Part C networks in place for those designated EGWP service areas outside of their individual plan service areas. However, access sufficient to meet the needs of enrollees must be in place once an applicant enrolls members of an employer or union group residing in geographical areas outside of its individual plan service area.
Network PFFS Applicants:
If applicant is seeking to offer individual plans in any part of a state may designate statewide service areas and provide coverage to employer group members residing anywhere in the entire state.
For Local CCP Applicants:
If applicant is seeking to offering individual plans in any part of a state may designate statewide service areas and provide coverage to employer group members residing anywhere in the entire state.
However, to enable employers and unions to offer CCPs to all their Medicare eligible retirees wherever they reside, an MAO offering a local CCP in a given service area (i.e., a state) can extend coverage to an employer or union sponsor’s beneficiaries residing outside of that service area when the MAO, either by itself or through partnerships with other MAOs, is able to meet CMS provider network adequacy requirements and provide consistent benefits to those beneficiaries. Applicants who are eligible for this waiver at the time of application or who may become eligible at any time during the contract year are strongly encouraged to designate their service area broadly (e.g., multiple states, national) to allow for the possibility of enrolling members during the contract year if adequate networks are in place. No mid-year service area expansions will be permitted. Applicants offering both individual and “800 series” plans will not initially be required to have Part C or D networks in place for those designated EGWP service areas outside of their individual plan service areas. However, access sufficient to meet the needs of enrollees must be in place once an applicant enrolls members of an employer or union group residing in geographical areas outside of its individual plan service area.
RPPO Applicants:
Applicants offering individual plans in any region may provide coverage to employer group members residing throughout the entire region (i.e., RPPOs must have the same service area for its EGWPs as for its individual plans).
I certify that I am an authorized representative, officer, chief executive officer, or general partner of the business organization that is applying for qualification to offer EGWPs in association with my organization’s Medicare Advantage (MA) contract with CMS. I have read, understand, and agree to comply with the above statement about service areas. If I need further information, I will contact one of the individuals listed in the instructions for this application.
{Entity MUST complete to be considered a complete application.}
2. Certification
Note: Any specific certifications below that reference Part D are not applicable to MAO applicants applying to offer Regular MSAs or Demonstration MSAs because these entities cannot offer Part D under these contracts. Entities can offer Part D benefits through a separate standalone Prescription Drug Plan (PDP); however, a separate application is required to offer “800 series” PDPs.
Applicant understands that dissemination/disclosure materials for its EGWPs are not subject to the requirements contained in 42 CFR 422.80 or 42 CFR 423.50 to be submitted for review and approval by CMS prior to use. Applicant also understands CMS reserves the right to review these materials in the event of beneficiary complaints or for any other reason it determines to ensure the information accurately and adequately informs Medicare beneficiaries about their rights and obligations under the plan. (Section 3.14.A.1 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)For new MAO applicants, this appendix, along with the 2011 Medicare Advantage Application and the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors, if applicable, comprise the entire EGWP application for MAOs. All provisions of the 2011 Medicare Advantage Application and the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors apply to all employer/union-group waiver plan benefit packages offered by MAOs except where the provisions are specifically modified and/or superseded by particular employer/union-only group waiver guidance, including those waivers/modifications set forth below.
For existing MAOs, this appendix comprises the entire “800 series” EGWP application for
MAOs. All provisions of the MAO’s existing contract with CMS will apply to all employer/union-group waiver plan benefit packages offered by the MAO except where the provisions are specifically modified and/or superseded by particular employer/union-only group waiver guidance, including those waivers/modifications set forth below.
I, the undersigned, certify to the following:
Applicant is applying to offer new employer/union-only group waiver (“800 series”) plans and agrees to be subject to and comply with all CMS employer/union-only group waiver guidance.
In order for new MAO applicants to be eligible for the CMS employer group waiver that allows certain MA Organizations (Regular MSA or Demonstration MSA) to offer employer/union-only group waiver plan benefit packages without offering plans to individual beneficiaries, Applicant understands and agrees that it must complete and submit the 2011 Medicare Advantage Application and, if applicable, the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors in addition to this application. The 2011 Medicare Advantage Application, the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors, if applicable, and this document comprise new MA Organization’s entire application.
In order for new MAO applicants to be eligible for the CMS employer group waiver that allows Regular MSA or Demonstration MSA plans to offer employer/union-only group waiver plan benefit packages without offering plans to individual beneficiaries, MAO Applicant understands and agrees that it must be licensed in at least one state. (Section 1.3 of the 2011 Medicare Advantage Application)
Applicant understands and agrees that it is not required to submit a 2011 Part D bid (i.e., bid pricing tool) in order to offer its EGWPs. (Section 3.2.6.A.1[current citation?] of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
MAO applicants applying to offer EGWPs and plans to individual beneficiaries understand and agree that it will not initially be required to have Part C or Part D networks in place for those designated EGWP service areas outside of their individual plan service areas or to submit Part D GeoNetworks® retail pharmacy reports (Appendix IX [current citation?]- Retail Pharmacy Network Access Instructions) and other pharmacy access submissions (mail order, home infusion, long-term care, I/T/U) required in Section 3.5[current citation?] of the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors for its designated EGWP service area. However, Part C and Part D access sufficient to meet the needs of enrollees must be in place once an Applicant enrolls members of an employer or union group residing in particular geographic locations outside of its individual plan service area. (Sections 2.2, and 2.3 of the 2011 Medicare Advantage Application; Section 3.5 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
In order to be eligible for the CMS retail pharmacy access waiver of 42 CFR 423.120(a)(1) (i.e., application of “TRICARE” standards), applicant attests that its retail pharmacy network is sufficient (or will be sufficient prior to enrollment) to meet the needs of its enrollees throughout the employer/union-only group waiver service area, including situations involving emergency access, as determined by CMS. Applicant acknowledges and understands that CMS may review the adequacy of the plan’s pharmacy networks and potentially require expanded access in the event of beneficiary complaints or for other reasons it determines in order to ensure that the plan’s network is sufficient to meet the needs of its employer group population. (Section 3.5.1.B of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant agrees to restrict enrollment in its EGWPs to those Medicare eligible individuals eligible for the employer’s/union’s employment-based group coverage. (Section 1.13.2.A.2 of the 2011 Medicare Advantage Application)
Applicant understands that its EGWPs will not be included in the processes for auto-enrollment (for full-dual eligible beneficiaries) or facilitated enrollment (for other low income subsidy eligible beneficiaries). (Section 3.6.A.2 of the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant understands that its EGWPs will not be subject to the requirements contained in 42 CFR 422.64 and 42 CFR 423.48 to submit information to CMS, including the requirements to submit information (e.g., pricing and pharmacy network information) to be publicly reported on www.medicare.gov, Medicare Prescription Drug Plan Finder (“MPDPF”) and the Medicare Options Compare. (Sections 3.8.A and 3.17.A.17 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant understands that dissemination/disclosure materials for its EGWPs are not subject to the requirements contained in 42 CFR 422.80 or 42 CFR 423.50 to be submitted for review and approval by CMS prior to use. However, applicant agrees that it will submit these materials to CMS at the time of use in accordance with the procedures outlined in Chapter 9 of the Medicare Managed Care Manual (MMCM). Applicant also understands CMS reserves the right to review these materials in the event of beneficiary complaints or for any other reason it determines to ensure the information accurately and adequately informs Medicare beneficiaries about their rights and obligations under the plan. (Section 3.14.A.1 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant understands that its EGWPs will not be subject to the requirements regarding the timing for issuance of certain disclosure materials, such as the Annual Notice of Change/ Evidence of Coverage (ANOC/EOC), Summary of Benefits (SB), Formulary, and LIS rider when an employer’s or union’s open enrollment period does not correspond to Medicare’s Annual Coordinated Election Period. For these employers and unions, the timing for issuance of the above disclosure materials should be appropriately based on the employer/union sponsor’s open enrollment period. For example, the Annual Notice of Change/Evidence of Coverage (ANOC/EOC), Summary of Benefits (SB), LIS rider, and Formulary are required to be received by beneficiaries no later than 15 days before the beginning of the employer/union group health plan’s open enrollment period. The timing for other disclosure materials that are based on the start of the Medicare plan (i.e., calendar) year should be appropriately based on the employer/union sponsor’s plan year. (Section 3.14.A.10 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant understands that the dissemination/disclosure requirements set forth in 42 CFR 422.111 and 42 CFR 423.128 will not apply to its EGWPs when the employer/union sponsor is subject to alternative disclosure requirements (e.g., the Employee Retirement Income Security Act of 1974 (“ERISA”)) and complies with such alternative requirements. Applicant agrees to comply with the requirements for this waiver contained in employer/union-only group waiver guidance, including those requirements contained in Chapter 9 of the MMCM. (Sections 3.14.A.1-2, 8 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant understands that its EGWPs will not be subject to the Part D beneficiary customer service call center hours and call center performance requirements. Applicant attests that it will ensure that a sufficient mechanism is available to respond to beneficiary inquiries and that it will provide customer service call center services to these members during normal business hours. However, CMS may review the adequacy of these call center hours and potentially require expanded beneficiary customer service call center hours in the event of beneficiary complaints or for other reasons in order to ensure that the entity’s customer service call center hours are sufficient to meet the needs of its enrollee population. (Section 3.14.A.5 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
Applicant understands that CMS has waived the requirement that the EGWPs must provide beneficiaries the option to pay their premiums through Social Security withholding. Thus, the premium withhold option will not be available for enrollees in Applicant’s EGWPs. (Sections 3.6.A.9 and 3.24.A.2-4 of the 2011 Solicitation for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors)
In order to be eligible for the CMS service area waiver for Local CCPs that allows an MAO to extend coverage to employer group members outside of its individual plan service area, applicant attests it has at the time of application or will have at the time of enrollment, Part C networks adequate to meet CMS requirements and is able to provide consistent benefits to those beneficiaries, either by itself or through partnerships with other MAOs. If applicant is also applying to offer Part D, applicant attests that such expanded service areas will have convenient Part D pharmacy access sufficient to meet the needs of these enrollees.
Regular MSA or Demonstration MSA employer/union-only group waiver plan applicants understand that that they will be permitted to enroll members through a Special Election Period (SEP) as specified in Chapter 2, Section 30.4.4.1, of the Medicare Managed Care Manual (MMCM).
This Certification is deemed to incorporate any changes that are required by statute to be implemented during the term of the contract, and any regulations and policies implementing or interpreting such statutory provisions.
I have read the contents of the completed application and certify that the information contained herein is true, correct, and complete. If I become aware that any information in this application is not true, correct, or complete, I agree to notify CMS immediately and in writing.
I authorize CMS to verify the information contained herein. I agree to notify CMS in writing of any changes that may jeopardize my ability to meet the qualifications stated in this application prior to such change or within 30 days of the effective date of such change. I understand that such a change may result in termination of the approval.
I understand that in accordance with 18 U.S.C.§ 1001, any omission, misrepresentation or falsification of any information contained in this application or contained in any communication supplying information to CMS to complete or clarify this application may be punishable by criminal, civil, or other administrative actions including revocation of approval, fines, and/or imprisonment under Federal law.
I acknowledge that I am aware that there is operational policy guidance, including the forthcoming 2011 Call Letter, relevant to this application that is posted on the CMS website and that it is continually updated. Organizations submitting an application in response to this solicitation acknowledge that they will comply with such guidance should they be approved to offer EGWPs in association with the organization’s MA contract with CMS.
I certify that I am an authorized representative, officer, chief executive officer, or general partner of the business organization that is applying for qualification to offer EGWPs in association with my organization’s MA contract with CMS. I have read and agree to comply with the above certifications.
{Entity MUST check box to be considered a complete application.}
{Entity MUST create 800-series PBPs during plan creation and designate EGWP service areas.}
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) provides employers and unions with a number of options for providing medical and prescription drug coverage to their Medicare-eligible employees, members, and retirees. Under the MMA, these options include making special arrangements with Medicare Advantage Organizations (MAOs) and Section 1876 Cost Plans to purchase customized benefits, including drug benefits, for their members; purchasing benefits from sponsors of standalone prescription drug plans (PDPs) and directly contracting with CMS to become a Direct Contract PFFS MAO or PDP sponsor themselves. Each of these approaches involves the use of CMS waivers authorized under Section 1857(i) or 1860D-22(b) of the Social Security Act (SSA). Under this authority, CMS may waive or modify requirements that “hinder the design of, the offering of, or the enrollment in” employer or union-sponsored group plans.
Which Applicants Should Complete This Appendix?
This appendix is to be used by employers or unions seeking to contract directly with CMS to become PFFS MAOs (“Direct Contract PFFS MAOs”) for their Medicare-eligible active employees and/or retirees. Please follow the application instructions below and submit the required material in support of your application to offer a Direct Contract PFFS MAO.
All Direct Contract PFFS MAO applicants must complete and submit the following:
(1) The 2011 Medicare Advantage Application. This portion of the appendix is submitted electronically through the Health Plan Management System (HPMS).
(2) The 2011 Part C Financial Solvency & Capital Adequacy Documentation for Direct Contract PFFS MAO Applicants. This portion of the appendix is submitted electronically through HPMS.
(3) The 2011 Direct Contract PFFS MAO Attestations. This portion of the appendix is submitted electronically through HPMS. A copy of these attestations is included with this application.
(4) The 2011 Request for Additional Waiver/Modification of Requirements (Optional). This portion of the application is submitted electronically through HPMS. This submission is optional and should be submitted only if the Direct Contract PFFS MAO applicant is seeking new waivers or modifications of CMS requirements.
All of the above enumerated submissions will comprise a completed application for new Direct Contract PFFS MAO applicants. Failure to complete and submit item numbers 1 through 3 above will result in a denial of the Direct Contract PFFS MAO application (item number 4 is optional, as noted above).
Please note that in addition to this application, all Direct Contract PFFS MAOs seeking to contract directly with CMS to offer Part D coverage (i.e., PFFS MA-PDs) must also complete the 2011 Solicitation for Applications for New Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors and the 2011 Solicitation for Applications for New Employer/Union Direct Contract Medicare Advantage Prescription Drug Plan (MA-PD) Sponsors.
Applicants may submit individual waiver/modification requests to CMS. The applicant should submit these additional waiver/modifications via hard copy in accordance with the instructions above.
These requests must be identified as requests for additional waivers/modifications and must fully address the following items:
Specific provisions of existing statutory, regulatory, and/or CMS policy requirement(s) the entity is requesting to be waived/modified (please identify the specific requirement (e.g., “42 CFR 422.66,” or “Section 40.4 of Chapter 2 of the Medicare Managed Care Manual (MMCM)”) and whether you are requesting a waiver or a modification of these requirements);
How the particular requirements hinder the design of, the offering of, or the enrollment in, the employer-sponsored group plan;
Detailed description of the waiver/modification requested including how the waiver/modification will remedy the impediment (i.e., hindrance) to the design of, the offering of, or the enrollment in, the employer-sponsored group plan;
Other details specific to the particular waiver/modification that would assist CMS in the evaluation of the request; and
Contact information (contract number, name, position, phone, fax and email address) of the person who is available to answer inquiries about the waiver/modification request.
Direct Contract PFFS MAO Attestations For Contract _________
I. SERVICE AREA REQUIREMENTS
In general, MAOs can cover beneficiaries only in the service areas in which they are licensed and approved by CMS to offer benefits. CMS has waived these requirements for Direct Contract MAOs. Direct Contract PFFS MAO applicants can extend coverage to all of their Medicare-eligible employees/retirees, regardless of whether they reside in one or more other MAO regions in the nation. In order to provide coverage to retirees wherever they reside, Direct Contract PFFS MAOs must set their service area to include all areas where retirees may reside during the plan year (no mid-year service area expansions will be permitted).
Direct Contract PFFS MAOs that offer Part D (i.e., MA-PDs) will be required to submit pharmacy access information for the entire defined service area during the application process and demonstrate sufficient access in these areas in accordance with employer group waiver pharmacy access policy.
I certify that I am an authorized representative, officer, chief executive officer, or general partner of the business organization that is applying for qualification to offer Direct Contract PFFS MAO plan. I have read, understand, and agree to comply with the above statement about service areas. If I need further information, I will contact one of the individuals listed in the instructions for this application.
{Entity MUST check box for their application to be considered complete.}
2. Certification
All provisions of the 2011 Medicare Advantage Application apply to all plan benefit packages offered by Direct Contract PFFS MAO except where the provisions are specifically modified and/or superseded by particular employer/union-only group waiver guidance, including those waivers/modifications set forth below (specific sections of the 2011 Medicare Advantage Application that have been waived or modified for new Direct Contract PFFS MAOs are noted in parentheses).
I, the undersigned, certify to the following:
1) Applicant is applying to offer new employer/union Direct Contract PFFS plans and agrees to be subject to and comply with all CMS employer/union-only group waiver guidance.
2) Applicant understands and agrees that it must complete and submit the 2011 Medicare Advantage Application in addition to this 2011 Initial Application for Employer/Union Direct Contract PFFS Medicare Advantage Organization application in its entirety. The 2011 Medicare Advantage Application along with Section 2.7 (Part C Financial Solvency & Capital Adequacy Documentation for Direct Contract PFFS MAO applicants) and Section 2.4 (Request for Additional Waiver/Modification of Requirements (Optional)) of the 2011 Initial Application for Employer/Union Direct Contract PFFS Medicare Advantage Organization and this attestation comprise a new Direct Contract PFFS MAO applicant’s entire application.
3) In general, an MAO must be organized and licensed under state law as a risk-bearing entity eligible to offer health insurance or health benefits coverage in each state in which it offers coverage (42 CFR 422.400). However, CMS has waived the state licensing requirement for all Direct Contract PFFS MAOs. As a condition of this waiver, applicant understands that CMS will require such entities to meet the financial solvency and capital adequacy standards contained in this application. (Section 1.3 of the 2011 Medicare Advantage Application)
4) Applicant agrees to restrict enrollment in its Direct Contract PFFS plans to those Medicare-eligible individuals eligible for the employer’s/union’s employment-based group coverage. (Section 1.13.2.A.2 of the 2011 Medicare Advantage Application)
5) In general, MAOs must meet minimum enrollment standards as set forth in 42 CFR 422.514(a). Applicant understands that it will not be subject to the minimum enrollment requirements set forth in 42 CFR 422.514(a). (Section 1.14 of the 2011 Medicare Advantage Application)
6) Applicant understands that dissemination/disclosure materials for its Direct Contract PFFS plans are not subject to the requirements contained in 42 CFR 422.80 to be submitted for review and approval by CMS prior to use. However, applicant agrees that it will submit these materials to CMS at the time of use in accordance with the procedures outlined in Chapter 9 of the Medicare Managed Care Manual (MMCM). Applicant also understands that CMS reserves the right to review these materials in the event of beneficiary complaints or for any other reason it determines to ensure the information accurately and adequately informs Medicare beneficiaries about their rights and obligations under the plan. (Section 1.13.1.1 of the 2011 Medicare Advantage Application)
7) Applicant understands that its Direct Contract PFFS plans will not be subject to the requirements regarding the timing for issuance of certain disclosure materials, such as the Annual Notice of Change/ Evidence of Coverage (ANOC/EOC), Summary of Benefits (SB), Formulary, and LIS rider when an employer’s or union’s open enrollment period does not correspond to Medicare’s Annual Coordinated Election Period. For these employers and unions, the timing for issuance of the above disclosure materials should be appropriately based on the employer/union sponsor’s open enrollment period. For example, the Annual Notice of Change/Evidence of Coverage (ANOC/EOC), Summary of Benefits (SB), LIS rider, and Formulary are required to be received by beneficiaries no later than 15 days before the beginning of the employer/union group health plan’s open enrollment period. The timing for other disclosure materials that are based on the start of the Medicare plan (i.e., calendar) year should be appropriately based on the employer/union sponsor’s plan year. (Section 1.13.1.7 of the 2011 Medicare Advantage Application)
8) Applicant understands that the dissemination/disclosure requirements set forth in 42 CFR 422.111 will not apply to its Direct Contract PFFS plans when the employer/union sponsor is subject to alternative disclosure requirements (e.g., ERISA) and complies with such alternative requirements. Applicant agrees to comply with the requirements for this waiver contained in employer/union-only group waiver guidance, including those requirements contained in Chapter 9 of the MMCM. (Sections 1.13.1.1-2 of the 2011 Medicare Advantage Application)
9) Applicant understands that its Direct Contract PFFS plans will not be subject to the MA beneficiary customer service call center hours and call center performance requirements. Applicant attests that it will ensure that a sufficient mechanism is available to respond to beneficiary inquiries and will provide customer service call center services to these members during normal business hours. However, CMS may review the adequacy of these call center hours and potentially require expanded beneficiary customer service call center hours in the event of beneficiary complaints or for other reasons in order to ensure that the entity’s customer service call center hours are sufficient to meet the needs of its enrollee population. (Section 1.13.1.5 of the 2011 Medicare Advantage Application)
10) Applicant understands that its Direct Contract PFFS plans will not be subject to the requirements contained in 42 CFR 422.64 to submit information to CMS, including the requirements to submit information (e.g., pricing and provider network information) to be publicly reported on http://www.medicare.gov (Medicare Options Compare).
11) Applicant understands that the management and operations requirements of 42 CFR 422.503(b)(4)(i)-(iii) are waived if the employer or union (or to the extent applicable, the business associate with which it contracts for benefit services) is subject to ERISA fiduciary requirements or similar state or federal law standards. However, such entities (or their business associates) are not relieved from the record retention standards applicable to other MAOs set forth in 42 CFR 422.504(d). (Sections 1.2.8-10 of the 2011 Medicare Advantage Application)
12) In general, MAOs must report certain information to CMS, to their enrollees, and to the general public (such as the cost of their operations and financial statements) under 42 CFR 422.516(a). Applicant understands that in order to avoid imposing additional and possibly conflicting public disclosure obligations that would hinder the offering of employer sponsored group plans, CMS will modify these reporting requirements for Direct Contract PFFS MAOs to allow information be reported to enrollees and to the general public to the extent required by other laws (including ERISA or securities laws), or by contract.
13) In general, MAOs are not permitted to enroll beneficiaries who do not meet the MA eligibility requirements of 42 CFR 422.50(a), which include the requirement to be entitled to Medicare Part A. (42 CFR 422.50(a)(1)). Applicant understands that under certain circumstances, as outlined in section 30.1.4 of Chapter 9 of the MMCM, Direct Contract PFFS MAOs are permitted to enroll beneficiaries who are not entitled to Medicare Part A into Part B-only plan benefit packages. (Section 1.13.2.A.3 of the 2011 Medicare Advantage Application)
14) In general, MAOs are not permitted to enroll beneficiaries who have end-stage renal disease (ESRD). Applicant understands that under certain circumstances, as outlined in section 20.2.3 of Chapter 2 of the MMCM, Direct Contract PFFS MAOs are permitted to enroll beneficiaries who have ESRD. (Section 1.13.2.A.3 of the 2011 Medicare Advantage Application)
15) This Certification is deemed to incorporate any changes that are required by statute to be implemented during the term of the contract, and any regulations and policies implementing or interpreting such statutory provisions.
16) I have read the contents of the completed application and the information contained herein is true, correct, and complete. If I become aware that any information in this application is not true, correct, or complete, I agree to notify CMS immediately and in writing.
17) I authorize CMS to verify the information contained herein. I agree to notify CMS in writing of any changes that may jeopardize my ability to meet the qualifications stated in this application prior to such change or within 30 days of the effective date of such change. I understand that such a change may result in termination of the approval.
18) I understand that in accordance with 18 U.S.C.§ 1001, any omission, misrepresentation or falsification of any information contained in this application or contained in any communication supplying information to CMS to complete or clarify this application may be punishable by criminal, civil, or other administrative actions including revocation of approval, fines, and/or imprisonment under Federal law.
19) I acknowledge that I am aware that there is operational policy guidance, including the forthcoming 2011 Call Letter, relevant to this application that is posted on the CMS website and that it is continually updated. Organizations submitting an application in response to this solicitation acknowledge that they will comply with such guidance should they be approved to offer employer/union-only group waiver plans in association with the organization’s MA contract with CMS.
I certify that I am an authorized representative, officer, chief executive officer, or general partner of the business organization that is applying for qualification to offer a Direct Contract PFFS MAO plan. I have read and agree to comply with the above certifications.
{Entity MUST check box for their application to be considered complete.}
Background and Instructions
An MAO generally must be licensed by at least one state as a risk-bearing entity (42 CFR 422.400). CMS has waived the requirement for Direct Contract PFFS MAOs. Direct Contract PFFS MAOs are not required to be licensed, but must meet CMS Medicare Advantage (MA) Part C financial solvency and capital adequacy requirements. Each Direct Contract PFFS MAO applicant must demonstrate that it meets the financial solvency requirements set forth in this application and provide all required information set forth below. CMS has the discretion to approve, on a case-by-case basis, waivers of such requirements if the Direct Contract PFFS MAO can demonstrate that its fiscal soundness is commensurate with its financial risk and that through other means the entity can assure that claims for benefits paid for by CMS and beneficiaries will be covered. In all cases, CMS will require that the employer’s/union’s contracts and sub-contracts provide beneficiary hold-harmless provisions.
The information required in this Appendix must be submitted in hardcopy in accordance with the instructions above.
EMPLOYER/UNION ORGANIZATIONAL INFORMATION
A. Complete the information in the table below.
INDENTIFY YOUR ORGANIZATION BY PROVIDING THE FOLLOWING INFORMATION: |
|
Type of DIRECT CONTRACT MEDICARE ADVANTAGE PLAN requested (Check all that apply):
Open Access (Non-Network) PFFS Plan
Contracted Network PFFS Plan |
|
Organization’s Full Legal Name:
|
|
Full Address Of Your Organization’s Headquarters (Street, City, State, Zip):
|
|
Tax Status: For Profit Not For Profit |
Is Applicant Subject To ERISA? Yes No |
Type Of Entity (Check All That Apply) : Employer Labor Union Fund Established by One or More Employers or Labor Organizations Government Church Group
Publicly-Traded Corporation Privately-Held Corporation Other (list Type) _____________________________________________
|
|
Name of Your Organization’s Parent Organization, if any:
|
|
State in Which your Organization is Incorporated or Otherwise Organized to do Business:
|
|
B. Summary Description
Briefly describe the organization in terms of its history and its present operations. Cite significant aspects of its current financial, general management, and health services delivery activities. Please include the following:
The size of the Medicare population currently served by the applicant, and if any, the maximum number of Medicare beneficiaries that could be served by a Direct Contract PFFS MAO.
The manner in which benefits are currently provided to the current Medicare population served by the applicant, and if any, the number of beneficiaries in each employer sponsored group option currently made available by the Direct Contract PFFS MAO applicant and how these options are currently funded (i.e., self-funded or fully insured).
The current benefit design for each of the options described in B above, including premium contributions made by the employer and/or the retiree, deductibles, co-payments, or co-insurance, etc. (Applicant may attach a summary plan description of its benefits or other relevant materials describing these benefits.)
Information about other Medicare contracts held by the applicant, (i.e., 1876, fee for service, PPO, etc.). Provide the names and contact information for all CMS personnel with whom applicant works on their other Medicare contract(s).
The factors that are most important to applicant in deciding to apply to become a Direct Contract PFFS MAO for its retirees and how becoming a Direct Contract PFFS MAO will benefit the applicant and its retirees.
C. If the applicant is a state agency, labor organization, or a trust established by one or more employers or labor organizations; applicant must provide the required information listed below:
State Agencies:
If applicant is a state agency, instrumentality or subdivision, please provide the relationship between the entity that is named as the Direct Contract PFFS MAO applicant and the state or commonwealth with respect to which the Direct Contract PFFS MAO applicant is an agency, instrumentality or subdivision. Also, applicant must provide the source of applicant’s revenues, including whether applicant receives appropriations and/or has the authority to issue debt.
Labor Organizations:
If applicant is a labor organization, including a fund or trust, please provide the relationship (if any) between applicant and any other related labor organizations such as regional, local or international unions, or welfare funds sponsored by such related labor organizations. If applicant is a jointly trusted Taft-Hartley fund, please include the names and titles of labor-appointed and management-appointed trustees.
Trusts:
If applicant is a trust such as a voluntary employee beneficiary association under Section 501(c)(9) of the Internal Revenue Code, please provide the names of the individual trustees and the bank, trust company or other financial institution that has custody of applicant’s assets.
D. Policymaking Body (42 CFR 422.503(b)(4)(i)-(iii)
In general, an entity seeking to contract with CMS as a Direct Contract PFFS MAO must have policymaking bodies exercising oversight and control to ensure actions are in the best interest of the organization and its enrollees, appropriate personnel and systems relating to medical services, administration and management, and at a minimum an executive manager whose appointment and removal are under the control of the policymaking body.
An employer or union directly contracting with CMS as a Direct Contract PFFS MAO may be subject to other, potentially different standards governing its management and operations, such as the Employee Retirement Income Security Act of 1974 (“ERISA”) fiduciary requirements, state law standards, and certain oversight standards created under the Sarbanes-Oxley Act. In most cases, they will also contract with outside vendors (i.e., business associates) to provide health benefit plan services. To reflect these issues and avoid imposing additional (and potentially conflicting) government oversight that may hinder employers and unions from considering applying to offer Direct Contract MA Plans, the management and operations requirements under 42 CFR 422.503(b)(4)(i)-(iii) are waived if the employer or union (or to the extent applicable, the business associate with which it contracts for health benefit plan services) is subject to ERISA fiduciary requirements or similar state or federal laws and standards. However, such entities (or their business associates) are not relieved from the record retention standards applicable to other MAOs. In accordance with the terms of this waiver, please provide the following information:
List the members of the organization's policymaking body (name, position, address, telephone number, occupation, term of office and term expiration date). Indicate whether any of the members are employees of the applicant.
If the applicant is a line of business rather than a legal entity, does the Board of Directors of the corporation serve as the policymaking body of the organization? If not, describe the policymaking body and its relationship to the corporate board.
Does the Federal Government or a state regulate the composition of the policymaking body? If yes, please identify all Federal and state regulations that govern your policymaking body (e.g., ERISA).
II. FINANCIAL SOLVENCY
Please provide a copy of the applicant’s most recent independently certified audited statements.
Please submit an attestation signed by the Chairman of the Board, Chief Executive Officer and Chief Financial Officer or Trustee or other equivalent official attesting to the following:
1. The applicant will maintain a fiscally sound operation and will notify CMS within 10 business days if it becomes fiscally unsound during the contract period.
2. The applicant is in compliance with all applicable Federal and state requirements and is not under any type of supervision, corrective action plan, or special monitoring by the Federal or state government or a state regulator. NOTE: If the applicant cannot attest to this compliance, a written statement of the reasons must be provided.
III. FINANCIAL DOCUMENTATION
Minimum Net Worth at the Time of Application - Documentation of Minimum Net Worth
At the time of application, the applicant must demonstrate financial solvency through furnishing two years of independently audited financial statements to CMS. These financial statements must demonstrate a required minimum net worth at the time of application of the greater of $3.0 million or the number of expected individuals to be covered under the Direct Contract PFFS MAO Plan times (X) $800.00. Complete the following:
Minimum Net Worth: $
Number of expected individuals to be covered under the Direct Contract PFFS
MAO Plan times (X) $800.00 = $______________________.
NOTE: If the Direct Contract PFFS MAO applicant is also applying to offer a Direct Contract PFFS MAO that provides Part D coverage (i.e., MA-PD), it must complete and submit the corresponding Direct Contract MA-PD application with this application and meet the Part D Minimum Net Worth requirements stated in the separate Direct Contract MA-PD application.
If the applicant has not been in operation at least twelve months, it may choose to: 1) obtain independently audited financial statements for a shorter time period; or 2) demonstrate that it has the minimum net worth through presentation of un-audited financial statements that contain sufficient detail to allow CMS to verify the validity of the financial presentation. The un-audited financial statements must be accompanied by an actuarial opinion from a qualified actuary regarding the assumptions and methods used in determining loss reserves, actuarial liabilities and related items.
A “qualified actuary” for purposes of this application means a member in good standing of the American Academy of Actuaries, a person recognized by the Academy as qualified for membership, or a person who has otherwise demonstrated competency in the field of actuarial science and is satisfactory to CMS.
If the Direct Contract PFFS MAO applicant’s auditor is not one of the 10 largest national accounting firms in accordance with the list of the 100 largest public accounting firms published by the CCH Public Accounting Report, the applicant should enclose proof of the auditor’s good standing from the relevant state board of accountancy.
Minimum Net Worth On and After Effective Date of Contract
The applicant must have net worth as of the effective date of the contract of the greatest of the following financial thresholds; $3.0 Million; or, an amount equal to eight percent of annual health care expenditures, using the most recent financial statements filed with CMS; or the number of expected individuals to be covered under the Direct Contract PFFS MAO Plan times (X) $800.00.
Liquidity at the Time of Application ($1.5 Million)
The applicant must have sufficient cash flow to meet its financial obligations as they become due. The amount of the minimum net worth requirement to be met by cash or cash equivalents is $1.5 Million. Cash equivalents are short-term highly liquid investments that can be readily converted to cash. To be classified as cash equivalents, investments must have a maturity date not longer than three months from the date of purchase.
NOTE: If the Direct Contract PFFS MAO applicant is also applying to offer a Direct Contract MA PFFS Plan that provides Part D coverage (i.e., MA-PD), it must complete and submit the corresponding Direct Contract MA-PD application and meet the Part D Liquidity requirements stated in the separate Direct Contract MA-PD application.
Liquidity On and After Effective Date of Contract
After the effective date of the contract, an applicant must maintain the greater of $1.5 Million or 40 percent of the minimum net worth requirement outlined in Section III.B above in cash or cash equivalents.
In determining the ability of an applicant to meet the requirements of this paragraph D, CMS will consider the following:
The timeliness of payment;
The extent to which the current ratio is maintained at 1:1 or greater, or whether there is a change in the current ratio over a period of time; and
The availability of outside financial resources.
CMS may apply the following corresponding corrective remedies:
If a Direct Contract PFFS MAO fails to pay obligations as they become due, CMS will require the Direct Contract PFFS MAO to initiate corrective action to pay all overdue obligations.
CMS may require the Direct Contract PFFS MAO to initiate corrective action if either of the following is evident:
The current ratio declines significantly; or
There is a continued downward trend in the current ratio.
The corrective action may include a change in the distribution of assets, a reduction of liabilities, or alternative arrangements to secure additional funding to restore the current ratio to at least 1:1.
If there is a change in the availability of outside resources, CMS will require the Direct Contract PFFS MAO to obtain funding from alternative financial resources.
Methods of Accounting
A Direct Contract PFFS MAO applicant generally must use the standards of Generally Accepted Accounting Principles (GAAP). GAAP are those accounting principles or practices prescribed or permitted by the Financial Accounting Standards Board. However, a Direct Contract PFFS MAO whose audited financial statements are prepared using accounting principles or practices other than GAAP, such as a governmental entity that reports in accordance with the principles promulgated by the Governmental Accounting Standards Board (GASB), may utilize such alternative standard.
Bonding and Insurance
An applicant may request a waiver in writing of the bonding and/or insurance requirements set forth at 42 CFR 422.503(b)(4)(iv) and (v). Relevant considerations will include demonstration that either or both of the foregoing requirements are unnecessary based on the entity’s individualized circumstances, including maintenance of similar coverage pursuant to other law, such as the bonding requirement at ERISA Section 412. If the waiver request is based on the existence of alternative coverage, the applicant must describe such alternative coverage and enclose proof of the existence of such coverage.
Additional Information
A Direct Contract PFFS MAO applicant must furnish the following financial information to CMS to the extent applicable:
Self-Insurance/Self Funding- If the Direct Contract PFFS MAO applicant’s PFFS Plan(s) will be self-insured or self-funded, it must forward proof of stop-loss coverage (if any) through copies of policy declarations.
Trust- If the Direct Contract PFFS MAO applicant maintains one or more trusts with respect to its health plan(s), a copy of the trust documents, and if the trust is intended to meet the requirements of Section 501(c)(9) of the Internal Revenue Code, the most recent IRS approval letter.
Forms 5500 and M-1- The two most recent annual reports on Forms 5500 and M-1 (to the extent applicable) for the Direct Contract PFFS MAO applicant’s health plans that cover prescription drugs for individuals who are Part D eligible.
ERISA Section 411(a) Attestation- The Direct Contract PFFS MAO (including a Direct Contract PFFS MAO that is exempt from ERISA) must provide a signed attestation that no person serves as a fiduciary, administrator, trustee, custodian, counsel, agent, employee, consultant, adviser or in any capacity that involves decision-making authority, custody, or control of the assets or property of any employee benefit plan sponsored by the Direct Contract PFFS MAO applicant, if he or she has been convicted of, or has been imprisoned as a result of his or her conviction, of one of the felonies set forth in ERISA Section 411(a), for 13 years after such conviction or imprisonment (whichever is later).
Defined Benefit Pension Plan- If the Direct Contract PFFS MAO applicant sponsors one or more defined benefit pension plans (within the meaning of ERISA Section 3(35)) that is subject to the requirements of Title IV of ERISA, the latest actuarial report for each such plan.
Multi-Employer Pension Plan- If the Direct Contract PFFS MAO applicant is a contributing employer with respect to one or more multi-employer pension plans within the meaning of ERISA Section 3(37), the latest estimate of contingent withdrawal liability.
Tax-Exempt Direct Contract PFFS MAOs Only- a copy of the most recent IRS tax-exemption.
IV. INSOLVENCY REQUIREMENTS
A. Hold Harmless and Continuation of Coverage/Benefits.
The Direct Contract PFFS MAO shall be subject to the same hold harmless and continuation of coverage/benefit requirements as other MA contractors.
B. Deposit Requirements - Deposit at the Time of Application
A Direct Contract PFFS MAO generally must forward confirmation of its establishment and maintenance of a deposit of at least $1.0 Million to be held in accordance with CMS requirements by a qualified U. S. financial institution. A “qualified financial institution” means an institution that:
Is organized or (in the case of a U.S. office of a foreign banking organization) licensed, under the laws of the United States or any state thereof; and
Is regulated, supervised, and examined by the U.S. Federal or state authorities having regulatory authority over banks and trust companies.
The purpose of this deposit is to help assure continuation of services, protect the interest of Medicare enrollees, and pay costs associated with any receivership or liquidation. The deposit may be used to satisfy the minimum net worth requirement set forth in Section III above.
A Direct Contract PFFS MAO may request a waiver in writing of this requirement.
NOTE: In addition to the requirements in this Application, if the Direct Contract PFFS MAO is also applying to offer a Direct Contract MA PFFS Plan that provides Part D coverage (i.e., MA-PD), it must complete and submit the corresponding Direct Contract MA-PD application with this application and meet the Part D Deposit requirements stated in the separate Direct Contract MA-PD application.
Deposit On and After Effective Date of Contract
Based on the most recent financial statements filed with CMS, CMS will determine the adequacy of the deposit under this Section and inform the Direct Contract PFFS MAO as to the necessity for any increased deposit. Factors CMS will consider shall include the total amount of health care expenditures during the applicable period, the amount of expenditures that are uncovered, and the length of time necessary to pay claims.
Rules Concerning Deposit
The deposit must be held in trust and restricted for CMS’ use in the event of insolvency to pay related costs and/or to help assure continuation of services.
All income from the deposit are considered assets of the Direct Contract PFFS MAO and may be withdrawn from the deposit upon CMS’ approval. Such approval is not to be withheld unreasonably
On prior written approval from CMS, a Direct Contract PFFS MAO that has made a deposit under this Section may withdraw such deposit or any part thereof if:
a substitute deposit of cash or securities of equal amount and value is made;
the fair market value of the assets held in trust exceeds the required amount for the deposit; or
the required deposit is reduced or eliminated.
V. GUARANTEES (only applies to an applicant that utilizes a Guarantor)
A. General policy
The Direct Contract PFFS MAO, or the legal entity of which the Direct Contract PFFS MAO is a component, may apply to CMS to use the financial resources of a Guarantor for the purpose of meeting the requirements of a Direct Contract PFFS MAO set forth above. CMS has the sole discretion to approve or deny the use of a Guarantor.
B. Request to Use a Guarantor
To apply to use the financial resources of a Guarantor, a Direct Contract PFFS MAO must submit to CMS:
Documentation that the Guarantor meets the requirements for a Guarantor under paragraph (C) of this section; and
The Guarantor's independently audited financial statements for the current year-to-date and for the two most recent fiscal years. The financial statements must include the Guarantor's balance sheets, profit and loss statements, and cash flow statements.
C. Requirements for Guarantor
To serve as a Guarantor, an organization must meet the following requirements:
1. Be a legal entity authorized to conduct business within a state of the United States.
2. Not be under Federal or state bankruptcy or rehabilitation proceedings.
3. Have a net worth (not including other guarantees, intangibles and restricted reserves) equal to three times the amount of the Direct Contract PFFS MAO guarantee.
4. If a state insurance commissioner or other state official with authority for risk-bearing entities regulates the Guarantor, it must meet the net worth requirement in Section III above with all guarantees and all investments in and loans to organizations covered by guarantees excluded from its assets.
5. If the Guarantor is not regulated by a state insurance commissioner or other similar state official, it must meet the net worth requirement in Section III above with all guarantees and all investments in and loans to organizations covered by a guarantee and to related parties (subsidiaries and affiliates) excluded from its assets.
D. Guarantee Document
If the guarantee request is approved, a Direct Contract PFFS MAO must submit to CMS a written guarantee document signed by an appropriate Guarantor. The guarantee document must:
State the financial obligation covered by the guarantee;
Agree to:
(a) Unconditionally fulfill the financial obligation covered by the guarantee; and
(b) Not subordinate the guarantee to any other claim on the resources of the Guarantor;
Declare that the Guarantor must act on a timely basis, in any case not more than five business days, to satisfy the financial obligation covered by the guarantee; and
Meet any other conditions as CMS may establish from time to time.
E. Ongoing Guarantee Reporting Requirements
A Direct Contract PFFS MAO must submit to CMS the current internal financial statements and annual audited financial statements of the Guarantor according to the schedule, manner, and form that CMS requires.
F. Modification, Substitution, and Termination of a Guarantee
A Direct Contract PFFS MAO cannot modify, substitute or terminate a guarantee unless the Direct Contract PFFS MAO:
Requests CMS' approval at least 90 days before the proposed effective date of the modification, substitution, or termination;
Demonstrates to CMS' satisfaction that the modification, substitution, or termination will not result in insolvency of the Direct Contract PFFS MAO; and
Demonstrates how the Direct Contract PFFS MAO will meet the requirements of this Section.
G. Nullification
If at any time the Guarantor or the guarantee ceases to meet the requirements of this section, CMS will notify the Direct Contract PFFS MAO that it ceases to recognize the guarantee document. In the event of this nullification, a Direct Contract PFFS MAO must:
Meet the applicable requirements of this section within 15 business days; and
If required by CMS, meet a portion of the applicable requirements in less than the 15 business days in paragraph (G.1.) of this section.
VI. ONGOING FINANCIAL SOLVENCY/CAPITAL ADEQUACY REPORTING REQUIREMENTS
An approved Direct Contract PFFS MAO is required to update the financial information set forth in Sections III and IV above to CMS on an ongoing basis. The schedule, manner, form and type of reporting, will be in accordance with CMS requirements.
In addition to the CFR citations provided above, the following contract provisions are required in agreements between MA organizations and provider and suppliers of health care as stated in Chapter 11 of the Medicare Managed Care Manual Section 100.4.
In addition to the CFR citations provided above, the following contract provisions are required in agreements between MA organizations and provider and suppliers of health care as stated in Chapter 11 of the Medicare Managed Care Manual Section 100.4.
September 14, 2009
DRAFT Page
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | PART 1 GENERAL INFORMATION |
| Author | Emmanuelle Goodrich |
| File Modified | 0000-00-00 |
| File Created | 2021-02-03 |
© 2026 OMB.report | Privacy Policy