ALS Registry Protocol
ALS registry protocol clean.doc
National Amyotrophic Lateral Sclerosis (ALS) Registry
ALS Registry Protocol
OMB: 0923-0041
Proposal for the National ALS Registry
INTRODUCTION
Residents of communities living near hazardous waste sites have expressed concerns about elevated rates of selected neurological diseases such as amyotrophic lateral sclerosis (ALS). The absence of population-based surveillance systems or registries for neurological diseases from which estimates of prevalence and incidence could be obtained, as well as enumerating cases within a specific community, makes it difficult to address these concerns. Attempts to address these questions have resulted in efforts to identify all the cases within a specific geographic area by working with local neurologists and other medical care providers. This is both time consuming and costly. Because of previous experience by the Agency for Toxic Substances and Disease Registry (ATSDR), as well as other researchers trying to obtain information directly from medical care providers, it was decided to first identify existing surveillance systems, registries, and databases for selected neurological diseases and then use this information to examine additional ways to identify affected individuals to be included in a population-based surveillance system/registry. Four pilot projects were conducted which evaluated the feasibility of accurate identification of ALS cases using administrative data from the Centers for Medicaid and Medicare Services (CMS), the Veterans Health Administration (VHA), and the Veterans Benefits Administration (VBA) when compared with the medical records.
This protocol describes the methodology for developing the National ALS Registry/population-based surveillance using existing administrative data and self-registration of affected individuals. The primary objective of this surveillance system/registry is to obtain reliable information on the incidence and prevalence of ALS and to better describe the demographic characteristics (age, race, sex, and geographic location) of those with ALS.
BACKGROUND
Disease Description and Epidemiology
In 1869, the French neurologist Jean-Martin Charcot described a unique condition characterized by deterioration of both lower and upper motor neurons, and this condition was termed amyotrophic lateral sclerosis (ALS).1 Many people know ALS as Lou Gehrig’s disease, named after the famous baseball player who, in 1939, retired because of his illness.
Reports from the United States and other countries indicate an annual incidence rate of 0.2 to 2.4 per 100,000 population and a prevalence of 0.8 to 7.3 per 100,000 population.2 The onset of ALS is age-related with the highest rate of onset occurring between 55 and 75 years of age.2-4 Prognosis also appears to be age-related with slightly better survival occurring among those with a younger age at onset. The average survival time after onset of symptoms is approximately three years, and only a small proportion of patients survive beyond five years.2 ALS is more common in males than females by a ratio of 1.5 – 2 to 1,4, 5 but recent studies have suggested that this sex difference is decreasing over time.4, 6
In addition to ALS, several other less common conditions are classified under the general term of motor neuron disease, but ALS accounts for 85 percent or more of all motor neuron cases. Most individuals who are initially diagnosed with these other conditions will ultimately progress to include both upper and lower motor neurons and thus will be diagnosed as having ALS.2, 7
Differential diagnosis of ALS requires a neurological exam as well as neurophysiological tests and other tests to rule out non-motor neuron diseases and other motor neuron diseases with restricted presentations. False-negative rates can be high in the early stages of the disease8, 9 and false-positive misdiagnoses have been shown to occur in 7 to 8 percent of cases.10, 11 The diagnosis of ALS will become more uniform worldwide as the World Federation of Neurology El Escorial criteria and its subsequent revision are utilized.12, 13
Uncertainty about the incidence and prevalence of ALS, as well as the role of the environment in the etiology of ALS, supports the need for a surveillance system for these diseases.14, 15 In addition, such a system could provide an unbiased source from which to recruit patients to participate in future research studies.
Surveillance
Public health surveillance is defined as “the ongoing, systematic collection, analysis, and interpretation of health data essential to the planning, implementation, and evaluation of public health practice, closely integrated with the timely dissemination of these data to those who need to know. The final link of the surveillance chain is the application of these data to prevention and control. A surveillance system includes a functional capacity for data collection, analysis, and dissemination linked to public health programs.”16 Surveillance is important to monitor changes in incidence and prevalence of a condition as well as to provide a source for patients with specific conditions that can be asked to participate in research studies. Surveillance data can also be used in planning for health care needs, detecting changes in health practices, and assessing the burden of disease. For chronic diseases, monitoring the burden of disease (morbidity, disability, and mortality) may be very important.17 To date, national disease surveillance systems have been related primarily to infectious diseases with cancer and birth defects being the two exceptions. In 1992, directors of the World Health Organization (WHO) non-communicable disease collaborating centers and key officials in centers for non-communicable diseases advocated for the increased surveillance of non-communicable diseases. This recommendation was based on the lack of incidence data for non-communicable diseases.18
Traditionally, surveillance systems have relied on physicians and other health care providers “reporting” to a specified entity, usually the state or local health department; that information can then be relayed to the next level as appropriate. The designation of “reportable” is conferred by the Council of State and Territorial Epidemiologists (CSTE), which was established in the 1950s. Once a disease has been designated reportable, each state must decide if current health department authorities would include the new disease or whether new legislation must be sought. Historically no non-communicable diseases have been made reportable by CSTE, including cancer. Cancer is reportable in most states; however, this was accomplished by Congress passing Public Law 102-515, Cancer Registries Amendment Act. This legislation required the authorization of a statewide registry under state law before receiving Federal funds.
Unfortunately, physicians have historically been poor reporters of disease; for that reason laboratory and hospital reporting have been built into surveillance systems.19 Because physicians do not make good “reporters,” and the history of making diseases nationally reportable has mostly excluded non-communicable diseases, this does not appear to be the best strategy for surveillance of selected neurological diseases.
As a more feasible strategy, Thacker and Stroup describe a comprehensive public health surveillance system which would be a network of health information systems linked electronically. Data for this system would be collected from many sources including population-based systems (e.g., vital statistics), provider-based systems (e.g., physician, laboratory, and hospital records), and payer systems (e.g., Medicare or Medicaid).20
Sources of data and reliability of coding
Increasingly, electronically available data collected for purposes other than research, such as claims data, are being used in epidemiological studies. A great deal of research has gone into the reliability of coding in large datasets such as Medicare and Medicaid. The issue is how reliable are these data for studies given that the information was collected for other uses such as claims. Most of the research has focused on identifying a specific disease or procedure using codes and comparing that with the medical record which is considered the gold standard.21-24 One study comparing the accuracy of Medicare hospital claims with the hospital records found that for Diseases of the Nervous System and Sense Organs (ICD-9-CM: 320-389), the agreement between the coding was 91.4%.25
Causes for an erroneous code in a claims database can range from computer entry errors to lack of sufficient clinical information to accurately code the claim.26 Changes in reimbursement using Diagnosis Related Group (DRG) may also cause some coding inconsistencies. When multiple codes are allowed, it is important to understand the uses of each and ascertain if there are differences in reliability. It is also important to understand the value of using multiple codes. For example, many chronic conditions such as diabetes might not be the “reason” for the encounter but may be listed as a contributing or comordid condition.27 Likewise, individuals with ALS might be seen for symptoms such as trouble walking or swallowing.
Usually only one database is used for a particular study. It is important to understand the limitations of the dataset being used.28 It is also important to evaluate the dataset on a macro level to assess gaps.29 Several researchers have pointed out that to obtain accurate information it is important not to rely on just one encounter/report because there can be changes in diagnosis30 and existing chronic conditions may not be listed in each encounter.31 It could be necessary for some types of research to obtain additional information from another dataset. In a review of strengths and limitations of Medicaid data for epidemiologic research, the authors point out that it may be hard to identify incident conditions. 32 The first mention of a condition does not necessarily indicate that it was diagnosed on that date but might just merely indicate the first time it was documented. If additional procedures are required to be documented along with a diagnosis, this can assist in determining whether the case is incident.33 In a study of hip fracture, investigators developed an algorithm that defined hip fractures using both diagnosis and procedure codes and a combination of information from both hospital claims and Part B claims (outpatient) for Medicare recipients. Even for a condition that is almost certainly treated as an inpatient, some claims would have been missed without using the outpatient information. The authors also point out the importance of including information from the Veterans Administration (VA) because some Medicare recipients might receive care at a VA facility which would not be reflected in the Medicare files.34 Therefore, using multiple data sets, creating an algorithm to identify cases which includes inpatient and outpatient information, as well as using a combination of diagnoses and procedures can also increase the certainty of the diagnosis.
In addition, it is important in chronic diseases to look at multiple years of data. In a study by Pope et al, the prevalence of MS increased with the length of observation. The prevalence estimate for one year of claims for the privately insured population was 18 per 10,000 enrollees, 29 per 10,000 Medicare enrollees, and 53 per 10,000 Medicaid disabled enrollees. The prevalence estimated increased with two years of data to 24, 36 and 71 per 10,000 enrollees respectively.35 In another study examining the accuracy of Medicare claims data for identifying Alzheimer’s disease, the authors determined that a minimum of three years of data were needed to identify the patients. More years of data increased the number identified but only slightly. In addition, hospital files alone only identified 29% of the patients, whereas only physician encounters and institutional outpatient files identified 75% of the patients. Using 5 years of inpatient and outpatient data, 79% of the cases were identified. An analysis of clinical data on the patients revealed that those with less severe disease were less likely to be identified.36
The particular use of data from Health Maintenance Organizations (HMO) comes from studies conducted by the Kaiser Permanente Center for Health Research (CHR). CHR has participated in several surveillance projects related to asthma and infectious diseases. In one such study, an algorithm is being developed and validated for identifying individuals with prevalent (pre-existing) asthma and an algorithm for identifying cases of incident asthma from the subset of members who do not already have prevalent asthma. The study will estimate the incidence of asthma in various age-sex strata, and the cost of an ongoing surveillance system for incident cases based on these tools.37
Legislative Mandate
A bill to amend the Public Health Service Act to provide for the establishment of an Amyotrophic Lateral Sclerosis Registry, S. 1382: ALS Registry Act, was signed into law on October 10, 2008 by President Bush and became Public Law No: 110-373. The purpose of the registry as described in the bill, is to: (1) better describe the incidence and prevalence of ALS in the United States; (2) examine appropriate factors, such as environmental and occupational, that might be associated with the disease; (3) better outline key demographic factors (such as age, race or ethnicity, gender, and family history of individuals who are diagnosed with the disease) associated with the disease; and (4) better examine the connection between ALS and other motor neuron disorders that can be confused with ALS, misdiagnosed as ALS, and in some cases progress to ALS. The registry will collect personal health information that may provide a basis for further scientific studies of potential risks for developing ALS.
RATIONALE
A number of private databases have been created to study ALS which have included a large amount of clinical data and has been used to study the natural history of the disease as well as to monitor health care decisions. Each database was created to answer a specific research question; therefore, it is likely to be difficult to get agreement on what clinical information should be universally collected.
In general, existing databases are valuable for research and for surveillance as long as the researcher recognizes the limitations of the data, limitations in quality of the original data collection, and any biases that might arise in variations in ascertainment or treatment of the disease being studied.38 The use of multiple existing databases such as Medicare, Medicaid, and Veterans Administration should be a feasible way to identify patients with ALS.
The dilemma of what clinical information to collect and how to standardize the collection and classification of this information could be eliminated by creating a surveillance system that collected a minimal amount of information on each patient. The more detailed information about the patient with ALS would reside with the source of the information. The minimal data would be used to describe the prevalence and incidence of ALS. It would also be used to identify cases to contact for consent to participate in research studies. It would be necessary to request the additional information from the source of the case if a patient agreed to participate in a study. Some data not available in administrative/claims data will be collected through voluntary surveys. (See Survey section)
PILOT PROJECTS
To evaluate the feasibility of using existing administrative data to identify cases of ALS, ATSDR funded four geographically diverse pilot projects including tertiary care facilities for ALS, HMOs, and state based organizations. These four pilot projects matched data from Medicare, Medicaid, the Veterans Health Administration, and Veterans Benefits Administration to data available within the four pilot project sites administrative and clinical databases for a 5-year time period (January 1, 2001 – December 31, 2005). ATSDR provided the pilot projects with individual encounters with an ICD-9 code for any MND (335.2-335.29) for the specific project catchment area. Pilot projects completed a standardized spreadsheet for each individual found in any database indicating in which database(s) a record was located, ICD-9 code recorded for the encounter, as well as the years and types of providers seen. Medical records were abstracted and diagnoses verified. A deidentified dataset was sent to ATSDR for analysis. All individuals who were identified with a possible ALS diagnosis, as indicated by ICD-9 code for any MND, and had their medical record reviewed by a neurologist from the four pilot projects were combined. Approximately 4400 medical records were reviewed. It was possible to develop algorithms using variables from the administrative data that identified true cases of ALS (verified by a neurologist) (Table 1). Similar results were found in the individual pilot project analyses. These pilot projects were determined to be not human subjects research at ATSDR because only deidentified data were provided by the partners.
Table 1 – Best algorithm for determining true cases of ALS from administrative data1
ALS |
Not ALS |
A l g o r i t h m |
|
Neurologist Review |
|
* In same database |
|
ALS |
Not ALS |
||
ALS |
1282 |
265 |
|||
Not ALS |
233 |
1531 |
|||
Sensitivity = 0.85 |
|||||
Specificity = 0.85 |
|||||
PPV = 0.83 |
|||||
NPV = 0.87 |
|||||
National Databases include Veterans Health Administration, Veterans Benefits Administration, Medicare, and Medicaid.
Death Certificate includes ICD-10 code G12.2 for MND. Death Certificates are not an independent database because there is not a specific code for ALS.
Rilutek is the only prescription medication specifically used to treat ALS.
One or more visits for an MND other than ALS.
Any individual not falling into the ALS or Not ALS category is in the possible ALS category. For example, an ALS code in 2 years but no visit to a neurologist would be in the possible ALS category and reevaluated as new data become available.
OBJECTIVES
The
objectives of this project are to develop a population-based
surveillance system/registry for ALS. The primary goal of the
surveillance system/registry is to obtain reliable information on the
incidence and prevalence of ALS and to better describe the
demographic characteristics (age, race, sex, and geographic location)
of those with ALS. The secondary goal of the surveillance
system/registry is to collect additional information on potential
risk factors for ALS including, but not limited to, family history of
ALS, smoking history, and military service.
PROPOSED
SURVEILLANCE DESIGN AND METHODS
A population-based surveillance system/registry for ALS will be created by identifying persons with ALS from existing administrative databases and self-registration by interested ALS patients. A flowchart illustrating surveillance system/registry inclusion can be found in Attachment A. A minimal amount of data will be contained in the surveillance system/registry including:
Name
City
State
Last 5 digits of the Social Security Number
Month and Year of birth
Sex
Race
Date first in database
Database reporting/self-registration
Vital status
Date of death
Data Base Descriptions
A number of databases will be used including Medicare, Medicaid, Veterans Health Administration, and Veterans Benefits Administration. These national databases cover approximately 90 million people.
Veterans Health Administration data includes inpatient, outpatient, and pharmacy records for veterans receiving health care benefits. Approximately 20% of veterans qualify for this benefit.
Veterans Benefits Administration data includes records for veterans receiving pensions or compensation for disabilities considered service related. ALS is considered service related if it is diagnosed within 1 year of separation from active duty.
Medicare data includes inpatient, outpatient, pharmacy, and long-term care records for individuals receiving this benefit. Medicare is United States government provided insurance for people age 65 or older, some disabled people under age 65, and people of all ages with End-Stage Renal Disease (permanent kidney failure treated with dialysis or a transplant). Individuals approved for Social Security Administration, Disability Insurance Benefit or Supplemental Security Income because of ALS can begin receiving Medicare without a 24 month waiting period.
Medicaid data includes inpatient, outpatient, and pharmacy records for individuals receiving this benefit. Medicaid is the United States health program for individuals and families with low incomes and resources. It is an entitlement program that is jointly funded by the states and federal government, and is managed by the states.
Initial Identification of Possible Cases of ALS
Anyone in an administrative data base with ICD-9-CM codes of 335.2 -335.29 will be identified. All individuals with any Motor Neuron Disease (MND) will be evaluated because other MNDs can be confused with ALS. Because of the short clinical course of ALS, it is necessary to differentiate between individuals seen once to rule out ALS and those with only one occurrence of an ALS code because they died soon after diagnosis.
Algorithm
Using data from the pilot projects, ATSDR developed algorithms which will be used on each database to identify “cases” of ALS from all those identified by the methods mentioned previously. Applying the algorithm to possible cases will sort individuals into three categories, ALS, possible ALS, and definitely not ALS. Only those identified as having ALS will be entered into the registry. As new data become available, the algorithm will be rerun looking for new cases and reevaluating the possible ALS cases to see if the new information clarifies their case status. It is anticipated that new data will be available on a yearly basis and that the algorithms will be rerun with those individuals identified as possible ALS cases to see if case status is clarified.
Self-registration
All cases of ALS are not identified from existing administrative data for a variety of reasons, including eligibility requirements for the various entitlement programs, miscoding, and misdiagnosis. The preliminary data analysis of the pilot data suggests that individuals with ALS can be identified from existing administrative datasets. However, younger, self payees, and those who died sooner after diagnosis are more likely to be missed in the existing data, therefore, individuals will be allowed to self-identify for inclusion in the registry. We would like all ALS patients to register so that they can take part in the collection of data not available in administrative data (See Survey Section). ALS patients will be consented prior to registering.
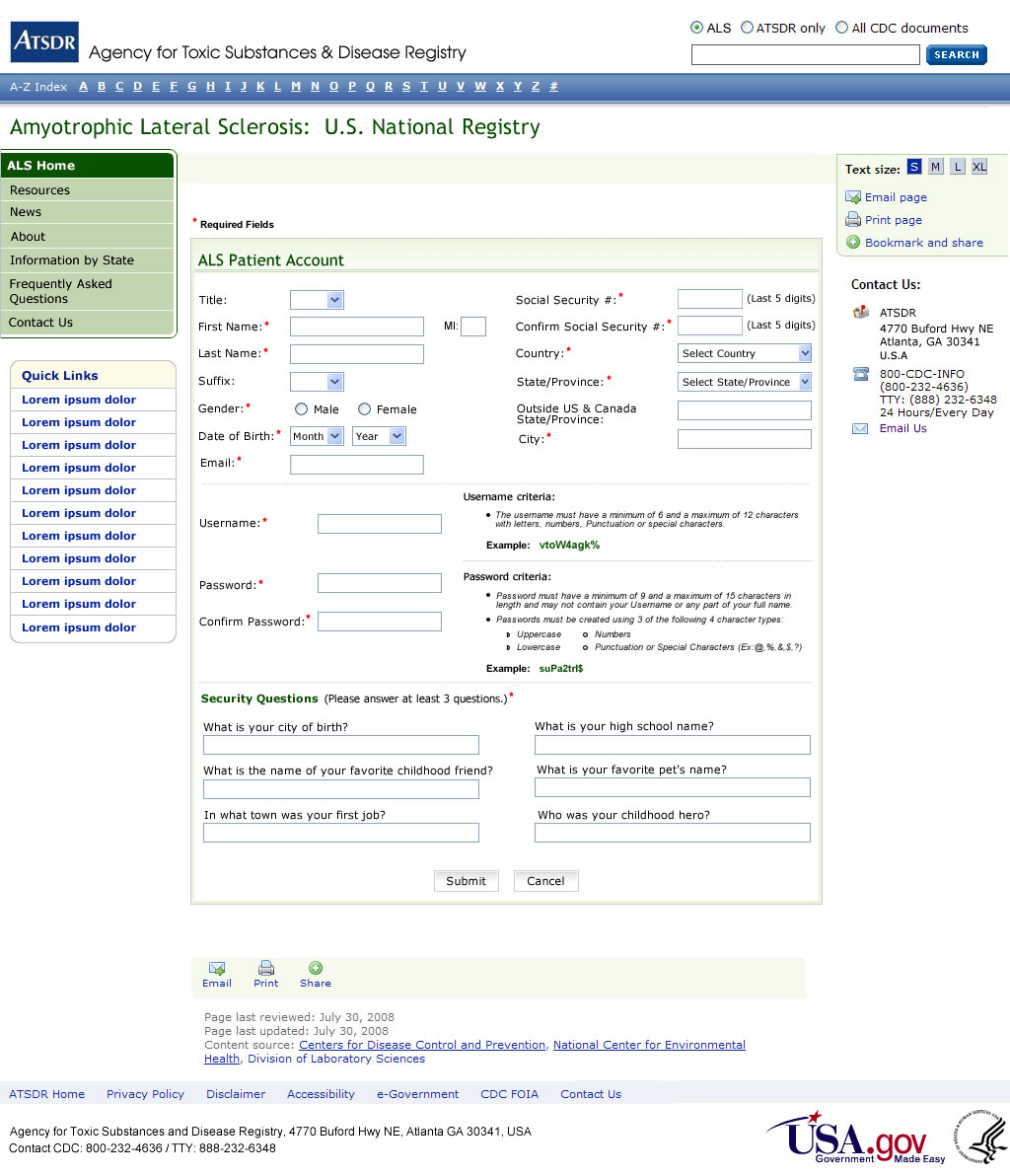
The VA ALS registry used six validation questions to screen possible individuals with ALS. Of the 98.7% of the veterans who passed the screening, 93.4% were confirmed to have ALS or another MND based on neurologist medical record review.39 We will use these six questions as part of the registration process (Attachment B). Once a person self-identifies, he/she will be asked to complete the validation questions. If the answers indicate the person has ALS, he/she will be allowed to create an account to become part of the registry (Attachment C) after consenting (Attachment D). Technical assistance will be available for ALS patients because they may have difficulty with the computer because of physical disabilities related to their disease. The information collected during the registration process is primarily used to make sure there are not duplicates in the registry given that case ascertainment will come from multiple sources. The matching algorithm relies primarily on Social Security Number (SSN). In an evaluation of more than 300,000 records received from CMS, no duplicates were identified using the last 5 digits of the SSN. In addition to SSN, we will have first and last name which should make duplicates easily identifiable.
It is unknown who many individuals will self-register, however this is a motivated group. The VA ALS registry allowed self-referral and 18% of the individuals evaluated for inclusion in the registry were from self-identification, although a number of these individuals had also been identified via records review.39 Self-registration will be encourage so that the registrant can participate in additional data collection activities. Two advocacy groups, the ALS Association and the Muscular Dystrophy Association, ALS Division, have expressed their intention to advertise the existence of the registry to their constituents.
Death Data
The National Death Index (NDI) is a central computerized index of death record information on file in the State vital statistics offices. The NDI is a national file of identifying death record information (beginning with 1979 deaths) compiled from computer files submitted by State vital statistics offices. Death records are added to the NDI file annually, approximately 12 months after the end of a particular calendar year. Cases of ALS identified by the registry will be sent to the NDI to determine vital status. Information on vital status will be maintained in the registry.
On a yearly basis ATSDR will ask the Mortality Statistics Branch at the National Center for Health Statistic to search their data for death certificates mentioning ALS. Since 2003, searches can be made on the text of the death certificate so that the search will be specific for ALS and synonyms for ALS such as Lou Gehrig’s disease, and is not dependent on the ICD-10 code of G12.2. Currently ICD-10 codes do not include a specific code for ALS. Rather ICD-10 G12.2 codes for all MNDs. The death certificate number will be submitted to the state and a death certificate purchased. This information will be used to verify diagnosis as well as identify cases that may have been missed by the registry.
Surveys
Congress anticipated that two additional purposes for the registry would be to examine appropriate factors, such as environmental and occupational, that might be associated with the disease and to better outline key demographic factors (such as age, race or ethnicity, gender, and family history of individuals who are diagnosed with the disease) associated with the disease. The information necessary to examine these demographic and potential risk factors for disease are not usually part of a registry or public health surveillance system. To enable the collection of additional information from registrants who volunteer, a series of short survey modules will be available for completion via a secure web portal. We are using a survey validated by the ALS Consortium of Epidemiologic Studies (ACES).41 The survey has been divided in to short modules because of the physical limitations of the study population (Attachment E). All surveys are designed to be answered only once except for the symptoms survey which can be answered every 6 months. The ALS Functional Rating Scale-Revised (ALSFRS-R) is a standard set of questions used by physicians to measure functioning overtime. Researchers have developed and tested a self-administered version of the ALSFRS-R which showed excellent reliability to change over time.40 The published version of the self-administered ALSFRS-R was slightly modified to make the question responses more user friendly. Individuals will be consented prior to registering with the National ALS Registry and completing any survey modules. (See human subjects section)
Although the generalizability of the survey data will be dependent on the number of individuals who choose to participate, these data can be used to inform risk factor specific study protocols.
Human Subjects Protection
ATSDR is requesting a waiver of consent for including the data obtained from the existing data sources, including Medicare, Medicaid, Veterans Health Administration, and Veterans Benefits Administration. The research involves no more than minimal risk to the subjects because the information has already be collected, the waiver will not adversely affect the rights and welfare of the subjects as there is no interaction with the participants; the research could not practicably be carried out without the waiver because there are more then 15,000 individuals ho would need to be contacted and contact information is not up-to-date; and the ATSDR website being created for the ALS registry will provide additional pertinent information after participation.
ATSDR is also requesting a waiver of consent for the six validation questions. The research involves no more than minimal risk to the subjects because the information other than date of diagnosis is not retained and only used to determine eligibility, the waiver will not adversely affect the rights and welfare of the subjects as there is penalty for not providing the information and we will not know who chooses not to participate; the research could not practicably be carried out without the waiver because we would be obtaining consent for people who were not eligible and the would be the only information collected about them; and the ATSDR website being created for the ALS registry will provide additional pertinent information after participation.
Registration and participation in providing additional information by completing surveys is entirely voluntary. Prior to determining eligibility a privacy statement will be displayed explaining the reasons for the data collection (Attachment F). They will also be shown a consent statement (Attachment D). If the person decides to proceed, they will follow the procedures outlined in self-registration. Once registered, an individual has the opportunity to provide additional information through survey modules. Because all information is collected electronically, the individual will have to agree with the consent statement prior to proceeding with registration. We are requesting a waiver of documentation of consent for this project. The research presents no more than minimal risk of harm to subjects and involves only the collection of survey data. The greatest risk to participants is from a breach of confidentiality. To minimize this risk, we have developed extensive data security procedures outlined in the data security section. Collection of this type of survey information (smoking history, family history of disease, occupation history, etc) is often collected outside of the research context without consent.
Data security
Creating an account
External Users (ALS Patients / External Researchers) must self-register before accessing the ALS Web Portal. Personal information is collected during this registration process and users are allowed to create their own unique username and password. Users are also required to answer security questions which are used as alternative authentication credentials if their password is forgotten. Upon successful registration, users are required to login into their account using their username and password. External Users are authenticated against a backend SQL encrypted database.
Internal Users (CDC Employees / System Administrators) are required to be pre-approved by ATSDR management before accessing the ALS Intranet Web Portal. Once a user is approved, ATSDR management sends a request to the System Administrator to create a user account. The request must include the user’s CDC User ID, First Name, Last Name, Gender, City, State, Country, and Email in order for the System Administrator to add the user to the ALS System. Users must first log into the CDC network to access the ALS Intranet Web Portal and are authenticated using Active Directory. No login is required.
The ALS system creates a sequential unique identifier in the database every time a user account is created. This unique identifier identifies each user and is used to link user information inside the system. Another unique identifier (Last 5 of SSN) will be used to verify patient data outside of the ALS system.
Login procedures
For authentication purposes, users will be verified using their unique username along with their password. External Users are allowed to self-register online and create their own username. Duplicate checks are implemented during registration to ensure uniqueness of usernames and emails.
Password management
External users are allowed to change or reset their passwords, but are not allowed to retrieve their password. Passwords can be changed via the user’s account after the user has been authenticated by providing the old password and can only be changed once every 6 days. If a user forgets his/her password, the password can be reset by providing alternate authentication credentials. These credentials include the user’s username, registered email address, and a security question. Passwords are required to be reset every 60 days. Users will be given a 2 week email notice before their password expires. Users will be directed to reset their expired password if they attempt to login after their password has expired.
Usernames are unique and can not be changed. Users must contact the System Administrator by phone to retrieve their username. The System Administrator is required to ask verification questions before releasing any information to the user; which can include the user’s first and last name, month & day of birth, City, State, Country, and two security questions.
The status of an account will change to inactive if the user has not logged into his/her account in 6 or more months. Users will be given a 2 week email notice before their account is inactivated. Users will be required to contact the System Administrator by phone to re-activate their account. The System Administrator will be required to verify the user by asking verification questions which include the user’s First and Last Name, Date of Birth: Month & Year (ALS Patients only), Address: City, Province/State, Country, and 2 security questions.
No personal information or credentials can be sent to a user’s email, only notices or confirmations.
User accounts can not be removed and remain in the database permanently. Only the account status can change.
Encryption
Information in Identifiable Form (IIF) fields will be masked on the Graphical User Interface because of the sensitivity of the data. For example, month and year of birth will be masked.
All Private Indentifying Information (PII) data which includes the last 5 digits of the SSN will be encrypted using AES_256 (Advance Encryption Standard 256 bit) encryption, the strongest encryption standard supported by SQL Server 2005.
To encrypt/decrypt data in database columns designed to hold PII data, a user must be given access to open and close a symmetric key.
Minimize collection of identifiable information
The information required for registration has been limited to only that needed to make sure that an individual truly has ALS and is not already part of the registry. Address information has been limited to city and state, birth information has been limited to month and year of birth, and only the last five digits of the SSN will be collected.
Physical Controls
Production and test servers are stored in a server room secured by the CDC. Access tools are in place to secure entry into CDC buildings (Guards, ID Badges, Key Card, Cipher Locks, and Closed Circuit TV).
Data management
On a quarterly basis, data will be downloaded from the web-based portal and provided to ATSDR. ATSDR will merge the self-identified individuals into the registry after first checking for duplicates. The registry will be maintained on a secure server or stand-alone hard-drive. Access to the data will be limited to approved study personnel. Deidentified data sets will be used for data analysis.
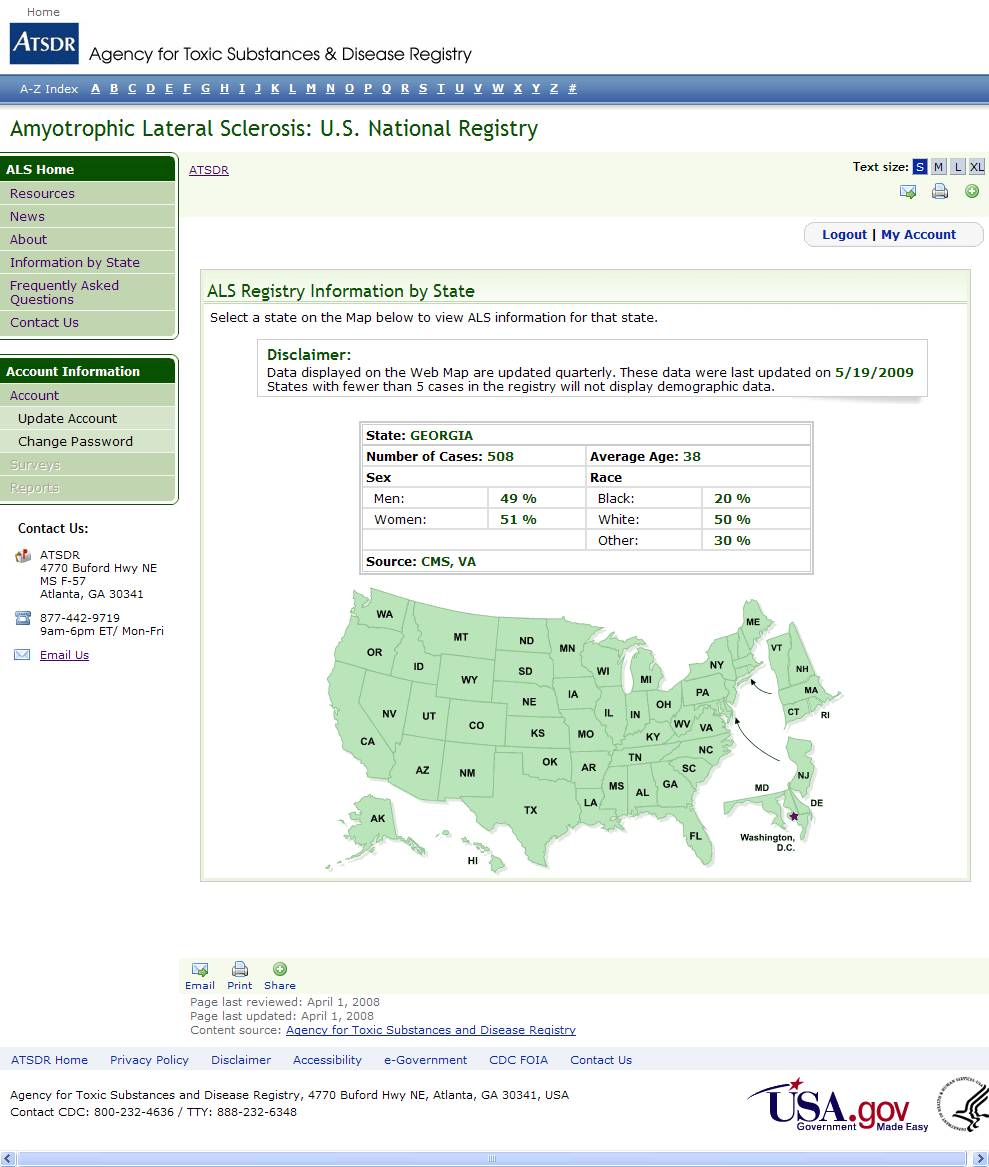
On a quarterly basis, ATSDR will provide back to the web-based portal a dataset which is deidentified, including only state, age, race, and sex. This dataset will be used to populate the surveillance/registry map available on the website (Attachment G)
Evaluation
It will be important to evaluate the completeness of the surveillance/registry. Information will be captured each time a case is identified and from which database so that capture-recapture statistical techniques can be used to estimate the number of people missed.42, 43 It is also important to identify the level of service, e.g., primary practice or neurologist, for each identified record as it assists in the evaluation of the reliability of the diagnosis. If the evaluation identifies groups of individuals underrepresented in the registry, additional case finding strategies will be developed.
CONCLUSION
There is a public health need for accurate estimates of people affected by neurodegenerative diseases to better assess the health care needs of the population, detect changes in health care practices, and assess the burden of disease. Although the idea of a comprehensive public health surveillance system using existing data was described more than 10 years ago, 20 there have been no attempts to initiate such a system on a national level. This endeavor will provide needed information on ALS which can be used by others.
REFERENCES
Bobowick AR, Brody JA. Epidemiology of motor-neuron diseases. N Engl J Med 1973;288:1047-55.
Mitsumoto H, Chad DA, Pioro EP. (1998). Amyotrophic Lateral Sclerosis. Philadelphia, PA: F.A. Davis Company.
Eisen A. Amyotrophic lateral sclerosis is a multifactorial disease. Muscle Nerve 1995;18:741-52.
Chio A. Risk factors in the early diagnosis of ALS: European epidemiological studies. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1 Suppl 1:S13-8.
Kondo K. Motor neuron disease: changing population patterns and clues for etiology. Adv Neurol 1978;19:509-43.
Maasilta P, Jokelainen M, Loytonen M, Sabel CE, Gatrell AC. Mortality from amyotrophic lateral sclerosis in Finland, 1986-1995. Acta Neurol Scand 2001;104:232-5.
Norris F, Shepherd R, Denys E, U K, Mukai E, Elias L, Holden D, Norris H. Onset, natural history and outcome in idiopathic adult motor neuron disease. J Neurol Sci 1993;118:48-55.
Belsh JM. Diagnostic challenges in ALS. Neurology 1999;53:S26-30; discussion S35-6.
Belsh JM, Schiffman PL. Misdiagnosis in patients with amyotrophic lateral sclerosis. Arch of Intern Med 1990;150:2301-5.
Davenport RJ, Swingler RJ, Chancellor AM, Warlow CP. Avoiding false positive diagnoses of motor neuron disease: lessons from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry 1996;60:147-51.
Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman O. Amyotrophic lateral sclerosis mimic syndromes: a population-based study. Arch Neurol 2000;57:109-13.
Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994;124 Suppl:96-107.
Miller RG, Munsat TL, Swash M, Brooks BR. Consensus guidelines for the design and implementation of clinical trials in ALS. World Federation of Neurology committee on Research. J Neurol Sci 1999;169:2-12.
Litt J, Tran N, Malecki KC, Neff R, Resnick B, Burke T. Identifying priority health conditions, environmental data, and infrastructure needs: a synopsis of the Pew Environmental Health tracking project. Environ Health Perspect 2004;112:1414-8.
Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect 2005;113:1250-6.
Centers for Disease Control and Prevention (CDC). Comprehensive plan for epidemiologic surveillance: Centers for Disease Control, August 1986. Atlanta, GA: CDC, 1986.
Thacker SB, Stroup DF, Rothenberg RB. Public health surveillance for chronic conditions: a scientific basis for decisions. Stat Med 1995;14:629-41.
Shanghai declaration on non-communicable diseases. WHO Directors of Non-Communicable Disease Collaborating Centres and Key Officials.
BMJ 1993;306:588.
Birkhead GS and Maylahn CM. (2000). State and Local Public Health Surveillance. In: Teutsch SM and Churchill RE (Eds.), Principles and Practice of Public Health Surveillance – Second Edition (pp. 253-286). New York: Oxford University Press.
Thacker SB, Stroup DF. Future directions for comprehensive public health surveillance and health information systems in the United States.
Am J Epidemio. 1994;140:383-97.
Benesch C, Witter DM, Wilder AL, Duncan PW, Samsa, GP, Matchar DB. Inaccuracy of the International Classification of Diseases (ICD-9-CM) in identifying the diagnosis of ischemic cerebrovascular disease. Neurology 1997;49:660-4.
Tennis P, Bombardier C, Malcolm E, Downey W. Validity of rheumatoid arthritis diagnoses listed in the Saskatchewan hospital separation database. J Clin Epidemio 1993;46:675-83.
Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, Lindsey SM, Roberts WN, Sheff DM, Spencer RT, Weaver AL, Baron JA. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum 1997;40:1594-1600.
Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA. The Sensitivity of Medicare claims data for case ascertainment of six common cancers. Med Care 1999;37:436-44.
Fisher ES, Whaley FS, Krushat M, Malenka DJ, Fleming C, Baron JA, Hsia DC. The accuracy of Medicare’s hospital claims data: progress has been made, but problems remain. Am J Public Health 1992;82:243-8.
Peabody JW, Luck J, Sharad J, Bertenthal D, Glassman P. Assessing the accuracy of administrative data in health information systems. Med Care 2004;11:1066-70.
Humphries KH, Rankin JM, Carere RG, Buller CE, Kiely FM. Spinelli JJ. Co-morbidity data in outcomes research: Are clinical data derived from administrative databases a reliable alternative to chart review? J Clin Epidemio 2000;53:343-9.
Green J, Wintfeld N. How accurate are hospital discharge data for evaluating effectiveness of care? Med Care 1993;31:719-31.
Hennessy S, Bilker WB, Weber A, Strom BL. Descriptive analyses of the integrity of a US Medicaid claims database. Pharmacoepidemiol Drug Saf 2003;12:103-111.
Worth RM, Mytinger RE. Medical insurance claims as a source of data for research: accuracy of diagnostic coding. Hawaii Med J 1996;55:9-11.
Szeto HC, Coleman RK, Gholami P, Hoffman BB, Goldstein MK. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care 2002;8:37-43.
Wang PS, Walker A, Tsuang M, Orav EJ, Levin R, Avorn J. Strategies for improving comorbidity measures based on Medicare and Medicaid claims data. J Clin Epidemiol 200;53:571-8.
Bright RA, Avorn J, Everitt DE. Medicaid data as a resource for epidemiologic studies: strengths and limitations J Clin Epidemiol. 1989;10:937-45.
Fisher ES, Baron JA, Malenka DJ, Barrett J, Bubolz TA. Overcoming potential pitfalls in the use of Medicare data for epidemiologic research. Am J Public Health 1990;80:1487-90.
Pope GC, Urato CJ, Kulas ED, Kronick R, Gilmer T. Prevalence, expenditures, utilization, and payment for persons with MS in insured populations. Neurology 2002;58:37-43.
Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol 2000;55:929-37.
Kaiser Permanente Center for Health Research Website. Available at http://www.kpchr.org/public/default.asp. Accessed March 29, 2006.
Kuller H. The use of existing databases in morbidity and mortality studies (editorial). Am J Public Health 1995;85:1198-1200.
Allen KD, Kasarskis EJ, Bedlack RS, Rozear MP, Morgenlander JC, Sabet A, Sams L, Lindquist JH, Harrelson ML, Coffman CJ, Oddone EZ. The national registry of veterans with amyotrophic lateral sclerosis. Neuroepidemiology 2008;30:180-190.
Montes J, Levy G, Albert S, Kaufmann P, Buchsbaum R, Gordon PH, Mitsumoto H. Development and evaluation of a self-administered version of the ALSFRS-R. Neurology 2006;67:1294-6.
See http://aces.stanford.edu/
Coffman CJ, Horner RD, Grambow SC, Lindquist J. Estimating the Occurrence of Amyotrophic Lateral Sclerosis among Gulf War (1990-1991) Veterans Using Capture-Recapture Methods: An Assessment of Case Ascertainment Bias. Neuroepidemiology 2005;24:141-150.
Preux P, Druet-Cabanac M, Couratier P, Debrock C, Truong T, Marcharia W, Vallat J, Dumas M, Boutros-Toni F. Estimation of the amyotrophic lateral sclerosis incidence by capture-recapture method in Limousin region of France. J Clin Epidemio 2000;53:1025-29.
A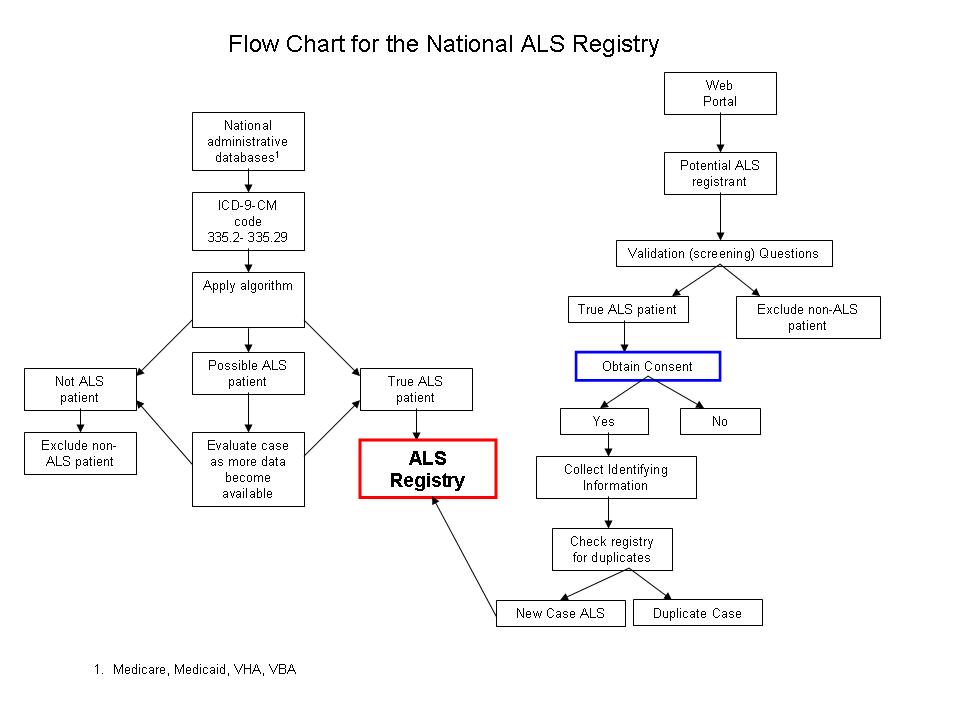 TTACHMENT
A
TTACHMENT
A
ATTACHMENT B
Validation Questions
Q1: |
Were you ever told by a health professional that you might have ALS or Lou Gehrig’s disease? |
|
a. Yes (Go to Q2) |
|
b. No (Go to Q3) |
|
|
Q2: |
Were you clinically diagnosed with ALS? |
|
a. Yes (Go to Q5) |
|
b. No (Go to Q3) |
|
|
Q3: |
Is there another diagnosis that you have been given by a health professional? |
|
a. Yes (Go to Q4) |
|
b. No (Go to Q5) |
|
|
Q4: |
What was the diagnosis? |
|
a. Possible ALS (not yet determined/diagnosed) (Go to Q5, then Q6) |
|
b. Primary lateral sclerosis (Go to Q5, then Q6) |
|
c. Progressive bulbar palsy (Go to Q5, then Q6) |
|
d. Progressive muscular atrophy (Go to Q5, then Q6) |
|
e. Other (please list) (Go to Q5, then Q6) |
|
|
Q5: |
Have you been seen by a neurologist? |
|
a. Yes (Go to Q6 if Q2 = Yes, or Q3 = Yes) |
|
b. No (Go to Q6 if Q2 = Yes, or Q3 = Yes) |
|
|
Q6: |
What was the date of your diagnosis? |
|
__ __/__ __/__ __ __ __ |
|
M M D D Y Y Y Y |
ATTACHMENT C
Create an Account
ATTACHMENT D
Consent Form
National ALS Registry
Background – ALS is the most common motor neuron disease which causes the deterioration of the upper and motor neuron. Motor neurons send signals to the muscles. There are a lot of questions about the number of people who have ALS and what causes it.
Purpose – The purpose of this research is to get a better picture of who gets ALS or other motor neuron diseases. The information could also be used to design studies about what causes ALS. You are being asked to take part because you have ALS. If you decide to register, you will be asked for information about you and where you live. If you take part in any of the survey modules, you will be asked to answer some questions about who you are, where you lived or worked, family history of ALS, and how you are coping with your diseases. You will only be asked to answer most questions one time. You will be asked to complete questions on how you are coping with your ALS twice a year. The survey modules can be done whenever you want. You can do them all at once or over a period of time.
Risks – The major risk of taking part is someone getting your information. To keep this from happening, we will limit who can see your information. We will also have computer security that keeps your information safe.
Benefits – There are no direct benefits to you. In the future, your information could help others with ALS.
Confidentiality – Your information will be kept confidential to the extent allowed by law. Only authorized individuals will have access to your information. Your information will be stored in a secure location with limited access. Any information that is published about people in the registry will not identify you.
Results – The website where you registered will have reports about what we learn from people who take part in the National ALS Registry and surveys.
Voluntary – Taking part is up to you. You do not have to take part and you can stop taking part at any time. You will not lose any benefits to which you are entitled if you do not take part or chose to quit.
If you have any questions about the surveys, you can contact Dr. Oleg Muravov at XXX. If you have any questions about your rights as a research participant you can contact XXX.
By clicking on ACCEPT, if you agree to take part in the surveys.
Accept – (Go to list of Surveys)
Reject – (Go to ALS registry home page)
Flesch-Kincaid Reading Level 7.5
ATTACHMENT E
Surveys
ONE TIME SURVEYS
Questionnaire to be divided into 6 SURVEY modules by topic
The purpose of this questionnaire is to obtain some general information about yourself, as well as information on lifestyle factors.
1.1.1.1.1General Instructions
Please read these questions carefully and answer to the best of your knowledge.
When answering choice questions, click on the box(es)
1.1.2SURVEY 1 : BACKGROUND INFORMATION
1. What is your date of birth?
Month Day Year
2. How old are you today? years old
3. How old were you when you were told by a neurologist that you had ALS. years old
4. What is your gender? 1 Male 2 Female
5. What is your current marital status?
1 Never married 2 Married 3 Separated
4 Divorced 5 Widowed 6 Living with partner
6. How many years of schooling have you completed? years
7. What is the highest level of education that you have completed?
1 Grade school (grades 1-8) 2 High school diploma
3 Technical or trade school diploma 4 College diploma
5 Graduate school diploma 6 Other (specify):________________
8. Do you consider yourself Spanish, Hispanic, or Latino/Latina?
1 No 2 Yes, Puerto Rican
3 Yes, Mexican, Mexican American, Chicano 4 Yes, Cuban
5 Yes, other Spanish, Hispanic, or Latino/Latina (specify): ______________________
8. What do you consider to be your race or ethnic group? If you belong to more than one of these groups, please indicate all groups that apply to you.
1 White 2 Black or African-American
3 Native American or Alaska Native 4 Asian Indian
5 Chinese 6 Filipino
7 Japanese 8 Korean
9 Vietnamese 10 Other Asian (specify):_______________
11 Native Hawaiian 12 Guamanian or Chamorro
13 Samoan
14 Other Pacific Islander (specify):_______________
99 Don’t know
9. In what country were you born?
________________________________________
10. What is your current height? (FT) (IN)
11. What is your current weight? (LBS)
12. What is your height at age 40 years? (FT) (IN)
13. What is your weight at age 40 years? (LBS)
1.1.3 LIFESTYLE INFORMATION
We are now going to ask you to answer a few questions about your occupation and other lifestyle factors.
SURVEY 2
OCCUPATION
14. What is your current employment status?
1 Full-time employed 2 Part-time employed
3 Retired 4 Disabled
5 Full-time student 6 Homemaker
7 Unemployed 8 Other (specify):________________
15. If currently employed, what is your occupation? Please indicate your job title and the industry in which you worked.
_________________________________________________________________________
JOB TITLE
________________________________________
INDUSTRY
15a. For how many years were you employed in this occupation? years
16. In which occupation were you employed for the longest period of time? Please indicate your job title and the industry in which you worked.
_________________________________________________________________________
JOB TITLE
_________________________________________________________________________
INDUSTRY
16a. For how many years were you employed in this occupation? years
SURVEY 3
MILITARY HISTORY
17. Were you ever a member of the armed forces?
1
Yes 2
No (go to question 15) 9
Don’t know (go to question 15)
Yes 2
No (go to question 15) 9
Don’t know (go to question 15)
 17a.
If yes, in which branch of service were you employed?
17a.
If yes, in which branch of service were you employed?
1 Army 2 Navy 3 Marines
4 Air Force 5 Reserves/National Guard
6 Coast Guard
17b. Were you ever deployed to a war arena?
1 Yes 2 No (go to question 15)

 17c.
If yes, to which war arena were you deployed? Please specify
all arenas (for example, WWII Europe, Vietnam).
17c.
If yes, to which war arena were you deployed? Please specify
all arenas (for example, WWII Europe, Vietnam).
1 _______________________
2 _______________________
3 _______________________
4 _______________________
SURVEY 4
SMOKING
18. Have you ever smoked one or more cigarettes per day for six months or longer?
1
Yes 2
No (go to question 16) 9
Don’t know (go to question 16)
Yes 2
No (go to question 16) 9
Don’t know (go to question 16)
 18a.
If yes, how old were you when you first started smoking one or more
cigarettes per day?
years old
18a.
If yes, how old were you when you first started smoking one or more
cigarettes per day?
years old
18b. Are you still a cigarette smoker?
1
Yes 2
No 9
Don’t know
Yes 2
No 9
Don’t know
1 8c.
If no, at what age did you last stop smoking cigarettes?
8c.
If no, at what age did you last stop smoking cigarettes?
year old
18d. During periods when you smoked, for how many years in total did you smoke cigarettes? years
18e. During periods when you smoked, how many cigarettes did you usually smoke in a day? One pack contains 20 cigarettes. number cigarettes per day
ALCOHOL
19. Did you ever drink alcoholic beverages such as wine, beer and spirits at least once a month for
6 months or more?
1 Yes 2 No (go to question 17) 9 Don’t know (go to question 17)

1 9a.
Are you still drinking alcoholic beverages at least once per
month?
9a.
Are you still drinking alcoholic beverages at least once per
month?
1 Yes 2 No
19b. During periods when you were drinking alcoholic beverages, for how many years in total did you drink alcoholic beverages? years
19b. During periods when you were drinking, how many alcoholic beverages did you usually have in a week OR month? A drink is 12 oz. beer, 4 ounces of wine or a drink containing 1 oz. of liquor.
Please check one
number of drinks per 1 week OR 2 month
SURVEY 5
PHYSICAL ACTIVITY
20. Have you ever engaged in vigorous leisure-time physical activity for at least 10 minutes that caused
heavy sweating or large increases in breathing or heart rate?
1
Yes 2
No 9
Don’t know
Yes 2
No 9
Don’t know
2 0a.
If yes, please indicate the number of times per week, month OR
year that you engaged in vigorous activity for at least 10 minutes
for each age period (up
to your current age period).
If you did not
engage in vigorous activity for any age period (up to your current
age period), fill in the number of times as 00.
0a.
If yes, please indicate the number of times per week, month OR
year that you engaged in vigorous activity for at least 10 minutes
for each age period (up
to your current age period).
If you did not
engage in vigorous activity for any age period (up to your current
age period), fill in the number of times as 00.
Age period |
Engaged in Physical Activity |
Number of Times |
Please check one Week Month Year |
15-24 years |
1 Yes 2 No |
|
1 2 3 |
25-34 years |
1 Yes 2 No |
|
1 2 3 |
35-44 years |
1 Yes 2 No |
|
1 2 3 |
45-54 years |
1 Yes 2 No |
|
1 2 3 |
55-64 years |
1 Yes 2 No |
|
1 2 3 |
65 years or older |
1 Yes 2 No |
|
1 2 3 |
SURVEY 6
FAMILY HISTORY
The following questions relate to biological family members including parents, sisters and brothers (including half siblings) and children. Please do not include adopted relatives.
21. How many biological sisters (including half-sisters) do you have, living or deceased?
number
21. How many biological brothers (including half-brothers) do you have, living or deceased?
number
22. How many biological children do you have, living or deceased?
number
Please complete a few questions about each of your immediate relatives with respect to particular medical conditions they may have had.
Among your biological relatives, including your parents, sisters, brothers and children, has anyone ever been diagnosed by a physician with any of the following conditions?
YOUR BIOLOGICAL PARENTS:
Relationship |
Is the family member living? |
What is the family member’s current age or the age at his/her death? |
Has the family member ever been diagnosed by a physician with any of the following medical conditions? |
At what age was he/she diagnosed with the condition? |
Mother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
Father |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
YOUR BIOLOGICAL SIBLINGS:
Relationship |
Is the family member living? |
What is the family member’s current age or the age at his/her death? |
Has the family member ever been diagnosed by a physician with any of the following medical conditions? |
At what age was he/she diagnosed with the condition? |
1 Sister 2 Brother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Sister 2 Brother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Sister 2 Brother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
YOUR BIOLOGICAL SIBLINGS:
Relationship |
Is the family member living? |
What is the family member’s current age or the age at his/her death? |
Has the family member ever been diagnosed by a physician with any of the following medical conditions? |
At what age was he/she diagnosed with the condition? |
1 Sister 2 Brother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Sister 2 Brother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Sister 2 Brother |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
YOUR BIOLOGICAL CHILDREN:
Relationship |
Is the family member living? |
What is the family member’s current age or the age at his/her death? |
Has the family member ever been diagnosed by a physician with any of the following medical conditions? |
At what age was he/she diagnosed with the condition? |
1 Daughter 2 Son |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Daughter 2 Son |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Daughter 2 Son |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
YOUR BIOLOGICAL SIBLINGS:
Relationship |
Is the family member living? |
What is the family member’s current age or the age at his/her death? |
Has the family member ever been diagnosed by a physician with any of the following medical conditions? |
At what age was he/she diagnosed with the condition? |
1 Daughter 2 Son |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Daughter 2 Son |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
1 Daughter 2 Son |
1 Yes 2 No 9 Don’t know |
years old |
Amyotrophic lateral sclerosis: 1 Yes 2 No 9 Don’t know Alzheimer’s disease: 1 Yes 2 No 9 Don’t know Parkinson’s disease: 1 Yes 2 No 9 Don’t know |
Age Don’t know
Age Don’t know
Age Don’t know |
Self-Administered Rating Scale
The following rating scale is used to assess changes in physical functioning in persons with ALS and other motor neuron diseases.
The questions refer to how you are currently functioning at home. Please read each item carefully and base your answers on your functioning today compared to the time before you had any symptoms of ALS. Please choose the answer that best fits your functional status today. Place an “x” in the box next to your answer.
Compared with the time before you had symptoms of ALS or motor neuron disease:
1. Have you noticed any changes in your speech?
-
no change
I have a noticeable speech difference.
My speech has changed. I am asked often to repeat words or phrases.
My speech has changed. I sometimes need the use of alternative communication methods (i.e. computer, writing pad, letter board or eye chart).
I am unable to communicate verbally.
2. Have you noticed any changes (increases) in the amount of saliva in your mouth (regardless of any medication use)?
-
no change
I have slight but definite excess of saliva with or without night time drooling.
I have moderate amounts of excessive saliva with or without minimal day time drooling.
I have marked amounts of excessive saliva with some daytime drooling.
I have marked excessive saliva with marked drooling requiring a constant tissue or handkerchief.
3. Have there been any changes in your ability to swallow?
-
no changes for all foods and liquids
I have some changes in swallowing or occasional choking episodes (including coughing during swallowing).
I am unable to eat all consistencies of food and have modified the consistency of foods eaten.
I use a feeding tube (PEG) to supplement what is eaten by mouth.
I do not eat anything by mouth and receive all nutrition through a feeding tube (PEG).
4. Has your handwriting changed? Please choose the best answer that describes your handwriting with your dominant (usual) hand without a cuff or brace.
-
no changes
My handwriting is slower and/or sloppier but all the words are legible.
Not all my words are legible.
I am able to hold a pen but unable to write.
I am unable to hold a pen.
5. The following question refers to your ability to cut foods and handle utensils (feed yourself).
a. Is most of your nutrition through a feeding tube (PEG)?
-
Yes – Skip to II
No – Skip to b
b. Do you eat most of your meals by mouth?
-
Yes – Skip to I
I. Cutting food and handling utensils:
-
no change
My cutting food or handling utensils is somewhat slow and clumsy (or different than before) but I do not need assistance or adaptive equipment.
I sometimes need help with cutting more difficult foods.
My food must be cut by someone else but I can feed myself slowly without assistance.
I need to be fed.
II. Using a feeding tube (PEG)
-
I use a PEG without assistance or difficulty.
I use a PEG without assistance however I may be slow and /or clumsy.
I require assistance with closures and fasteners.
I provide minimal assistance to a caregiver.
I am unable to perform any of the manipulations.
6. Has your ability to dress and perform self-care activities (i.e. bathing, teeth brushing, shaving, combing your hair, other hygienic activities) changed?
-
no change
I perform self-care activities without assistance but with increased effort or decreased efficiency.
I require intermittent assistance or use different methods (i.e. sit down to get dressed, fasten buttons with a fastener or your non-dominant hand).
I require daily assistance.
I do not perform self-care activities and am completely dependent on caregiver.
7. Has your ability to turn in bed and adjust the bed clothes (i.e.. cover yourself with the sheet or blanket) changed?
-
no change
I can turn in bed and adjust the bed clothes without assistance but it is slower or more clumsy.
I can turn in bed or adjust the bedclothes without assistance but with great difficulty.
I can initiate turning in bed or adjusting the bed clothes but require assistance to complete the task.
I am helpless in bed.
8. Has your ability to walk changed?
-
no change
My walking has changed but I do not require any assistance or devices (i.e. foot brace, cane, or walker).
I require assistance to walk (i.e. cane, walker, foot brace or hand held assistance).
I can move my legs or stand up but am unable to walk from room to room.
I cannot walk or move my legs.
9. Has your ability to climb stairs changed?
-
no change
I am slower.
I am unsteady and/or more fatigued.
I require assistance (i.e. using the handrail, cane or person).
I cannot climb stairs.
10. Do you experience shortness of breath or have difficulty breathing?
-
no change
I have shortness of breath only with walking.
I have shortness of breath with minimal exertion (i.e. talking, eating, bathing or dressing).
I have shortness of breath at rest while either sitting or lying down.
I have significant shortness of breath (all of the time) and considering using mechanical ventilation.
11. Do you experience shortness of breath or have difficulty breathing while lying down on your back?
-
no change
I occasional have shortness of breath while lying on back but don’t routinely use more that two (2) pillows to sleep.
I have shortness of breath while lying on back and require more than two pillows (or an equivalent) to sleep.
I can only sleep sitting up due to shortness of breath.
I require the use of respiratory (breathing) support (BiPAP® or invasive ventilation via tracheostomy) to sleep and do not sleep without it.
12. Do you require respiratory (breathing) support?
-
I need no respiratory support.
I need intermittent use of BiPAP®.
I need continuous use of BiPAP® at night.
I need continuous use of BiPAP® at night and during the day (nearly 24 hours per day).
I need mechanical ventilation by intubation or tracheostomy.
13. Please indicate who completed this survey:
-
I completed the survey (patient).
I completed the survey with assistance.
I completed the survey with assistance from caregiver or family member.
The caregiver completed the survey alone.
14. What is your current weight? __ __ __ lbs
15. Have you been hospitalized in the past 6 months? Yes No
15a. If yes, how many times were you in the hospital? __ __
15b. How many days were you hospitalized? __ __ (total number of days)
16. Have you gone to the Emergency Room in the past 6 months? Yes No
16a. If yes, how many times have you visited the Emergency Room? __ __
ATTACHMENT F
Privacy Statement
The purpose of this screen is to collect personal contact information to include you in the National ALS Registry and make sure we do not already have your information. While providing this information is voluntary, failure to do so may result in your not being included in the ALS National Registry. Your decision to provide the requested information on the National ALS Registry screen constitutes your implicit [explicit] consent that the ATSDR [CDC] may share this information with appropriate ATSDR [CDC] administrative staff, scientists, and researchers in order to facilitate creation of the National ALS Registry and further research on ALS. The information requested here is collected under the authority ALS Registry Act, Public Law No: 110-373.
NOTE: your personal information is not accessible by anyone other than authorized individuals for official business. The ONLY information viewable by the general population is information on ALS and aggregate information.
ATTACHMENT G
Web Surveillance/Registry Map
| File Type | application/msword |
| File Title | BACKGROUND |
| Author | wendy e kaye |
| Last Modified By | wek1 |
| File Modified | 2010-07-07 |
| File Created | 2009-10-13 |
© 2026 OMB.report | Privacy Policy