Passback_Attachment_Biomarker Study Protocol 2010-02-25
Passback_Attachment_Biomarker Study Protocol 2010-02-25.doc
Agricultural Health Study: A Prospective Cohort Study of Cancer and Other Diseases Among Men and Women in Agriculture (NCI)
Passback_Attachment_Biomarker Study Protocol 2010-02-25
OMB: 0925-0406
Technical Evaluation of Protocols Committee
Protocol for a Molecular Epidemiology Study of MGUS and Biospecimen Collection for Biomarker Studies of Pesticide Exposures in the Agricultural Health Study Cohort
OEEB Approval: February 24, 2009
OEEB Branch Chief Signature: __________________________________________________
Debra Silverman Date
SAG Approval: July 20, 2009
Investigators and Other Research Personnel
Core Research Team:
Senior Investigators: Michael Alavanja (PI), Laura Beane Freeman (CO-PI), Ola Landgren (CO-PI), Neil Caporaso (CO-PI), Sharon Savage (CO-PI), Jonathan Hofmann (CO-PI).
Investigators: Kent Thomas (EPA), Jay Lubin, Shelia Zahm, Stella Koutros, Gabriella Andreotti, Carol Christensen (NCI/EPA), Dale Sandler (NIEHS), and Cynthia Hines (NIOSH).
Study-Specific Extramural Researchers:
MGUS and Telomere Measurement Extramural Team: Robert Kyle (Mayo Clinic), Jerry Katzmann (Mayo Clinic), Vincent Rajkumar (Mayo Clinic), Andrea Baccarelli (Harvard University, University of Milan), Intramural PIs (Landgren and Savage, lead).
DNA methylation Extramural Research Team: Andrea Baccarelli (Harvard University, University of Milan), Lifang Hou (Northwestern University). Paolo Ghia, (Universita Vita-Salute San Raffaele), Intramural PIs.(Caporaso and Alavanja, lead)
Current Support Services
Westat Coordinating Center: Kate Torres, Marsha Dunn
Iowa Site Personnel: Chuck Lynch (Iowa Director), Ellen Heywood (Study Coordinator), Dan Scaffinger (computer programmer), Gayle Robertson (contract administrator)
North Carolina Site Personnel: Charles Knott (North Carolina Director), Margaret Hayslip (Study Coordinator)
February 2010
Table of Contents
Page
SUMMARY ..................................................................................................................... 3
BACKGROUND.............................................................................................................. 4
OBJECTIVES.................................................................................................................. 6
METHODS ...................................................................................................................... 7
Study Design and Location…………………………………………………………… 7
Sample Selection and Eligibility.................................................................................... 7
Subject Recruitment....................................................................................................... 9
Introductory Letter and Follow-up Phone Call........................................................... 10
Additional Mailings and Contacts with Consenters................................................... 10
Data Collection, Informed Consent, and Compensation………………….................... 13
Interview .................................................................................................................... 13
Blood and Urine Samples........................................................................................... 13
Subject Compensation................................................................................................ 14
Shipment of Blood and Urine Samples........................................................................... 14
Monitoring of Study Progress ........................................................................................ 15
Quality Control............................................................................................................... 16
Exposure Assessment………………………………………………………………..... 16
Telomere Length and DNA Methylation Assays………………………………………. 16
Outcome Ascertainment................................................................................................. 16
Data Analysis.................................................................................................................. 17
Sample Size and Study Power ....................................................................................... 18
Personnel......................................................................................................................... 20
Human Subjects and Confidentiality.............................................................................. 21
Time Schedule................................................................................................................ 23
Budget............................................................................................................................ 23
LIST OF APPENDICES................................................................................................... 26
REFERENCES………………………………………………………………………….. 26
SUMMARY
This study has two primary objectives. First, we propose to determine the prevalence and study the etiology of monoclonal gammopathy of undetermined significance (MGUS) in a sample of 1,600 cancer-free, Agricultural Health Study (AHS) pesticide applicators over the age of 50, with well-characterized occupational exposures and lifestyle factors. MGUS has been observed to precede all cases of multiple myeloma in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. To achieve this objective we will compare the prevalence of MGUS in the AHS cohort with the prevalence in two general population-based cohorts (i.e., Olmsted County and NHANES III) with well-characterized MGUS prevalence levels. We will also examine the associations between MGUS and specific pesticides within the AHS cohort, and determine whether selected biomarkers are associated with excess MGUS and whether these biomarkers are significantly associated with specific pesticides.
The second objective will establish a resource with the remaining biospecimens collected from the participants for the MGUS study that will be used to evaluate the biological plausibility and the mechanism-of-action of associations between pesticides and cancers observed in earlier AHS studies. Many of these pesticides are non-genotoxic and their mechanism of carcinogenesis has not been determined. The biospecimen resource will include blood and urine samples. Junior investigators will be encouraged to pursue funding via IRAs, molecular epidemiology class awards, and technology development awards to utilize this resource. Of immediate interest is the use of the first 400 bio-specimens in a pilot evaluation of the prevalence of monoclonal B-cell lymphocytosis (MBL) in the AHS cohort and its potential link to specific pesticides. MBL has been shown to precede Chronic Lymphocytic Leukemia (CLL) in the PLCO study and several pesticides in current widespread use in the AHS have been linked to leukemia.
BACKGROUND
Multiple myeloma (MM) is a largely incurable neoplasm of differentiated B-cells (plasma-cells) characterized by an overproduction of monoclonal immunoglobulins, causing approximately 11,000 deaths per year. Recently, Dr. Ola Landgren has observed that MM was always preceded by a premalignant disorder, monoclonal gammopathy of undetermined significance (MGUS) in the PLCO study (Landgren, 2009). This finding establishes a key role for MGUS in the pathway to multiple myeloma. Although the etiology of MGUS and MM remain largely unclear, previous cohort and case-control studies have reported an elevated risk of MM among farmers and other agricultural workers (Perrotta, 2008). However, most prior investigations have been hampered by small numbers and limited exposure assessment.
In the Agricultural Health Study (AHS), a prospective cohort of 57,310 private and commercial pesticide applicators with comprehensive pesticide exposure assessment, we found a standardized incidence ratio (SIR) of 1.34 (95% confidence interval CI=0.97-1.81) for MM (Alavanja, 2005). Permethrin, a widely-used insecticide on farms and home gardens is significantly associated with MM in the AHS (Rusiecki, 2009). Other pesticides possibly associated with MM in the AHS cohort include glyphosate (De Roos, 2005) and atrazine (Rusiecki, 2004), and possibly other pesticides that have not yet been evaluated for MM. In a preliminary AHS study of MGUS prevalence we observed a significant excess compared to Olmsted County (Landgren, 2009, Appendix 1, Table 1). In the same study we also observed associations between MGUS and several widely-used pesticides (Landgren, 2009, Appendix 1, Table 2). These preliminary findings are in need of replication in a larger sample of the AHS cohort, but they suggest that pesticide exposure associated with MGUS risk might be an underlying explanation of the previously observed excess multiple myeloma risk among persons exposed to pesticides in a variety of other studies.
Telomeres consist of long nucleotide repeats (5’-TTAGGG-3’) and a protein complex at chromosome ends. Telomeric attrition can result in critically short telomeres prompting genomic instability. Previous studies have reported telomere length in MM cases that are approximately 40% of those in controls (Cottliar, 2003; Shammas, 2003; Wu, 2003; Wu, 2005), but telomere length has not been evaluated with MGUS status. In a preliminary AHS study, telomere length was measured in DNA from buccal cells using the Cawthon method (Cawthon, 2002) in the laboratory of Dr. Andrea Baccarelli. We found that telomeres were significantly shorter among subjects exposed to a number of pesticides including permethrin and 2,4-D (Appendix 1, Table 3). Given these preliminary findings one of our primary objectives is to investigate the association between pesticide exposure, telomere length and MGUS status.
DNA methylation is an important component of gene expression and genome stability. It is often altered in cancer cells compared to normal cells. In MGUS and MM several tumor suppressor genes are silenced via hyper-methylation of the promoter regions. In addition, altered global genomic hypo-methylation has also been observed in MM and thus may be involved in the early and precancerous stages of the disease. Specifically, data from Dr. Andrea Baccarelli’s lab showed that MM cases exhibited low methylation in Alu and LINE-1 sequences compared to the standard results of pyrosequencing analysis for both Alu and LINE-1 (Bollati, 2009). Dr. Baccarelli’s data suggest that repetitive DNA hypo-methylation is a feature of MM and possibly in MGUS.
Several other biological markers in plasma may be related to MGUS and risk of progression to MM. When immunoglobulin molecules are normally produced by plasma cells in the bone marrow, the heavy chains (G, A, M, D, or E) and the light chains (kappa or lambda) are produced separately. When a clonal proliferation of plasma cells starts to develop, free light chain (FLC) concentrations in the serum increase along with abnormal kappa to lambda ratios. Approximately one-third of MGUS patients have abnormal FLC and these patients have a higher rate of progression to MM. We believe identifying this subset of MGUS positive cases with abnormal FLC may be an important tool for etiological research.
In this study we can more comprehensively investigate the etiology of MGUS, and the biological mechanisms of the associations between pesticides and MGUS, by evaluating telomere length and DNA methylation, as well as polyclonal proteins and free circulating kappa and lambda immunoglobulin chains. These more specific assays coupled with MGUS may have even stronger relationships with selected pesticides. Identifying specific exposures responsible for myelomagenesis will help us to better understand chemical carcinogenesis in humans, and help us to reduce the risk of diseases by identifying and quantifying potential disease risks associated with particular exposures.
In addition to our interest in MGUS and multiple myeloma, we are also interested in evaluating the link between pesticide exposures and leukemia. Several pesticides (e.g., diazinon, fonofos, metribuzin, EPTC and possibly permethrin, glyphosate, atrazine and alachlor) have been shown to be associated with leukemia in the AHS cohort. In this study we will evaluate risk of monoclonal B-cell lymphocytosis (MBL), which is a premalignant disorder associated with chronic lymphocytic leukemia (CLL). Healthy persons with a small number of B-cell clones circulating in the peripheral blood are designated as having MBL; Rawstron et al. (2008) reported that the prevalence of MBL among UK hospital out patients between 40-90 years of age with no history or suspicion of cancer was 5.0%. In another study of MBL among a residential population in Italy referred for routine blood tests, Dagklis et al. (2009) found a higher prevalence of MBL (>6%). Hospital-based series indicate an excess risk of CLL among those with MBL. Recently, Landgren et al. (2009) showed that 98 % of CLL cases are preceded by MBL (up to 6.4 years before CLL diagnosis) in cryopreserved peripheral whole blood samples from participants in the PLCO Cancer Screening Trial. Identifying specific exposures associated with MBL would be important to better understand chemical carcinogenesis in humans and to reduce the risk of disease by taking appropriate public health action. More detailed background information regarding CLL, MBL and related biologic markers is provided in Appendix 2.
In terms of other cancers, the AHS has identified 11 pesticides (i.e., diazinon, chlorpyrifos, carbofuran, fonofos, metribuzin, EPTC, imazethapyr, metolachlor, pendimethalin, trifluralin and dicamba) in current wide-spread use that have significant exposure-response relationships with other cancers (Appendix 1, Table 4). Suggestive evidence of an association has also been seen with several other pesticides (Appendix 1, Table 4). These findings, although not conclusive, indicate that these chemicals deserve further evaluation. In addition to their use on farms, some of these pesticides (e.g., 2,4-D, glyphosate, permethrin, pendimethalin, dichlorvos) have wide-spread use in the general population as home and garden pesticides used by millions of Americans with no occupational exposures to pesticides. Since many of these pesticides have not previously been hypothesized to cause cancer and since many of these pesticides are non-genotoxic, further research is needed to evaluate the etiological significance of our work.
OBJECTIVES
This study has two primary objectives. First, we propose to determine the prevalence and study the etiology of monoclonal gammopathy of undetermined significance (MGUS), a precursor biomarker of multiple myeloma (MM), in a sample of 1,600 cancer-free, Agricultural Health Study (AHS) pesticide applicators over the age of 50, with well-characterized occupational exposures and lifestyle factors. For the second objective, we will establish a resource with the remaining biospecimens collected from the participants for the MGUS study that will be used for future molecular studies, including pilot studies of MBL prevalence among AHS participants and hematologic alterations following recent exposure to diazinon, an organophosphate insecticide associated with leukemia in a previous analysis within the AHS cohort (Beane Freeman et al. 2005). Specific aims for each of these primary objectives are as follows:
Prevalence and etiological studies of MGUS
Use serum samples from 1,600 AHS study subjects to determine the prevalence of MGUS among AHS cohort members over 50 years of age and compare to the prevalence among individuals of similar age in Olmsted County, MN and in the NHANES III survey sample.
Evaluate the relationship between specific pesticide exposures and the prevalence of MGUS.
Determine if telomere length differs between the positive MGUS cases and those study subjects without MGUS.
Determine if specific pesticides are associated with telomere length in an exposure-response pattern.
Determine if positive MGUS cases are associated with global hypo-methylation and gene specific hyper-methylation compared to those study subjects without MGUS.
Evaluate polyclonal proteins and free circulating kappa and lambda chains to determine if these more specific assays have even a stronger relationship with specific pesticides.
Establishing a biospecimen resource for future molecular studies including pilot studies of MBL prevalence and hematotoxicity of diazinon
Collect blood, a first morning urine void and questionnaire data from 1,600 AHS study subjects for future molecular studies of pesticide exposures shown to be associated with cancer (i.e., multiple myeloma, leukemia and cancers of the prostate, lung, colon, rectum, pancreas, bladder, and possibly NHL) and other chronic diseases in the AHS cohort.
In a pilot investigation, determine if MBL is elevated in the AHS cohort.
Determine if recent exposure to diazinon (previously associated with leukemia in the AHS cohort) produces hematologic changes [i.e., alterations in peripheral blood cell counts measured in a complete blood count (CBC) and CD4+ T-cells or other lymphocyte subsets] in a pilot study with serial measurements obtained before and after exposure from 50 recently exposed subjects.
As a secondary objective of this study, we will also collect information about cancer screening practices (e.g., history of digital rectal exam, PSA testing, and colonoscopy/sigmoidoscopy) among the AHS participants who are contacted for this study. This information will be used for a variety of research purposes, including an assessment of whether participants in this study are more or less likely to undergo screening evaluations than the general population (for which general survey screening rates are available). This comparison will help investigators assess whether selection bias may be affecting study results.
Relevance to the mission of OEEB and DCEG
Investigating the etiology of cancers that occur excessively among farmers, pesticide applicators and other workers has been one of the primary focuses of the OEEB for the past 30 years. The proposed study is consistent with that mission by establishing a biospecimen resource within the AHS to help identify human carcinogens in the agricultural environment and in some consumer products used by many Americans. Only a fraction of the material collected from each individual will be used for the MGUS research, MBL pilot investigation, and markers of hematologic alterations discussed in this protocol. The remaining biospecimens will be used in separately proposed etiological studies to be conducted in future years. This project is also responsive to the recommendations of the most recent OEEB site visit and the follow-up statements of the Board of Scientific Counselors which urged OEEB investigators to pursue molecular epidemiology within the AHS to determine the carcinogenic mode-of-action of these widely used and economically important chemicals. While the site-visit team recommended collection of blood on all or a majority of the AHS cohort, we believe our more economical protocol can achieve some of the most important desired objectives of mechanistic analyses.
METHODS
Study Design and Location
This is a cross-sectional study among a subset of participants in the AHS cohort. Following is a detailed description of the study design in terms of identifying and recruiting eligible participants, collecting biospecimens and questionnaire data, conducting biomarker assays, and performing analyses. An overview of the study design and plans for sample processing, testing, and storage in the repository is shown in Figure 1 below. The study will be conducted state-wide in Iowa and North Carolina, the locations of the larger AHS cohort.
Sample Selection and Eligibility
A total of 1,600 AHS participants (1,072 from Iowa and 528 from North Carolina) will be enrolled in this study, including a subset of 50 participants (33 from Iowa and 17 from North Carolina) with recent exposure to diazinon (separate selection criteria and study procedures for this group are described below). The eligibility criteria for all study subjects are as follows:
Male private pesticide applicator;
Alive as of the latest update of the National Death Index (NDI) and over 50 years of age at the time of initial contact;
Cancer free as of the most recent linkage with the Cancer Registry;
Completed phase I, II and III interviews (these study subjects have the most comprehensive exposure evaluation and also will be the most interested in the study).
Individuals who meet any of the following criteria will be ineligible for this study:
Deceased or no longer residing in Iowa or North Carolina;
Ever diagnosed with any type of cancer other than non-melanoma skin cancer;
Unable to speak English;
Have a blood clotting disorder such as hemophilia; or
Registered with the AHS as a “no contact”.
The 50 Recently Exposed subjects will meet these same inclusion and exclusion criteria. For the Recently Exposed group, we will select subjects who mix, load, or apply diazinon for agricultural use during the current growing season. We will focus on recent diazinon exposure because lifetime diazinon use has been previously associated with leukemia risk among participants in the AHS (Beane Freeman, 2005). Candidates for this group will be designated during the sample selection process based on phase III interview data, but their eligibility to remain in this group will be determined during the contact procedures, which are described below. Because of possible logistic challenges in identifying a sufficient number of applicators who currently use diazinon and conducting home visits according to the specified time frame for recent diazinon use, we consider this aspect of the protocol to be a pilot investigation. Selection criteria and procedures for enrolling participants in the Recently Exposed group will be reviewed and modified as needed after Year 2.
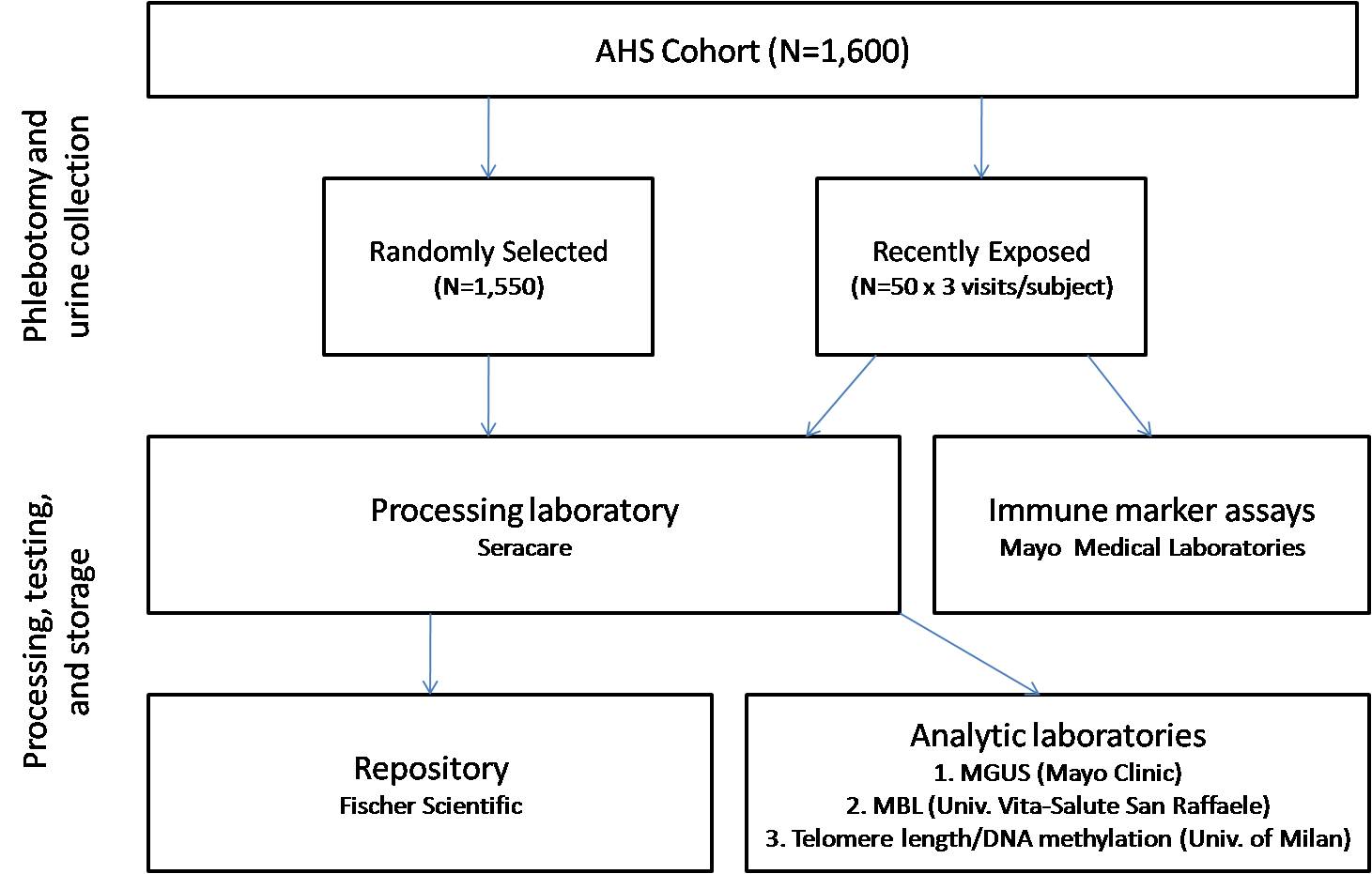
Figure 1. Overview of study design and plans for specimen collection, processing, testing, and storage in the repository
Subject Recruitment
The progression and timing of procedures from the identification of potentially eligible subjects through completion of the home visit is illustrated in Figure 2. Experience from previous substudies within the AHS cohort suggests that we should be able to enroll at least one-third of the subjects who are eligible for this study. We anticipate that we will have to contact an estimated 4,800 individuals to meet our planned enrollment of 1,600 AHS subjects. It should be noted that this is far fewer than the total number of AHS subjects who appear to be eligible for this study based on preliminary analyses. The coordinating center will generate a list of potentially eligible subjects annually (approximately 1,200 each year) based on registry and phase III interview data.
Recruitment and study procedures (including interviews and biospecimen collection) will be pilot tested in March 2010. Pilot testing in both Iowa and North Carolina is necessary to account for different possible barriers to participation among subjects in these two populations and to determine whether state-specific modifications to the protocol are needed to ensure study feasibility. In accordance with Office of Management and Budget regulations, we will include nine pilot subjects from each state. Recruiting subjects from more rural areas may prove to be logistically challenging; consequently, pilot subjects will be recruited from counties (one in each state) that are distant from urban centers. The pilot phase of this study will be used to evaluate the protocol for scheduling home visits, conducting interviews, collecting and shipping biospecimens, and laboratory processing. Time from blood and urine collection to delivery at the laboratory and temperature of samples upon receipt will be recorded to assess biospecimen shipping procedures. The study protocol will be amended as needed after reviewing the results of the pilot phase with the coordinating center and study centers in each state.
Introductory letters and calls to potential subjects will begin upon completion of the pilot phase (anticipated in May 2010). Subjects will be recruited and enrolled throughout the year. Field stations will use maps, as well as scheduling and tracking procedures to manage contacts and phlebotomy.
For participants in the Recently Exposed group, we will conduct a series of home visits with interviews and sample collection at the following three time points: 1) in the off-season prior to exposure; 2) on the day after cessation of diazinon use; and 3) 21 days (±3 days) after diazinon use. The pre-season home visit will take place in the winter (i.e., January-March). We anticipate that diazinon may be applied between April-August; we will plan to collect the two post-exposure blood samples during this time period. To schedule subsequent visits after diazinon exposure, we will follow up with these subjects on a regular basis before and during the growing season to determine the timing of diazinon applications and to schedule home visits accordingly. To assist with planning for scheduling home visits with participants in the Recently Exposed group, we will also consult with local extension agents in Iowa and North Carolina on a regular basis during the growing season in order to anticipate when participants are likely to apply diazinon.
Introductory Letter and Follow-up Phone Call
Each potentially eligible subject will be mailed an introductory letter and study brochure explaining the study and indicating that a follow-up call will occur later. Letters will be sent out on a monthly basis.
During the follow-up call, the interviewer will verify that the subject received the introductory letter, address subjects’ questions, determine eligibility via a short questionnaire (including eligibility for a blood draw), assess interest in participation, obtain verbal consent, provide additional instructions, and schedule the home visit. Additionally, all of the AHS participants who are contacted by phone (including those who decline to participate in the Biomarker Study or are ineligible) will be asked for permission to collect some information about their cancer screening practices. If they verbally consent, we will ask three questions regarding their history of cancer screening tests, including digital rectal exams, PSA testing, and colonoscopies and sigmoidoscopies.
Additional Mailings and Contacts with Consenters
Four weeks prior to the scheduled home visit, subjects who verbally consent to participate will receive a mailing that includes the home visit confirmation letter, copies of the consent forms, a reminder card for key elements of the questionnaire (particularly recent pesticide use), and the urine collection kit.
The phlebotomist will conduct a follow-up call 2-3 weeks prior to the home visit to introduce him/herself, verify receipt of mailed materials, address any questions or concerns, and verify the date and time of home visit. Postcards with the scheduled visit date and phone numbers for the study team (toll-free number) and the phlebotomist (cell phone number) will be mailed to all subjects shortly after the follow-up call.
Finally, two to three days before the scheduled home visit, all scheduled subjects will receive a reminder call from the phlebotomist to confirm the date and time, reiterate that questionnaire item responses will be needed, and remind subjects about the morning void urine collection. For subjects in the Recently Exposed group, this call will also serve to verify that the subject has recently mixed, loaded, or applied diazinon.
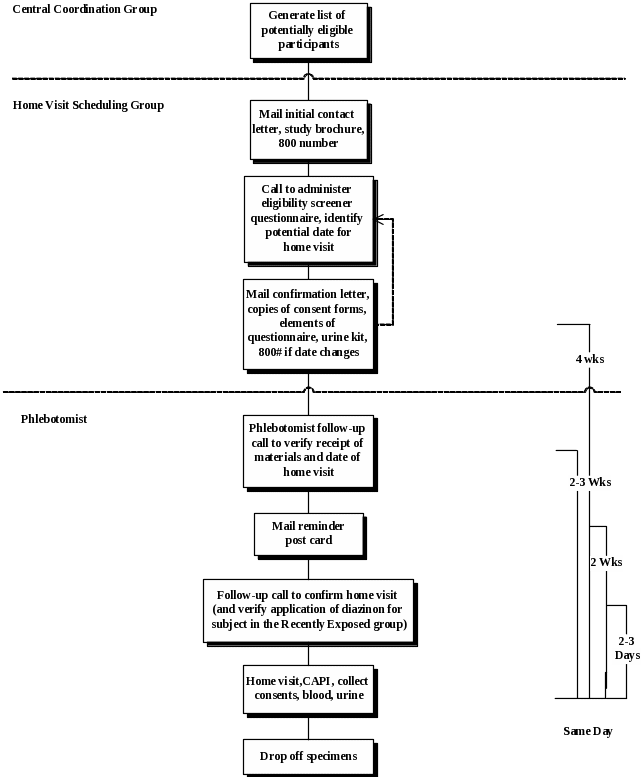
Figure 2. Overview of the Timeline for Recruitment and Data Collection
Data Collection, Informed Consent, and Compensation
The phlebotomist will travel to the subject’s home to: 1) recheck eligibility, 2) obtain signed consents for the interview and biospecimen collection, 3) administer a questionnaire via a computer-assisted personal interview (CAPI), 4) recheck to be sure the subject does not have an inherited blood clotting problem, 5) collect the blood samples, and 6) collect the urine sample.
To reduce travel time for phlebotomists, the states will be divided into geographic subareas for the AHS Biomarker Study. Maps illustrating the distribution of AHS cohort members in Iowa and North Carolina by county are provided in Appendix 3.
In order to validate residential information in the AHS cohort and facilitate future environmental studies through linkage to existing data sources, the phlebotomist will use a handheld Global Positioning System (GPS) receiver to record the exact location of the home of each study participant at the time of the home visit.
Interview
As indicated above, the mailing sent four weeks prior to the visit will include copies of the consent forms, and a list of key elements on the questionnaire to help the subject prepare for the interview. Before the interview begins, the phlebotomist will review the consent form with the subject. The phlebotomist will collect one signed copy and ask the subject to retain the other signed copy for his records.
If the subject agrees to the interview, the phlebotomist will administer the CAPI, which will be audio recorded with the subject’s consent. The CAPI will elicit information about recent medication use, medical conditions, smoking status, alcohol consumption, and pesticide use during the current/previous growing season. We will ask about the duration of pesticide use (number of days and hours per day), dates of recent applications, the product formulation (liquid or other), handling activities and method of application, and use of personal protective equipment. The interview is expected to take 20 minutes, on average.
Blood and Urine Samples
If the subject agrees to the blood sample, the phlebotomist will collect a 44.0-mL blood sample by venipuncture using all standard procedures for safety. The following blood samples will be collected from all participants: one 10.0-mL serum tube, one 6.0-mL heparin tube, one 6.0-mL EDTA tube, two 8.5-mL acid citrate dextrose (ACD) tubes, and two 2.5-mL PAXgene tubes. For the subjects in the Recently Exposed group, these tubes will be collected at the pre-season home visit; an additional 6.0-mL EDTA tube will also be collected from Recently Exposed subjects for the hematologic assays (i.e., CBC, lymphocyte subset measures).
For the subsequent post-exposure home visits among subjects in the Recently Exposed groups, the following blood samples will be collected: two 6.0-mL EDTA tubes (one for the repository and one for the hematologic assays), two 8.5-mL ACD tubes, and one 2.5-mL PAXgene tube.
For all participants, a 1.0-L urine collection kit will be sent to the home prior to the phlebotomy visit with instructions for collecting the first morning void on the day of the phlebotomy visit. The urine sample will be collected to quantify and validate pesticide exposures in a separately funded effort and to provide biospecimens for emerging analytical technologies. The subject will be asked to collect the entire void volume, and to record the time of sample collection and the previous void time. Also, we will request that the subject refrigerates the sample and gives it to the phlebotomist at the scheduled visit. Participants in the Recently Exposed group will be asked to provide a urine sample for each scheduled visit.
If the subject forgot to collect the urine sample, the phlebotomist will make sure that the subject has the necessary supplies, request that he collect the urine sample the following morning, and make sure that he understands all that is required of him to collect and ship the sample with the shipping materials provided.
Subject Compensation
Subjects will be reimbursed $75 for completing each home visit. Personal checks will be cut prior to the scheduled visits, so that reimbursement can be immediately provided as the phlebotomist completes the home visit.
Shipment of Blood and Urine Samples
The blood tubes and urine sample that will be collected from all participants will be shipped via Overnight Service to the processing laboratory (SeraCare, Frederick, MD). The serum tube, heparin tube, EDTA tube, and PAXgene tubes will be shipped cold (i.e., with frozen ice packs), and the ACD tubes will be shipped at ambient temperature. The second EDTA tube collected from subjects in the Recently Exposed group will be shipped at ambient temperature to the Mayo Medical Laboratories (Rochester, MN).
The phlebotomist will take the biologic samples collected at the home visit and drop them off at a nearby express mailing site for shipment via Overnight Service with the intention of getting these samples to the processing laboratory and the Mayo Medical Laboratories on the day following collection (within 24 hours whenever possible). The phlebotomist will conduct home visits on Monday through Thursday only, so that the biological samples can be delivered Tuesday through Friday (i.e., no weekend delivery), and processed by the recipient laboratories on the day following collection. To accomplish this, the phlebotomist will need to know the location of all express sites and their end-of-day pickup times in the geographic area.
When shipments are received by the processing laboratory, the biospecimens will be processed immediately and frozen at -80ºC, with the exception of the cryopreserved lymphocytes, which will be stored at a colder temperature in a liquid nitrogen freezer. Time of delivery and any deviations from the specimen collection protocol (e.g., low blood volume in any tube, incorrect specimen temperature) will be recorded upon receipt at the processing laboratory. The serum tube will be centrifuged; serum will be aliquoted into 1.0 mL samples and the clot will be saved. The heparin and EDTA tubes will be centrifuged and separated into samples of plasma (0.5-mL aliquots), buffy coat, and RBCs. Blood from the ACD tubes will be mixed upon receipt and DMSO will be added to a final concentration of 10%. These samples will be stored as 1.0-mL aliquots of whole blood. They will be cryopreserved using a controlled rate freezer to -90ºC, and will be stored in a liquid nitrogen freezer. The PAXgene tubes do not require processing; they will be frozen at -20ºC for 24 hours before being transferred for storage at -80ºC. For the urine specimen, the processing laboratory will measure the total void volume upon receipt. A 105-mL sample will be retained (ten aliquots of 10-mL, and five aliquots of 1-mL). The remainder of the urine specimen will be discarded. After processing and temporary storage, biospecimens will be transferred every six months from the processing laboratory to the repository (ThermoFischer Scientific, Frederick, MD) for long-term storage and the analytic laboratories for specific assays (i.e., MGUS, MBL, telomere length, and DNA methylation).
The additional EDTA tube collected from participants in the Recently Exposed group will be used for the hematological assays (i.e., CBC, lymphocyte subset assays). These assays will be performed immediately upon receipt at the Mayo Medical Laboratories. Assays will be performed on the day following collection (within 24 hours of collection whenever possible). Time from sample collection to analysis will be recorded to evaluate possible sample degradation and account for any time-related effects in the analysis if necessary.
A detailed overview of collection, shipment, laboratory processing, and storage procedures is provided in Appendix 4. It should be noted that procedures for specimen collection and processing are modeled after protocols that have been used successfully to manage biospecimens collected in the NCI-SEER non-Hodgkin’s lymphoma study and the U.S. Kidney Cancer Case-Control Study.
Monitoring of Study Progress
The field stations will use existing computer software to establish computerized data management systems to schedule and track mailings and telephone contacts, and home visit outcomes (i.e., interviews and specimen collection), as well as the progress of the study in their state. The coordinating center will track overall progress; additionally they will use a biospecimen sample tracking software system to track biospecimen shipment and receipt. The field station and coordinating center databases will be similar to other AHS tracking databases to ensure linkability to other AHS research activities in which each has participated.
The response rates for in-person interviews and biospecimen collection will be reviewed weekly. Feedback to the study centers will be provided through monthly conference calls among principal investigators at NCI, the coordinating center, and field stations, weekly telephone calls between the study manager at the coordinating center and study centers, and annual study meetings.
Quality Control
The following quality control procedures will be conducted for each phase of the study:
All phlebotomists and study coordinators will attend a standardized training.
A field manual will be developed.
All interviews will be recorded (with the subjects’ consent). For each interviewer/phlebotomist, a field supervisor will review tapes of his/her first five interviews, 10% of his/her interviews over the next three months, and 5% of interviews thereafter.
Field problems and solutions will be discussed at regular supervisor-phlebotomist meetings, weekly phone calls of study coordinators, and monthly phone calls of study investigators, and will be documented.
Standardized protocols for collecting and processing biospecimens will be used.
External quality control samples (10% of samples) will be included with each batch of laboratory samples for assays.
Exposure Assessment
To characterize lifetime exposure to pesticides among study participants for the MGUS analysis, we will utilize the extensive exposure data available from the phase I, phase II, and phase III interviews. These data will be available for all AHS subjects included in this study as per the eligibility criteria. For the analyses of short-term markers of hematologic alterations, we will characterize exposure to diazinon by measuring urinary metabolite concentrations and using information collected via questionnaire at the time of the first post-application home visit (i.e., the day after cessation of diazinon use).
Telomere Length and DNA Methylation Assays
The telomere length and DNA methylation assays will be performed by the laboratory of Dr. Andrea Baccarelli at the University of Milan (Milan, Italy) using DNA extracted from buffy coat. Telomere length will be measured using a quantitative polymerase chain reaction (QPCR) assay based on a method developed by Cawthon (2009). Telomere (T) and single gene copy (S) signals are measured in separate wells on parallel plates, and adjusted in comparison to standard reference DNA. The standardized T/S ratio characterizes differences in telomere length. Global DNA methylation will be quantified using the method described by Yang et al (2004). Briefly, DNA will be treated with bisulfite and DNA repetitive element (Alu and LINE-1 sequences) will be PCR amplified; the PCR product will be analyzed using pyrosequencing to quantify DNA methylation.
Outcome Ascertainment
The primary outcome of MGUS will be determined based on results of assays performed by the laboratory of Dr. Robert Kyle at the Mayo Clinic (Rochester, MN). All of the AHS participants in this study will be tested for MGUS. Additionally, MBL (a precursor of CLL) will be evaluated among AHS participants in a pilot study in collaboration with the laboratory of Dr. Paolo Ghia at the Universita Vita-Salute San Raffaele (Milan, Italy). Short-term hematological markers – including cell types and characteristics measured by the CBC and analysis of the major lymphocyte subsets (i.e., CD4+ T-cell, CD8+ T-cell, B-cell, and NK-cell counts) – will be evaluated among Recently Exposed participants; assays for these markers will be performed by the Mayo Medical Laboratories (Rochester, MN).
Data Analysis
The analyses will be conducted by the core research team and study-specific extramural researchers. For the MGUS studies, the prevalence of MGUS in the sample from the AHS cohort will be compared to the prevalence in Olmsted County, MN as well as to the prevalence among NHANES III study subjects whose questionnaire data indicate that their primary job was not farming or pesticide application. We will also determine if there are significant associations with specific pesticides and MGUS. Odds ratios estimating the risk of MGUS in relation to specific pesticides will be calculated using multiple logistic regression with adjustment for known and suspected MGUS risk factors (e.g., age, obesity, education, medication use, and family history of cancer). We will also begin to explore some of the molecular mechanisms involved in the etiology of MGUS. These initial efforts will focus on telomere length, altered DNA methylation, and measurements of free light chain immunoglobulins. For these analyses, markers will be analyzed as categorical variables (tertiles based on the distribution among participants without MGUS). Other molecular mechanisms will be considered as hypotheses are developed. Also, as a secondary analysis we will evaluate risk of MGUS in a pooled sample of 2,155 subjects from the proposed study and the previous MGUS study by Landgren et al. (2009) if there does not appear to be any heterogeneity of effect between the two studies.
For the MBL Pilot Study, we will determine the feasibility of measuring MBL (monoclonal B-cell lymphocytosis), a precursor of Chronic Lymphocytic Leukemia (CLL), from blood collected in the field from the AHS cohort, cryopreserved, and shipped to Dr. Paolo Ghia’s laboratory. We will also determine the prevalence of MBL in the AHS cohort, and determine factors that may influence any excess risk observed. Several pesticides used by the AHS cohort have shown significant associations with an excess risk of leukemia. Evaluating the feasibility of conducting an adequately sized etiological investigation will be a key objective of this pilot study.
In addition to the MGUS and MBL studies, we will also evaluate short-term biomarkers of hematologic changes that may be related to the etiology of leukemia among the Recently Exposed subjects. Measures of immune suppression and immune system perturbation are of particular interest (i.e., CBC, levels of lymphocyte subsets). Hematologic testing will be performed on fresh blood samples assessed on the day following collection. Because some hematologic alterations may be short-lived, we will compare measurements in samples obtained the day after diazinon use to pre-season baseline measurements. However, differences between measurements obtained immediately following exposure and 21 days after exposure will be characterized to evaluate whether hematologic alterations are transient or persistent. Multiple linear regression will be used to evaluate these markers in relation to diazinon exposure. In addition to using these serial blood samples to evaluate hematologic alterations, we anticipate that they will also be a useful resource for other biomarkers in future analyses (e.g., DNA adducts, chromosomal aberrations).
Sample Size and Study Power
The use of individual pesticides varies widely in the AHS cohort. For simplicity of computation, we have calculated the expected statistical power for the MGUS analysis based on the estimated proportion of the cohort that has ever been exposed to selected pesticides (Table 1). Power calculations were based on an expected MGUS prevalence of 3.7% in this study population, which is the observed prevalence among men over 50 years of age in Olmsted County, MN (Landgren et al. 2009). The statistical power is greater than 80% to detect a 2-fold difference in MGUS risk when the prevalence of exposure is between 30-60%. For pesticides used by 20-80% of the cohort, we will have at least 92% power to detect a 2.5 fold excess risk. These categories include many of the pesticides of particular interest for MM and MGUS. It should be noted that these power estimates are likely to be conservative because they are based on an estimated background prevalence of 3.7%, which is much lower than the 6.8% prevalence of MGUS that was observed in a previous analysis within the AHS cohort (Landgren et al. 2009). Also, if there does not appear to be any heterogeneity of effect between the previous MGUS study by Landgren et al. and the proposed study, we will perform a secondary pooled analysis with the combined sample of 2,155 AHS subjects.
Table 1. Statistical power to detect various levels of excess prevalence of MGUS by frequency of pesticide exposure (N=1,600)
Pesticide exposure |
Statistical power to detect the indicated MGUS odds ratios* |
|||
(exposed vs. unexposed) |
3.0 |
2.5 |
2.0 |
1.8 |
90% exposed vs. 10% unexposed1 |
0.86 |
0.63 |
0.34 |
0.24 |
80% exposed vs. 20% unexposed2 |
0.99 |
0.92 |
0.63 |
0.46 |
70% exposed vs. 30% unexposed3 |
>0.99 |
0.98 |
0.78 |
0.61 |
60% exposed vs. 40% unexposed4 |
>0.99 |
0.99 |
0.85 |
0.69 |
50% exposed vs. 50% unexposed5 |
>0.99 |
0.99 |
0.87 |
0.72 |
40% exposed vs. 60% unexposed6 |
>0.99 |
0.99 |
0.86 |
0.71 |
30% exposed vs. 70% unexposed7 |
>0.99 |
0.98 |
0.82 |
0.67 |
20% exposed vs. 80% unexposed8 |
0.99 |
0.94 |
0.73 |
0.58 |
10% exposed vs. 90% unexposed9 |
0.92 |
0.79 |
0.54 |
0.42 |
* 2-tail test, alpha=0.05, baseline probability of disease=3.7%
1glyphosate; 22,4-D; 3atrazine; 4none as of Phase III questionnaire; 5alachlor, malathion; 6chlorpyrifos, carbaryl, terbufos; 7carbofuran, pendimethalin; 8metribuzin, fonofos, EPTC, diazinon; 9permethrin, paraquat, coumaphos, chlorothalonil
Since one of our specific objectives is to examine the association between telomere length and MGUS risk, we have calculated the statistical power for this analysis (Table 2). Again assuming a background prevalence of MGUS of 3.7%, we expect to have 94% statistical power to detect a linear trend of MGUS risk increasing from a 1.6-fold excess in the middle tertile of telomere length to a 2.5-fold excess risk in the lowest tertile of telomere length, respectively, compared to the highest tertile.
Table 2. Statistical power to detect various levels of excess prevalence of MGUS
by tertiles of telomere length (N=1,600)
|
Statistical power to detect the indicated linear trend in MGUS odds ratios* |
||
Linear trend examined (Telomere length as exposure variable) |
1.0 (ref, highest tertile), 1.7 (middle tertile), 3.0 (lowest tertile) |
1.0 (ref, highest tertile), 1.6 (middle tertile), 2.5 (lowest tertile) |
1.0 (ref, highest tertile), 1.4 (middle tertile), 2.0 (lowest tertile) |
Power |
0.99 |
0.94 |
0.71 |
* Assuming 2-tail test with alpha of 0.05 and baseline probability of disease of 3.7%; the highest tertile of telomere length will be used as the reference category.
Power calculations for risk of MBL in relation to pesticide exposures are shown in Table 3 below. As with the MGUS analysis, we calculated the statistical power based on a range of values for the estimated proportion of AHS subjects who have ever been exposed to selected pesticides. We estimated a background probability of MBL of 5.0% based on the prevalence reported by Rawstron et al. (2008). Based on these calculations, we estimate that we will have >80% power to detect an odds ratio of 1.8 or greater if the prevalence of exposure is between 40-60% and an odds ratio of 2.0 or greater if the exposure prevalence is between 20-70%.
Table 3. Statistical power to detect various levels of excess prevalence of MBL by frequency of pesticide exposure (N=1,600)
Pesticide exposure |
Statistical power to detect the indicated MBL odds ratios* |
|||
(exposed vs. unexposed) |
3.0 |
2.5 |
2.0 |
1.8 |
90% exposed vs. 10% unexposed1 |
0.95 |
0.79 |
0.47 |
0.33 |
80% exposed vs. 20% unexposed2 |
>0.99 |
0.98 |
0.77 |
0.60 |
70% exposed vs. 30% unexposed3 |
>0.99 |
>0.99 |
0.89 |
0.74 |
60% exposed vs. 40% unexposed4 |
>0.99 |
>0.99 |
0.93 |
0.81 |
50% exposed vs. 50% unexposed5 |
>0.99 |
>0.99 |
0.95 |
0.83 |
40% exposed vs. 60% unexposed6 |
>0.99 |
>0.99 |
0.94 |
0.83 |
30% exposed vs. 70% unexposed7 |
>0.99 |
>0.99 |
0.91 |
0.78 |
20% exposed vs. 80% unexposed8 |
>0.99 |
0.98 |
0.83 |
0.69 |
10% exposed vs. 90% unexposed9 |
0.96 |
0.87 |
0.64 |
0.50 |
* 2-tail test, alpha=0.05, baseline probability of disease=5.0%
1glyphosate; 22,4-D; 3atrazine; 4none as of Phase III questionnaire; 5alachlor, malathion; 6chlorpyrifos, carbaryl, terbufos; 7carbofuran, pendimethalin; 8metribuzin, fonofos, EPTC, diazinon; 9permethrin, paraquat, coumaphos, chlorothalonil
Following are the estimated power calculations for the pilot study to evaluate hematologic alterations in relation to recent exposure to diazinon (Table 4). With a total of 50 subjects, we will have sufficient power to detect relatively small hematologic alterations comparing pre- and post-exposure measurements.
Table 4. Power calculations for the analysis of hematologic alterations among participants with recent diazinon exposure using serial measurements (i.e., pre- vs. post-exposure measurements)
|
Expected values |
|
Minimum detectable difference in the mean response of matched pairs (pre- vs. post-exposure measurements)1 |
|||||
Measurement |
Mean2 |
Within-person SD3 |
|
N=30 |
|
N=40 |
|
N=50 |
|
|
|
|
|
|
|
|
|
WBC count (per µL blood) |
6480 |
609 |
|
322 |
|
277 |
|
246 |
|
|
|
|
|
|
|
|
|
Lymphocyte count (per µL blood) |
2130 |
262 |
|
139 |
|
119 |
|
106 |
|
|
|
|
|
|
|
|
|
1 Estimated based on a two-sided paired t-test with alpha=0.05 and 80% power
2 Cell count per µL blood, based on observed values among controls in Lan et al. (2004)
3 Estimated based on within-subject coefficients of variation reported in Dot et al. (1992)
Personnel
Study Investigators
Intramural:
Dr. Michael Alavanja PI.
Dr. Laura Beane-Freeman, Co-PI.
Dr. Jonathan Hofmann, Co-PI.
Drs. Neil Caporaso and Ola Landgren, Co-PIs and experts in the MGUS/MM and MBL/CLL.
Dr. Sharon Savage, Co-PI and expert in telomere length aberrations.
Dr. Jay Lubin, project statistician
Ms. Cynthia Hines (NIOSH) and Mr. Kent Thomas (USEPA) are exposure assessors with the AHS.
Dr. Shelia Zahm, senior epidemiologist.
Dr. Dale Sandler is the NIEHS lead investigator on the AHS.
Drs. Stella Koutros and Gabriella Andreotti are post-doctoral fellows with the AHS.
Ms. Carol Christensen (EPA) is a staff epidemiologist the USEPA and a doctoral student at JHU.
Extramural:
Drs. Robert Kyle (Mayo), Jerry Katzmann (Mayo), and Vincent Rajkumar (Mayo) are leading experts in the MGUS/MM field. The Protein Immunology lab at Mayo Clinic has agreed to conduct the MGUS analysis for the minimal cost of the reagents and other minor laboratory costs.
Dr. Andrea Baccarelli (University Milan) has conducted the pilot investigations of telomere length and DNA methylation alterations in the AHS. He would be interested in continuing this work as a collaborator on this project.
Dr. Paolo Ghia, Unit of Lymphoid Malignancies, Department of Oncology, Università Vita-Salute San Raffaele and Istituto Scientifico San Raffaele, Milano, Italy. Dr. Ghia is a leading experts in MBL/CLL and he is personally interested in participating in the proposed AHS project. His laboratory is a high throughput MBL facility.
Human Subjects and Confidentiality
Subjects in this proposed study will be adult male residents of the study areas in the United States. We anticipate that most participants will be non-Hispanic whites, but we will not restrict on the basis of race in this study. Due to the low prevalence of women among the applicators in the AHS cohort (2%), women will be excluded from this study. Children will not be included (the AHS cohort does not include children).
All research personnel will have completed the required education in protection of human research participants and the NIH Computer Security Awareness training course. The interviewer/phlebotomists will explain the informed consent and study procedures to potential participants and obtain signed consent prior to interviewing and collection of biological samples. The subjects will be informed that their medical care will not be affected by their decision with regard to participation in this study.
The risks and benefits of participation also will be explained. Given that this is an observational epidemiologic study, no adverse effects are expected among participants. Data collected as part of the study will include an in-person interview, blood sample, and urine sample. The home interview will take approximately 20 minutes. The physical risks associated with the study are minimal and only include those associated with blood collection. All blood samples will be collected by experienced phlebotomists. During the blood drawing, the subject may feel a little pain or get a bruise at the place on the arm where the blood is drawn; it is possible, but unlikely, that there may be swelling or bleeding.
All study subjects will be compensated for their time and effort ($75 per home visit). The subjects generally will not benefit directly from the results of this study, except for the psychological rewards involved in contributing to medical knowledge which may help other people to reduce their risk of disease. Findings from this study will occasionally be summarized in regular updates that are distributed to AHS cohort members.
For the participants in the Recently Exposed group (N=50), we will be performing CBC/lymphocyte subset assays because we expect that there may be some immune perturbations related to farming exposures that are of possible research interest. However, we anticipate that few, if any, of the participants in this group will have abnormal CBC/lymphocyte subset assay results of any clinical significance. Nonetheless, a clinician on the research team (Dr. Neil Caporaso) will review the CBC/lymphocyte subset results for these participants, and we will send a letter to any participants with abnormal results that may have clinical significance. The language in this letter will be revised as needed depending on the specific assay results that are observed (e.g., if we observe severe anemia or a striking elevation of the white blood cell count, then we would include a stronger statement regarding medical follow-up). Dr. Caporaso will be available by phone to answer any questions that participants may have about the assay results.
With the exception of the CBC/lymphocyte subset assay results for participants in the Recently Exposed group, we will not report individual test results to participants for any of the other assays performed in this study for several reasons. First, these assays will be conducted for research purposes, and will not necessarily be performed under conditions required by the Clinical Laboratory Improvement Amendments (CLIA) for diagnostic tests. Second, the average rate of progression from MGUS to multiple myeloma is 1% per year (Kyle, 2002). Consequently, the vast majority of the individuals who test positive for MGUS in this study will not go on to develop multiple myeloma in their lifetime. It should also be noted that no therapies are available for MGUS or MBL that would prevent progression to multiple myeloma or chronic lymphocytic leukemia, respectively. Moreover, previous studies indicate that there is no added benefit in terms of disease prognosis of early therapy relative to therapy at presentation of symptomatic disease (Ola Landgren, personal communication). Current clinical guidelines do not recommend screening for MGUS or MBL in the population (Ola Landgren, personal communication).
Confidentiality of patient data and electronic records will be maintained at all times. A study number will identify each subject in the database. Subject records and computer files will be stored in securely locked cabinets when not in use. Training sessions and annual signed confidentiality pledges will emphasize and remind research personnel the importance of keeping all data strictly confidential. All blood and urine containers will only be labeled with a study ID number. All statistical analyses and publication of study results will involve grouped data.
Requests from participants who elect to withdraw their data or consent for their specimens from the AHS are honored without question. A request to be withdrawn from the main study by a cohort member who is also part of this Biomarker Study population will result in
removal of the affected data from the Biomarker Study as well.
Time Schedule
Table 3: Schedule for the AHS Biomarker Study commencing in March 2010
Activity |
Time |
Executive Committee Review |
January 7th-February 6th, 2009 |
SAG review |
March 2009 |
TEP Review |
November 2009 |
OMB change request submission |
December 2009 |
Develop study materials |
March through November 2009 |
Submit IRB applications |
December 2009-January 2010 |
Train study personnel |
February-March 2010 |
Begin pilot testing for field work |
March-May 2010 |
Modify study procedures as needed |
June 2010 |
Begin fully developed field work |
July 2010 |
Budget
Annual cost estimates for the AHS Biomarker Study for FY 2009-2014
|
1st year (2009) |
2nd year (2010) |
3rd year (2011) |
4th year (2012) |
5th year (2013) |
6th year (2014) |
Total |
USEPA |
300,000 |
250,000 |
250,000 |
250,000 |
250,000 |
100,000 |
$1,400,000 |
NCI |
0 |
195,708 |
195,708 |
195,708 |
195,708 |
72,293 |
$855,126 |
Total cost estimates of each task for FY 2010-2014
A. Phlebotomy, Shipping and Laboratory Processing
Item |
No. Tubes Collected |
No. Vials Processed |
Unit cost |
Sample size1 |
Cost |
Phlebotomy Kits (per made). |
|
|
$37.36 |
1,700 |
63,512 |
Phlebotomy, urine and blood shipping including remuneration to study subjects. |
|
|
$460 |
1,700 |
782,000 |
Receipt control |
|
|
$60 |
1,700 |
102,000 |
Laboratory supplies |
|
|
|
1,700 |
44,000 |
Lavender top (EDTA) |
1 |
|
$47.52 |
1,700 |
80,784 |
PAXgene tube |
2 |
|
$3.55 |
1,700 |
6,035 |
Red top (serum) |
1 |
|
$47.52 |
1,600 |
76,032 |
Yellow top (ACD) |
2 |
|
$10.45 |
1,700 |
35,530 |
Green top (heparin) |
1 |
|
$47.52 |
1,600 |
76,032 |
Urine kit |
1 |
1 |
$5.00 |
1,700 |
8,500 |
Short term storage at SERACARE |
|
28,000 vials (from aliquots) |
$0.49/vial/yr |
1,700 |
13,720 |
Shipment to repository |
|
|
$1,832/320 persons/yr |
5 yrs |
9,160 |
Receipt control at repository |
|
|
$120.00/year |
5 yrs |
600 |
Long term storage at repository (5 years) |
|
|
$208/month & freezer & racks |
60 months & $7,441 freezer & $1,600 |
21,521 |
Total field & processing |
|
|
|
|
$ 1,319,426 |
1 Samples will be obtained from 1,600 participants; serial samples (3/subject) will be collected from the 50 participants in the “recently exposed” pilot study
B. Study management
Item |
Cost |
Study manager |
84,500 |
--Field work coordinator (IA and NC) |
232,700 |
--Computer programmer |
39,300 |
--Clerks &Technicians |
35,400 |
--Blaise license and materials |
33,500 |
--Hotel, per diem, and mileage reimbursement as necessary |
87,900 |
Total Study management |
$ 513,300 |
C. Proposed analyses and handling costs
Item |
|
Cost |
Shipping cost to Mayo Clinic laboratory |
$300 for one shipment/year X 5 years |
1,500 |
MGUS analysis (NCI shared cost with Mayo Clinic) including quality control runs on 10% of the samples |
|
60,000 |
DNA extraction |
1,600 X $50 |
80,000 |
DNA-telomere length Including quality control runs on 10% of the samples |
1,760 X $10 |
17,600 |
DNA-methylation (shared cost) Including quality control runs on 10% of the samples |
1,760 X $45 |
79,200 |
Total analyses and handling |
|
$238,300 |
D. MBL Pilot study
Item |
|
|
Shipping cost to Dr. Paolo Ghia’s laboratory |
One shipment/year X 5 years |
3,600 |
Leukemia-MBL analysis (NCI shared cost with Dr. Ghia’s laboratory |
$80/sample X 1,600 samples |
128,000 |
Total |
|
$ 131,600 |
E. Hematologic biomarkers (Mayo Medical Laboratories)
Item |
|
Cost |
Fedex & Receipt control |
3 samples/subject for 50 subjects X $70 |
10,500 |
CBC and lymphocyte subset analysis plus 10% QC runs |
$110 X 150 samples |
16,500 |
Total |
|
$ 27,000 |
F. Measurement of urinary pesticide metabolites among Recently Exposed subjects (CDC)
Item |
|
Cost |
Shipping cost to CDC laboratory |
One shipment |
500 |
Measurement of urinary metabolite concentrations |
$500 X 50 samples |
25,000 |
Total |
|
$ 25,500 |
Total: $2,255,126
List of Appendices
Appendix 1: Supporting tables for background
Appendix 2: Detailed background information for CLL, MBL, and other related biomarkers
Appendix 3: Distribution of AHS cohort members in Iowa and North Carolina by county (maps)
Appendix 4: Blood and urine collection, shipment, and processing overview
REFERENCES
Alavanja MC, Ward MH, Reynolds P. Carcinogenicity of agricultural pesticides in adults and children. Journal of Agromedicine 2007;12(1):39-56.
Alavanja MCR, Sandler DP, Lynch CF, Knott C, Lubin JH, Tarone R, Thomas K, Dosemeci M, Barker J, Hoppin JA, Blair A. Cancer Incidence in the Agricultural Health Study. Scand J Work Environ Health 2005;31(S1):39–45.
Alavanja MCR, Dosemeci M, Samanic C, Lubin J, Lynch CF, Knott C, Barker J, Hoppin JA, Sandler DP, Coble J, Thomas K, and Blair A. Pesticides and Lung Cancer Risk in the Agricultural Health Study Cohort. American Journal of Epidemiology 2004;160:876-885.
Alavanja MCR, Samanic C, Dosimeci M, Lubin J, Tarone R, Lynch CF, Knott C, Thomas K, Hoppin JA, Barker J, Coble J, Sandler DP, Blair A. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. American Journal of Epidemiology 2003;157(9):800-814.
Anderson KC. Multiple myeloma: how far have we come? Mayo Clin Proc 2003;78:15-17.
Artandi SE. Telomeres, telomerase, and human disease. N Engl J Med 2006;355:1195-1197.
Barbullushi M, Dioni L, Bonzini M, Pestatori AC, Fustinoni S, Cavallo D, Marinelli B, Schwartz J, Bertazzi PA, Baccarelli A. Accelerated telomere shortening and traffic exposure (submitted).
Beane Freeman LE, Bonner MR, Blair A, Hoppin JA, Sandler DP, Lubin JH, Dosemeci M, Lynch CF, Knott C, and Alavanja MCR. Cancer Incidence among Male Pesticide Applicators in the Agricultural Health Study Cohort Exposed to Diazinon. American Journal of Epidemiology 2005;162(11):1070-1079.
Bollati V, Fabris S, Pegoraro V, Ronchetti D, Mosca L, Deliliers GL, Motta V, Bertazzi PA, Baccarelli A, Neri A. Differential repetitive DNA methylation in multiple myeloma molecular subgroups. Carcinogenesis 2009;30:1330-1335.
Bonner MR, Coble J, Blair A, Beane Freeman LE, Hoppin JA, Sandler DP, and Alavanja MCR. Malathion Exposure and the Incidence of Cancer in the Agricultural Health Study. American Journal of Epidemiology 2007;166(9):1023-1034.
Bonner MR, Lee WJ, Sandler DP, Hoppin JA, Dosemeci M, and Alavanja MCR. Occupational Exposure to Carbofuran and the Incidence of Cancer in the Agricultural Health Study. Environmental Health Perspectives 2005;113(3):285-289.
Botchkina GI, Kim RH, Botchkina IL, Kirschenbaum A, Fricher Z, Adler HL. Noninvasive Detection of Prostate Cancer bt Quantitative Analysis of Telomerase Activity. Clin Cancer Res 2005;11(9):3243-3249.
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47.
Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009;37:e21.
Costa DB, Shin B, Cooper DL. Pneumococcemia as the presenting feature of multiple myeloma. Am J Hematol 2004;77:277-281.
Cottliar A, Pedrazzini E, Corrado C, Engelberger MI, Narbaitz M, Slavutsky I. Telomere shortening in patients with plasma cell disorders. Eur J Haematol 2003;71:334-340.
Curwin BD, Hein MJ, Sanderson WT, Nishioka MG, Reynolds SJ, Ward EM, Alavanja MC. Pesticide contamination inside farm and nonfarm homes. J Occup Environ Hyg 2005;2(7):357-367.
Dagklis A, Fazi C, Scarfo L, et al. Monoclonal B lymphocytosis in the general population. Leuk Lymphoma 2009;50:490-2.
De Roos AJ, Blair A, Rusiecki JA, Hoppin JA, Svec M, Dosemeci M, Sandler DP, and Alavanja MC. Cancer Incidence among Glyphosate-Exposed Pesticide Applicators in the Agricultural Health Study. Environmental Health Perspectives 2005;113(1):49-54.
Dennis LK, Lowe JB, Lynch C, Alavanja M. Cutaneous melanoma and obesity in the Agricultural Health Study. Annals of Epidemiology 2008;18(3):214-221.
de Tute R, Yuille M, Catovsky D, Houlston RS, Hillmen P, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL) in CLL families: substantial increase in relative risk for young adults. Leukemia 2006;20:728-729.
Dot D, Miro J, Fuentes-Arderiu X. Biological variation of the leukocyte differential count quantities. Scand J Clin Lab Invest 1992;52:607-11.
Engel L, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, and Alavanja MC. Pesticide Use and Breast Cancer Risk among Farmers' Wives in the Agricultural Health Study. American Journal of Epidemiology 2005;161:121-135.
Flower KB, Hoppin JA, Lynch CF, Blair A, Knott C, Shore DL, Sandler DP. Cancer risk and parental pesticide application in children of Agricultural Health Study participants. Environmental Health Perspectives 2004;112:631-635.
French Cooperative Group on Chronic Lymphocytic Leukaemia. Natural history of stage A chronic lymphocytic leukaemia untreated patients. Br J Haematol 1990;76:45-57.
Ghia P, Prato G, Scielzo C, Stella S, Geuna M, Guida G, Caligaris-Cappio F. Monoclonal CD5+ and CD5- B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood 2004;103:2337-2342.
Han T, Ozer H, Gavigan M, Gajera R, Minowada J, Bloom ML, Sadamori N, Sandberg AA, Gomez GA, Henderson ES. Benign monoclonal B cell lymphocytosis--a benign variant of CLL: clinical, immunologic, phenotypic, and cytogenetic studies in 20 patients. Blood 1984;64:244-252.
HinesCJ, Deddens JA, Jaycox LB, Andrew RN, Striley CA, Alavanja M. Captan exposure and evaluation of a pesticide exposure algorithm among orchard pesticide applicators in the Agricultural health Study. Ann Occup Hyg 2008;52(3):153-166.
Hou L, Lee WJ, Rusiecki JA, Hoppin JA, Blair A, Bonner MR, Lubin JH, Samanic C, Sandler DP, Dosemeci M, and Alavanja MC. Pendimethalin exposure and cancer incidence among pesticide applicators. Epidemiology 2006;17(3):1-6.
Kang D, Park SK, Beane Freeman L, Lynch CF, Knott CE, Sandler DP, Hoppin JA, Docemeci M, Coble J, Lubin J, Blair A. Cancer incidence among pesticide applicators exposed to trifluralin in the Agricultural Health Study. Environmental Research 2008;107(2):271-276.
Koutros S, Cross AJ, Sandler DP, Hoppin JA, Ma X, Zheng T, Alavanja MC, Sinha R. Meat and meat mutagens and risk of prostate cancer in the agricultural health study. Cancer Epidemiol Biomarkers Prev 2008;17(1):80-87.
Koutros S, Mahajan R, Zheng T, Hoppin JA, Ma X, Lynch CF, Blair A, Alavanja MC. Dichlorvos exposure and human cancer risk: results from the Agricultural Health Study. Cancer Causes Control 2008;19(1):59-65.
Kyle RA, Therneau TM, Rajkumar V, Larson DR, Plevak MF, Offord JR, Dispenziere A, Katzmann JA, Melton LJ. Prevalence of Monoclonal Gammopathy of Undetermined Significance. New England Journal of Medicine 2006;354:1362-1369.
Lan Q, Zhang L, Li G, et al. Hematotoxicity in workers exposed to low levels of benzene. Science 2004;306:1774-6.
Landgren O, Kyle RA, Hoppin JA, Beane Freeman LE, Cerhan JR, Katzmann JA, Rajkumar SV, Alavanja MC. Pesticide exposure and the risk of monoclonal gammopathy of undertermined significance in the Agricultural Health Study. Blood 2009;113(25)6386-6391.
Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV. Monoclonal gammopathy of undertermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 2009;113(22):5412-5417.
Landgren O, Albitar M, Ma W, Abbasi F, Hayes RB, Marti GE, Caporaso NE. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med 2009;360(7):659-667.
Landgren O, Kyle RA. Multiple myeloma, chronic lymphocytic leukaemia and associated precursor diseases. British Journal of Haematology 2007;139:717-723.
Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR. Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: A population-based case-control study. Int J Cancer 2006;118:3095-3098.
Lee WJ, Sandler DP, Blair A, Samanic C, Cross AJ, and Alavanja MCR. Pesticide use and colorectal cancer risk in the Agricultural Health Study. Int J Cancer 2007;121:339-346.
Lee WJ, Blair A, Hoppin JA, Lubin JH, Rusiecki JA, Sandler DP, Dosemeci M, Alavanja MC. Cancer incidence among pesticide applicators exposed to chlorpyrifos in the Agricultural Health Study. Journal of the National Cancer Institute 2004;96(23):1781-1789.
Lee WJ, Hoppin JA, Blair A, Lubin JH, Dosemeci M, Sandler DP, Alavanja MCR. Cancer incidence among pesticide applicators exposed to alachlor in the Agricultural Health Study. American Journal of Epidemiology 2004;159:373-380.
Lynch SM, Rusiecki JA, Blair A, Dosemeci M, Lubin J, Sandler DP, Hoppin JA, Lynch CF, Alavanja MCR. Cancer incidence among pesticide applicators exposed to cyanazine in the Agricultural Health Study. Environmental Health Perspectives 2006;114(8):1248-1252.
Mahajan R, Blair A, Coble J, Lynch CF, Hoppin JA, Sandler DP, Alavanja MC. Carbaryl exposure and incident cancer in the Agricultural Health Study. Int J Cancer 2007;121(8):1799-1805.
Mahajan R, Blair A, Lynch CF, Schroeder P, Hoppin JA, Sandler DP, Alavanja MCR. Fonofos exposure and cancer incidence in the Agricultural Health Study. Environmental Health Perspectives 2006;114:1838-1842.
Mahajan R, Bonner MR, Hoppin JA, Alavanja MCR. Phorate exposure and incidence of cancer in the Agricultural Health Study. Environmental Health Perspectives 2006;114(8):1205-1209.
Marti GE, Faguet GB, Stewart C, Branham P, Carter PH, Washington GC, Bertin P, Muller J, Zenger V, Caporaso N, et al. Evolution of leukemic heterogeneity of human B-CLL lymphocytes between and within patients. Curr Top Microbiol Immunol 1992;182:303-311.
Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, Schleinitz TA, Caporaso N; International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol 2005;130:325-332.
Marti GE, Carter P, Abbasi F, Washington GC, Jain N, Zenger VE, Ishibe N, Goldin L, Fontaine L, Weissman N, Sgambati M, Fauget G, Bertin P, Vogt RF Jr, Slade B, Noguchi PD, Stetler-Stevenson MA, Caporaso N. B-cell monoclonal lymphocytosis and B-cell abnormalities in the setting of familial B-cell chronic lymphocytic leukemia . Cytometry B Clin Cytom 2003;52:1-12.
Meid FH, Gygi CM, Leisinger HJ, Bosman FT, Benchattar J. The use of telomerase activity for the detection of prostate cancer cells after prostatic massage. J Urol 2001;165:1802-1805.
Montserrat E, Vinolas N, Reverter JC, Rozman C. Natural history of chronic lymphocytic leukemia: on the progression and progression and prognosis of early clinical stages. Nouv Rev Fr Hematol 1988;30:359-361.
Noort D, van Zuylen A, Fidder A , van Ommen B, Hulst AG. Protein Adduct Formation by Glucuronide Metabolites of Permethrin. Chem Res Toxicol 2008;21:1396–1406.
Perrota C, Staines A, Cocco P. Multiple Myeloma and Farming. A Systematic review of 30 years of research. Where next? J Occup Med Toxicol 2008;3(1):27.
Purdue MP, Hoppin JA, Blair A, Dosemeci M, Alavanja MC. Occupational exposure to organochlorine insecticides and cancer incidence in the Agricultural Health Study. International Journal of Cancer 2007;120(3):642-649.
Rawstron AC, Green MJ, Kuzmicki A, Kennedy B, Fenton JA, Evans PA, O'Connor SJ, Richards SJ, Morgan GJ, Jack AS, Hillmen P. Monoclonal B lymphocytes with the characteristics of "indolent" chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood 2002;100:635-639.
Rawstron AC. Prevalence and characteristics of monoclonal B-cell lymphocytosis (MBL) in healthy individuals and the relationship with clinical disease. J Biol Regul Homeost Agents 2004;18:155-160.
Rawstron AC, Jones RA, Ferguson C, Hughes G, Selby P, Reid C, Dalal S, Howard M, Smith G, Hillmen P, Owen RG, Jack AS. Outreach service for monitoring patients with indolent B cell and plasma cell disorders. Leuk Lymphoma 2007;48:S88-89.
Rawstron AC, Bennett FL, O'Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008;359:575-83.
Rusiecki JA, De Roos A, Lee WJ, Dosemeci M, Lubin JH, Hoppin JA, Blair A, Alavanja MCR. Cancer incidence among pesticide applicators exposed to Atrazine in the Agricultural Health Study. Journal of the National Cancer Institute 2004;96(18):1375-1382.
Rusiecki JA, Hou L, Lee WJ, Blair A, Dosemeci M, Lubin JH, Bonner MR, Samanic C, Hoppin JA, Sandler DP, and Alavanja MC. Cancer incidence among pesticide applicators exposed to metolachlor in the Agricultural Health Study. International Journal of Cancer 2006;118(12):3118-3123.
Rusiecki JA, Blair A, Dosemeci M, Lubin JH, Bonner MR, Samanic C, Hoppin JA, Sandler DP, and Alavanja MC. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environmental Health Perspectives 2009:117(4):581-586.
Samanic C, Rusiecki JA, Dosemeci M, Hou L, Hoppin JA, Sandler DP, Lubin J, Blair A, Alavanja MCR. Cancer incidence among applicators exposed to dicamba in the Agricultual Health Study. Environmental Health Perspectives 2006;114:1521-1526.
Shammas MA, Shmookler Reis RJ, Akiyama M, Koley H, Chauhan D, Hideshima T, Goyal RK, Hurley LH, Anderson KC, Munshi NC. Telomerase inhibition and cell growth arrest by G-quadruplex interactive agent in multiple myeloma. Mol Cancer Ther 2003;2:825-833.
Shim YK, Vogt RF, Middleton D, Abbasi F, Slade B, Lee KY, Marti GE. Prevalence and natural history of monoclonal and polyclonal B-cell lymphocytosis in a residential adult population. Cytometry B Clin Cytom 2007;72(5)344-353.
Van Bemmel D, Beane-Freeman L, Lubin JH, Hoppin JA, Sandler DP, and Alavanja MC. S-ethyl-N,N-dipropylthiocarbamate exposure and cancer incidence among male pesticide applicators in the agricultural health study: a prospective cohort. Environmental Health Perspectives 2008;116(11):1541-1546.
Vogt RF, Meredith MNK, Powell J et al. Results in eleven individuals with B-CLL-like phenotypes detected in environmental health studies. In: Marti GE, Vogt RF, Zenger VE, eds. Proceedings of a USPHS Workshop on Laboratory Approaches to Deternining the Role of Environmental Exposures as Risk Factors for B-Cell Chronic Lymphocytic leukemia and Other B-cell Lymphoproliferative Disorders. Atlanta, GA: 1995;19-35.
Wang Z, Ramin SA, Tsai C, Lui P, Herbert PJ, Kyeyune-Nyombi E, Ruckle HC, Beltz RE, Sands JF. Detection of telomerase activity in prostate fluid specimens. Urol Oncol 2000;6:4-9.
Wayne G, Carter WG, Tarhoni M, Rathbone AJ, Ray DE. Differential protein adduction by seven organophosphorus pesticides in both brain and thymus. Human & Experimental Toxicology 2007;26:347-354.
Wu KD, Orme LM, Shaughnessy J Jr, Jacobson J, Barlogie B, Moore MA. Telomerase and telomere length in multiple myeloma: correlations with disease heterogeneity, cytogenetic status, and overall survival. Blood 2003;101:4982-4989.
Wu KD, Moore MA. Determination of telomerase activity and telomere length. Methods Mol Med 2005;113:207-223.
Yang AS, Estecio MR, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 2004;32:e38.
| File Type | application/msword |
| Author | Administrator |
| Last Modified By | Hofmann, Jonathan (NIH/NCI) |
| File Modified | 2010-04-21 |
| File Created | 2010-02-25 |
© 2026 OMB.report | Privacy Policy