CMS-10298 Supporting Statement A
CMS-10298 Supporting Statement A.docx
Data Collection for Developing Outpatient Therapy Payment Alternatives (DOTPA)
OMB: 0938-1096
The Centers for Medicare & Medicaid Services' Office of Research, Development, and Information (ORDI) strives to make information available to all. Nevertheless, portions of our files including charts, tables, and graphics may be difficult to read using assistive technology.
Persons with disabilities experiencing problems accessing portions of any file should contact ORDI through e-mail at [email protected].
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
CENTERS FOR MEDICARE AND MEDICAID SERVICES
DATA COLLECTION FOR DEVELOPING OUTPATIENT THERAPY PAYMENT ALTERNATIVES
OFFICE OF MANAGEMENT AND BUDGET
CLEARANCE PACKAGE SUPPORTING STATEMENT
February 19, 2010
Page
A. Background 1
A.1 Purpose 1
A.2 General Background 1
B. Justification 2
B.1 Need and Legal Basis 2
B.2 Information Users 3
B.3 Improved Technology and Burden Reduction 3
B.4 Efforts to Identify Duplication of Similar Information 3
B.5 Impact on Small Businesses or Other Small Entities 3
B.6 Consequences of Collecting the Information Less Frequently 3
B.7 Special Circumstances 4
B.8 Federal Register Notice 4
B.9 Explanation of Any Payment or Gift to Respondents 5
B.10 Confidentiality 5
B.11 Sensitive Questions 5
B.12 Respondent Burden 6
B.13 Annualized Cost for Respondents (Non-hour burden) 7
B.14 Annualized Cost to the Federal Government 7
B.15 Program Changes 7
B.16 Publication and Tabulation Schedule 7
B.17 Expiration Date 7
B.18 Certification Statement 7
C. Collection of Information Employing Statistical Methods 7
C.1 Respondent Universe and Response Rates 7
C.2 Procedures for the Collection of Information 8
Statistical Methodology for Stratification and Sample Selection 8
Statistical Power and Target Number of Outpatient Therapy Episodes 8
Survey Eligibility 11
C.3 Maximizing Response Rates 11
C.4 Contacts 11
References 12
LIST OF TABLES
Page
Table B-1 Individuals Outside the U.S. Department of Health and Human Services Consulted on This Data Collection 5
Table B-2 Estimated Response Burden 6
APPENDICES
Appendix A: Data Collection Instruments
A. Background
A.1 Purpose
The Centers for Medicare & Medicaid Services (CMS), a division of the U.S. Department of Health & Human Services (DHHS), seek approval for data collection for a project entitled Developing Outpatient Therapy Payment Alternatives. The purposes of this project are to identify, collect, and analyze therapy-related information tied to beneficiary need and the effectiveness of outpatient therapy services that is currently unavailable to CMS. The ultimate goal is to develop payment method alternatives to the current financial cap on Medicare outpatient therapy services.
Appendix A contains a copy of the data collection instruments.
A.2 General Background
CMS has awarded a five year contract to RTI International (RTI) to collect data, conduct analyses, and report the results. To complete the contract, RTI will: (1) develop a data collection strategy, including the recruitment of therapy providers to participate in data collection, (2) analyze the resulting data to identify payment alternatives to therapy caps, and (3) engage with the stakeholder community closely throughout the project.
Outpatient therapy services are furnished in such diverse settings as hospital outpatient facilities, nursing facilities (SNF), comprehensive outpatient rehabilitation facilities (CORF), and outpatient rehabilitation facilities (ORF). In addition, outpatient therapy services may be furnished by individual practitioners including physical therapists in private practice (PTPP), occupational therapists in private practice (OTPP), speech-language pathologists in private practice (SLPP), physicians, and specified non-physician practitioners (NPP) as permissible by state law. In order to compare beneficiary needs across these settings, it is important to collect these data using similar items across settings and disciplines. Although there currently exist assessment instruments for outpatient therapy, the item definitions and rating scales differ across these instruments and these instruments have not been tested in the full range of Medicare patients and therapy settings. These differences in the existing assessment instruments make it difficult to compare patient function and clinical characteristics across them.
The four data collection instruments for this study balance collecting an identical set of data on all patients against creating sets of items specific to each setting that would cover only the variation in patient case mix in that setting. The need for separate data collection instruments is driven by budget constraints that impose a contractually required paper-based data collection. Ideally, there would be a single data collection instrument where items are completed only for a patient when relevant. The range of case mix attributes of patients receiving outpatient therapy is wide; items relevant for some patient populations may exhibit ceiling or floor effects for others. Were data collection electronic, as in the Post-Acute Care Payment Reform Demonstration, a single data collection instrument featuring complex skip patterns would be feasible. However, to avoid complex skip patterns in a paper-based tool, the complete set of assessment items has been divided into two sets: admission and discharge instruments for community-based settings (CARE-C), and admission and discharge instruments for patients in day rehabilitation programs and residents of nursing facilities (CARE-F). Although named “community” and “facility” these instruments differ to assess, without ceiling or floor effects, the expected medical, functional, and cognitive conditions of the populations that typically present in these settings. Thus beneficiaries in day rehabilitation programs will be assessed using the CARE-F instrument because of the greater medical, functional, and cognitive impairments of these patients although they reside in the community rather than a facility. The instruments are attached in Appendix A.
The assessment instruments include items measuring case mix at “admission” (the beginning of an episode of therapy) and explaining expected resource use and outcomes, given the individual characteristics of the patient. The tool collects information related to:
Administrative status, such as provider, beneficiary, and payer characteristics;
Admission, including prior history and functioning;
Current medical items such as diagnoses and comorbid conditions;
Functional assessment, including basic mobility, daily activities, and cognitive function;
Screens for particular conditions with supplemental items to provide more refined information about specific limitations and impairments; and
Discharge, including change in status and caregiver information.
The data collection instruments include items from several sources: (1) administrative items and items related to measuring case mix and outcomes from the CARE tool; (2) assessment items from the Activity Measure for Post Acute Care (AM-PAC) Adaptive Short Form item pool; (3) assessment items derived from selected communication and cognition function items from the National Outcomes Measurement System (NOMS) instrument; and (4) additional items developed with assistance from clinical and measurement experts. Key stakeholder groups were consulted during the data collection instrument development process through a Technical Expert Panel, an Open Door Forum, regular conference calls with a panel of nominated experts from the three principal therapy professional organizations, and ad hoc discussions.
B. Justification
B.1 Need and Legal Basis
In Section 545 of the Benefits Improvement and Protection Act (BIPA) of 2000 (Pub. L. 106-554), the Congress required the Secretary of the Department of Health and Human Services to report on the development of standardized assessment instruments for outpatient therapy. As part of the response to this requirement, CMS envisions a new method of paying for outpatient therapy services that is based on classifying individual beneficiary’s needs and the effectiveness of therapy services, e.g., diagnostic category, functional status, health status. Currently, CMS cannot evaluate or implement this type of approach because CMS does not currently collect the appropriate data elements.
B.2 Information Users
The data collected using the project assessment tool will be used by CMS to assess alternative payment methods for outpatient therapy provided to Medicare beneficiaries. CMS will use the data to characterize patient severity of illness and level of function and to examine the degree to which items can be used to predict beneficiary resource use and beneficiary outcomes.
B.3 Improved Technology and Burden Reduction
The project assessment tool will be fielded as a hard copy form for maximum flexibility within each clinical setting. As a result, no electronic data collection system needs to be developed. The collected forms will be scanned into a secure server and processed into a relational database using Cardiff TeleForm form processing software.
B.4 Efforts to Identify Duplication of Similar Information
This information collection does not duplicate any other effort and the information cannot be obtained from any other source. Over 75 instruments exist to measure functional status for patients receiving therapy services, with four instruments receiving the most support in the provider community. However, it is necessary to develop a new tool for this study because each of these four leading instruments has sufficient shortcomings for some subset of patients receiving outpatient therapy.
B.5 Impact on Small Businesses or Other Small Entities
Providers participating in the project data collection will potentially include small physician and therapist private practices, outpatient rehabilitation facilities, and skilled nursing facilities as well as larger hospital outpatient departments and other post-acute providers. Provider participation in the demonstration is voluntary. Providers viewing the data collection as a burden due to organizational size can refuse to participate. During the development phase of the data collection instruments, consideration was made at every stage to minimize burden on small outpatient therapy clinics. Owners and other clinicians working in small outpatient therapy providers were included in the Technical Expert Panel advising on the content of the data collection instruments.
B.6 Consequences of Collecting the Information Less Frequently
This is a one-time data collection effort restricted to two years during the five-year study period of the Developing Outpatient Therapy Payment Alternatives project. Data collection will be limited to providers volunteering to participate in the demonstration. Each provider will not be requested to collect data for the entire two years, only for six months or until the target number of responses is reached whichever comes first. Our estimates suggest most providers will need about four months to collect the target number of responses. Less frequent data collection could jeopardize the generality or validity of the analyses.
B.7 Special Circumstances
The project assessment tool will be administered on all Medicare patients at admission and discharge from participating providers. Each provider will collect these data over a period lasting up to six months. Data coordinators at participating providers will administer the data collection, including assigning each patient a unique study ID number; provide the tool to the patient, proxy, or clinician as appropriate; and submit completed tools to the research contractor, RTI. Frequency of data reporting will depend on the volume of Medicare admissions and discharges at participating providers.
B.8 Federal Register Notice
The 60-day Federal Register notice was published on October 9, 2009, 12 comments were received.
Key stakeholder groups were consulted during the data collection instrument development process. These consultations included: (1) convening a Technical Expert Panel composed of external clinical and measurement experts; (2) holding an Open Door Forum to engage interested members of the public, provide information about the project, and seek input on the data collection; (3) nominees of the three principal therapy professional organizations in a consultative panel that met regularly to guide the data collection instrument development process; and (4) ad hoc discussions with key stakeholder organizations, including conference calls, in-person meetings, and presentations at annual member meetings. During these extensive consultations, we asked outside experts and stakeholders, particularly participants in the Technical Expert and consultative panels, to address the following data collection issues:
The specific content and format of the data collection instruments;
The ability of clinicians to provide the requested assessment data;
The frequency of patient assessment that will be asked of participants.
Individuals outside the United States Department of Health and Human Services who have been consulted about the data collection instruments include those shown in Table B-1.
Table B-1
Individuals Outside the U.S. Department of Health
and Human Services Consulted on This Data Collection
Project Staff and Consultants |
Technical Expert Panelists |
RTI and Subcontractor Staff Edward M. Drozd, Ph.D. (RTI International) Barbara J. Gage, Ph.D. (RTI International) Shulamit Bernard, Ph.D., RN (RTI International) Roberta Constantine, Ph.D., RN (RTI International) Alan M. Jette, Ph.D., PT (Boston University) Trudy R. Mallinson, Ph.D., OTR/L (Rehabilitation Institute of Chicago) Project Consultants Gerben DeJong, Ph.D. (National Rehabilitation Hospital) Dennis L. Hart, Ph.D., PT (FOTO, Inc.) Margaret Stineman, MD (University of Pennsylvania) |
Janet Brown, CCC-SLP (American Speech-Language-Hearing Association) Susan Coppola, MS, OTR/L, BCG, FAOTA (University of North Carolina at Chapel Hill) Edelle C. Field-Fote, Ph.D., PT (University of Miami) Bruce Gans, MD (Kessler Institute for Rehabilitation) Kathleen Gleason (MossRehab) Noreen Giovannone, MPT (Shore Rehabilitation Institute) Michael P. Johnson, PT, Ph.D., OCS (Mercy Rehab Associates) James Kelley, PT (Uniform Data Systems for Medical Rehabilitation) Carole Bernstein Lewis, Ph.D., DPT, FAPTA, GCS (Professional Sportscare and Rehabilitation) Nancy Richman, OTR/L, FAOTA (Glantz/Richman Rehabilitation Associates) Margaret A. Rogers, Ph.D. (American Speech-Language-Hearing Association) Elizabeth A. Skidmore, Ph.D., OTR/L (University of Pittsburgh) Jamie Stark, Ph.D., CSCS (Select Medical Corporation) Mary Van de Kamp, MS, CCC-SLP (Peoplefirst Rehabilitation) |
B.9 Explanation of Any Payment or Gift to Respondents
At this time, for budgetary reasons, CMS cannot provide any stipends for data collection.
B.10 Confidentiality
The data collected in the project assessment tool will be kept confidential by RTI and CMS. Only authorized staff at participating providers will record and transmit the completed instruments to RTI. Only project staff at CMS and RTI will have access to respondents’ data. Paper forms mailed to RTI from providers will be stored in locked areas, and electronic data created from those forms will be stored in a secure format, meeting all federal privacy guidelines. To protect beneficiary confidentiality, the subject’s name will not be linked to his/her individual data. For identification purposes, a unique project ID number will be assigned to each sample member.
All patient-level data will be protected from public dissemination in accordance with the Privacy Act of 1974, as amended. The information collected will be protected and held confidential in accordance with 20 CFR 401.3. Data will be treated in a confidential manner, unless otherwise compelled by law.
B.11 Sensitive Questions
The information collected in the project assessment tool is considered to be confidential personal health information. This individual level data is considered sensitive and all necessary protections will be employed to keep the data secure and confidential. Though this information is considered to be personal health information, similar information is currently collected in other CMS instruments. The items on the project assessment tool are being collected for the purposes of providing alternative payment systems for CMS.
B.12 Respondent Burden
CMS estimates the average time to complete the data collection instruments to be 15 minutes for the version of the instrument used in community-based settings and 30 minutes for the version used in nursing facilities and day rehabilitation programs. This estimate is based on internal RTI and external expert experience with similar assessment instruments and with extensive data collection experience using a comparable instrument, the CARE tool in the Post Acute Care Payment Reform Demonstration. In addition, CMS estimates the time necessary for recordkeeping to be 5 minutes per assessment, so that total burden for nursing facilities and day rehabilitation programs is estimated to be 35 minutes (0.58 hours) and for other providers to be 20 minutes (0.33 hours). Thus, as shown in Table B-2 below, the total estimated time burden is the provider- specific time per response multiplied by number of assessments summed across all provider types, or 14,271 hours. The increase in burden relative to the 60-day notice is due to a change in instruments for day rehabilitation programs made in response to commenter’s concerns that the CARE-C instrument did not assess properly the medical and functional conditions of complex patients.
Table B-2
Estimated Response Burden
Provider type |
Target number of participating providers |
Number of assessments |
Estimated time to complete (hours) |
Estimated time burden |
Hospital outpatient departments |
|
|
|
|
Non Day rehabilitation program |
22 |
7,700 |
0.33 |
2,541 |
Day rehabilitation program |
7 |
2,450 |
0.58 |
1,421 |
Skilled nursing facilities |
45 |
3,638 |
0.58 |
2,110 |
CORFs/ORFs/HHAs |
29 |
10,150 |
0.33 |
3,350 |
Private practices (PT only) |
29 |
3,046 |
0.33 |
1,005 |
Private practices (OT only or PT & OT) |
29 |
6,766 |
0.33 |
2,233 |
Private practices (any SLP) |
29 |
4,882 |
0.33 |
1,611 |
Totals |
190 |
38,632 |
- |
NOTE: The number of assessments assumes each patient receives an admission assessment, that most patients receive a discharge assessment (assuming approximately 15 percent of patients will not receive a discharge because of not attending their last scheduled visit), and that some patients (approximately 15 percent) receive an additional assessment as they reach the therapy cap but are allowed an exception. The row titled, “Totals” gives the sum of the rows above (except for estimated time to complete). Burden estimates assume 7 of the hospital outpatient departments are day rehabilitation programs using the CARE-F instrument, and 22 use the CARE-C instrument.
B.13 Annualized Cost for Respondents (Non-hour burden)
There are no fixed or variable non-hour costs incurred by respondents for participating in the survey. Respondents in the data collection will be given a toll-free telephone number and Web site address to contact an RTI-staffed help desk if they have questions about the study. Respondents will be provided shipping labels to use when returning their completed data collection instruments and so will not incur costs for postage.
B.14 Annualized Cost to the Federal Government
This 18 month data collection process is to occur once for research purposes. The total cost for this data collection is $841,194 over the contract period of performance. There is no additional cost burden to the federal government beyond what has been contractually allocated for the conduct of the study.
B.15 Program Changes
There are no program changes or adjustments.
B.16 Publication and Tabulation Schedule
There are no publications and tabulations associated with this collection.
B.17 Expiration Date
CMS will display the date for OMB approval on the data collection instruments.
B.18 Certification Statement
There are no exceptions to this certification statement.
C. Collection of Information Employing Statistical Methods
C.1 Respondent Universe and Response Rates
The target population for this project is fee-for-service Medicare beneficiaries receiving Part B-covered (“outpatient”) therapy services in hospital outpatient departments (HOPDs), nursing facilities, outpatient rehabilitation facilities (ORFs), comprehensive outpatient rehabilitation facilities (CORFs), home health agencies (HHAs), and by clinicians in private practices. Only patients of providers participating in this study will have assessment data provided by or for them. RTI and CMS estimate that approximately 38,632 assessments will be collected from 19,316 Medicare beneficiaries treated by up to 190 participating providers recruited from across the United States. Each provider will participate for at least four months in the data collection process, though not all simultaneously (Table B-2).
C.2 Procedures for the Collection of Information
Statistical Methodology for Stratification and Sample Selection
The sample design for this study is a stratified clustered design with the following hierarchy of design elements. We will first assemble a set of providers engaged in outpatient therapy using Medicare claims data. Claims will be aggregated by provider ID (using NPIs as well as legacy OSCAR and UPIN identifiers). Claims will also be used to indicate which providers have higher Medicare volume, so that these providers can be targeted for sampling (for greater data collection efficiency), instead of only using provider program participation files.
The next step in the sampling process is stratifying providers by the six provider type strata mentioned above, defined using the provider program participation data. We will use the volume information from claims data to identify the providers within each stratum with Medicare volumes above the median—however, if this criterion results in excluding a disproportionate number of providers in low density population areas, we will adjust the volume threshold. We will develop a sample of these providers and begin the recruitment process.
During the recruitment process we will determine, through discussion with leadership in the sampled organizations, the relevant organizational characteristics of each provider, for example the patient case mix, service specialization, and divisions into units or offices. Stratifying by categories of one or more of these organization characteristics will produce very small cell sizes, potentially unduly increasing statistical error. However, during recruitment we will attempt to balance the sample in these and other (e.g., geography) characteristics to produce a nationally representative sample.
Within each stratum, our sample of patient-episodes1 is not a simple random sample. The reason is clear. It would be too costly and impractical to select a simple random sample of patient-episodes because of data collection logistics—providers must be recruited. As a result, the patient-episodes within each stratum are clustered into 190 primary sampling units based on the 190 proposed facilities/practices. Since patients may have multiple episodes with a particular provider, the sample of episodes can be viewed as clustered by patient; however, we believe the principal source of sampling error to be provider-based clustering.
Statistical Power and Target Number of Outpatient Therapy Episodes
Power gives the probability that a true, real difference from zero (or other specific reference value) will be identified as a significant difference. Because of random variation, a measured difference may in fact be zero, or close to it, despite a true nonzero difference. A high-powered test of a case mix coefficient in a payment model is less sensitive to this random error and is more likely to identify a true nonzero difference of that case mix coefficient from the population average.
Our estimated total sample size is driven by the need for tests of case mix group coefficients in regression models of outpatient therapy episode payments to be reasonably powerful to identify meaningful differences from the average payment for reasonably-sized groups as statistically significant. Conditional on the difference from the mean, or “effect size,” of the case mix group (which determines the case mix weight) and the underlying program cost homogeneity of that group, the power of the test of the case mix weight from 1.0 will be determined by the proportion of patients in that group. Alternatively, conditional on the proportion of patients in that group and the underlying program cost homogeneity of that group, the power of the test of the case mix weight from 1.0 will be determined by the effect size of the case mix group.
Power analysis for a regression model is based on an F-test of coefficient estimates (Taylor and Muller, 1995). For this power analysis, we consider the special case of a test of a single regression coefficient (i.e. for a single case-mix group adjustor in a model of log episode payment). The power of the F-test of a coefficient estimate is given by
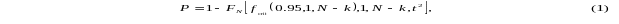
where FN
is the cumulative distribution function of the non-central F
distribution evaluated at
 ,
the critical F value for the test; with 1 numerator degree of
freedom, corresponding to the single coefficient being tested, and
,
the critical F value for the test; with 1 numerator degree of
freedom, corresponding to the single coefficient being tested, and
 denominator degrees of freedom, corresponding to the number of
degrees of freedom in the model; and a non-centrality parameter
denominator degrees of freedom, corresponding to the number of
degrees of freedom in the model; and a non-centrality parameter
 equal to the value of the F test statistic. N
represents the sample size, and k is the number of regressors
in the model.
equal to the value of the F test statistic. N
represents the sample size, and k is the number of regressors
in the model.
When the only restriction tested is for a single
coefficient, the F statistic can be simplified to the square
of the t statistic for the regression coefficient (Greene,
1993). However, because of the clustered sample in this study, an
adjustment must be made to coefficient standard errors to account
for the design effect D; the adjusted standard error will be
equal to the standard error, assuming simple random sampling,
multiplied by the square root of the design effect. Also, it will be
useful to express a case mix group coefficient b as a
percentage p of mean expenditures
 ,
so that
,
so that
 .
.
As a result, the
F statistic
 in equation (1) can be expressed as:
in equation (1) can be expressed as:
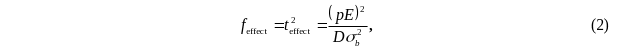
where b is the
estimated demonstration effect regression coefficient, and
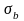 is the standard error of the estimate. Substituting (2) into (1),
the power of the test is given by
is the standard error of the estimate. Substituting (2) into (1),
the power of the test is given by
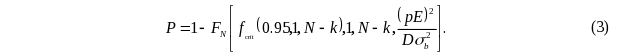
Using equation (3), to estimate the total episode
sample size N to achieve a desired power of 80 percent of
tests of case mix coefficients, it is necessary to provide several
inputs into that equation. For the number of regression
coefficients, we assume k will equal 100 to approximate the
models included in the Ciolek and Hwang (2004) episode-based payment
model report and to provide a conservative (high) estimate for the
number of regressors in the models estimated for this study.
Consistent with power estimates underlying the sample size estimates
for the Post-Acute Care Payment Reform Demonstration, we assume a
design effect of 3 for this study. This design effect estimate is
based on average design effects encountered in the Psychiatric
Inpatient Routine Cost Analysis project (Cromwell, et al., 2003),
which collected primary assessment and resource use data on 838
Medicare patients in 40 inpatient psychiatric facilities. Estimates
of effect sizes (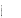 )
and standard errors
)
and standard errors
 are based on selected diagnosis groups in the Ciolek and Hwang
(2004) model report. However, we assume the regression model will
reduce standard errors by 17 percent, as in the log episode payment
models estimated by Ciolek and Hwang (2004). In addition, based on
findings from the Cromwell, et al. (2003) study, we assume
regression
are based on selected diagnosis groups in the Ciolek and Hwang
(2004) model report. However, we assume the regression model will
reduce standard errors by 17 percent, as in the log episode payment
models estimated by Ciolek and Hwang (2004). In addition, based on
findings from the Cromwell, et al. (2003) study, we assume
regression
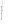 values will be further improved by 25 percent from using assessment
characteristics not currently included in administrative data versus
a completely claims-based model. Based on these assumptions, we
estimate that there will be sufficient power (80 percent) to
identify case mix groups equal to 15 percent of the total outpatient
therapy population with a 10 percent effect size, or case mix groups
equal to three percent of the population with a 20 percent effect
size, with a sample of 19,316 outpatient therapy episodes.
values will be further improved by 25 percent from using assessment
characteristics not currently included in administrative data versus
a completely claims-based model. Based on these assumptions, we
estimate that there will be sufficient power (80 percent) to
identify case mix groups equal to 15 percent of the total outpatient
therapy population with a 10 percent effect size, or case mix groups
equal to three percent of the population with a 20 percent effect
size, with a sample of 19,316 outpatient therapy episodes.
We estimate that 84.1 percent of episodes will have a PT component, 26.6 percent will have an OT component, and 15.9 percent will have an SLP component (these percentages sum to over 100 percent because some episodes have clinicians of multiple disciplines contributing to the patient’s care). This represents a 50 percent oversampling of SLP services and 20 percent for OT services (and a 10 percent under sampling of PT services). This deviation of our expected sample from the national distribution of outpatient therapy services will improve statistical power and accuracy for OT and SLP services, which are smaller proportions of all outpatient therapy services than are PT services. Sample weights will be computed for computing nationally representative estimates of utilization and cost of outpatient therapy services across disciplines and settings.
Assuming a data collection period of four to six months (averaging five months, with a length of data collection time based on a provider’s volume), we calculate the total number of providers required to achieve 19,316 episodes to be 190. This assumption is based on the average number of episodes per month for providers with above-median volume, but capped at 35 per month assuming that is the maximum number of assessments per month providers will be willing to collect (based on experience in the Post Acute Care Payment Reform Demonstration). This volume count can be achieved with: 29 hospital outpatient departments; 45 skilled nursing facilities; 29 CORFs, ORFs, and home health agencies; and 29 each of practices that are PT-only, PT plus OT, and any with SLPs. Furthermore, to capture more complex patients in day rehabilitation programs, 7 of the 29 targeted hospital outpatient departments will be day rehabilitation programs, identified during recruitment.
During the data collection period, we will request that an admission and discharge assessment be submitted for each Medicare beneficiary beginning an episode of care during the data collection window. Participating providers will be asked to complete the admission assessment within the first two visits of an episode and attempt to complete a discharge assessment within the last two expected visits. Episodes beginning near the end of the four-month data collection period will be included, and we will ask that providers submit discharge assessments for these beneficiaries if it occurs after that data collection period is over.
Survey Eligibility
Individual and institutional providers providing outpatient therapy services will be eligible for this data collection. The relevant patient population is all patients receiving outpatient therapy during the data collection period.
C.3 Maximizing Response Rates
The strategy for provider recruitment needs to consider several competing objectives, including practice setting, provider and geographic variation. Unlike the development of the CARE tool, we are not wedded to markets and as long as we can conduct regional training we can accommodate rural providers in the study. We do need to make sure that we include providers from different practice types, and that the data collection is not overly burdensome so that small provider practices can participate.
We will make every attempt to obtain geographic variation and will map the locations of proposed practices for CMS’ consideration and to illustrate the geographic location of proposed provider sites. In considering provider participation we will use the strata illustrated in Exhibit 1 to make sure we have sufficient sample for each practice setting.
We propose to begin provider recruitment by taking advantage of the extensive network available through our subcontractors. We will try to engage the support of the various associations, including AOTA, APTA, and ASHA, to gain their support for participating in the data collection effort. In addition, through stakeholder involvement, particularly through conference calls and the project Web site, we hope to solicit provider interest, particularly for unaffiliated providers. We will make available an e-mail address and a telephone number so that following a planned upcoming Open Door Forum, providers can contact us if they have questions about participation. As with the development of the CARE tool, we found that speaking at national meetings heightens awareness of the project and is an excellent forum for soliciting provider participation.
C.4 Contacts
David M.
Bott, Project Officer, Developing Outpatient Therapy Payment
Alternatives
Office of Research Development and
Information
Phone: (410) 786-0249
e-mail:
[email protected]
Edward
M. Drozd, Project Director
RTI International
Phone: (781)
434-1716
e-mail: [email protected]
References
Ciolek, D. and Hwang, W. Development of a Model Episode-Based Payment System for Outpatient Therapy Services: Feasibility Analysis Using Existing CY 2002 Claims Data. Final Report. PSC Contract No. 500-99-0009/0009. November, 2004.
Cromwell, J., Gage, B., Drozd, E.M., Maier, J., Osber, D.J., Evensen, C., Richter, E.M., and Goldman, H.H.: Psychiatric Inpatient Routine Cost Analysis, Final Report. Waltham, MA: RTI International. CMS Contract No. 500-95-0058, T.O. 13, February 2005.
Taylor, D.J. and Muller, K.E. “Computing Confidence Bounds for Power and Sample Size of the General Linear Univariate Model,” The American Statistician 49(1): 43–47, February 1995.
1 Note that a patient-episode may involve therapists from a single discipline or from multiple disciplines.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Data Collection for Developing Outpatient Therapy Payment Alternatives Office of Management and Budget Clearance Package Support |
| Author | Centers for Medicare & Medicaid Services |
| File Modified | 0000-00-00 |
| File Created | 2021-02-02 |
© 2026 OMB.report | Privacy Policy