Attachment 2 - 2007 CSSC Email Survey Report
Attachment 2 - 2007 CSSC Email Survey Report.doc
Survey of Health Care Professionals' Awareness and Perceptions of the National Cancer Institute's Intramural Clinical Trials (NCI)
Attachment 2 - 2007 CSSC Email Survey Report
OMB: 0925-0619
Second Annual Survey to Assess Health Care Professionals’ Awareness and Perceptions of the National Cancer Institute’s
Intramural Clinical Trials
Results from Electronic Surveys with Health Care Professionals
And
Comparison to 2006 Survey Data
Prepared for:
The National Cancer Institute’s Center for Cancer Research
September 2007
Table of Contents
Statement of Limitations and Strengths of the Research 4
Comparison of 2006 Survey Data 10
IV. Tables 13
I.Executive Summary
Introduction and Background
The National Cancer Institute (NCI) Center for Cancer Research (CCR) is the largest component of the Institute’s intramural (i.e., in-house) research program. One of its goals is to disseminate information about available cancer clinical trials to health care providers and to the public. Its outreach efforts focus on creating awareness of the CCR’s clinical trial program within the health care professional community, with the ultimate goal of increasing physician referrals to ongoing clinical trials.
Efforts began in 2006 to assess the effectiveness of MMG’s strategies aimed at increasing the awareness and understanding of the National Institutes of Health (NIH) Clinical Center among health care professionals. To assess respondents’ awareness and knowledge of NCI and measure awareness of NCI clinical trials at the NIH Clinical Center in Bethesda, Md., a survey was developed in 2006 by MMG and sent electronically to respondents who treat cancer patients. The purpose of the survey is to identify ways in which the presence of NCI among health care professionals can be increased and to understand the perceptions or any misconceptions about NCI’s role in clinical research.
The survey was first sent in July of 2006 to members of the American Medical Association (AMA) with a primary specialty of gastroenterology, medical oncology, radiation oncology, hematology/oncology, thoracic surgery, colon & rectal surgery, gynecological oncology, surgical oncology, or head & neck surgery. The survey was slightly revised in 2007 and then sent to members of the same AMA primary specialty categories.
The data in this report includes an in-depth analysis of the 2007 survey data, as well as a comparison to the 2006 data to identify trends in health care professionals’ knowledge and awareness of NCI.
Key Findings
85% of respondents know NCI sponsors clinical trials
82% of respondents reported being either “somewhat familiar” or “very familiar” with the functions of NCI
The South was the region with the largest percentage of respondents reporting they had ever referred a cancer patient to a clinical trial at NCI
80% of respondents know NCI conducts clinical trials
97% of respondents were aware of NCI before participating in this survey
Respondents who treat 11 or more cancer patients per month are more likely to be “very familiar” with NCI than respondents who treat 10 or less patients per month
As in 2006, respondents learn about clinical research opportunities mainly from colleagues, conferences/seminars, and the internet
E-mail, conferences/seminars, and the internet continue to be respondents’ preferred methods of learning about clinical trials
Compared to the survey data collected in 2006:
3% fewer respondents reported ever referring a patient to a cancer clinical trial
An additional 8% of respondents know NCI conducts clinical trials
An additional 7% of respondents know NCI provides physicians and patients with access to thought leaders in cancer research
An additional 6% of respondents know NCI conducts research for rare cancers typically not investigated by the private sector
An additional 11% of respondents have ever referred a patient to a clinical trial on the NIH campus in Bethesda
A
Recommendations
Respondents are most receptive to receiving clinical trial information by e-mail, conferences/seminars, and the internet; continue to rely heavily on these methods of communication. Limit the dissemination of information by mail, interdepartmental meetings, and Grand Rounds as those were respondents’ least preferred methods of receiving clinical trial information.
Most specifically, continue to increase the amount of e-mail outreach. As in 2006, a larger percentage of respondents wanted to receive more clinical trials information via email than they currently receive. Respondents seem to welcome receiving information about clinical trials via e-mail.
Because many respondents currently learn about clinical trials from their colleagues, sending “Dear Colleague” letters from NCI telling physicians about clinical trial opportunities may be an effective way to spread information by word of mouth.
Continue to promote greater familiarity with the activities of NCI; the data shows that respondents who are very familiar with the endeavors of NCI may be more likely to refer cancer patients to an NCI clinical trial
slightly larger percentage (4%) of respondents reported they had not referred any cancer patients to a clinical trial at NCI in the past year
II.Methodology
Recruitment of Participants
To recruit research participants, an e-mail was sent on July 24, 2007, to selected members of the AMA inviting them to participate in a survey about their knowledge and opinions of NCI. The e-mail was sent to 13,793 members representing the nine Primary Specialty categories listed in Table 1. The survey was sent to these same specialty categories in 2006. See Appendix A for the text of the e-mail invitation that was sent.
Table 1
AMA Primary Specialty Category |
Members |
Gastroenterology |
4,625 |
Medical oncology |
2,346 |
Radiation oncology |
2,210 |
Hematology/oncology |
1,864 |
Thoracic surgery |
1,660 |
Colon and rectal surgery |
563 |
Gynecological oncology |
280 |
Surgical oncology |
152 |
Head and neck surgery |
93 |
Of the 13,793 e-mails sent, 13,451 were successfully delivered. Ten percent (1,411) of those e-mails were opened. The e-mail was opened a total of 1,710 times, indicating that some of the messages were opened multiple times by the same recipient. Of those who received the e-mail, 327 individuals (2.4%) participated in the survey.
Figure 1 displays the metrics related to the survey invitation e-mails.
Figure 1

Statement of Limitations and Strengths of the Research
The data represent a self-selected group of health care providers with access to the internet and e-mail; this selection bias may have an impact on the survey findings. Because the same AMA specialty categories as last year were used, it can be assumed that some respondents participated in the 2006 and 2007 surveys. Also, people who were already familiar with NCI or clinical trials may have opted to participate while those not as familiar may have deferred because they were not confident in the subject matter. Respondents were allowed to skip questions, resulting in varying sample size from question to question as indicated in the tables. Findings should be considered valid from the respondents’ points of view; however, findings cannot be generalized to represent the opinions of other health care professionals.
III.Detailed Findings
Survey questions 12–16 gather demographic information about the respondents. Respondents were most likely to select the following categories:
Professional status of “MD” (92%)
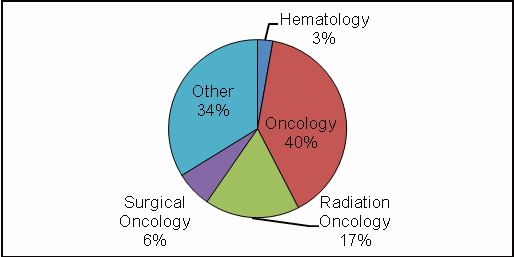
Specialize in oncology (40%)
Sees 31 or more cancer patients per month (45%)
In private practice (47%)
Primary practice located in the South (33%)
Have been working with cancer patients more than 20 years (44%)
The complete data set is listed in Tables 2–5. Figures 2 and 3 show the regional and occupational specialty breakdowns of all respondents.
Figure 2: Primary Practice Regions
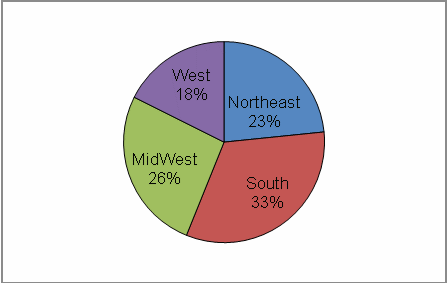
Figure 3: Occupational Specialty
Methods of Disseminating Clinical Trial Information
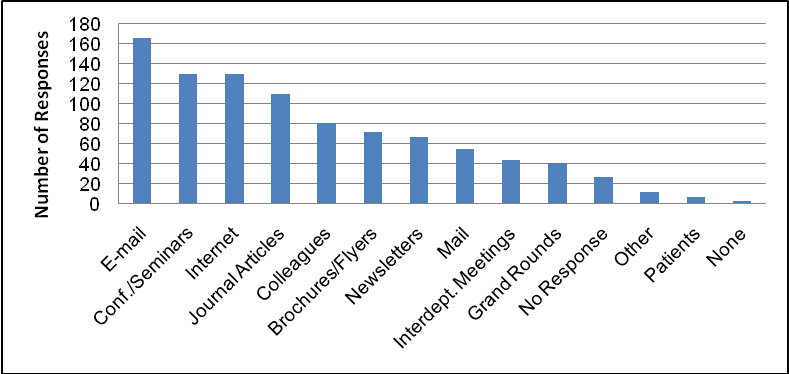
When respondents were asked how they learn about clinical research opportunities for their patients, the largest percentage (54%) said “conferences/seminars,” followed closely by “colleagues” (51%) and internet (45%) (Table 6). The top responses changed slightly when respondents were asked how they prefer to learn about clinical research opportunities for their patients (Table 7). The largest percentage (51%) preferred “e-mail” followed closely by “conferences/seminars” and “internet” (both 40%).
Respondents referring to NIH clinical trials in the past year are more inclined than those who have not referred in the past year to learn about clinical trials from colleagues, conferences/seminars, and the internet. They prefer to learn about clinical trial opportunities via e-mail and the internet (Table 8).
Respondents referring more than 20 patients to clinical research studies in the past year are more inclined than respondents who refer less patients to learn about opportunities via colleagues, conferences/seminars, interdepartmental meetings, mail, and patients. Sixty one percent prefer to learn about clinical trial opportunities via e-mail (Table 9).
Forty percent of the respondents who preferred to learn about clinical trial opportunities by e-mail did not list e-mail as a method for which they are currently receiving this information (Table 10). This indicated that a large group of respondents are not receiving information via e-mail even though they would prefer it. This offers an opportunity for NCI to increase e-mail outreach efforts.
Figure 4: Preferred Method of Learning About Clinical Trials (All Respondents)
Referrals to General Clinical Trials
Seventy five percent of respondents reported ever referring a patient to a cancer clinical trial. While many respondents have referred a cancer patient in the past, the general trend seems to be that most respondents refer only a few patients per year. The largest percentage (56%) of respondents reported referring 1–10 patients to a clinical trial in the past year (Table 11).

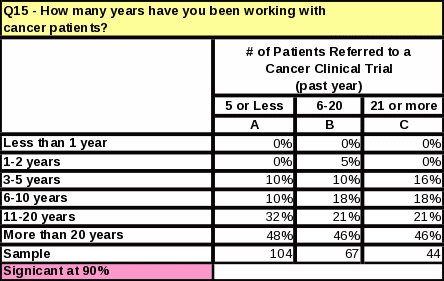
The length of time that respondents have worked with cancer patients does not impact the number of referrals made to clinical trials (Table 12). However, respondents who referred the most cancer patients to clinical research trials in the past year tend to see more cancer patients than those who referred less (Table 13). They are also more likely to know that NCI distributes cancer information to patients and physicians (Table 14).
Respondents in the university setting refer more patients to clinical trials than any other practice setting. See Table 15 for supporting data.
Awareness of NCI
Awareness of NCI is high among all respondents, regardless of frequency of referrals to clinical trials (Table 16). Figure 6 shows that 97% of respondents were aware of NCI before participating in this survey. Of the 10 respondents who were not aware of NCI, all were MDs; seven of them had referred a patient to a clinical trial before. This may mean that MDs referring patients to general clinical trials would be willing to also refer patients to NCI trials if they were made aware of opportunities at NCI.
Figure 5: Awareness of NCI

Knowledge of NCI’s Functions
Eighty two percent of respondents reported being either “somewhat familiar” or “very familiar” with the functions of NCI (Table 17).
80% of
respondents know NCI conducts clinical trials
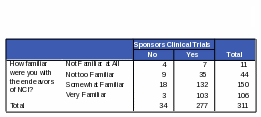
The data showed a relationship between familiarity with NCI and knowing that NCI sponsors and conducts clinical trials. The percentage of respondents correctly reporting that NCI conducts and sponsors clinical trials decreased as respondents’ reported knowledge of NCI decreased. For the complete counts, see Tables 19 and 20.
Respondents who are “somewhat familiar” or “very familiar” with NCI are more likely than those “not familiar” or “not very familiar” to know that NCI conducts and sponsors clinical trials. Respondents more familiar with NCI were 72% more likely to know NCI conducts clinical trials and 70% more likely to know NCI sponsors clinical trials. However, some respondents who said they were “very familiar” with NCI did incorrectly identify some of its functions. For example, 42% of the respondents who thought that NCI maintains regulatory oversight over pharmaceutical and biotechnology companies also said they were “very familiar” with the functions of NCI. Thirty five percent of respondents who said NCI defines standards of care for cancer patients also said they were “very familiar” with NCI. See Tables 21 and 22 for the complete counts.
Factors Related to Knowledge of NCI’s Functions:
The survey data showed a link between familiarity with NCI and the number of cancer patients treated per month. Table 23 shows that respondents who treat 11 or more cancer patients per month are more likely to be “very familiar” with NCI than respondents who treat 10 or less. Respondents who see a large amount of cancer patients per month are more likely to report a high familiarity with NCI.
Characteristics
of Respondents “Very Familiar” With the Functions of
NCI:
Treats
11+ cancer patients per month
More
than 20 years experience treating cancer patients
Primarily
practice in the West
Refers
more patients to clinical trials
Respondents
who refer more patients to clinical trials (both general and NCI)
are more familiar with the endeavors of NCI and give NCI credit for
providing more services (Tables 14 and 23). When respondents were
asked how many cancer patients they had referred to an NCI clinical
trial on the Bethesda campus in
the past year,
57% said “none”. In comparison, 52% of those who were
“very familiar” with NCI also said they had not referred
any patients to NCI in the past year. The 5% difference may mean
that increasing healthcare providers’ familiarity with NCI
could increase the numbers of referrals to NCI trials.
Respondents who were “very familiar” with NCI were 53% more likely than respondents who were “not familiar” or “not very familiar” to have referred a patient to an NCI-sponsored trial at a site other than NCI in Bethesda. This may indicate that referrals to NCI trials at institutions increase as respondents’ familiarity with NCI increases.
Respondents who have ever referred a patient to a cancer clinical trial were more likely than those who have never referred to a cancer clinical trial to know that NCI conducts and sponsors clinical trials. Eighty-eight percent of respondents who have ever referred to a cancer clinical trial also correctly reported that NCI conducts clinical trials and 94% of these respondents correctly reported that NCI sponsors clinical trials (Tables 24 and 25).
Experience with treating cancer patients is also related to increased familiarity with NCI. Fifty-five percent of respondents who reported being “very familiar” with NCI also reported “more than 20 years” of experience with treating cancer patients (Table 26).
Patient Referrals to NCI
Forty percent of respondents had never referred a cancer patient to an NCI clinical trial on the NIH campus (Table 27). More respondents, however, are making referrals to NCI-sponsored trials conducted elsewhere: Only 17% had never referred a patient to an NCI-sponsored clinical trial that was conducted at an institution other than NCI in Bethesda (Table 28).
Forty two percent of respondents who have ever referred a patient to a clinical trial at NCI in Bethesda had done so in the past year (Table 29).
Those referring to NCI trials in the past year have been working with cancer patients longer than those who have not referred patients to NCI trials in the last year (Table 30).
Those respondents who have referred more patients to clinical trials in general also referred more patients to NCI trials. Of the respondents who have ever referred a patient to a cancer clinical trial, 43% have also referred to an NCI clinical trial in Bethesda. A larger percentage (65%) had referred to an NCI-sponsored trial conducted at an institution other than Bethesda. The larger percentage of referrals to an institution other than Bethesda can most likely be explained by considering proximity; respondents seem to be referring patients to the closest facility.
Regional Referral Habits and Perceptions of NCI
Awareness of NCI does not vary by location of the respondent; however, the Northeast had the lowest percentage of respondents reporting they were “very familiar” or “somewhat familiar” with NCI (Table 32).
As shown in Table 34, respondents in the South and Midwest are more likely than respondents in the West to know NCI conducts clinical trials.
Analyses of the respondents’ geographical location revealed that the South had the highest percentage of respondents who had ever referred a cancer patient to a clinical trial at NCI in Bethesda (38%) (Table 31). Respondents from the South are more likely to refer patients to NCI clinical trials than respondents from the Northeast or West. However, respondents in the West who do refer to general clinical trials seem to do so in large numbers. The West had the largest proportion of respondents referring “16 or more” patients to a cancer clinical trial in the past year (Table 33). Respondents in the other regions were more likely to refer a smaller number of patients per respondent.
Profile of Respondents with High Referral Rates to NCI Clinical Trials
Those referring to NCI trials have been working with cancer patients longer than those who do not refer. Sixty six percent of all respondents who had referred to an NCI trial had been working with cancer patients more than 20 years (Table 35).
Profile
of Highest Referring Respondents to NCI Primary
practice located in the South Long
history of working with cancer patients
Treats
21 or more cancer patients per month Learn
about clinical trials through colleagues, conferences/seminars,
and the internet Also
refer more patients to clinical trials in general Familiar
with the endeavors of NCI
Table 36 shows that respondents who have referred “16 or more” patients to a clinical trial in the past year are more likely to have referred a patient to an NCI trial at Bethesda than respondents who referred fewer patients. Respondents referring 6 or more patients to a clinical trial in the past year were more likely than those referring fewer patients to have referred a patient to a trial conducted at an institution other than Bethesda (Table 37). This may indicate that respondents who refer a smaller number of cancer patients per year are more likely to refer their patients to NCI trials close to their practice rather than a clinical trial being conducted at NIH in Bethesda.
Miscellaneous
The final question of the survey asked whether the respondent would like to receive the Bethesda Trials News e-newsletter. Table 38 shows that the e-mail survey successfully placed 60% of respondents onto the Bethesda Trials News e-newsletter mailing list. Eleven percent of the respondents were already receiving the e-newsletter.
Comparison of 2006 Survey Data
Below are highlights of differences between the 2006 and 2007 survey data.
Methods of Disseminating Clinical Trial Information
Respondents continue to learn about clinical trial opportunities via conferences/seminars, colleagues, and the internet. They continue to prefer to learn about opportunities via e-mail, conferences/seminars, and the internet. In particular, a greater percentage of respondents now prefer email as their source of receiving information about clinical trials (51% in 2007 compared to 44% in 2006) (Table 41).
Referrals to Clinical Trials (General)
Three percent fewer respondents reported ever referring a patient to a cancer clinical trial (78% in 2006 versus 75% in 2007).
The total number of patients respondents have referred to a cancer clinical trial in the past year has gotten smaller. Eleven percent fewer respondents referred “11 or more” patients in the past year, and 10% more respondents report referring 1–10 patients in the past year.
Respondents in the university setting continue to refer more patients to clinical trials than any other practice setting.
Awareness and Knowledge of NCI’s Functions
Just as in 2006, this year’s respondents reported a high awareness and familiarity with NCI. The percentage of respondents reporting being “somewhat familiar” or “very familiar” with NCI has remained steady (83% in 2006 compared to 82% in 2007). See Figure 6 for the both years’ percentages.
Figure 6: Familiarity With NCI 2006 Versus 2007 (Percentage of All Respondents)
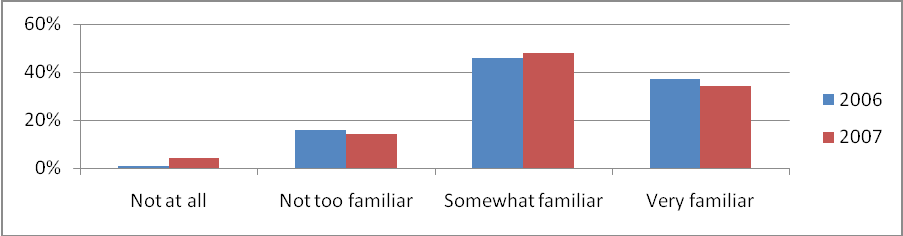
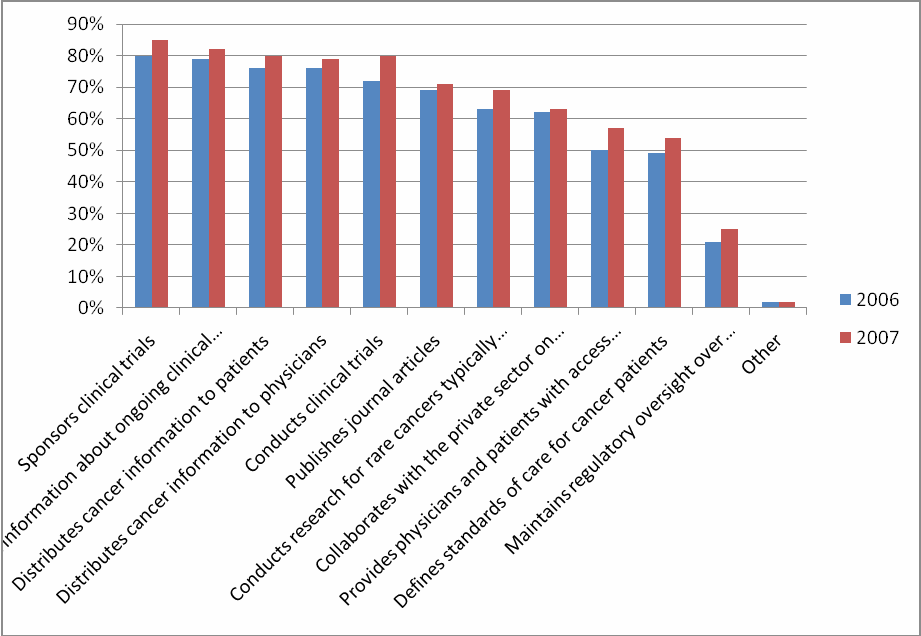
For the question “What activities do you think NCI conducts or provides?” every activity received a higher percentage than in 2006—even for those activities that are not actually a function of NCI.
Eight
percent more respondents know NCI conducts clinical trials
(compared to 2006)
Of those respondents who were “very familiar” with NCI, 2% more respondents know NCI conducts and sponsors clinical trials (Table 40).
Figure 7: Comparison of NCI’s Functions: 2006 and 2007
Patient Referrals to NCI
Fewer respondents reported never referring a patient to a clinical trial on the NIH campus in Bethesda (51% in 2006 compared to 40% in 2007). However, referrals to NCI in the past year are slightly lower: 4% more respondents reported not referring any cancer patients to a clinical trial at NCI in Bethesda in the past year (53% in 2006 versus 57% in 2007).
Regional Referral Habits and Perceptions of NCI
The region with the largest percentage of respondents who have ever referred to NCI is now the South. In 2006, the Northeast had the largest percentage of respondents who have ever referred a cancer patient to NIH in Bethesda, but that percentage dropped by 25% in 2007 (Table 42), making the South the region with the largest percentage of respondents making NCI referrals.
IV.Tables
Table 2: Respondents’ Professional Status
Survey Options |
Number of Respondents |
Percentage of Respondents |
MD |
269 |
92% |
Other |
14 |
5% |
RN |
6 |
2% |
NP |
3 |
1% |
RPh |
0 |
0% |
PharmD |
0 |
0% |
RD |
0 |
0% |
Total |
292 |
89% |
Table 3: Respondents’ Current Practice Setting
Survey Options (check all that apply) |
Number of Respondents |
Percentage of Respondents |
Private practice |
138 |
47% |
University |
58 |
20% |
Hospital-based outpatient |
32 |
11% |
Hospital-based clinic |
25 |
8% |
Hospital inpatient |
21 |
7% |
Other |
21 |
7% |
Total |
295 |
90% |
Table 4: Number of Years Spent Working With Cancer Patients
Survey Options |
Number of Respondents |
Percentage of Respondents |
More than 20 years |
127 |
44% |
11-20 years |
74 |
26% |
6-10 years |
43 |
15% |
3-5 years |
33 |
12% |
1-2 years |
7 |
2% |
Less than 1 year |
2 |
1% |
Total |
286 |
87% |
Table 5: Number of Cancer Patients Treated per Month
Survey Options |
Number of Respondents |
Percentage of Respondents |
31 or more |
131 |
45% |
1-5 |
43 |
15% |
6-10 |
38 |
13% |
21-30 |
34 |
12% |
11-20 |
29 |
10% |
None |
19 |
6% |
Total |
294 |
90% |
Table 6: Ways in Which Respondents Learn About Clinical Research Opportunities for Their Patients
Survey Options (check all that apply) |
Number of Respondents |
Percentage of Respondents |
Conferences/seminars |
177 |
54% |
Colleagues |
166 |
51% |
Internet |
147 |
45% |
Journal articles |
140 |
43% |
130 |
40% |
|
Brochures/flyers |
104 |
32% |
Newsletters |
75 |
23% |
74 |
23% |
|
Interdepartmental meetings |
69 |
21% |
Grand rounds |
58 |
18% |
Patients |
42 |
13% |
Other |
29 |
9% |
None/don’t learn about clinical trials |
8 |
2% |
Table 7: Ways in Which Respondents Prefer to Learn About Clinical Research Opportunities for Their Patients
Survey Options (check all that apply) |
Number of Respondents |
Percentage of Respondents |
166 |
51% |
|
Conferences/seminars |
130 |
40% |
Internet |
130 |
40% |
Journal articles |
110 |
34% |
Colleagues |
81 |
25% |
Brochures/flyers |
72 |
22% |
Newsletters |
67 |
21% |
55 |
17% |
|
Interdepartmental meetings |
44 |
14% |
Grand rounds |
41 |
13% |
Other |
12 |
4% |
Patients |
7 |
2% |
None/don’t learn about clinical trials |
3 |
1% |
Table
8: Statistical Significance
Table
9: Statistical Significance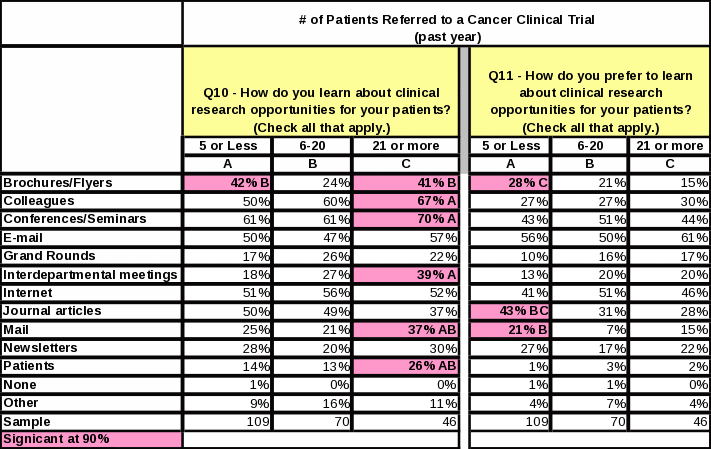
Table 10: Respondents who Learn About Clinical Research Opportunities for Their Patients by E-Mail / Respondents who Prefer to Learn About Clinical Research Opportunities for Their Patients by E-Mail
|
Prefer- E-mail |
Total |
||
No |
Yes |
|||
Learn- E-mail |
No |
131 |
66 |
197 |
Yes |
30 |
100 |
130 |
|
Total |
161 |
166 |
327 |
|
Table 11: Number of Patients Respondents Have Referred to a Cancer Clinical Trial in the Past Year
Survey Options |
Number of Respondents |
Percentage of Respondents |
None |
28 |
12% |
1-5 |
81 |
36% |
6-10 |
46 |
20% |
11-15 |
14 |
6% |
16-20 |
10 |
4% |
21 or more |
46 |
20% |
Total |
225 |
69% |
Table 12: Statistical Significance
Table
13: Statistical Significance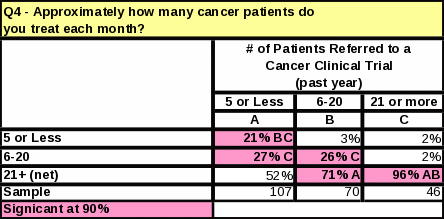
Table
14: Statistical Significance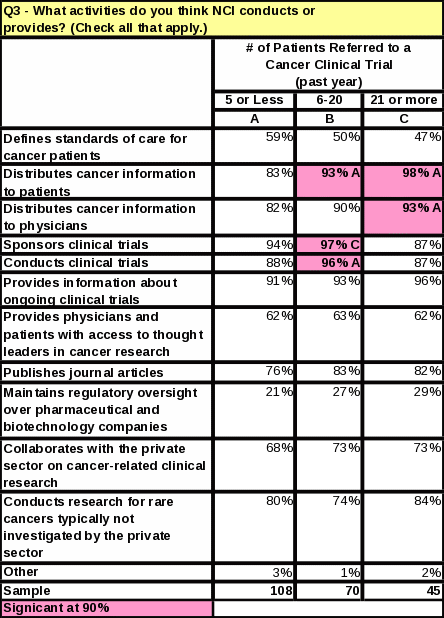
Table 15: Statistical Significance

Table 16:
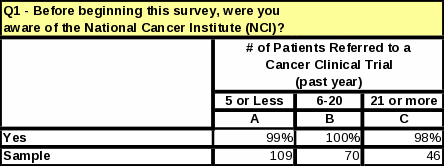
Table 17: Respondents’ Familiarity with NCI
|
Frequency |
Percent |
Valid Percent |
Cumulative Percent |
|
Valid |
Not Familiar at All |
11 |
3.4 |
3.5 |
3.5 |
Not too Familiar |
44 |
13.5 |
14.1 |
17.7 |
|
Somewhat Familiar |
150 |
45.9 |
48.2 |
65.9 |
|
Very Familiar |
106 |
32.4 |
34.1 |
100.0 |
|
Total |
311 |
95.1 |
100.0 |
|
|
Missing |
System |
16 |
4.9 |
|
|
Total |
327 |
100.0 |
|
|
|
Table 18: Activities Respondents Think NCI Conducts or Provides
Survey Options (check all that apply) |
Number of Respondents |
Percentage of Respondents |
Sponsors clinical trials |
278 |
85% |
Provides information about ongoing clinical trials |
267 |
82% |
Distributes cancer information to patients |
261 |
80% |
Conducts clinical trials |
260 |
80% |
Distributes cancer information to physicians |
258 |
79% |
Publishes journal articles |
231 |
71% |
Conducts research for rare cancers typically not investigated by the private sector |
227 |
69% |
Collaborates with the private sector on cancer-related research |
206 |
63% |
Provides physicians and patients with access to thought leaders in cancer research |
186 |
57% |
Defines standards of care for cancer patients |
175 |
54% |
Maintains regulatory oversight over pharmaceutical and biotechnology companies |
81 |
25% |
Other |
6 |
2% |
Table 19: Count: Respondents’ Familiarity with NCI / Activities of NCI: “Conducts Clinical Trials”

Table 20: Count: Respondents’ Familiarity with NCI / Activities of NCI: “Sponsors Clinical Trials”
T
 able
21: Count: Respondents’ Familiarity with NCI / Activity of
NCI: “Maintains Regulatory Oversight Over Pharmaceutical and
Biotechnology Companies”
able
21: Count: Respondents’ Familiarity with NCI / Activity of
NCI: “Maintains Regulatory Oversight Over Pharmaceutical and
Biotechnology Companies”
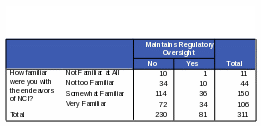
Table 22: Count: Respondents’ Familiarity with NCI / Activities of NCI: “Defines Standards of Care for Cancer Patients”
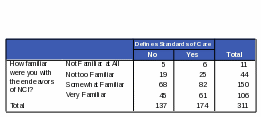
Table 23: Count: Respondents’ Familiarity with NCI / Number of Cancer Patients Treated per Month
|
How familiar were you with the endeavors of NCI? |
Total |
||||
Not Familiar at All |
Not too Familiar |
Somewhat Familiar |
Very Familiar |
|||
Approximately how many cancer patients do you treat each month? |
None |
2 |
6 |
6 |
4 |
18 |
1-5 |
6 |
9 |
16 |
8 |
39 |
|
6-10 |
0 |
9 |
24 |
3 |
36 |
|
11-20 |
1 |
1 |
13 |
14 |
29 |
|
21-30 |
0 |
7 |
17 |
9 |
33 |
|
31 or more |
1 |
7 |
58 |
63 |
129 |
|
Null |
1 |
4 |
8 |
3 |
16 |
|
No response |
0 |
1 |
8 |
2 |
11 |
|
Total |
11 |
44 |
150 |
106 |
311 |
|
Table 23: Statistical Significance
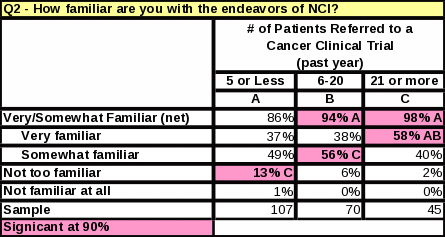
Table 24: Count: Number of Respondents Who Have Ever Referred a Patient to a Cancer Clinical Trial / Activities of NCI: “Conducts Clinical Trials”

Table 25: Count: Number of Respondents Who Have Ever Referred a Patient to a Cancer Clinical Trial / Activities of NCI: “Sponsors Clinical Trials”
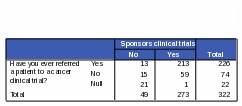
Table 26: Count: Years Working With Cancer Patients/Familiarity With NCI
|
How familiar were you with the endeavors of NCI? |
Total |
||||
|
Not Familiar at All |
Not too Familiar |
Somewhat Familiar |
Very Familiar |
|
|
How many years have you been working with cancer patients? |
Less than 1 year |
0 |
0 |
2 |
0 |
2 |
|
1-2 years |
1 |
3 |
3 |
0 |
7 |
|
3-5 years |
3 |
5 |
20 |
4 |
32 |
|
6-10 years |
0 |
9 |
20 |
10 |
39 |
|
11-20 years |
3 |
8 |
37 |
25 |
73 |
|
More than 20 years |
3 |
11 |
53 |
58 |
125 |
|
Null |
1 |
7 |
11 |
6 |
25 |
|
No response |
0 |
1 |
4 |
3 |
8 |
Total |
11 |
44 |
150 |
106 |
311 |
|
Table 27: Respondents Who Have Ever Referred a Cancer Patient to an NCI Clinical Trial on the National Institutes of Health (NIH) Campus in Bethesda, MD (Regardless of Whether the Patient was Eligible and/or Participated)
Survey Options |
Number of Respondents |
Percentage of Respondents |
Yes |
98 |
30% |
No |
130 |
40% |
Don’t Know |
96 |
30% |
Total |
324 |
70% |
Table 28: Respondents Who Have Ever Referred a Patient to an NCI-Sponsored Clinical Trial that was Conducted at an Institution Other than NCI in Bethesda, MD (Regardless of Whether the Patient was Eligible and/or Participated)?
Survey Options |
Number of Respondents |
Percentage of Respondents |
Yes |
147 |
65% |
No |
38 |
17% |
Don’t know |
42 |
19% |
Total |
227 |
69% |
Table 29: Count: Number of Respondents who have Ever Referred a Cancer Patient to an NCI Clinical Trial in Bethesda/Number of Cancer Patients Respondents Have Referred to a Clinical Trial at NCI in Bethesda, MD in the Past Year
|
In the past year, approximately how many cancer patients have you referred to a clinical trial at NCI in Bethesda, MD? |
Total |
||||||
None |
1-2 |
3-5 |
11 or more |
Null |
No response |
|||
Have you ever referred a cancer patient to an NCI clinical trial on the NIH campus in Bethesda, MD? |
Yes |
53 |
34 |
4 |
1 |
0 |
6 |
98 |
No |
0 |
0 |
0 |
0 |
0 |
127 |
127 |
|
Don't Know |
0 |
0 |
0 |
0 |
96 |
0 |
96 |
|
No response |
0 |
1 |
0 |
0 |
0 |
2 |
3 |
|
Total |
53 |
35 |
4 |
1 |
96 |
135 |
324 |
|
Table 30: Count: In the Past Year, How Many Cancer Patients Have you Referred to a Clinical Trial at NCI in Bethesda?/How Many Years Have You Been Working With Cancer Patients?
|
How many years have you been working with cancer patients? |
Total |
||||||||
Less than 1 year |
1-2 years |
3-5 years |
6-10 years |
11-20 years |
More than 20 years |
Null |
No response |
|||
In the past year, approximately how many cancer patients have you referred to a clinical trial at NCI in Bethesda, MD? |
None |
0 |
0 |
0 |
3 |
6 |
41 |
1 |
2 |
53 |
1-2 |
1 |
0 |
1 |
6 |
10 |
15 |
0 |
2 |
35 |
|
3-5 |
0 |
0 |
0 |
1 |
1 |
2 |
0 |
0 |
4 |
|
11 or more |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
|
Null |
1 |
4 |
9 |
12 |
15 |
25 |
27 |
3 |
96 |
|
No response |
0 |
3 |
22 |
21 |
40 |
43 |
3 |
3 |
135 |
|
Total |
2 |
7 |
32 |
43 |
73 |
126 |
31 |
10 |
324 |
|
Table 31: Statistical Significance
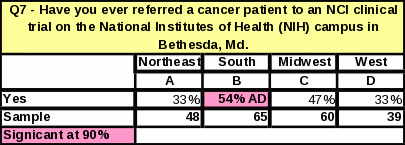
Table 32: Statistical Significance
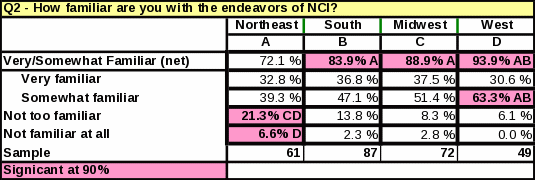
Table 33: Statistical Significance

Table 34: Statistical Significance

Table 35: Count: Years Working with Cancer Patients / Ever Referred a Cancer Patient to an NCI Clinical Trial on the NIH Campus in Bethesda
|
Have you ever referred a cancer patient to an NCI clinical trial on the NIH campus in Bethesda, MD? |
Total |
||||
Yes |
No |
Don't Know |
No response |
|||
How many years have you been working with cancer patients? |
Less than 1 year |
1 |
0 |
1 |
0 |
2 |
1-2 years |
0 |
3 |
4 |
0 |
7 |
|
3-5 years |
1 |
23 |
9 |
0 |
33 |
|
6-10 years |
12 |
19 |
12 |
0 |
43 |
|
11-20 years |
18 |
39 |
15 |
2 |
74 |
|
More than 20 years |
61 |
41 |
25 |
0 |
127 |
|
Null |
1 |
3 |
27 |
0 |
31 |
|
No response |
4 |
2 |
3 |
1 |
10 |
|
Total |
98 |
130 |
96 |
3 |
327 |
|
Table 36: Statistical Significance

Table 37: Statistical Significance

Table 38: Respondents Wishing to Receive the Bethesda Trials News E-Newsletter
Survey Options |
Number of Respondents |
Percentage of Respondents |
Yes |
175 |
60% |
No |
86 |
29% |
I currently receive this e-newsletter |
33 |
11% |
Total |
294 |
90% |
Table 39: Statistical Significance: Comparison of 2006 and 2007
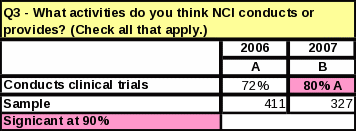
Table
40: Statistical Significance: Comparison of 2006 and 2007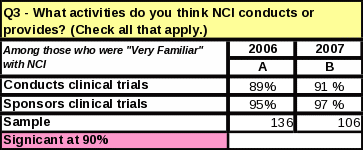
Table 41: Statistical Significance: Comparison of 2006 and 2007

Table 42: Statistical Significance: Comparison of 2006 and 2007
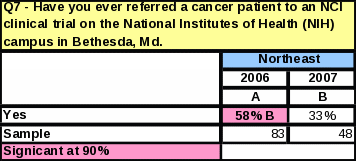
V.Appendices
Appendix A: Survey Invitation E-mail Content
Appendix B: Survey Format

Dear Health Professional:
The National Cancer Institute’s (NCI) Center for Cancer Research (CCR) is conducting a survey to gain a deeper understanding of the perception that health professionals who work with cancer patients have of NCI and the services the institute provides.
We would like to invite you to participate in a brief survey about your knowledge and awareness of NCI and would greatly value any information you could provide.
This survey should take approximately 5 minutes to complete. Please be assured that your responses will be kept confidential and will not be disclosed to anyone outside of NCI or its contractor, Matthews Media Group, Inc. (MMG), except as otherwise required by law. Data will be provided to NCI in aggregate form only, with any potentially identifying information removed.
To participate, please click on the following link:
http://www.matthewsgroup.com/nci-aware/
Thanks for your consideration. If you have any questions or would like to know the results of the survey, please contact Marita Lynott at [email protected] or 301-348-1639.
Warm Regards,
Susan McMullen
Outreach Coordinator
National Cancer Institute
Center for Cancer Research
Appendix B: Survey Format
NCI Center for Cancer Research Survey to Assess Health Care Professionals’ Awareness of NCI Intramural Clinical Trials
The National Cancer Institute’s (NCI) Center for Cancer Research (CCR) is conducting this survey with physicians and nurses in order to gain a deeper understanding of the perception that health professionals have of NCI and the services the institute provides. The results from this survey will be used to evaluate the level of awareness and understanding of NCI’s Center for Cancer Research and to develop new programs to educate and inform healthcare professionals of their activities.
Your participation in this survey is completely voluntary. Please be assured that your responses will be kept confidential and will not be disclosed to anyone outside NCI or its contractor, Matthews Media Group (MMG), except as otherwise required by law. Data will be provided to the NCI in aggregate form only, with any potentially identifying information removed. You may skip any questions that you prefer not to answer. This survey should take approximately 5 minutes to complete.
If
you have any questions or would like to know the results of the
survey, please contact Marita Lynott at [email protected] or
301-348-1639.
Yes No
|
If yes, continue; if no, skip to #4
|
Very Familiar Somewhat familiar Not too familiar Not familiar at all
|
Record and proceed to #3
|
Defines standards of care for cancer patients Distributes cancer information to patients Distributes cancer information to physicians Sponsors clinical trials Conducts clinical trials Provides information about ongoing clinical trials Provides physicians and patients with access to thought leaders in cancer research Publishes journal articles Maintains regulatory oversight over pharmaceutical and biotechnology companies Collaborates with the private sector on cancer-related clinical research Conducts research for rare cancers typically not investigated by the private sector Other__________________
|
Record and proceed to #4
|
None 1-5 6-10 11-20 21-30 31 or more
|
Record and proceed to #5
|
|
If yes, continue; if no, skip to #10
|
None 1-5 6-10 11-15 16-20 21 or more
|
Record and proceed to #7
|
Yes No
|
If yes continue; if no, skip to #8
|
None 1-2 3-5 6-10 11 or more
|
Record and proceed to #9
|
Yes No Don’t know
|
Record and proceed to #10
|
Brochures/Flyers Colleagues Conferences/Seminars E-mail Grand Rounds Interdepartmental meetings Internet Journal articles Mail Newsletters Patients None/Don’t learn about clinical trials Other___________________
|
Record and proceed to #11
|
Brochures/Flyers Colleagues Conferences/Seminars E-mail Grand Rounds Interdepartmental meetings Internet Journal articles Mail Newsletters Patients None Other___________________
|
Record and proceed to #12
|
Please tell us about yourself. |
|
RN RD NP PharmD RPh MD Other________________
|
Record and proceed to #13
|
Hematology Oncology Radiation Oncology Surgical Oncology Other_________________________________________________________________
|
Record and proceed to #14
|
City:_______________________________________________________ State:______ Outside the United States
|
Record and proceed to #15
|
Less than 1 year 1-2 years 3-5 years 6-10 years 11-20 years More than 20 years
|
Record and proceed to #16
|
Hospital inpatient Hospital-based outpatient Hospital-based clinic University Private practice Other____________
|
Record and proceed to #17
|
Thank you for taking the time to complete the survey. Your answers have been submitted. If you have any questions or would like to know the results of this survey, please contact Marita Lynott at 301-348-1639 or [email protected]. The National Cancer Institute's Center for Cancer Research distributes a free quarterly e-newsletter, BethesdaTrials News, which helps community physicians stay informed of investigational approaches to treating, diagnosing, and preventing cancer. Each issue features one or more clinical trials in process at NCI on the NIH campus in Bethesda, Md.
(Check only one.) I currently receive this e-newsletter
|
If yes, continue; if no or I currently receive, skip to closing
|
To sign up for BethesdaTrials News, please click on the link below, which will redirect you to The National Cancer Institute's Center for Cancer Research. The contact information you provide will not be linked to this questionnaire. Note that the National Cancer Institute's Center for Cancer Research does not sell or share its mailing list
http://bethesdatrials.cancer.gov/professionals/mailinglist.asp Thank you again for completing this survey. |
|
| File Type | application/msword |
| File Title | CSSC Email Survey |
| Author | MMG Profile |
| Last Modified By | jwalmsley |
| File Modified | 2010-03-15 |
| File Created | 2010-03-15 |
© 2026 OMB.report | Privacy Policy
