0920-09AU_SS_Sect B Revised 11_22
0920-09AU_SS_Sect B Revised 11_22.doc
Preventing HIV Risk Behaviors Among Hispanic Adolescents
OMB: 0920-0871
“Preventing HIV Risk Behaviors among Hispanic Adolescents”
Supporting Statement
Part B
Contact Information
Project Officer(s):
Leigh A. Willis, PhD, MPH
Minority HIV/AIDS Research Initiative
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
Division of HIV/AIDS Prevention/Epidemiology Branch
Centers for Disease Control and Prevention
1600 Clifton Road NE, Mailstop E-45
Atlanta, GA 30333.
Voice: (404) 639-8447
Fax: (404) 639-6127
Email: [email protected].
Date: November 22, 2010
B. Statistical Methods
1. Respondent Universe and Sampling Methods
The respondents providing the information for the proposed project are 400 Hispanic youth and their primary care-givers.
Participants will be Hispanic ninth graders (and their primary caregivers) selected from Miami Senior High and Coral Gables Senior High School, two of the 37 public High Schools, in Miami-Dade County, Florida. Participants who meet the following inclusion criteria will be eligible to participate in the study.
(a) Female and male adolescents of Hispanic immigrant origin, defined by at least one parent born in a Spanish speaking country of the Americas.
(b) Adolescents attending 9th grade at Miami Senior High and Coral Gables Senior High Schools, at the time of the baseline assessment. Adolescents who were held back in school and are older than traditional 9th graders would still be eligible to participate.
(c) Adolescents living with an adult primary caregiver who is willing to participate.
(d) Families living within the catchment areas of the two high schools included in this study at the time of the baseline assessment.
Participants who meet the following exclusion criteria will not be eligible to participate in the study.
(a) Prior psychiatric hospitalization by adolescent or caregiver (During the screening process, facilitators will ask both parents and youth whether or not they have ever been hospitalized for psychiatric reasons)
(b) Family reports to have (tentative or firm) plans to move out of the South Florida area during the two years of the study.
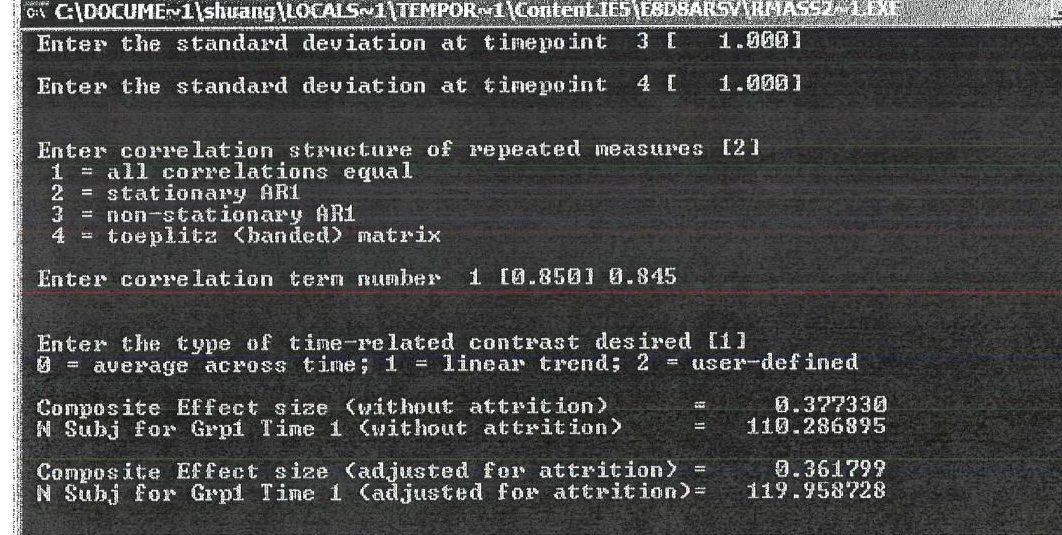
The estimated total number of participants needed to detect and intervention effect and number that will be randomized to one of the two conditions is 480 (i.e., 240 adolescents and 240 primary caregivers). The participants will be allocated as follows: 120 families (consisting of 120 adolescents and 120 primary caregivers) in the Familias Unidas intervention + Community Practice control condition and 120 families (consisting of 120 adolescents and 120 primary caregivers) in the Community Practice control only condition. Based on previously conducted research, the study should have an attrition rate of 5% and a response rate of 90% (Prado et al., 2007; Pantin et al., in press). Through a statistical power analysis it has been determined that a minimum sample size of 206 (103 dyads in each condition) will be needed to detect a moderate intervention effect (d=.34).
Table 1. Respondents and data collection instruments used in this study.
Respondent Type |
Number Participating |
Data Collection Instrument (Attachment 3) |
Corresponding Row(s) in Exhibit A.12.A |
Hispanic Adolescents Primary Caregivers |
400 |
5b,5c,5d,5e |
1 |
Primary Caregivers of Hispanic Adolescents and Hispanic Adolescents |
800 |
3a |
2 |
Primary Caregivers of Hispanic Adolescents |
240 |
3b,3d |
3 |
Hispanic Adolescents |
240 |
3c,3e |
4 |
A convenience sample of 240 dyads will be derived from two local high schools in Miami-Dade County. Individual youth and an individual from their family serve as the sampling unit and the unit of analysis for their segment of the study. Data collection for Hispanic adolescents and caregivers will take place at start of the study (Baseline), 4 months post-intervention, 12 months post-intervention and 24 months post-intervention. An individual Primary Caregiver of each adolescent participant will also provide the information contained in Attachment 3b and if necessary Attachment 3d, a total of four times. Individual Primary Caregivers of adolescents will spend approximately 45 minutes completing each assessment for a total of 3 hours over the duration of the project. Adolescent participants will provide the information contained in Attachment 3c and if necessary Attachment 3e, a total of four times (0, 4 months post-intervention, 12 months post-intervention, 24 months post-intervention). Individual adolescents will spend approximately 60 minutes completing each assessment for a total of 4 hours over the duration of the project. The average number of minutes necessary to complete adolescent and parent questionnaires was estimated based on prior prevention research (Prado et al., 2007; Pantin et al., in press).
2. Procedures for the Collection of Information
Recruiting of Participants
The principals of Miami Senior High and Coral Gables Senior High Schools will send invitation letters to all parents of 9th grade students. The letters will inform the parents that the University of Miami is conducting a study to prevent substance use and HIV risks in Hispanic youth and instructing the parents who wish to learn more about the project, to include their phone number or address, sign the response letter and have the student return the signed letter to the homeroom teacher. Parents indicating that they are interested in learning more about the study will be contacted by one of the study’s facilitators.
The facilitators will then explain the study to the parent over the phone and set up an initial appointment to meet with the parent and the target youth. During this initial meeting, the facilitator will screen both the parent and the youth for eligibility. To avoid potential coercion, parents and youth will be screened for inclusion/exclusion criteria separately (i.e. in a different office) by the facilitator. If a youth refuses to participate in the study, the facilitator will not tell the parent that their son/daughter refused to participate. Instead, the facilitator will tell the parent that the family is not eligible to participate in the study. If a family meets the eligibility criteria, they will be given and informed consent (parents) and an informed assent (youth).
The recruitment and screening process will continue until the target number of 240 youth-caregiver dyad is reached. Incorporating the attrition of 5% at each assessment observed in previous efficacy test of Familias Unidas, this study will enroll 240 participant dyads. Once the 240 participants are indentified and screened, they will be randomly assigned to either the experimental (Familias Unidas) or control condition (standard school district mandated HIV prevention education). Through a statistical power analysis it has been determined that a minimum sample size of 206 (103 dyads in each condition) will be needed to detect a moderate intervention effect (d=.34).
In addition to a baseline assessment, upon completion of the experimental and control programs participants will be assessed at 4 months post-intervention, 12 months post-intervention and 24 months post-intervention for a total of 4 assessments over a 2 year period. All adolescent self-report measures will be administered via Questionnaire Design Studio’s Audio Computer Assisted Interviewer Program (A-CASI:Resnick et al., 1997) in conjunction with direct entry onto a laptop and all parent self-report measures will be administered using the A-CASI method in conjunction with touch-screen technology. Parents will use the touch-screen technology instead of a laptop because in earlier prevention studies with a similar population (i.e. Hispanic immigrant parents), pilot studies indicated that the parent participants overwhelmingly preferred to answer questionnaires on tablets with the touch-screen over laptop computers. Research staff will be trained to turn on the computers for the participants for assessments and in answering any questions the participants may have. All data collection will take place in secure office space at the University of Miami.
Data will be analyzed using a latent growth curve approach to Hierarchical Linear Modeling (HLM) using Mplus (Bryk & Raudenbush, 1992; Muthen & Muthen, 1998-2006). HLM is also known as random coefficients regression, mixed or multi-level models. To evaluate the effectiveness of Familias Unidas (relative to the control condition), the study will test for differences in trajectories of drug use and unprotected sexual behavior over the 4 assessment time points between the 2 conditions. As recommended by Bryk & Raudenbush (1992), tests will be performed to determine if there is significant variation in individual trajectories over time across all conditions. A similar test will be conducted to examine whether Familias Unidas has a significant impact (increase) on family functioning, our proposed mechanism of action. Thus, if there is a significant reduction in drug use and/or unprotected sexual behavior trajectories (relative to the control condition), the study will be considered the Familias Unidas intervention efficacious. Familias Unidas will also be considered efficacious if we observe improvements in family functioning (relative to the control condition). Family functioning will be measured using the items from the Parenting Practices (Loeber et al., 1999), (2) the Family Relations Scale (Tolan, Gorman-Smith, Zelli, & Huesmann, 1997), the Parent-Adolescent Communication Scale (Barnes & Olson, 1982), and the Parent-Adolescent Communication about Sex (1999).
3. Methods to Maximize Response Rates and Deal with Nonresponse
Attrition will be a challenge for this study. Based on previous experience with this population, The University of Miami estimates 5% attrition rate which is reflected in the burden tables. Non-response rates will be less of a challenge for this study due to the To maximize response rates, in addition to their own contact information, all participants will be asked to provide the names of 3 persons who will always know where they can be reached. These names will help us maintain contact with the families in case they move or their telephone lines become disconnected. The investigators have also found that regular, cordial contact with the families improves retention rates.
To maximize study retention in the assessments participants will be provided with a token of appreciation for their participation. Participants who complete all assessments will receive $190, distributed as follows: $40 for completing baseline, $45 for post-intervention (4-months post-intervention), $50 (12-months post-intervention), and $55 (24-months post-intervention). The investigators found this token to be effective in attaining a 90% response rate for previously conducted studies with this target population.
In order to minimize non-response bias data will be analyzed using an intent-to-treat design so that all participants (regardless of whether they complete the intervention) will be analyzed. Furthermore, Hierarchical Linear Modeling will be used to analyze data, all participants will be included in the analyses (regardless of whether they have one or more missing data points). Due to the software that is used to estimate our hierarchical linear models use an EM algorithm, cases with missing data can be included.
4. Test of Procedures or Methods to be Undertaken
No novel tests or procedures will be used in this request.
5. Individuals Consulted on Statistical Aspects and Individuals Collecting and/or Analyzing Data
Individuals consulted on statistical aspects are the lead scientists on this project from the University of Miami, Miller School of Medicine, 1425 N.W. 10th Ave., Suite 312,Miami, FL 33136.
Name |
Degree(s) |
Role |
Institution |
Guillermo Prado |
Ph.D. |
Principal Investigator |
University of Miami Miller School of Medicine University of Miami, 1425 N.W. 10th Avenue, Suite 312, Miami, FL 33136. [email protected] 305-243-2748 |
Hilda Pantin |
Ph.D. |
Co-Principal Investigator/Local Mentor |
University of Miami Miller School of Medicine University of Miami, 1425 N.W. 10th Avenue, Suite 312, Miami, FL 33136. [email protected] 305-243-2343 |
The other individual consulted on the statistical aspects is Dr. Kim Miller, the project officers from Centers for Disease Control and Prevention, 1600 Clifton Rd., MS E-45, Atlanta, GA 30333.
Name |
Degree(s) |
Role |
Institution |
Kim S. Miller |
Ph.D. |
Investigator/CDC Mentor |
Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention 1600 Clifton Road, MS E-45 Atlanta, GA 404-639-6160 |
Individuals who will collect and analyze the data are from the University of Miami
Name |
Degree(s) |
Role |
Institution |
Guillermo Prado |
Ph.D. |
Principal Investigator |
University of Miami Miller School of Medicine University of Miami, 1425 N.W. 10th Avenue, Suite 312, Miami, FL 33136. [email protected] 305-243-2748 |
Hilda Pantin |
Ph.D. |
Co-Principal Investigator/Local Mentor |
University of Miami Miller School of Medicine University of Miami, 1425 N.W. 10th Avenue, Suite 312, Miami, FL 33136. [email protected] 305-243-2343 |
References:
Pantin, H., Coatsworth, D., Feaster, D.J., Newman, F.L., Briones, E., Prado, G., Schwartz, S., Szapocznik, J. (2003). Familias Unidas: The efficacy of an intervention to increaseparental investment in Hispanic immigrant families. Prevention Science, 4, 189-201.
Prado, G., Pantin, H., Briones, E., Schwartz, S., Feaster, D., Huang, S., Sullivan, S., Tapia, M.,Sabillon, E., Lopez, B., & Szapocznik, J. (2007). A randomized controlled trial of a family-centered intervention in preventing substance use and HIV risk behaviors in Hispanic adolescents. Journal of Consulting and Clinical Psychology, 75 (6), 914-926.
| File Type | application/msword |
| File Title | Title |
| Author | vbs6 |
| Last Modified By | DHHS |
| File Modified | 2010-11-22 |
| File Created | 2010-11-22 |
© 2026 OMB.report | Privacy Policy