1884ss05 rev
1884ss05 rev.doc
Addendum to Partial Update of the TSCA Section 8(b) Inventory Data Base, Production and Site Reports
OMB: 2070-0162
July 19, 2010 [NPRM: RIN 2070-AJ43]
Supporting Statement for a Request for OMB Review under
The Paperwork Reduction Act
1. Identification of the Information Collection
1(a) Title of the Information Collection
Title: Proposed Rule Addendum to Partial Update of the TSCA Section 8(b) Inventory Data Base, Production and Site Reports
EPA ICR No.: 1884.05 OMB Control No.: 2070-0162
1(b) Short Characterization/Abstract
The following information collection request (ICR) addendum addresses the paperwork requirements contained in a proposed rule (RIN 2070-AJ43) that would amend the information collection activities of the Inventory Update Reporting (IUR) program under the Toxic Substances Control Act (TSCA) (40 CFR Part 710). Under TSCA section 8(a) (15 USC 2607), the Environmental Protection Agency (EPA) is authorized to collect certain information on chemicals manufactured or processed in the United States. In addition, under TSCA section 8(b), the Agency is required to compile and keep current, via periodic inquiry, the Inventory of Chemical Substances in Commerce (TSCA Inventory). The TSCA Inventory is a listing of chemical substances manufactured, imported, and processed for commercial purposes in the United States. The Office of Pollution Prevention and Toxics (OPPT) has used the IUR to update the basic chemical production information for selected larger volume chemicals in the TSCA Inventory six times (every four years), beginning in 1986, and to collect additional information relating to the manufacture, processing, and use of those chemicals, beginning in 2006.
EPA is proposing to amend the current IUR requirements in order to clarify the reporting requirements, improve the quality and utility of the data submitted, better match data collected with the Agency’s overall information needs, and where possible, reduce the paperwork burden on both regulated entities and EPA. Manufacturers (including importers) would be required to use e-IURweb (the Agency-provided reporting tool) to submit a completed Form U (EPA Form 7740-8) electronically via the Internet. As proposed, the 2011 IUR would include additional manufacturing, processing, and use exposure-related data elements, including the volume of the chemical used on the reporting site; the volume of the chemical directly exported; whether the chemical is being recycled, remanufactured, reprocessed, reused, or reworked; and the production volumes for the previous years since the last principal reporting year. Additional amendments to the 2011 IUR would include changes to the reporting frequency from every five years to every four years; modifications to the method used to determine whether a manufacture (including importer) is subject to IUR reporting for reporting cycles subsequent to the 2011 reporting cycle; modifications to reporting thresholds, including eliminating the upper threshold; revisions to industrial classifications used; revisions to the list of commercial and consumer product categories; and modifications to situations in which confidentiality may be claimed and to the process for making such claims.
OPPT will use the updated IUR data in its chemical risk-management efforts. Individual sites manufacturing, including importing, chemicals will submit the required information. The information will be stored electronically for reference by EPA staff and others. Within the constraints of confidentiality claims, the information will be made public through the Agency’s IUR website (www.epa.gov/iur). Further discussion of how the information is used, stored, and collected is included in this document.
The collection is expected to involve an average of approximately 4,221 respondents at an annual cost of $40.3 million during the ICR addendum period. The details of the paperwork burden and cost estimates are discussed in this document.
2. Need for and use of the Collection
2(a) Need/Authority for the Collection
Under TSCA, EPA is required to identify, assess, and control risks of injury to human health and the environment posed by commercial chemicals. Under TSCA section 8(b), EPA is required to compile and keep current a complete list of chemical substances manufactured or processed in the United States. TSCA section 8(a) authorizes the Administrator to promulgate rules to provide for the maintenance and collection of records from manufacturers, importers, and processors of commercial chemicals. Sections 8(a)(1) and (2) of TSCA also authorize the Agency to collect information on the chemical manufacturing and importing industry. EPA possesses broad discretion in determining the information to be reported under TSCA section 8(a). The IUR rule currently is codified at 40 CFR 710. EPA is proposing to move the IUR rule to a new Part at 40 CFR 711.
EPA is proposing now to modify the IUR rule1 to meet four primary goals: (1) to tailor the information collected to better meet the Agency’s overall information needs; (2) to increase its ability to effectively provide public access to the information; (3) to obtain new and updated information relating to potential exposures to a subset of chemical substances listed on the TSCA Inventory; and (4) to improve the usefulness of the information reported. EPA believes requiring electronic reporting; collecting comprehensive information for most reported chemicals; and adjusting the specific reported information, the frequency the information is reported, and confidential business information (CBI) claim requirements would accomplish these goals.
The IUR provides basic exposure-related manufacturing, processing and use information used by EPA and others in a wide range of Agency activities. EPA’s efforts to use the 2006 IUR data have identified areas where further improvements are needed to improve risk-screening capabilities. Screening level data need not be precise, but should be accurate and reliable enough for the Agency to develop screening-level exposure assessments. The 2011 IUR would provide more detailed information needed to better understand and interpret the IUR data, further enhancing the Agency’s ability to identify, screen, and manage potential chemical risks. Because exposure is a key component of risk, the IUR exposure-related information would allow OPPT to screen chemicals based on the potential for risk in order to protect human health and the environment, as required by TSCA. The exposure-related data on manufacturing, processing, and use potentially allow the Agency and others to avoid more burdensome regulatory requirements. These enhanced data would allow EPA to conduct a more effective and efficient screening level review of chemicals to identify candidates for further evaluation.
2(b) Practical Utility/Users of the Data
The proposed modifications associated with reporting methods and changes to the reporting software would ensure the information reported to EPA is accurate and in compliance with the IUR requirements.
e-IURweb Reporting Software
Beginning with the 2011 submission period, EPA is proposing to require electronic reporting for all IUR submissions, including joint submissions and amendments. Persons submitting information under the IUR rule would be required to use e-IURweb, the Agency-provided, web-based software to complete Form U (the IUR reporting form). The information would be submitted electronically via the Internet, through EPA’s Central Data Exchange (CDX). Users of CDX are required to register with the system, including submitting an authorized signature agreement to EPA.
The 2011 submission process will be similar to that available for the 2006 IUR, when EPA provided an opportunity for IUR respondents to send their reports electronically, through CDX, on a voluntary basis. Approximately one-third of the 2006 submissions were filed through CDX, using the 2006 e-IUR downloadable software. The elimination of paper-based submissions in favor of electronic reporting, including the use of e-IURweb, is expected to greatly improve the reporting process, both for EPA and for manufacturers (including importers).
The 2011 e-IURweb would feature several improved capabilities over the 2006 e-IUR software. These improvements include an enhanced validation system, which would alert users when a required field on the form is either missing information or contains certain kinds of potentially incorrect information. EPA expects other updates to include automated chemical identity checks, automated company and site identity checks, and the facilitation of joint submissions and amendments.
Electronic submission would also ensure that IUR data would have completed a basic validation check, could be quickly incorporated into a database and ready for immediate Agency use, and would eliminate subsequent data entry errors which occurred with paper submissions. Furthermore, EPA believes the mandatory use of e-IURweb and CDX would reduce the reporting burden on industry by reducing both the cost and the time required to review, edit and transmit data to the Agency.
CDX Registration
Each IUR submission must have an associated authorized official. The authorized official signs the certification statement and submits the IUR report via CDX.. During the 2006 IUR, respondents were required to complete an electronic signature agreement (ESA) and to submit the agreement in hard copy with a wet ink signature to EPA in order to complete the CDX registration. For 2011 IUR reports, EPA is modifying the 2006 ESA to identify more clearly the individual(s) required to sign the agreement. To register in CDX, the CDX registrant (also referred to as “Electronic Signature Holder”) would download two forms: the Electronic Signature Agreement and the Verification of Company Authorizing Official. For identity authentication, the registrant would complete the ESA, sign and date it, have the form notarized, and mail it to EPA. The Verification of Company Authorizing Official form requires the signature of an immediate supervisor or witnessing official verifying that the registrant is the person who should sign their name in the certification statement box on Form U. Once EPA receives both forms, EPA would activate the registrant’s CDX account and send a notification via email.
Respondents may need or desire to have more than one individual complete an electronic signature agreement, so that other individuals can add information to an original IUR submission. Persons submitting supplemental information for an IUR submission on behalf of a company would need to register with CDX by signing an ESA and Verification of Support Submitter form. The Verification of Support Submitter form requires the signature of an immediate supervisor or witnessing official from the company and anyone he/she authorizes to submit additional information. The company official can authorize an unlimited number of support respondents, but will have to complete a form for each support respondent. Support respondents can work with an unlimited number of company officials, but will need an agreement with each company official (even if the company officials work at the same company). A support respondent may be an employee of the company, an outside consultant for the company, or an authorized representative agent for the company. While this individual would not be able to sign the certification statement required for the initial IUR submission, he or she would be able to provide additional information, if needed, using CDX. For 2006 and prior IUR submissions, respondents were not able to provide additional information electronically.
Data Elements for IUR Submissions
The IUR information collection is the only mechanism through which EPA can collect basic information on commercial chemicals listed on the TSCA Inventory of Chemical Substances, including production volume and other manufacturing (including importing) processing, and use exposure-related data. This information collection is necessary because these data are not otherwise available. EPA would use the information it is proposing to collect in the following ways:
(1) Parent company identification information: Company identification information is collected already by the current IUR. EPA is proposing to require reporting of additional company identification information associated with the location of the company, and is clarifying that the company information is to be for the ultimate domestic parent company associated with the reporting plant site. These data would help ensure the company information is consistently provided.
(2) Manufacturing-related information:
The production volume for each of the years since the last principal reporting year: The one-year snapshot of production volume provided by the current IUR does not provide an accurate picture of the chemicals in commerce, and may provide an erroneous view of the exposure scenarios associated with a particular chemical substance. The high turn-over of reported chemicals from one submission period to the next indicates a dynamic, changing industry. EPA has received comments from industry concerning the year-to-year changes that occur for chemical production that may affect IUR reporting. EPA would use these data for chemical manufacturing, and processing and use trend analyses; and for the assessment of the effectiveness of Agency and public programs, among other uses.
The production volume of a manufactured (including imported) chemical substance used at the reporting site: This data element would replace the data element indicating that a chemical is site-limited. This new data element identifies whether a chemical is used by the reporting site. A domestically manufactured chemical would be identified as a site-limited chemical (or that a portion of the chemical is site-limited). An imported chemical would be identified as used by the importer. This information is related to potential exposures associated with the on-site volumes, and would provide the Agency with more accurate information for exposure assessments and other data analyses.
Whether an imported chemical is physically at the reporting site: This data element would enable the Agency and others to better assess manufacturing-related potential exposures, thereby enabling more accurate information for screening-level analyses and other uses of the IUR data.
The production volume directly exported and not domestically processed or used: This data element would allow EPA to better identify the completeness of the reported processing and use information, by indicating the proportion of the production volume potentially covered by the reported processing and use information. IUR processing and use information is required only for domestic use situations, and is not required for any volumes directly exported.
Whether a manufactured (including imported) chemical, such as a byproduct, is being recycled, remanufactured, reprocessed, reused, or reworked: This data element would provide valuable information for Agency and public programs that encourage industry to find uses for wastes rather than disposing of the wastes, such as in a landfill.
These data would also be used in other ways, such as in chemical exposure and risk screening, testing and/or priority setting, and exposure estimation required by the Interagency Testing Committee (ITC) under TSCA section 4; for EPA monitoring activities of newly manufactured substances that have completed PMN review under TSCA section 5(a); to support the development of TSCA regulations under section 6; and to measure potential human and environmental exposure under TSCA section 8(e). Each data element would correspond to a data point necessary for basic risk-screening.
Under the current IUR rule, only manufacturers (including importers) of larger-volume chemicals are required to report processing and use information. As proposed, respondents would be required to report processing and use information for all reported chemical substances, regardless of production volume, unless the chemical substance is partially exempt. This proposed change provides exposure-related processing and use information to EPA and others for moderate-volume chemicals, enabling the Agency to address such chemicals in the same manner as the higher-volume chemicals.
(3) Industrial processing and use data: This proposal does not add any industrial processing and use data elements. The industrial function categories would be revised to better identify the functions of the chemical substances. The North American Industrial Classification System (NAICS) codes would be replaced with industrial sector (IS) codes. The IS codes reduce the number of choices available to the respondent, thus streamlining the reporting process and making the data easier to use.
(4) Consumer and commercial end-use exposure data: EPA is proposing to add two data elements: an indication of consumer or commercial use and the number of commercial workers. These data would be used to determine exposure potential based on consumer or commercial populations. These two populations are very different from each other, and the ability to distinguish uses between the two enables better exposure-based screening of the chemical. The number of commercial workers is needed to better assess the size of the commercial population. In addition, the product categories would be revised to better identify the uses of the chemical substances.
Special Considerations for Joint Respondents
In certain situations IUR respondents currently are allowed to report the IUR information jointly with the supplier of the chemical substance for which the respondent is reporting. For example, importers may not know the specific chemical identity of the imported Inventory substance because the foreign supplier chooses to keep the information confidential. In addition, a manufacturer may not know the specific chemical identity of the substance being manufactured because the supplier of a reactant used to manufacture the substance chooses to keep the information confidential. In such situations, the manufacturer (including importer) is still responsible for ensuring that the IUR information is submitted to EPA and may do so by submitting a joint report. For example, in the case of an imported substance, the U.S. importer, as the primary respondent, completes the majority of the required information on Form U, and provides a trade name in Part II.2.A.4 to identify the chemical substance. The primary respondent then contacts the foreign supplier, who is the secondary respondent, to notify them of the need to report the specific chemical identity information directly to EPA in the joint submission section (Part IV) of Form U, using the e-IURweb reporting software.
For 2006 and prior IUR submissions, respondents were not able to submit joint reports electronically. EPA has added Part IV to Form U to accommodate joint submissions. Because signatures are required by each party of a joint submission, they must each register with CDX, complete their own sections on Form U, and submit their own sections of the same report electronically to EPA. The secondary respondent will not be able to access the information provided by the primary respondent and vice versa. The reporting tool will match both submissions based upon company and chemical information provided by the manufacturer (including importer), acting as a primary respondent, and by the supplier, acting as a secondary respondent. EPA will also use the identifying number assigned to the primary respondent during CDX registration to link the joint reports in an internal database. The information provided by the primary and secondary respondents will be saved and combined as one joint submission. EPA will process the joint submission once all Form Us are received and matched by the Agency.
This information collection would allow EPA to connect submissions from the primary respondent and secondary respondent and to request clarification from the secondary respondent if needed. The data EPA proposes to collect would be utilized in the following ways:
Joint Submission Information (primary respondent only): The primary respondent provides only a trade name or other designation to identify the chemical substance being reported. Therefore, the requested data are needed by the Agency to identify the chemical substance correctly and provide the company name and complete mailing address of the secondary respondent.
Secondary Company Identification Information: These data would identify the secondary respondent’s company name and the complete mailing address of the company. The information provided would help ensure that the company information is provided consistently, and would be used to associate the secondary respondent’s company with the primary respondent’s company and site plant.
Technical Contact Information: The company’s technical contact information would provide EPA with the name and complete mailing address of the person who would be able to answer questions EPA may have about the reported chemical substance.
Primary Company Identification Information: These data would provide the primary respondent’s parent company name and the complete address of the company to help ensure that the company information is provided consistently, and would be used to associate the primary respondent’s company and site plant with the secondary respondent’s company.
Trade Product Identification Information: These data would identify the primary respondent’s company name, site name, and site address, as well as the CAS Registry Number, the appropriate specific chemical identities, and product composition. These data are needed by EPA to combine the secondary respondent’s chemical-specific information with the primary respondent’s information to result in a complete IUR submission.
Information secured through the IUR collections is used increasingly by a wide variety of governmental and non-governmental users. Consistent with Congress’s intent that TSCA data be used to facilitate any government public health and environment efforts, IUR data have been used by EPA’s Office of Water, Office of Solid Waste and Emergency Response, and Office of Air and Radiation to identify and characterize particular chemical substances. Non-confidential IUR data are incorporated into a number of databases and products maintained by organizations including Right-To-Know-Net and INFORM2. IUR data were used to identify chemicals of particular concern for the National Institutes of Health. Non-confidential IUR data were also released to selected states to help them identify facilities manufacturing suspected endocrine disrupters.
Data reported under the 2011 IUR would enhance the capabilities of the Agency and other Federal agencies to ensure risk management actions are taken on chemicals posing the most concern. More in-depth reporting of the processing and use data, more careful consideration of the need for confidentiality claims, and adjustments to the specific data elements would better support a robust risk assessment and management program. By enhancing the data supplied to Agency risk-screening programs, EPA expects to more effectively and expeditiously identify and address potential risks posed by chemicals and provide improved access and information to the public.
EPA would also use the information submitted through the 2011 collection to update the Agency’s comprehensive chemical manufacturing, exposure, and use database, maintained as part of the Manage Toxic Substances (MTS) system. IUR data prior to the 2006 collection are maintained in a series of databases known as the Chemical Update System (CUS). The MTS IUR data, combined with CUS and the Chemicals in Commerce Information System (CICIS) database, serves as a primary source of information about the chemical industry for EPA, as well as other Federal Agencies. The MTS IUR data provide information about the chemicals used, where they are produced, how much is produced or imported, and how they are processed and used. The chemical industry is dynamic, as demonstrated by the approximately 30 percent change in chemicals reported from one IUR submission period to the next; therefore continual updating of the database is essential.
3. Non duplication, Consultations, and Other Collection Criteria
3(a) Non duplication
The data included in this information collection (i.e., production volume, chemical manufacture, exposure, and processing and use data) are not collected comprehensively or systematically at the national level. There are a variety of sources for certain data elements, but the sources are either incomplete or incompatible.
3(b) Public Notice Required Prior to ICR submission to OMB
The proposed rulemaking serves as the public notice for this ICR. Interested parties should submit comments referencing Docket ID No. EPA-HQ-OPPT-2009-0187 to the address listed at the end of this document. Responses will be taken into account in developing the final rulemaking.
3(c) Consultations
In addition to the public notice and comment period, OMB regulations, at 5 CFR 1320.8(d)(1), require agencies to consult with potential ICR respondents and data users about specific aspects of an ICR before the agency submits the ICR to OMB for review and approval. In accordance with this regulation, EPA will solicit consultation feedback from nine potential respondents and data users with respect to this final rule ICR.
3(d) Effects of Less Frequent Collection
EPA is proposing to return the reporting frequency to every four years, which was the frequency in effect from 1986 to 2006. In an effort to reduce the reporting burden associated with the 2003 amendments, EPA had changed the reporting frequency to every five years. While the less frequent reporting does reduce burden, a review of the 2006 IUR reporting has revealed an approximately 30 percent change in the chemicals that are reported from one submission period to the next. EPA now believes that reporting every five years does not provide data sufficiently current to meet Agency and public needs and now considers every four years to be the minimum acceptable frequency. In addition, the IUR Modifications proposed rule requests comment on even more frequent reporting. Comments from industry representatives have led EPA to believe that more frequent reporting creates efficiencies, both for the respondent and for EPA. With more frequent reporting, companies would be able to establish standard systems and practices to collect the required information.
The Agency needs to be able to make accurate chemical risk management decisions in a timely and cost effective manner, especially because alternative data sources do not exist for these data. The effect of less frequent collection of these data is to diminish significantly the Agency’s ability to understand the chemical industry and monitor the production levels of chemicals manufactured (including imported) in the United States. As described above, the IUR data demonstrate that chemical industry product lines and manufacturing in the United States change substantially from one submission period to the next. The Agency needs up-to-date information in order to fulfill its mandate to keep the TSCA Inventory current under section 8(b) of TSCA; collecting the data every five years means the Agency is working with data which are potentially six or more years old, and therefore, cannot be considered current.
Less frequent collection could result in EPA using outdated information in its decision making. For example, changing market conditions, batch processing, or the development of new uses for the chemical can cause production volumes or chemical uses to change from one year to the next. Companies buy and sell plant sites, and the chemicals produced at a site can change. Based on past IUR reporting, the Agency would be working with the IUR data which approximately 30 percent of the chemical substances known to be in commerce at volumes of 25,000 lb. or more may not actually be in commerce at those levels.
3(e) General Guidelines
This collection does not exceed any of the Paperwork Reduction Act guidelines at 5 CFR 1320.6, with the exceptions listed below.
The record retention period of this collection is five years, as specified in 40 CFR 710.57, exceeding the PRA maximum of three years. This is necessary to ensure companies retain records long enough to facilitate completion of Form U (EPA Form 7740-8) in the next collection, which is in four years (as proposed), and to allow EPA’s enforcement activities to overlap two IUR reporting cycles.
Confidential Business Information (CBI) claims limit access to the IUR data, especially by the public. EPA recognizes that some information submitted to the Agency is legitimately confidential business information; because of this, EPA’s review of CBI data is an inherently governmental function that EPA must perform to protect human health and the environment.
3(f) Confidentiality
Respondents may claim information reported to EPA under this rule as confidential if such information would reveal the respondents’ trade secrets or proprietary information as defined by TSCA section 14 and existing TSCA regulations. EPA has long-established procedures for handling, storing, processing, and disposing of TSCA confidential information. Transfers of this information to other governmental agencies can be made only if the other agency agrees to adhere to all TSCA confidentiality provisions. EPA will maintain standard CBI procedures to protect any confidential, trade secret, or proprietary information from disclosure in accordance with EPA’s confidentiality regulation, 40 CFR Part 2, Subpart B.
3(g) Sensitive Questions
This collection does not include questions of a sensitive nature.
4. The Respondents and the Information Requested
4(a) Respondents/NAICS Codes
The regulated community consists of companies manufacturing or importing chemicals listed on the TSCA Inventory and regulated under TSCA section 8. In general, the industry segments that compose the regulated community for the rule are those that produce or import organic and inorganic chemicals. Most respondents previously reported information under the IUR. The Agency’s previous experience with IUR collections has shown that the majority of the respondents affected by this collection activity are from the following North American Industrial Classification System (NAICS) code categories:
325 - Chemical Manufacturing (including importing)
324 - Petroleum and Coal Product Manufacturing (including importing)
The subsectors identified above represent the designation of sites that likely would be subject to IUR reporting. However, activities at these sites may vary, making identification of the regulated community more difficult. For example, NAICS codes reflect a site’s primary activity, omitting substantial participation a company may have in other industry activities. Second, NAICS codes selected by parent companies reflect the parent company’s primary activity, even though many parent companies are primarily holding companies with small subsidiaries. Each of these small subsidiaries may belong in a completely different industry classification based on its own primary activity. Information on parent company NAICS codes does not provide a very accurate characterization of the types of sites subject to reporting, and facilities that do not fall under these categories must still report if they meet the reporting criteria.
Generally, TSCA section 8 excludes small manufacturers (including importers) from reporting. EPA defines small manufacturers (including importers) for purposes of IUR and certain other reporting in 40 CFR 704.3.
In addition to the anticipated respondents listed above, manufacturers (including importers) of byproducts may be required to report under the IUR rule. Byproduct manufacturers (including importers) may be listed under a different primary activity for a site, such as NAICS codes 22, 322, 331, and 3344; e.g., utilities, paper manufacturing, primary metal manufacturing, and semiconductor and other electronic component manufacturing. For purposes of the IUR, a byproduct is a chemical substance produced without a separate commercial intent during the manufacture, processing, use or disposal of another chemical substance or mixture (40 CFR 704.3). Such a chemical substance, like any other manufactured chemical substance, is subject to IUR reporting if it is manufactured, is listed in EPA’s Master Inventory File, is not otherwise excluded from reporting, and its manufacturer is not specifically exempted from IUR reporting requirements. For instance, a manufacturer (including importer) that uses a chemical substance in the production of an article may produce a byproduct substance that is chemically different from the starting substance; the manufacturer (including importer) therefore may incur reporting obligations under the IUR for that byproduct. While some manufacturers (including importers) of byproducts may not have reported to the IUR in the past, they should be aware now of their reporting obligations under the IUR rule.
4(b) Information Requested
Data elements, including record keeping requirements
The IUR data elements are related to or indicative of three components of exposure. These components are: (1) the number of ecosystems or size of human populations potentially exposed, (2) the potential human or environmental exposure concentrations, and (3) the frequency and duration of potential exposures. The data enhances EPA’s ability to evaluate each of these components of exposure. Respondents are required to submit certain known or reasonably ascertainable manufacturing, processing, and use exposure-related information. For the 2006 IUR, respondents were required to submit the processing and use information to the extent it was readily obtainable; the IUR Modifications rule proposes to standardize all of the submitted information to the single “known to or reasonably ascertainable by” reporting standard.
Using e-IURweb, individuals would report the proposed data elements as follows (the following list identifies new or revised data elements and does not address the full data requirements):
Authorized Company Official’s e-mail Address. The e-mail address of the company official authorized to sign and submit the IUR Form U.
Parent Company Name and Address. The name and mailing address of the ultimate domestic parent company. The requirement that the ultimate parent company reported be the ultimate domestic parent company is part of the IUR Modifications proposal; the parent company name is already required by the IUR. This is not a new requirement; rather the change better identifies the correct company name.
Manufacturing Information. The production volume for each of the years since the last principal reporting year; the volume of the reported chemical substance used at the reporting site; whether an imported chemical is physically at the reporting site; the production volume directly exported and not domestically processed or used; and whether a manufactured (including imported) chemical, such as a byproduct, is being recycled, reused, reprocessed, remanufactured, or reworked.
Processing and Use Information. For the 2006 IUR, only those manufacturers (including importers) of chemicals with production volumes of 300,000 lb. or more were required to report processing and use information. The IUR Modifications rule proposes to require respondents to report this information for all reported chemicals, unless the chemical is specifically partially exempted.
Industrial Processing and Use Data. For the 2006 IUR, respondents were able to select up to 10 unique combinations of the type of process or use (code), NAICS code associated with the industrial use, and industrial function category. The IUR Modifications rule proposes to revise the list of industrial function categories and to replace the NAICS codes with Industrial Sectors.
Consumer and Commercial Use Data. For the 2006 IUR, respondents were able to select up to 10 consumer and commercial product categories. The IUR Modifications rule proposes to revise the list of consumer and commercial product categories. The respondent must indicate whether the use is consumer, commercial or both, and, if commercial, the number of reasonably likely to be exposed commercial workers must be reported as a range.
Joint Submissions
Joint submissions are allowed only in those instances where a supplier will not disclose to the respondent the specific chemical name of the imported Inventory substance or of a reactant used to manufacture the Inventory substance. This may happen, for instance, when a company is importing a mixture under a trade name, and the foreign manufacturer does not want to reveal the components in the mixture. (See Special Considerations for Joint Respondents in section 2b, above) Proposed changes to the IUR would make it easier for respondents to use electronic reporting for both the primary and the secondary portions of a joint submission. In addition to signing the certification statement and completing Parts I, II, and III, primary respondents would report on proposed data elements in Part II, Blocks 2.A.4 through 2.A.10, on Form U as follows (see Attachment A):
Joint Submission Information. Trade name of the chemical substance being reported, secondary company name, and complete mailing address (city, state, zip code, and country (if applicable)).
Secondary respondents would register with CDX and request to submit a joint report by selecting “Joint submission-as secondary respondent” on the main page of the e-IURweb reporting tool. They would provide the primary company’s information and the trade product name, supplied to them by the primary respondent, to gain access to the Form U containing information specific to the trade product name. After the secondary respondent is granted access to the form, it would report on proposed data elements in Part IV on Form U as follows (see Attachment A):
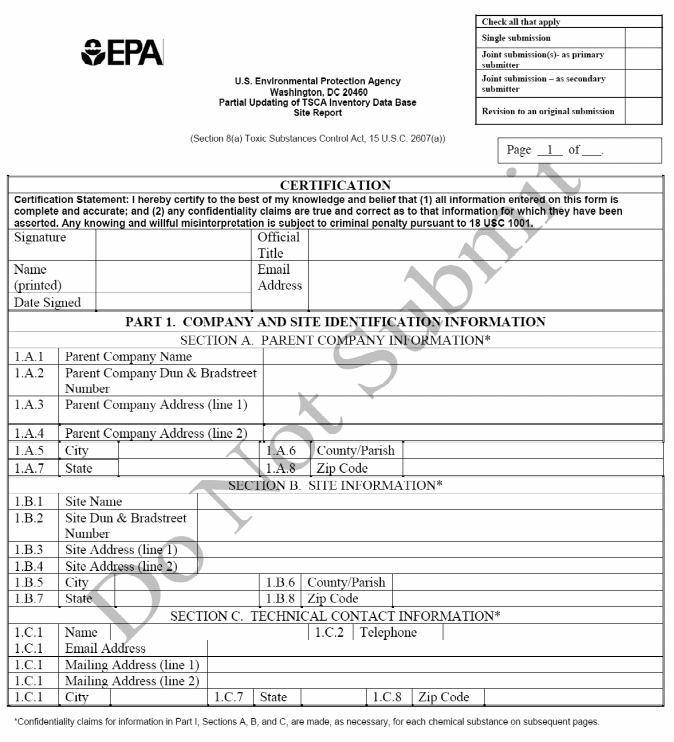
Certification. The company official must certify by signature and date that to the best of his/her knowledge and belief: 1) all information entered on Form U has been completed in compliance with the regulatory requirements; and 2) any confidentiality claims are true and correct as to that information for which they have been asserted.
Secondary Company Information. The secondary company name and complete mailing address (city, state, zip code, and country (if applicable)).
Secondary Technical Contact Information. The technical contact name, phone number, complete mailing address (city, state, zip code, and country (if applicable)), and email address.
Primary Company Information. The primary company name, site name, and complete mailing address ((city, state, and zip code).
Trade Product Information. The trade product name, chemical name, CAS Registry Number, ID code, and percentage of each chemical component of a trade product.
(ii) Respondent Activities/Information Collections (ICs)
EPA identified the following ICs in the currently-approved ICR (EPA ICR No. 1884.04) for activities that respondents would complete when complying with the existing rule:
Compliance Determination;
Rule Familiarization;
Report Preparation and Submission – Partial Reports;
Report Preparation and Submission - Full Reports; and
Recordkeeping.
As a result of this proposed rule, EPA would include a new IC addressing the initial paperwork activities a company would need to complete in order to be able to submit reports electronically through CDX. EPA also would incorporate recordkeeping activities into the ICs that address the preparation and submission of partial and full reports, rather than include them as a stand-alone IC (see Table 1).
Table 1: Information Collections (ICs) for IUR Reporting
Information Collection |
Description |
Compliance Determination |
Determine whether reporting is required for a chemical manufactured (including imported) at a particular site, based on the chemical’s production volume and the applicability of certain reporting exemptions.
Beginning with reporting cycles subsequent to the 2011 reporting cycle, the proposed rule would modify the method used to determine whether a manufacturer (including importer) is subject to IUR reporting. For example, for a 2015 submission period, reporting would be required if the production volume of a chemical substance met or exceeded the 25,000 lb. threshold in the 2014 principal reporting year for the 2015 submission period, and in any calendar year since the last the last principal reporting year (i.e., since 2010 for the 2011 reporting cycle) |
Rule Familiarization |
Become familiar with the full requirements of the rule, which entails reading the rule, understanding the various reporting and administrative requirements, and determining the manner in which reporting requirements will be met for each chemical. |
CDX Registration Activities |
The proposed rule would require electronic reporting, and therefore would require that all respondents complete the CDX registration process. As part of registering with CDX, each respondent would provide identifying information that would comprise all or most of the information requested in Part I of Form U (EPA Form 7740-8). This information will then be pre-populated whenever the respondent prepares a partial or full report.
In order to submit electronically to EPA via CDX, the respondent’s authorized official, who will be signing and submitting the site’s IUR Form U, must register with CDX and must submit an Electronic Signature Agreement (ESA) and a Verification of Company Authorizing Official form. This is the same process required for electronic submissions for the 2006 IUR.
Once the registration process is completed, the registered individual would receive a case number and be able to access the e-IURweb reporting software. |
Prepare and Submit Report, and Maintain Records- Partial Report |
Compile the required information, determine the CBI status of information and fulfill appropriate substantiation measures, and use e-IURweb to complete and submit only Parts I and II (and Part IV, if a joint submission) of Form U (EPA Form 7740-8) for chemicals specifically listed in the proposed regulation for which there is a partial reporting exemption. Retain all records related to the submission for five years after the submission period. |
Prepare and Submit Report, and Maintain Records- Full Report |
Compile the required information, determine the CBI status of information and fulfill appropriate substantiation requirements, and use e-IURweb to complete and submit Parts I, II, and III (and Part IV, if a joint submission) of Form U (EPA Form 7740-8) for all reportable chemical substances. Retain all records related to the submission for five years after the submission period. |
5. The Information Collected–Agency Activities, Collection Methodology, and Information Management
5(a) Agency Activities
The activities routinely conducted by EPA related to the processing, analysis and storage of the information collected under this rule include the following:
Review and verify forms as they are received;
Answer respondent questions and provide any necessary assistance;
Process submissions for inclusion in IUR database;
Review requests for confidentiality in the submissions;
Maintain the database; and
Distribute the data.
5(b) Collection Methodology and Management
The next IUR collection will occur in 2011. All manufacturers (including importers), except for those defined as “small manufacturers” by EPA’s regulations, are required to submit information on every substance subject to this proposed rule that they manufacture (including import) in quantities that meet or exceed the IUR thresholds. As proposed, the collection would occur every four years.
(i) Collection Methodology
Respondents would be required to submit information associated with this data collection electronically via the Internet using e-IURweb and CDX. The 2006 IUR allowed and encouraged electronic reporting, thus providing both respondents and the Agency the opportunity to test an electronic reporting system. Comments from respondents and lessons learned from the collection of paper, CD, and electronic submissions were used to develop the 2011 collection methodology. Some of the changes to this methodology are regulatory and are included in the IUR Modifications proposal; others are process-oriented or internal to EPA or the respondent.
Potential respondents would be notified of the need to report in three ways: (1) EPA plans to publish a Federal Register notice, (2) email notices will be sent to previous IUR respondents, and (3) articles will be published in the trade press. Reporting materials, including a non-submission version of the e-IURweb reporting software and a variety of guidance documents (Instruction Manual, Q&As, Case Studies), will be available on EPA’s IUR website. Respondents also can obtain these materials from the TSCA Hotline. Respondents obtain the submission version of the e-IURweb reporting software as part of the CDX registration process, as described in section 4 of this document.
As proposed, EPA will receive all IUR submissions electronically. The CDX registration process, required for all respondents, would provide a case number which the respondent would use to identify its submission and which the Agency would use to track any additional information or changes related to that submission. EPA believes this is an improvement over the 2006 IUR, which resulted in the generation of several identifying numbers by either the respondent or EPA.
Information quality and validation begins with the e-IURweb reporting tool, which will be programmed to help the respondent provide the information required, in the correct format as required by the IUR rule. Use of e-IURweb will eliminate many of the problems with incorrect chemical identifications experienced in the past by providing a current listing of the TSCA Inventory chemicals and their associated identification numbers. Other respondent-generated errors, such as incorrect codes, will also be eliminated due to the use of techniques such as drop-down menus, restrictions on the specific information that can be entered, and error-checking algorithms.
Mandatory IUR reporting via the Internet is the most efficient collection method for respondents and EPA. Respondents receive almost immediate notification that EPA has received their submission, and EPA is able to upload the information directly into the IUR database, which improves the efficiency of EPA’s data receipt and processing activities. This collection method also eliminates the introduction of errors by avoiding the need to scan or key-enter data submitted on paper or CD. Additional validations will be programmed into the data-entry system to further ensure the quality of the data.
To aid persons subject to this information collection, the Agency’s TSCA and CDX Hotlines are available to answer questions regarding the IUR requirements or submission process. When Hotline staff are unable to answer questions, the respondent is referred to OPPT’s Information Management Division (IMD) or Chemical Control Division (CCD), as appropriate. Other Divisions within OPPT or OEI are used as necessary.
(ii) Data Management
This section describes the Agency tasks required for efficiently processing submissions under the IUR. The tasks for which the Agency is responsible are presented under four main categories: database systems development, guidance document development, Form U processing, and additional tasks. The task descriptions presented below generally do not change from collection to collection.
IUR data is stored in a database managed by the Agency. Once updated, the IUR database is then available to EPA technical reviewers to search or export into their various analytical modeling systems and databases. The IUR database is also available for quick screening and other direct uses. The Agency makes publicly available as much information as possible, within the confines of protecting CBI.
Database Systems Development and Maintenance -- The Agency is responsible for having adequate information systems in place to support the database that serves as the primary data storage medium for IUR collections. File servers with appropriate backup are used to contain the IUR databases. Following the 2006 IUR collection, EPA updated the technology used to store the IUR data, storing it in a larger Manage Toxic Substances (MTS) database. In addition, IUR data are tracked via the correspondence tracking system utilized by the Confidential Business Information Tracking System (CBITS) located within the Confidential Business Information Center (CBIC).
Guidance Document Development -- The Agency is responsible for developing guidance to assist respondents in complying with IUR requirements. The guidance documents usually are developed by a contractor with oversight by Agency personnel.
Form U Processing -- The Agency is responsible for processing IUR Form U submissions. This includes developing standard operating procedures and documentation for all stages in the IUR document life cycle, document receipt and tracking, data input, quality control, file and database maintenance, information security, CBI aggregation policy, data dissemination, and staff training. For the 2011 IUR submission period, EPA will develop new processes to receive IUR submissions over the Internet, using CDX.
Additional Activities -- The Agency develops various supporting documents associated with the reporting software and makes them available on the Internet. . In addition, the Agency is responsible for providing the TSCA Hotline with standardized responses for frequently asked questions; preparing mailings, mailing lists, and labels; and developing outgoing information materials.
5(c) Small Entity Flexibility
The current IUR regulation provides ample flexibility to small entities. This regulation affects only businesses -- governmental jurisdictions and not-for-profit organizations are not required to take any action. Small manufacturers (including importers), in accordance with TSCA section 8(b) (40 CFR Sections 710.29 and 710.28), are exempt and therefore are generally not subject to any of the reporting or recordkeeping requirements. A manufacturer (including importer) is considered a small business if (1) the firm’s total annual sales when combined with those of its parent company (if any) are less than $40 million for the submission period and (2) its total production and/or importation of the chemical substances, mixture or category, for the reporting period, does not exceed 100,000 pounds (45,000 kilograms) at an individual site owned and controlled by the firm. The Economic Analysis for the Proposed Inventory Update Reporting Modifications Rule determined that the impact on these companies is, on average, significantly less than one percent of revenues (EPA 2009).
5(d) Collection Schedule
The submission period shall be from June 1, 2011 to September 30, 2011. This submission period/schedule follows the requirements of 40 CFR 710.53 (proposed 40 CFR 711.13).
6. Estimating the Burden and Cost of the Collection
This section presents the burden and costs estimates incurred by all affected entities as a result of the proposed IUR rule amendments. This addendum to the supporting statement covers the years 2011, 2012, and 2013. Therefore, it provides burden and cost estimates for the information collection corresponding to the 2011 reporting cycle (which is the first submission after the proposed amendments to the IUR rule are finalized), and also for the information collection corresponding to the next reporting cycle, for which respondents will be preparing during 2012 and 2013. Even though reporting occurs only once per reporting cycle (once every five years for the 2011 cycle and, as proposed, every four years in the future), EPA believes that rule compliance and data collection activities, and thus, costs and burdens, are incurred over the course of the reporting cycle. Therefore, for purposes of this analysis, the burden and cost for one reporting cycle are averaged over the number of years in the reporting cycle and are presented here as average annual figures. All costs are presented in year 2008 dollars. The IUR requires reporting on a “per site” basis rather than a “per company” basis. Therefore, each site is considered a respondent and is likely to submit one Form U containing one or more chemical-specific reports. EPA estimates that a total of 4,085 respondents will respond to this information collection in the 2011 reporting cycle, and 4,289 respondents will respond in future reporting cycles.
Burden and cost calculations are based on the assumption that EPA will receive an average of 29,253 full reports and 618 partial reports during the first reporting cycle, and 30,716 full reports and 649 partial reports in future reporting cycles. Each report is for a single chemical/site combination. Each site is expected to submit an average of 7.16 full reports and 0.15 partial reports. The average burden per respondent, which is one site, is estimated to be 792 hours for the 2011 reporting cycle, or 158 hours annually over a five-year cycle. For future four-year reporting cycles, the average burden per respondent is estimated to be 615 hours per cycle, or 154 hours annually, over a four-year cycle. Given that this ICR addendum covers a time period that spans both the first and a future reporting cycle, the average annual burden over the three year period of 2011 through 2013 is 155 hours. This is higher than the burden estimated for the currently approved ICR) (EPA ICR No. 1884.04, due to both program changes and an adjustment in reporting requirement criteria, including additional data requirements and modifications to reporting thresholds.
6(a) Estimating Respondent Burden
For the 2011 reporting cycle, each manufacturing site (including importers) must submit a Form U if the site meets or exceeds the 25,000 lb. threshold for at least one chemical in the 2010 principal reporting year. In future cycles, this reporting threshold will apply for any calendar year since the last principal reporting year. For purposes of the ICR, one manufacturing site is equivalent to one respondent. Form U contains four Parts. Part I contains basic site identification information and must be completed by all sites. Part II contains manufacturing data (production volumes, etc.) specific to each chemical, which also must be completed by all sites. Together, Part I and Part II are considered a “partial report.” Part III contains processing and use information. Under the proposed amendments, Part III would be completed for all chemicals unless the chemical is specifically exempted from the requirement to do so. Part IV contains secondary company identification information and specific information identifying a chemical substance. Part IV is completed only by a secondary respondent (see Section 2(b)). For purposes of this analysis, burden and costs associated with Part IV are considered part of the burden and costs estimates of Part I (respondent identification) and the beginning of Part II (chemical identification), and therefore were not separately calculated. Together, Parts I, II, and III (and Part IV, when applicable) are considered a “full report.” Each report is submitted for a unique chemical/site combination; that is, a site must complete a separate report for each applicable chemical, but Part I of Form U is completed only once per site. EPA anticipates that it will be completed when respondents register with CDX. To comply with the regulation, manufacturers (including importers) must complete the following activities in Table 2. Table 2 also provides a cross-walk of the related Information Collection that corresponds to each activity.
Table 2: Cross-Walk between Industry Activities and Related Information Collections (ICs)
Activity |
Description |
Related IC(s) |
Compliance Determination |
Site staff must determine whether reporting is required for a chemical manufactured (including imported) at a particular site, based on the chemical’s production volume and the applicability of certain reporting exemptions. This involves determining whether the production volume of a chemical substance met or exceeded the 25,000 lb. threshold in the 2010 principal reporting year for the 2011 submission period. In future reporting cycles, site staff must determine whether the production volume met or exceeded the threshold in the associated principal reporting cycle and in any calendar year since the last principal reporting year in future reporting cycles. |
Compliance Determination |
Rule Familiarization |
Site staff must familiarize themselves with the requirements of the rule. Staff from sites that reported previously must become familiar with new requirements, and staff from sites new to reporting must become familiar with all requirements. This entails reading the rule, understanding the various reporting and administrative requirements, and determining the manner in which the reporting requirements will be met. |
Rule Familiarization |
CDX Registration |
Before submitting Form U, all respondents must register with CDX. In addition, respondents must complete an Electronic Signature Agreement form, which is signed, dated, and notarized; complete a Verification of Company Authorizing Official form, which is signed by an immediate supervisor or witnessing official; and mail both forms back to EPA. |
CDX Registration Activities |
Preparation of Reports |
Site staff must collect all of the required information, and complete partial and/or full reports using a Form U report form, for each of the reportable chemicals at that site. The information must be reviewed, and submitted to EPA. This task involves any research necessary to identify the correct information to report, the act of completing Form U (technical and clerical burden), and managerial review. Once Form U is completed, company staff must submit it electronically to EPA via CDX. |
Prepare and Submit Report, and Maintain Records- Partial Report
Prepare and Submit Report, and Maintain Records – Full Report |
Recordkeeping |
Respondents must keep records supporting their submissions for five years. |
Prepare and Submit Report, and Maintain Records - Partial Report
Prepare and Submit Report, and Maintain Records- Full Report |
Burden estimates were derived originally from a survey conducted by EPA in 1996 (under OMB Control No. 2070-0034) to assess the potential burden associated with the IUR, as amended at that time. The survey was distributed to previous IUR respondents selected from the IUR database. Burden estimates were updated for a 2005 amendment to the rule as described in Economic Analysis of IUR Modifications Final Rule (EPA, 2005)3. Burden estimates for new reporting elements in the current proposal were derived as described in the Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b).
Table 3 and Table 4 illustrate the burden for a typical respondent on a per-activity basis, including time required to complete each section of Form U. Because IUR reporting for all respondents occurs within a required timeframe, which EPA is proposing to change from once every five years to once every four years, the Agency expects only one collection to occur, in 2001, during the three-year period covered by this ICR addendum. Therefore, burden hour estimates for 2011 (Table 3) are based on the first reporting cycle burdens in the Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b). Burden hour estimates for 2012 and 2013 (Table 4) are based on the future reporting cycle burdens as also described in the economic analysis (EPA, 2010b).4 The section-by-section industry burden estimates for report preparation also include the burden of compliance determination and rule familiarization. EPA estimates the total industry burden for completing and submitting one partial report to be 57.36 hours, and the estimated burden for completing and submitting one full report, to be 140.38 hours in the first reporting cycle. Each site is expected to submit an average of 7.16 full reports and 0.15 partial reports.
EPA calculated burden estimates for each element of Form U individually using the 1996 survey results, economic analyses for other rules with similar requirements (such as the Premanufacture Notification Electronic Reporting final rule), and EPA’s best professional judgment. More detailed information on the derivation of these estimates is found in the IUR EA (EPA, 2010b).
Table 3: Total Industry Burden, by Activity, First Reporting Cycle
Activity |
Clerical Burden (hours) |
Technical Burden (hours) |
Managerial Burden (hours) |
Total Burden (hours) |
||||||
(a) |
(b) |
(c) |
(d) = (a)+(b)+(c) |
|||||||
PREPARATION OF REPORT (Includes rule familiarization associated with each data element) |
||||||||||
Part I. Site Identification Information |
||||||||||
|
Certification |
0.00 |
0.85 |
1.01 |
1.86 |
|||||
|
Company Information (Parent Company Name, D&B Number, Mailing Address, Technical Contact, Technical Contact Mailing Address) |
0.00 |
0.04 |
0.02 |
0.06 |
|||||
|
Plant Site Identification (Site Name, D&B Number, Mailing Address) |
0.00 |
0.06 |
0.02 |
0.08 |
|||||
|
Total for Part I |
0.00 |
0.95 |
1.05 |
2.00 |
|||||
Part II. Manufacturing Information |
||||||||||
|
Site-Limited, Activity, Production Volume (lb.) |
0.00 |
2.28 |
0.56 |
2.84 |
|||||
|
Chemical Identification Upfront CBI Substantiation |
0.00 |
1.45 |
0.77 |
2.22 |
|||||
|
Plant Site Upfront Substantiation |
0.00 |
0.83 |
0.51 |
1.34 |
|||||
|
Total Number of Workers |
0.00 |
1.43 |
0.59 |
2.02 |
|||||
|
Maximum Concentration, Physical Form, Percent Volume of Production |
0.00 |
2.79 |
1.07 |
3.86 |
|||||
|
Production Volume for Each of the Years since Last Principal Reporting Year |
0.00 |
4.10 |
1.01 |
5.11 |
|||||
|
Production Volume Used On-Site |
0.00 |
0.20 |
0.05 |
0.25 |
|||||
|
Whether Imported Chemical is Physically at Reporting Site |
0.00 |
0.11 |
0.03 |
0.14 |
|||||
|
Volume Exported |
0.00 |
1.03 |
0.25 |
1.28 |
|||||
|
Whether a Chemical is to be Recycled, Remanufactured, Reprocessed, Reused, or Reworked |
0.00 |
0.11 |
0.03 |
0.14 |
|||||
|
Total for Part II |
0.00 |
14.34 |
4.85 |
19.19 |
|||||
Part III. Processing and Use Information |
||||||||||
|
|
Upfront Substantiation for Processing and Use Information CBI Claims |
0.00 |
0.43 |
0.26 |
0.69 |
||||
|
Industrial Processing and Use Exposure-Related Data |
|||||||||
|
|
Determination of Applicability |
0.00 |
1.01 |
0.28 |
1.29 |
||||
|
|
Industrial Function Category |
0.00 |
4.67 |
2.07 |
6.73 |
||||
|
|
Sector |
0.00 |
0.94 |
0.40 |
1.33 |
||||
|
|
Percent of Production Volume |
0.00 |
10.00 |
5.27 |
15.27 |
||||
|
|
Total Number of Processing and Use Sites |
0.00 |
9.14 |
3.52 |
12.67 |
||||
|
|
Total Number of Potentially Exposed Workers |
0.00 |
15.43 |
3.83 |
19.26 |
||||
|
Consumer and Commercial Use Exposure-Related Data |
|||||||||
|
|
Determination of Applicability |
0.00 |
0.94 |
0.25 |
1.19 |
||||
|
|
Identification of Production Category/Use by Children |
0.00 |
1.68 |
0.25 |
1.92 |
||||
|
|
Percent of Production Volume |
0.00 |
1.26 |
0.45 |
1.71 |
||||
|
|
Maximum Concentration by Category |
0.00 |
1.36 |
0.34 |
1.70 |
||||
|
|
Number of Commercial Workers Reasonably Likely to be Exposed |
0.00 |
15.43 |
3.83 |
19.26 |
||||
|
Total for Part III |
0.00 |
62.26 |
20.76 |
83.02 |
|||||
COMPLIANCE DETERMINATION |
||||||||||
|
Compliance Determination |
0.00 |
2.50 |
0.00 |
2.50 |
|||||
RULE FAMILIARIZATION |
||||||||||
|
Rule Familiarization |
0.00 |
19.00 |
9.00 |
28.00 |
|||||
CDX REGISTRATION ACTIVITIES |
||||||||||
|
|
CDX Registration |
0.00 |
0.73 |
0.18 |
0.92 |
||||
|
|
CDX Electronic Signature Agreement |
0.00 |
1.00 |
0.75 |
1.75 |
||||
|
Total for CDX Registration Activities |
0.00 |
1,73 |
0.93 |
2.67 |
|||||
RECORDKEEPING |
||||||||||
|
Recordkeeping |
0.75 |
1.50 |
0.75 |
3.00 |
|||||
TOTAL BURDEN |
||||||||||
Burden for one Partial Report (Parts I, II, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
0.75 |
38.29 |
15.65 |
57.36 |
||||||
Burden for one Full Report (Parts I, II, III, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
0.75 |
100.55 |
36.41 |
140.38 |
||||||
Note: Totals may not sum due to rounding. |
||||||||||
Source: Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b). |
||||||||||
Table 4: Total Industry Burden, by Activity, Future Reporting Cycles
Activity |
Clerical Burden (hours) |
Technical Burden (hours) |
Managerial Burden (hours) |
Total Burden (hours) |
||||||
(a) |
(b) |
(c) |
(d) = (a)+(b)+(c) |
|||||||
PREPARATION OF REPORT (Includes rule familiarization associated with each data element) |
||||||||||
Part I. Site Identification Information |
||||||||||
|
Certification |
0.00 |
0.85 |
1.01 |
1.86 |
|||||
|
Company Information (Parent Company Name,, D&B Number, Mailing Address, Technical Contact, Technical Contact Mailing Address) |
0.00 |
0.01 |
0.00 |
0.01 |
|||||
|
Plant Site Identification (Site Name, D&B Number, Mailing Address) |
0.00 |
0.01 |
0.00 |
0.02 |
|||||
|
Total for Part I |
0.00 |
0.95 |
1.05 |
2.00 |
|||||
Part II. Manufacturing Information |
||||||||||
|
Site-Limited, Activity, Production Volume (lb.) |
0.00 |
1.82 |
0.45 |
2.27 |
|||||
|
Chemical Identification Upfront CBI Substantiation |
0.00 |
1.16 |
0.61 |
1.77 |
|||||
|
Plant Site Upfront Substantiation |
0.00 |
0.66 |
0.41 |
1.07 |
|||||
|
Total Number of Workers |
0.00 |
1.14 |
0.47 |
1.61 |
|||||
|
Maximum Concentration, Physical Form, Percent Volume of Production |
0.00 |
2.23 |
0.86 |
3.09 |
|||||
|
Production Volume for Each of the Years since Last Principal Reporting Year |
0.00 |
3.28 |
0.81 |
4.09 |
|||||
|
Production Volume Used On-Site |
0.00 |
0.16 |
0.04 |
0.20 |
|||||
|
Whether Imported Chemical is Physically at Reporting Site |
0.00 |
0.09 |
0.02 |
0.11 |
|||||
|
Volume Exported |
0.00 |
0.82 |
0.20 |
1.02 |
|||||
|
Whether a Chemical is to be Recycled, Remanufactured, Reprocessed, Reused, or Reworked |
0.00 |
0.09 |
0.02 |
0.11 |
|||||
|
Total for Part II |
0.00 |
11.47 |
3.88 |
15.35 |
|||||
Part III. Processing and Use Information |
||||||||||
|
|
Upfront Substantiation for Processing and Use Information CBI Claims |
0.00 |
0.43 |
0.21 |
0.64 |
||||
|
Industrial Processing and Use Exposure-Related Data |
|||||||||
|
|
Determination of Applicability |
0.00 |
0.81 |
0.23 |
1.03 |
||||
|
|
Industrial Function Category |
0.00 |
3.53 |
1.65 |
5.18 |
||||
|
|
Sector |
0.00 |
0.75 |
0.32 |
1.06 |
||||
|
|
Percent of Production Volume |
0.00 |
8.00 |
4.22 |
12.22 |
||||
|
|
Total Number of Processing and Use Sites |
0.00 |
7.31 |
2.82 |
10.13 |
||||
|
|
Total Number of Potentially Exposed Workers |
0.00 |
12.34 |
3.06 |
15.41 |
||||
|
Consumer and Commercial Use Exposure-Related Data |
|||||||||
|
|
Determination of Applicability |
0.00 |
0.75 |
0.20 |
0.95 |
||||
|
|
Identification of Production Category/Use by Children |
0.00 |
0.67 |
0.20 |
0.87 |
||||
|
|
Percent of Production Volume |
0.00 |
1.01 |
0.36 |
1.37 |
||||
|
|
Maximum Concentration by Category |
0.00 |
1.09 |
0.28 |
1.36 |
||||
|
|
Number of Commercial Workers Reasonably Likely to be Exposed |
0.00 |
12.34 |
3.06 |
15.41 |
||||
|
Total for Part III |
0.00 |
49.03 |
16.60 |
65.63 |
|||||
COMPLIANCE DETERMINATION |
||||||||||
|
Compliance Determination |
0.00 |
2.50 |
0.00 |
2.50 |
|||||
RULE FAMILIARIZATION |
||||||||||
|
Rule Familiarization |
0.00 |
2.00 |
2.00 |
4.00 |
|||||
CDX REGISTRATION ACTIVITIES |
||||||||||
|
|
CDX Registration |
0.00 |
0.73 |
0.18 |
0.92 |
||||
|
|
CDX Electronic Signature Agreement |
0.00 |
1.00 |
0.75 |
1.75 |
||||
|
Total for CDX Registration Activities |
0.00 |
1.73 |
0.93 |
2.67 |
|||||
RECORDKEEPING |
||||||||||
|
Recordkeeping |
0.75 |
1.50 |
0.75 |
3.00 |
|||||
TOTAL BURDEN |
||||||||||
Burden for one Partial Report (Parts I, II, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
0.75 |
18.34 |
7.65 |
29.41 |
||||||
Burden for one Full Report (Parts I, II, III, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
0.75 |
67.37 |
24.26 |
95.04 |
||||||
Note: Totals may not sum due to rounding. |
||||||||||
Source: Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b). |
||||||||||
6(b) Estimating Cost
EPA multiplied burden estimates by standard wage rates for managerial, technical, and clerical levels developed from information published by the Bureau of Labor Statistics (BLS) and a method outlined in the document Wage Rates for Economic Analyses of the Toxics Release Inventory Program (EPA, 2002b). Wage data for the three occupational categories was gathered for manufacturing industries from Employer Costs for Employee Compensation Supplementary Tables: Historical Data December 2006 – December 2008 (BLS, 2009).
The cost of fringe benefits, such as health insurance and vacation, is taken for each labor category from the same ECEC series. Following the methodology outlined in (EPA, 2002b), fringe benefits are calculated as a percentage of total wages for each category. EPA added 17 percent to the wages in each category to account for overhead, based on information provided by the chemical industry and chemical industry trade associations in the Revised Economic Analysis for the Amended Inventory Update Rule: Final Report (EPA, 2002a). The wages for each of the three categories were then multiplied by benefits and overhead factors to estimate loaded, annual salaries in year 2008 dollars. Table 5 contains the loaded wage rates for the managerial, technical and clerical occupation categories.
Table 5: Derivation of Loaded Wage Rates for the Private Manufacturing Sector in 2008$
|
Wage1 |
Fringe Benefits1 |
Fringes as % of Wage |
Overhead % of Wage2 |
Fringe + Overhead Factor |
Loaded Wages |
(a) |
(b) |
(c) = (b)/(a) |
(d) |
(e)=(1)+(c)+(d) |
(f) = (a) x (e) |
|
Managerial |
$43.22 |
$19.46 |
48.37% |
17% |
1.62 |
$70.03 |
Technical |
$35.29 |
$17.55 |
47.58% |
17% |
1.67 |
$58.84 |
Clerical |
$17.22 |
$8.33 |
45.03% |
17% |
1.65 |
$28.48 |
1 Employer Costs for Employee Compensation Supplementary Tables: Historical Data December 2006 – December 2008, US Bureau of Labor Statistics, March 12, 2009 (BLS, 2009). 2 An overhead rate of 17 percent was estimated based on industry data gathered for the Revised Economic Analysis for the Amended Inventory Update Rule: Final Report (EPA, 2002a). |
||||||
Table 6 contains the cost per activity of completing Form U for one respondent in the first reporting cycle. Burden hours presented in Table 3 were multiplied by the corresponding loaded wage rage in Table 5. EPA estimates the total cost for completing and submitting one partial report in the first reporting cycle is $3,538 and the cost for completing and submitting one full report is $8,655. More information on the derivation of these costs is found in the IUR EA (EPA, 2010b).
Similarly, Table 7 contains the cost per activity of completing Form U for one respondent in future reporting cycles. Burden hours presented in Table 4 were multiplied by the corresponding loaded wage rage in Table 5. EPA estimates the total cost for completing and submitting one partial report in future reporting cycles is $1,636 and the cost for completing and submitting one full report is $5,684. More information on the derivation of these costs is found in the IUR EA (EPA, 2010b).
Table 6: Total Industry Cost, by Activity, First Reporting Cycle
Activity |
Clerical Cost (2008$) |
Technical Cost (2008$) |
Managerial Cost (2008) |
Total Cost (2008$) |
||||||||
(a) |
(b) |
(c) |
(d) = (a)+(b)+(c) |
|||||||||
PREPARATION OF REPORT (Includes rule familiarization associated with each data element) |
||||||||||||
Part I. Site Identification Information |
||||||||||||
|
Certification |
$0.00 |
$50.01 |
$70.73 |
$120.74 |
|||||||
|
Company Information (Parent Company Name, D&B Number, Mailing Address, Technical Contact, Technical Contact Mailing Address) |
$0.00 |
$2.35 |
$1.40 |
$3.75 |
|||||||
|
Plant Site Identification (Site Name, D&B Number, Mailing Address) |
$0.00 |
$3.53 |
$1.40 |
$4.93 |
|||||||
|
Total for Part I |
$0.00 |
$55.90 |
$73.53 |
$129.43 |
|||||||
Part II. Manufacturing Information |
||||||||||||
|
Site-Limited, Activity, Production Volume (lbs) |
$0.00 |
$134.15 |
$32.95 |
$167.10 |
|||||||
|
Chemical Identification Upfront CBI Substantiation |
$0.00 |
$85.32 |
$45.01 |
$130.33 |
|||||||
|
Plant Site Upfront Substantiation |
$0.00 |
$48.84 |
$30.01 |
$78.84 |
|||||||
|
Total Number of Workers |
$0.00 |
$84.14 |
$34.42 |
$118.56 |
|||||||
|
Maximum Concentration, Physical Form, Percent Volume of Production |
$0.00 |
$164.16 |
$62.96 |
$227.12 |
|||||||
|
Production Volume for Each of the Years since Last Principal Reporting Year |
$0.00 |
$241.48 |
$59.31 |
$300.79 |
|||||||
|
Production Volume Used On-Site |
$0.00 |
$11.71 |
$2.88 |
$14.59 |
|||||||
|
Whether Imported Chemical is Physically at Reporting Site |
$0.00 |
$6.71 |
$1.65 |
$8.36 |
|||||||
|
Volume Exported |
$0.00 |
$60.37 |
$14.83 |
$75.20 |
|||||||
|
Whether a Chemical is to be Recycled, Remanufactured, Reprocessed, Reused, or Reworked |
$0.00 |
$6.71 |
$1.65 |
$8.36 |
|||||||
|
Total for Part II |
$0.00 |
$843.58 |
$339.98 |
$1,183.56 |
|||||||
Part III. Processing and Use Information |
||||||||||||
|
|
Upfront Substantiation for Processing and Use Information CBI Claims |
$0.00 |
$25.12 |
$18.37 |
$43.49 |
||||||
|
Industrial Processing and Use Exposure-Related Data |
|||||||||||
|
|
Determination of Applicability |
$0.00 |
$59.27 |
$19.94 |
$79.21 |
||||||
|
|
Industrial Function Category |
$0.00 |
$274.49 |
$144.64 |
$419.13 |
||||||
|
|
Sector |
$0.00 |
$55.01 |
$27.68 |
$82.69 |
||||||
|
|
Percent of Production Volume |
$0.00 |
$588.41 |
$369.34 |
$957.75 |
||||||
|
|
Total Number of Processing and Use Sites |
$0.00 |
$537.89 |
$246.72 |
$784.62 |
||||||
|
|
Total Number of Potentially Exposed Workers |
$0.00 |
$907.74 |
$268.15 |
$1,175.90 |
||||||
|
Consumer and Commercial Use Exposure-Related Data |
|||||||||||
|
|
Determination of Applicability |
$0.00 |
$55.01 |
$17.56 |
$72.57 |
||||||
|
|
Identification of Production Category/Use by Children |
$0.00 |
$98.73 |
$17.26 |
$116.00 |
||||||
|
|
Percent of Production Volume |
$0.00 |
$74.02 |
$31.55 |
$105.57 |
||||||
|
|
Maximum Concentration by Category |
$0.00 |
$80.02 |
$24.11 |
$104.13 |
||||||
|
|
Number of Commercial Workers Reasonably Likely to be Exposed |
$0.00 |
$907.74 |
$268.15 |
$1,175.90 |
||||||
|
Total for Part III |
$0.00 |
$3,663.47 |
$1,453.48 |
$5,116.95 |
|||||||
COMPLIANCE DETERMINATION |
||||||||||||
|
Compliance Determination |
$0.00 |
$147.10 |
$0.00 |
$147.10 |
|||||||
RULE FAMILIARIZATION |
||||||||||||
|
Rule Familiarization |
$0.00 |
$1,117.95 |
$630.25 |
$1,748.19 |
|||||||
CDX REGISTRATION ACTIVITIES |
||||||||||||
|
|
CDX Registration |
$0.00 |
$43.15 |
$12.84 |
$55.99 |
||||||
|
|
CDX Electronic Signature Agreement |
$0.00 |
$58.84 |
$52.52 |
$111.36 |
||||||
|
Total for CDX Registration Activities |
$0.00 |
$101.99 |
$65.36 |
$167.35 |
|||||||
RECORDKEEPING |
||||||||||||
|
Recordkeeping |
$21.36 |
$88.26 |
$52.52 |
$162.14 |
|||||||
TOTAL COST |
||||||||||||
Cost for one Partial Report (Parts I, II, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
$21.36 |
$2,354.77 |
$1,161.63 |
$3,537.76 |
||||||||
Cost for one Full Report (Parts I, II, III, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
$21.36 |
$6,018.24 |
$2,615.11 |
$8,654.71 |
||||||||
Note: Totals may not sum due to rounding. |
||||||||||||
Source: Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b). |
||||||||||||
Table 7: Total Industry Cost, by Activity, Future Reporting Cycles
Activity |
Clerical Cost (2008$) |
Technical Cost (2008$) |
Managerial Cost (2008) |
Total Cost (2008$) |
||||||||
(a) |
(b) |
(c) |
(d) = (a)+(b)+(c) |
|||||||||
PREPARATION OF REPORT (Includes rule familiarization associated with each data element) |
||||||||||||
Part I. Site Identification Information |
||||||||||||
|
Certification |
$0.00 |
$50.01 |
$70.73 |
$120.74 |
|||||||
|
Company Information (Parent Company Name, D&B Number, Mailing Address, Technical Contact, Technical Contact Mailing Address) |
$0.00 |
$0.47 |
$0.28 |
$0.75 |
|||||||
|
Plant Site Identification (Site Name, D&B Number, Mailing Address) |
$0.00 |
$0.71 |
$0.28 |
$0.99 |
|||||||
|
Total for Part I |
$0.00 |
$51.19 |
$71.29 |
$122.48 |
|||||||
Part II. Manufacturing Information |
||||||||||||
|
Site-Limited, Activity, Production Volume (lbs) |
$0.00 |
$107.32 |
$31.37 |
$138.70 |
|||||||
|
Chemical Identification Upfront CBI Substantiation |
$0.00 |
$68.25 |
$42.86 |
$111.11 |
|||||||
|
Plant Site Upfront Substantiation |
$0.00 |
$39.07 |
$28.57 |
$67.64 |
|||||||
|
Total Number of Workers |
$0.00 |
$67.31 |
$32.77 |
$100.08 |
|||||||
|
Maximum Concentration, Physical Form, Percent Volume of Production |
$0.00 |
$131.33 |
$59.94 |
$191.27 |
|||||||
|
Production Volume for Each of the Years since Last Principal Reporting Year |
$0.00 |
$193.18 |
$56.47 |
$249.65 |
|||||||
|
Production Volume Used On-Site |
$0.00 |
$9.37 |
$2.74 |
$12.11 |
|||||||
|
Whether Imported Chemical is Physically at Reporting Site |
$0.00 |
$5.37 |
$1.57 |
$6.93 |
|||||||
|
Volume Exported |
$0.00 |
$48.30 |
$14.12 |
$62.41 |
|||||||
|
Whether a Chemical is to be Recycled, Remanufactured, Reprocessed, Reused, or Reworked |
$0.00 |
$5.37 |
$1.57 |
$6.93 |
|||||||
|
Total for Part II |
$0.00 |
$674.87 |
$271.98 |
$946.85 |
|||||||
Part III. Processing and Use Information |
||||||||||||
|
|
Upfront Substantiation for Processing and Use Information CBI Claims |
$0.00 |
$25.12 |
$14.70 |
$39.82 |
||||||
|
Industrial Processing and Use Exposure-Related Data |
|||||||||||
|
|
Determination of Applicability |
$0.00 |
$25.12 |
$15.95 |
$41.07 |
||||||
|
|
Industrial Function Category |
$0.00 |
$0.00 |
$115.71 |
$115.71 |
||||||
|
|
Sector |
$0.00 |
$47.41 |
$22.14 |
$69.56 |
||||||
|
|
Percent of Production Volume |
$0.00 |
$207.82 |
$295.47 |
$503.30 |
||||||
|
|
Total Number of Processing and Use Sites |
$0.00 |
$44.01 |
$197.38 |
$241.39 |
||||||
|
|
Total Number of Potentially Exposed Workers |
$0.00 |
$470.73 |
$214.52 |
$685.25 |
||||||
|
Consumer and Commercial Use Exposure-Related Data |
|||||||||||
|
|
Determination of Applicability |
$0.00 |
$47.41 |
$14.05 |
$61.46 |
||||||
|
|
Identification of Production Category/Use by Children |
$0.00 |
$207.82 |
$13.81 |
$221.63 |
||||||
|
|
Percent of Production Volume |
$0.00 |
$44.01 |
$25.24 |
$69.25 |
||||||
|
|
Maximum Concentration by Category |
$0.00 |
$470.73 |
$19.29 |
$490.01 |
||||||
|
|
Number of Commercial Workers Reasonably Likely to be Exposed |
$0.00 |
$430.32 |
$214.52 |
$644.84 |
||||||
|
Total for Part III |
$0.00 |
$2,884.62 |
$1,162.78 |
$4,047.40 |
|||||||
COMPLIANCE DETERMINATION |
||||||||||||
|
Compliance Determination |
$0.00 |
$147.10 |
$0.00 |
$147.10 |
|||||||
RULE FAMILIARIZATION |
||||||||||||
|
Rule Familiarization |
$0.00 |
$117.68 |
$140.05 |
$257.73 |
|||||||
CDX REGISTRATION ACTIVITIES |
||||||||||||
|
|
CDX Registration |
$0.00 |
$43.15 |
$12.84 |
$55.99 |
||||||
|
|
CDX Electronic Signature Agreement |
$0.00 |
$58.84 |
$52.52 |
$111.36 |
||||||
|
Total for CDX Registration Activities |
$0.00 |
$101.99 |
$65.36 |
$167.35 |
|||||||
RECORDKEEPING |
||||||||||||
|
Recordkeeping |
$21.36 |
$88.26 |
$52.52 |
$162.14 |
|||||||
TOTAL COST |
||||||||||||
Cost for one Partial Report (Parts I, II, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
$21.36 |
$1,079.09 |
$535.84 |
$1,636.29 |
||||||||
Cost for one Full Report (Parts I, II, III, Compliance Determination, Rule Familiarization, Recordkeeping and CDX Registration Activities) |
$21.36 |
$3,963.71 |
$1,698.63 |
$5,683.70 |
||||||||
Note: Totals may not sum due to rounding. |
||||||||||||
Source: Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b). |
||||||||||||
6(c) Estimating Agency Burden and Cost
EPA is responsible for the following activities associated with administering the IUR rule:
Document receipt and tracking;
Data entry and quality control of data entry;
Backup systems operation;
Data processing;
Systems development;
Contract oversight and management;
Publication and printing of forms and materials; and
Operation of the TSCA Hotline to handle IUR-related calls.
Costs related to EPA activities that involve using the data are not included.
EPA Staff Activities
Of the tasks listed above, Agency personnel are responsible for 1) quality control of data entry; and 2) data processing, systems development, and contract oversight and management. Contractors perform the other activities, as described below.
EPA estimates the total burden of completing Agency tasks to be one full-time equivalent at the GS 13 level for data processing, systems development, and contract oversight and management, per-reporting cycle. An estimated 0.181 FTEs are needed at the GS 12 level for quality control of data entry. Calculations of the Agency burden are presented in Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b).
EPA labor costs are based on annual federal wage rates published by the Office of Personnel Management for the Washington-Baltimore-Northern Virginia, DC-MD-PA-VA-WV locality pay area for 2008 (OPM, 2008). Wages are presented in terms of GS-level and step. Based on previous IUR economics analyses, a Step 3 is assumed for all FTEs (EPA, 2002a and EPA, 2005). Following the methodology outlined in Economic Analysis for the Amended Inventory Update Rule: Final Report (EPA, 2002a), EPA added 58 percent to the wage rate to account for fringe benefits and overhead costs.
8 shows the loaded wage rates for Agency staff at the GS-12 Step 3, and GS-13 Step 3 levels.
Table 8: Derivation of Loaded Agency Wage Rates (2008$)
Pay Grade |
Annual Salary |
Overhead and Fringe Benefits (% of wages) |
Overhead and Fringe Benefit Cost |
Total |
GS 12 Step 3 |
$77,416 |
58% |
$44,901 |
$122,317 |
GS 13 Step 3 |
$88,493 |
58% |
$51,326 |
$139,819 |
Source: The unloaded Federal salary for 2008 is from the Office of Personnel Management salary table for Washington-Baltimore-Northern Virginia (OPM, 2008). |
||||
Table 9 contains the burden and cost per report for all EPA staff activities. The activities performed by the GS-13 level staff member, including systems development, and contract oversight and management, are fixed costs and are not dependent on the number of reports submitted to EPA. Therefore, the total burden for systems development and contract oversight is one FTE at the GS-13 level, with a cost of $139,819. Quality control of data entry is performed by the GS-12 level staff member and is dependent on the number of reports received. The burden for quality control of data is approximately 0.000054 FTE per report and the total cost per-report is approximately $0.81. The burden and cost of processing each data element in Form U are derived in the Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b). EPA multiplied the burdens by the number of data elements in each section to estimate the total cost and burden of processing each Form U. For more detail on the derivation of these burdens, see the IUR EA (EPA, 2010b).
Table 9: EPA Staff Burden and Cost of Processing One Report
Activity |
Agency Burden per Activity (FTE) |
Agency Burden per Activity (hours) |
Agency Cost per Activity (2008$) |
GS-12 Step 3 per-Report Burden |
|||
Quality Control of Data for Part I |
0.0000009 |
0.0019 |
$0.11 |
Quality Control of Data for Part II |
0.0000027 |
0.0056 |
$0.33 |
Quality Control of Data for Part III |
0.0000030 |
0.0063 |
$0.37 |
Total GS-12 Burden, per report |
0.0000066 |
0.0138 |
$0.81 |
GS-13 Step 3 Fixed Cost Burden |
|||
Systems development, and contract oversight and management |
1 |
2,080 |
$139,819 |
Total GS-13 Burden, per reporting cycle |
1 |
2,080 |
$139,819 |
Contractor Activities
Agency costs also include payment for extramural tasks completed by contractors (this category includes costs to EPA, but not burden hours). Contractor activities include document receipt, tracking, data entry, maintaining backup systems, printing and publishing forms and materials, and managing the TSCA hotline, as presented in Table 10. With the exception of document receipt, tracking, and data entry, all contractor costs are fixed and are not dependent on the number of reports received. All fixed costs are taken from the last published ICR and were inflated from 2007 to 2008 dollars with an inflation factor calculated using the Employment Cost Index (ECI), seasonally adjusted, for white-collar occupations in private industry (BLS, 2009).
EPA calculated the cost estimate per report for document receipt, tracking, and data entry, $0.63, by starting with the cost estimated in the 2007 ICR, and updating it to reflect the new data elements and the electronic submission requirement in the proposed amendments. The reduction in burden caused by the electronic submission requirement is based on a study conducted by EPA, “A Business Case Analysis of EPA’s Central Data Exchange” (EPA, 2007), of the costs and benefits of the electronic Central Data Exchange (CDX) system. The study estimates an 88.6 percent decrease in EPA processing burden as a result of using the CDX system. For more information, see the Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b).
Table 10: Cost of Contractor Activities
Activity |
Annual Cost (2007$) |
Inflation Factor |
Annual Cost (2008$) |
Variable Costs |
|||
Document receipt, tracking, and data entry for Part I |
$0.78 |
n/a |
$0.09 |
Document receipt, tracking, and data entry for Part II |
$1.92 |
n/a |
$0.25 |
Document receipt, tracking, and data entry for Part III |
$2.45 |
n/a |
$0.29 |
Total Cost of Document receipt, tracking, and data entry, per full report |
$5.14 |
n/a |
$0.63 |
Fixed Costs |
|||
Maintaining and Operating Back-Up Systems |
$56,711 |
1.04 |
$59,145 |
Printing and Publishing Forms and Materials |
$5,298 |
1.04 |
$5,525 |
Managing the TSCA Hotline |
$42,855 |
1.04 |
$44,694 |
Total Fixed Cost |
$104,864 |
n/a |
$109,364 |
6(d) Bottom-Line Industry Burden and Cost Estimates
This section describes the estimated total social paperwork burden and cost of the IUR rule, including the proposed amendments. The next IUR submission period will occur in 2011 for chemicals manufactured (including imported) during the calendar year 2010. Even though reporting occurs only once per reporting cycle (once every five years for the 2011 cycle and, as proposed, once every four years in the future), EPA believes rule compliance and data collection activities, and thus, costs and burdens, are incurred over the course of the reporting cycle. Therefore, for purposes of this analysis, the burden and cost for one reporting cycle are averaged over the number of years in the reporting cycle and are presented here as average annual figures. This ICR addendum supporting statement is for the three-year period of 2011 through 2013; therefore, average annual figures for each of these three years are presented below.
Respondent tally
EPA calculated the numbers of sites and reports submitted based on submission information from the December 2008 version of the IUR database, which includes data from the most recent (2006) IUR collection. The EPA IUR database contains information collected under the IUR for previous submission periods and was used to generate estimates of expected reports for the 2011 reporting cycle. In the 2011 reporting year, EPA expects a total of 4,085 sites to submit 29,253 full reports (98 percent of all reports) and 618 partial reports (two percent of all reports) for a total of 29,871 reports. In future reporting cycles, EPA expects 4,289 sites to submit 30,716 full reports and 649 partial reports for a total of 31,365 reports (98 percent and two percent of all reports, respectively).
Total Industry Burden/Cost for First Reporting Cycle. EPA estimates the total industry burden for the first reporting cycle, including both current baseline burden and the burden resulting from the proposed amendments, to be 3.24 million hours. Given that this data collection is part of a five-year reporting cycle, EPA estimates the annual industry burden for 2011 to be 647,016 hours. As presented in Table 11, EPA estimates the total cost to industry would be $199 million, or an annual cost of $39.7 million.
Table 11: Estimated Respondent Burden and Cost Associated with the First Reporting Cycle (2011)
Activity |
Total Burden per Activity (hours) |
Total Number of Units |
Total Cost per Activity (2008$) |
Total Burden per Reporting Cycle (hours) |
Total Cost per Reporting Cycle (2008$) |
Annual Burden (hours) |
Annual Cost (hours) |
Compliance Determination (per Site) |
2.50 |
4,085 Sites |
$147.10 |
10,213 |
$600,896 |
2,043 |
$120,179 |
Rule Familiarization (per Site) |
28.00 |
4,085 Sites |
$1,748.19 |
114,380 |
$7,141,370 |
22,876 |
$1,428,274 |
CDX Registration Activities (per Site) |
2.67 |
4,085 Sites |
$111.36 |
10,893 |
$454,905 |
2,179 |
$90,981 |
Part I Preparation (per site) |
2.00 |
4,085 Sites |
$129.43 |
8,170 |
$528,706 |
1,634 |
$105,741 |
Partial Report Preparation (Part II, per Report) |
19.19 |
618 Partial Reports |
$1,183.56 |
11,861 |
$731,438 |
2,372 |
$146,288 |
Full Report Preparation (Part II and Part III, per Report) |
102.21 |
29,253 Full Reports |
$6,300.50 |
2,989,951 |
$184,308,643 |
597,990 |
$36,861,729 |
Recordkeeping (per Report) |
3.00 |
29,871 Reports |
$162.14 |
89,613 |
$4,843,211 |
17,923 |
$968,642 |
Total Industry Burden and Cost for the First Reporting Cycle |
3,235,080 |
$198,609,168 |
647,016 |
$39,721,834 |
|||
Total Industry Burden and Cost for Future Reporting Cycles. EPA estimates the total industry burden for future reporting cycles, including both current baseline burden and the burden resulting from the proposed amendments, to be 2.64 million hours. Given that this data collection will occur every four years, EPA estimates the annual industry burden for future years to be 659,742 hours. As presented in Table 12, EPA estimates the total cost to industry would be $162 million, or an annual cost of $40.5 million.
Table 12: Estimated Annual Respondent Burden and Cost Associated with Future Reporting Cycles
Activity |
Total Burden per Activity (hours) |
Total Number of Units |
Total Cost per Activity (2008$) |
Total Burden (hours) |
Total Cost per Reporting Cycle (2008$) |
Annual Burden (hours) |
Annual Cost (hours) |
Compliance Determination (per Site) |
2.50 |
4,289 Sites |
$147.10 |
10,723 |
$630,904 |
2,681 |
$157,726 |
Rule Familiarization (per Site) |
4.00 |
4,289 Sites |
$257.73 |
17,156 |
$1,105,419 |
4,289 |
$276,355 |
CDX Registration Activities (per Site) |
2.67 |
4,289 Sites |
$167.35 |
11,437 |
$717,751 |
2,859 |
$179,438 |
Part I Preparation (per Site) |
1.89 |
4,289 Sites |
$122.48 |
8,098 |
$525,309 |
2,024 |
$131,327 |
Partial Report Preparation (Part II, per Report) |
15.35 |
649 Partial Reports |
$946.85 |
9,964 |
$614,503 |
2,491 |
$153,626 |
Full Report Preparation (Part II and Part III, per Report) |
80.98 |
30,716 Full Reports |
$4,994.25 |
2,487,494 |
$153,403,359 |
621,873 |
$38,350,840 |
Recordkeeping (per Report) |
3.00 |
31,365 Reports |
$162.14 |
94,095 |
$5,085,444 |
23,524 |
$1,271,361 |
Total Industry Burden and Cost for Future Reporting Cycles |
2,638,966 |
$162,082,689 |
659,742 |
$40,520,672 |
|||
Annual Industry Burden and Cost for 2011 to 2013. As shown in Table 13, EPA calculated the estimated total annual respondent burden and cost associated with this ICR addendum by taking a weighted average of the first and future reporting cycles, to reflect that the period covered by this ICR, 2011 through 2013, spans two reporting cycles with different burdens and costs. The total annual burden associated with this ICR addendum is 655,500 hours. The total annual cost is $40.3 million.
Table 13: Estimated Annual Average Burden and Cost Associated with this ICR Addendum
Activity |
2011 |
2012 |
2013 |
2011-2013 Average |
||||
Annual Burden (hours) |
Annual Cost (2008$) |
Annual Burden (hours) |
Annual Cost (2008$) |
Annual Burden (hours) |
Annual Cost (2008$) |
Total Annual Burden (hours) |
Total Annual Cost (2008$) |
|
Compliance Determination (per Site) |
2,043 |
$120,179 |
2,681 |
$157,726 |
2,681 |
$157,726 |
2,468 |
$145,210 |
Rule Familiarization (per Site) |
22,876 |
$1,428,274 |
4,289 |
$276,355 |
4,289 |
$276,355 |
10,485 |
$660,328 |
CDX Registration Activities (per Site) |
2,179 |
$90,981 |
2,859 |
$179,438 |
2,859 |
$179,438 |
2,632 |
$149,952 |
Part I Preparation (per Site) |
1,634 |
$105,741 |
2,024 |
$131,327 |
2,024 |
$131,327 |
1,894 |
$122,798 |
Partial Report Preparation (Part II, per Report) |
2,372 |
$146,288 |
2,491 |
$153,626 |
2,491 |
$153,626 |
2,451 |
$151,180 |
Full Report Preparation (Part II and Part III, per Report) |
597,990 |
$36,861,729 |
621,873 |
$38,350,840 |
621,873 |
$38,350,840 |
613,912 |
$37,854,469 |
Recordkeeping (per Report) |
17,923 |
$968,642 |
23,524 |
$1,271,361 |
23,524 |
$1,271,361 |
21,657 |
$1,170,455 |
Total |
655,500 |
$40,254,393 |
||||||
Average Burden and Cost per Site. As shown in Table 14, the Agency estimates the typical respondent burden for this information collection activity to be 792 hours in the first reporting cycle and 615 hours in future reporting cycles. Given that the 2011 (first) collection is based on a five-year reporting cycle, the average annual burden for the first reporting cycle would be 158 hours. Given that a collection would occur once every four years under the proposed rule, the average annual burden for future cycles would be 154 hours. These burden estimates assume each site will submit an average of 7.16 full reports and 0.15 partial reports in both the next, and future, reporting cycle. This is a decrease of 2.01 partial reports per site and an increase of 2.98 full reports per site compared to the average number of reports completed per site presented in the currently approved ICR (EPA ICR No. 1884.04) .
Table 14: Average Burden per Site
Activity |
Burden Hours |
Total Hours per Activity |
Reports per Average Site |
Total Burden (hours per average site) |
Annual Burden (hours per average site) |
||
Managerial |
Technical |
Clerical |
|||||
First Reporting Cycle |
|||||||
Rule Familiarization. Compliance Determination, CDX Registration Activities and Part I Preparation (per Site) |
10.98 |
24.18 |
0.00 |
35.17 |
1.00 |
35.17 |
7.03 |
Partial Report Preparation (Part II, per Report) |
4.85 |
14.34 |
0.00 |
19.19 |
0.15 |
2.90 |
0.58 |
Full Report Preparation (Part II and Part III, per Report) |
25.61 |
76.60 |
0.00 |
102.21 |
7.16 |
732 |
146 |
Recordkeeping (per Report) |
0.75 |
1.50 |
0.75 |
3.00 |
7.31 |
21.9 |
4.39 |
Total Hours |
792 |
158 |
|||||
Future Reporting Cycles |
|||||||
Rule Familiarization. Compliance Determination, CDX Registration Activities and Part I Preparation (per site) |
3.95 |
7.10 |
0.00 |
11.05 |
1.00 |
11.05 |
2.76 |
Partial Report Preparation (Part II, per Report) |
3.88 |
11.47 |
0.00 |
15.35 |
0.15 |
2.32 |
0.58 |
Full Report Preparation (Part II and Part III, per Report) |
20.49 |
60.50 |
0.00 |
80.98 |
7.16 |
580 |
145 |
Recordkeeping (per Report) |
0.75 |
1.50 |
0.75 |
3.00 |
7.31 |
21.9 |
5.48 |
Total Hours |
615 |
154 |
|||||
Table 15 presents the average cost per site, by activity, for an IUR respondent. EPA estimates the average site will submit 7.16 full reports and 0.15 partial reports and incur a total cost of $48,678 during the first reporting cycle ($9,736 annually), and $37,790 during future reporting cycles ($9,448 annually) for Form U completion and submission.
Table 15: Average Cost per Site
Activity |
Cost (2008$) |
Total Cost per Activity |
Reports per Average Site |
Total Cost (2008$ per average site) |
Annual Cost (2008$ per average site) |
||
Managerial |
Technical |
Clerical |
|||||
First Reporting Cycle |
|||||||
Rule Familiarization. Compliance Determination, CDX Registration Activities and Part I Preparation (per site) |
$769.13 |
$1,423 |
$0.00 |
$2,192.06 |
1.00 |
$2,192 |
$438 |
Partial Report Preparation (Part II, per Report) |
$339.98 |
$843.58 |
$0.00 |
$1,183.56 |
0.15 |
$179 |
$35.82 |
Full Report Preparation (Part II and Part III, per Report) |
$1,793.45 |
$4,507 |
$0.00 |
$6,300.50 |
7.16 |
$45,122 |
$9,024 |
Recordkeeping (per Report) |
$52.52 |
$88.26 |
$21.36 |
$162.14 |
7.31 |
$1,186 |
$237 |
Total Cost |
$48,678 |
$9,736 |
|||||
Future Reporting Cycles |
|||||||
Rule Familiarization. Compliance Determination, CDX Registration Activities and Part I Preparation (per site) |
$276.70 |
$417.96 |
$0.00 |
$694.66 |
$1.00 |
$695 |
$174 |
Partial Report Preparation (Part II, per Report) |
$271.98 |
$674.87 |
$0.00 |
$946.85 |
$0.15 |
$143 |
$35.82 |
Full Report Preparation (Part II and Part III, per Report) |
$1,434.76 |
$3,559.49 |
$0.00 |
$4,994.25 |
$7.16 |
$35,767 |
$8,942 |
Recordkeeping (per Report) |
$52.52 |
$88.26 |
$21.36 |
$162.14 |
$7.31 |
$1,186 |
$296 |
Total Cost |
$37,790 |
$9,448 |
|||||
As shown in Table 16, EPA calculated the estimated burden and cost per site associated with this ICR addendum by taking a weighted average of the first and future reporting cycles to reflect that the period covered by this ICR addendum, 2011 through 2013, spans two reporting cycles with different burdens and costs. The average annual burden per site is 155 hours and the average annual cost per site is $9,544.
Table 16: Estimated Annual Burden and Cost per Site Associated with this ICR Addendum
Activity |
2011 |
2012 |
2013 |
2011-2013 Average |
||||
Annual Burden (hours) |
Annual Cost (2008$) |
Annual Burden (hours) |
Annual Cost (2008$) |
Annual Burden (hours) |
Annual Cost (2008$) |
Annual Burden (hours) |
Annual Cost (2008$) |
|
Rule Familiarization. Compliance Determination, CDX Registration Activities and Part I Preparation (per site) |
7.03 |
$438 |
2.76 |
$174 |
2.76 |
$174 |
4.19 |
$262 |
Partial Report Preparation (Part II, per Report) |
0.58 |
$35.82 |
0.58 |
$36 |
0.58 |
$36 |
0.58 |
$36 |
Full Report Preparation (Part II and Part III, per Report) |
146 |
$9,024 |
145 |
$8,942 |
145 |
$8,942 |
145 |
$8,969 |
Recordkeeping (per Report) |
4.39 |
$237 |
5.48 |
$296 |
5.48 |
$296 |
5.12 |
$277 |
Total |
155 |
$9,544 |
||||||
Table 17 presents the burden hours, organized by information collection, for both the first and future reporting cycle, and weighted, annual averages for the ICR addendum period, for IUR respondents.
Table 17: Information Collection Tally for First and Future Reporting Cycles
Information Collection |
No. of Respondents |
No. of Responses / Respondent |
Responses Subtotal |
Burden Hours per Response |
Burden Hours Subtotal |
First Reporting Cycle |
|||||
Compliance Determination |
4,085 |
1 |
4,085 |
2.50 |
10,213 |
Rule Familiarization |
4,085 |
1 |
4,085 |
28.00 |
114,380 |
CDX Registration Activities |
4,085 |
1 |
4,085 |
4.67 |
19,063 |
|
4,085 |
1 |
4,085 |
0.92 |
3,745 |
|
4,085 |
1 |
4,085 |
1.75 |
7,149 |
|
4,085 |
1 |
4,085 |
2.00 |
8,170 |
Prepare and Submit Report, and Maintain Records – Partial Report |
4,085 |
0.15 |
618 |
22.19 |
13,718 |
|
4,085 |
0.15 |
618 |
19.19 |
11,863 |
|
4,085 |
0.15 |
618 |
3.00 |
1,854 |
Prepare and Submit Report, and Maintain Records - Full Report |
4,085 |
7.16 |
29,255 |
105.21 |
3,077,924 |
|
4,085 |
7.16 |
29,255 |
102.21 |
2,990,159 |
|
4,085 |
7.16 |
29,255 |
3.00 |
87,765 |
Future Reporting Cycles |
|||||
Compliance Determination |
4,289 |
1 |
4,289 |
2.50 |
10,723 |
Rule Familiarization |
4,289 |
1 |
4,289 |
4.00 |
17,156 |
CDX Registration Activities |
4,289 |
1 |
4,289 |
4.55 |
19,535 |
|
4,289 |
1 |
4,289 |
0.92 |
3,932 |
|
4,289 |
1 |
4,289 |
1.75 |
7,506 |
|
4,289 |
1 |
4,289 |
1.89 |
8,098 |
Prepare and Submit Report, and Maintain Records – Partial Report |
4,289 |
0.15 |
649 |
18.35 |
11,911 |
|
4,289 |
0.15 |
649 |
15.35 |
9,964 |
|
4,289 |
0.15 |
649 |
3.00 |
1,947 |
Prepare and Submit Report, and Maintain Records - Full Report |
4,289 |
7.16 |
30,716 |
83.98 |
2,579,642 |
|
4,289 |
7.16 |
30,716 |
80.98 |
2,487,494 |
|
4,289 |
7.16 |
30,716 |
3.00 |
92,148 |
Average for ICR Addendum Period |
|||||
Compliance Determination |
4,221 |
1 |
4,221 |
2.50 |
10,553 |
Rule Familiarization |
4,221 |
1 |
4,221 |
12.00 |
49,564 |
CDX Registration Activities |
4,221 |
1 |
4,221 |
4.59 |
19,378 |
Prepare and Submit Report, and Maintain Records – Partial Report |
4,221 |
0.15 |
639 |
19.63 |
12,513 |
-- Part II of Form U |
4,221 |
0.15 |
639 |
16.63 |
10,597 |
-- Recordkeeping |
4,221 |
0.15 |
639 |
3.00 |
1,916 |
Prepare and Submit Report, and Maintain Records - Full Report |
4,221 |
7.16 |
30,229 |
91.05 |
2,745,736 |
--Part II and III of Form U |
4,221 |
7.16 |
30,229 |
88.06 |
2,655,049 |
--Recordkeeping |
4,221 |
7.16 |
30,229 |
3.00 |
90,687 |
Some burden estimate subtotals may not calculate due to rounding of unit burden estimates
Agency Tally
Table 18 presents the Agency costs associated with the IUR rule for the first reporting cycle. EPA multiplied the costs per report by the total number of Parts I, II, and III to calculate the total burden and cost associated with the number of reports EPA expects to be submitted. The total burden is 0.172 FTEs and the total cost is $37,396 in the first reporting cycle, for variable cost activities. The burden and cost of the fixed cost activities remains unchanged by the number of reports submitted; the total fixed burden is one FTE per reporting cycle and the cost is $249,184. The total Agency burden is 1.181 FTEs. The estimated total cost incurred by the Agency for the first reporting cycle, $286,580, was calculated by summing the Agency staff and contractor activities.
Table 18: Total Cost and Burden of Agency Activities (First Reporting Cycle)
Activity |
Staff |
Form U Section |
Total Burden per Activity (FTE) |
Total Number of Units |
Total Cost per Activity (2008$) |
Total Burden (FTE) |
Total Cost (2008$) |
|||
Variable Burdens and Costs |
||||||||||
Document receipt, tracking, and data entry
|
Contractor |
Part I |
N/A |
4,085 Sites |
$0.09 |
N/A |
$360 |
|||
Part II |
N/A |
29,871 Part IIs |
$0.25 |
N/A |
$7,550 |
|||||
Part III |
N/A |
29,253 Part IIIs |
$0.29 |
N/A |
$8,402 |
|||||
Quality Control of Data |
EPA Employee (GS-12 Step 3) |
Part I |
0.0000009 |
4,085 Sites |
$0.11 |
0.004 |
$465 |
|||
Part II |
0.0000027 |
29,871 Part IIs |
$0.32 |
0.080 |
$9.759 |
|||||
Part III |
0.0000030 |
29,253 Part IIIs |
$0.37 |
0.089 |
$10.860 |
|||||
Total Variable Cost and Burden |
0.172 |
$37,396 |
||||||||
Fixed Burdens and Costs |
|
|
||||||||
Data Processing, Systems Development, and Contract Oversight and Management |
EPA Employee (GS-13 Step 3) |
N/A |
N/A |
N/A |
N/A |
1.000 |
$139,819 |
|||
Maintaining and Operating Back Up Systems |
Contractor |
N/A |
N/A |
N/A |
N/A |
N/A |
$59,145 |
|||
Printing and Publishing Forms and Materials |
Contractor |
N/A |
N/A |
N/A |
N/A |
N/A |
$5,525 |
|||
Managing the TSCA Hotline |
Contractor |
N/A |
N/A |
N/A |
N/A |
N/A |
$44,694 |
|||
Total Fixed Cost and Burden |
1.000 |
$249,184 |
||||||||
Total Agency Cost and Burden |
1.172 |
$286,580 |
||||||||
6(e) Reasons for Change in Burden
EPA estimates industry will incur an increase of 245,015 hours in annual burden compared to the estimate in the information collection request most recently approved by OMB (from 410,485 hours currently approved as reported in the currently approved ICR (EPA ICR No. 1884.04) (EPA, 2008a), to 655,500 hours as estimated in Section 6(d) above). This increase is due to both program changes and an adjustment in reporting requirement criteria. Several of the proposed IUR amendments change the number of partial and/or full reports EPA expects to be submitted, based on the specific chemicals the amendments affect. Details on the proposed amendments and the burden and cost impacts of each amendment when taken individually and considered together (i.e., one proposed amendment may change the impact of another), are described in the Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule (EPA, 2010b).
6(f) Burden Statement
The annual public burden for this collection of information is estimated to average 155 hours per respondent. According to the Paperwork Reduction Act, “burden” means the total time, effort, or financial resources expended by persons to generate, maintain, retain, or disclose or provide information to or for a Federal agency. For this collection it includes the time needed to determine whether a site is subject to the rule (Compliance Determination); review and understand instructions; prepare and submit reports (including searching data sources); complete and review the collection of information; transmit the information; and keep records.
An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The OMB control number for this information collection appears above. The OMB control numbers for EPA's regulations in title 40 of the CFR, after appearing in the Federal Register when approved, are listed in 40 CFR Part 9, are displayed either by publication in the Federal Register or by other appropriate means, such as on the related collection instrument or form, if applicable. The display of OMB control numbers in certain EPA regulations is consolidated in 40 CFR Part 9.
To comment on the Agency’s need for this information, the accuracy of the provided burden estimates, and any suggested methods for minimizing respondent burden, including the use of automated collection techniques, EPA has established a docket for this ICR addendum under Docket ID No. EPA-HQ-OPPT-2009-0187 which is available for public viewing at the Pollution Prevention and Toxics Docket in the EPA Docket Center (EPA/DC), EPA West, Room 3334, 1301 Constitution Ave., NW, Washington, DC. The EPA Docket Center Public Reading Room is open from 8:30 a.m. to 4:30 p.m., Monday through Friday, excluding legal holidays. The telephone number for the Reading Room is (202) 566-1544 and the telephone number for the Pollution Prevention and Toxics Docket is (202) 566-0280.
An electronic version of this docket is available at http://www.regulations.gov/. Use the federal government wide electronic docket and comment system at www.regulations.gov to submit or view public comments, access the index listing of the docket contents, and to access those documents in the docket that are available electronically. Once in the system, select “advance search,” then key in the docket ID number identified above. Also, you can send comments to the Office of Information and Regulatory Affairs, Office of Management and Budget, 725 17th Street, NW, Washington, DC 20503, Attention: Desk Office for EPA. Please include the EPA Docket ID No. EPA-HQ-OPPT-2009-0187 and OMB control number 2070-0162 in any correspondence.
Sources
BLS 2009. U.S. Bureau of Labor Statistics. Employer Costs for Employee Compensation Supplementary Tables: Historical Data December 2006 – December 2008. (March12, 2009) http://www.bls.gov/ncs/ect/sp/ecsuphst.pdf.
EPA, 2002a. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch. Economic Analysis for the Amended Inventory Update Rule: Final Report (EPA-HQ-OPPT-2002-0054-0260). August 2002.
EPA, 2002b. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch. Wage Rates for Economic Analysis of the Toxics Release Inventory Program. June 10, 2002.
EPA, 2005. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch. Economic Analysis of IUR Modifications Final Rule (EPA-HQ OPPT 2004-0106-0055). July 2005.
EPA, 2006. US EPA, Office of Pollution Prevention and Toxics, Instructions for Reporting for the 2006 Partial Updating of the TSCA Chemical Inventory Database. Nov. 2006. Available at http://www.epa.gov/iur/pubs/2006_inst_tsca_cheminv.pdf.
EPA, 2007. U.S. EPA, Office of Environmental Information. A Business Case Analysis of EPA’s Central Data Exchange. March, 2007.
EPA, 2008a. ICR No. 1884.04. [Information Collection Request for] Partial Update of the TSCA Section 8(b) Inventory Data Base, Production and Site Report Supporting Statement for a Request for OMB Review under the Paperwork Reduction Act. September 5, 2008. EPA-HQ-OPPT-2008-0504-0002.
EPA, 2008b. U.S. EPA, Office of Pollution Prevention and Toxics, Information Management Division, 2006 IUR Database Statistics for IUR Modifications Rule. Washington, DC. December 17, 2008.
EPA, 2009a. U.S. EPA, Office of Pollution Prevention and Toxics. TSCA Section 4 Federal Register Enforceable Consent Agreements. http://www.epa.gov/oppt/chemtest/pubs/4eca.html. Washington, DC. Updated October 1, 2009.
EPA, 2009b. U.S. EPA, Office of Pollution Prevention and Toxics, Sunset Dates of Chemicals Subject to Final TSCA Section 4 and Related 12(b) Actions, Modified on September 1, 2009. http://www.epa.gov/oppt/chemtest/pubs/sunset.html. Washington, DC. Updated September 1, 2009.
EPA, 2010a. TSCA Section 5 Premanufacture and Significant New Use Notification Electronic Reporting; Revisions to Notification Regulations; Final rule. Federal Register (75 FR 773-790), January 6, 2010)
EPA, 2010b. U.S. EPA, Office of Pollution Prevention and Toxics, Economic and Policy Analysis Branch. Economic Analysis for the Proposed Inventory Update Reporting (IUR) Modifications Rule. July 2010.
OPM, 2008. Office of Personnel Management, Salary Table 2008-DCB, Washington Baltimore-Northern Virginia, DC-MD-PA-VA-WV. Accessed from http://www.opm.gov/oca/08tables/pdf/DCB.pdf.
Attachment A:2011 IUR Form U
Attachment B:
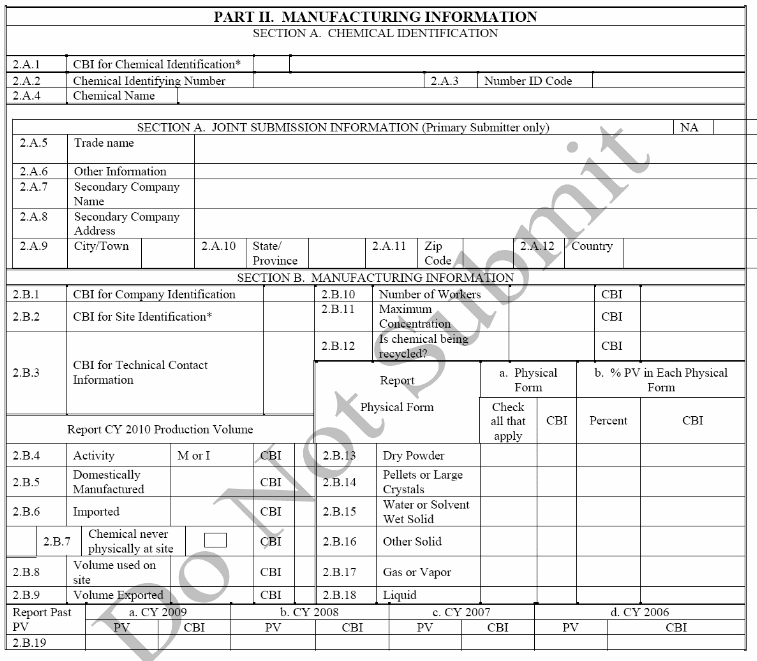


1 The proposed rule is titled “TSCA Inventory Update Modifications.”
2 INFORM is a nonprofit environmental research organization.
3 The economic analysis for the 2005 Amendments assumed a 15 percent reduction in burden for the completion of Part III because submitters were no longer required to report use and downstream processing information for exports.
4 EPA believes that the burden for future reporting cycles will be reduced because of efficiencies achieved through the establishment of compliance processes; as described in the IUR EA (EPA, 2010b). After a site’s first reporting cycle, the availability of data from previous reporting cycles and familiarity with reporting requirements will expedite the process for submitters who have previously submitted a Form U.
Page
| File Type | application/msword |
| File Title | • |
| Author | cfarquh |
| Last Modified By | ctsuser |
| File Modified | 2010-08-12 |
| File Created | 2010-08-12 |
© 2026 OMB.report | Privacy Policy