ACE OMB Supporting Statement A 100908
ACE OMB Supporting Statement A 100908.docx
The Medicare Acute Care Episode Demonstration
OMB: 0938-1117
The Centers for Medicare & Medicaid Services’ Office of Research, Development, and Information (ORDI) strives to make information available to all. Nevertheless, portions of our files including charts, tables, and graphics may be difficult to read using assistive technology.
Persons with disabilities experiencing problems accessing portions of any file should contact ORDI through e-mail at [email protected].

Evaluation of the
Medicare Acute Care Episode (ACE) Demonstration
HHSM-500-2009-00083G
OMB Supporting Statement – Part A
September 8, 2010
Oswaldo Urdapilleta, PhD, IMPAQ International, LLC
Annette Snyder, PhD, Hilltop Institute, UMBC
Jasmine Ainetchian, IMPAQ International, LLC
Cynthia Boddie-Willis, MD, Hilltop Institute, UMBC
Submitted to:
Centers for Medicare & Medicaid Services 7500 Security Boulevard Mailstop: C3-21-28 Baltimore, MD 21244-1850
Project Officer: Jesse M Levy, PhD, ORDI
|
Submitted by:
IMPAQ International, LLC 10420 Little Patuxent Pkwy. Suite 300 Columbia, MD 21044 Telephone: (443) 367-0088 Facsimile: (443) 367-0477
Project Director: Oswaldo Urdapilleta, PhD
|
Contents
3. Use of Information Technology 5
8. Federal Register/Outside Consultation 6
9. Payments/Gifts to Respondents 6
12. Burden Estimates (Hours and Wages) 7
14. Cost to Federal Government 10
The Acute Care Episode (ACE) Demonstration was designed to address the use of a global payment for an episode of care as an alternative approach to payment under traditional Medicare fee-for-service (FFS). The episode of care is defined as the bundle of Part A and Part B services provided during an inpatient stay for Medicare FFS beneficiaries for included MS-DRGs, which are hip/knee replacement or revision surgery and/or coronary artery bypass graft surgery or cardiac intervention procedure.
The evaluation of the ACE Demonstration will reveal whether the use of a bundled payment system will produce savings for Medicare for episodes of care involving the included DRGs, while increasing quality. Some of the data for the evaluation will come from key stakeholder/decision-maker interviews and focus groups of both the implementing staff (e.g., physicians, nurses, and other staff) and the Medicare beneficiaries participating in the demonstration. These interviews and focus groups will provide information about infrastructure changes and participants’ perspectives, experiences, and satisfaction. The impact of sharing savings resulting from the demonstration for PHOs, providers, and beneficiaries (e.g., gainsharing and premium rebates) will also be explored.
The Centers for Medicare & Medicaid Services (CMS) requests that the Office of Management and Budget (OMB) approve, under the Information Collection Request requirements of the Paper Work Reduction Act of 1995, its plan to collect information from stakeholders and Medicare beneficiaries participating in the Acute Care Episode (ACE) Demonstration. This data collection is a component of the evaluation of the ACE Demonstration that is being performed by IMPAQ International and its subcontractor, The Hilltop Institute, at the University of Maryland, Baltimore County, henceforth referred to as the “IMPAQ team,” under contract to CMS.
The statutory authority for the ACE Demonstration Project derives from Section 1866C of the Social Security Act (Attachment A) and the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (P.L. 108-173) (see Attachment B), whereby the Secretary of the Department of Health and Human Services may authorize demonstration projects to improve health care quality by offering incentives to encourage the delivery of efficient, effective and safe health care services. Further, the MMA of 2003 directs “broad and ongoing consultation with relevant stakeholders in … evaluations to support and improve the programs established under titles XVIII, XIX, and XXI of the Social Security Act.”
Specifically, the Solicitation for Applications for the ACE Demonstration states that “An independent evaluation will be conducted for this demonstration to evaluate the feasibility and cost effectiveness of the bundled payment methodology and the improvement in quality of care and other benefits to Medicare beneficiaries.” In its successful competitive bid for the evaluation contract, IMPAQ described a comprehensive plan that included a quantitative analysis of financial data, and clinical processes and outcomes, as well as a qualitative study of the impact on various stakeholders (beneficiaries, physicians, and hospital staff and administrators). This information collection request focuses on the qualitative analysis.
The qualitative data collection will include interviews with key stakeholders at each of the 11 hospitals; focus groups with personnel involved in various phases of the acute care episode; and focus groups with Medicare beneficiaries who received treatment at ACE Demonstration sites for ACE-related services. The structured guide questions and site visit correspondence for these activities are included in attachments C and D.
For CMS, the success of the demonstration will rest on reducing costs while simultaneously achieving improved quality (clinical effectiveness, safety, and efficiency). Care was taken in the development of the demonstration to consider ways to encourage participation by various stakeholders. Hospitals and providers were directly incentivized by allowing gainsharing. Also, as the only entity in its market area to participate in the demonstration and to be given the right to designate itself as a “Medicare Cardiac (or Orthopedic) Value-Based Care Center”, the successful applicant was given an opportunity to increase its visibility, credibility, and market share. In fact, this designation was intended to be used as an integral component of a marketing campaign, which applicants were required to describe in their proposal. Successful applicants are expected to utilize this process and to vet all marketing materials with CMS prior to implementation. CMS further agreed to make joint appearances and press releases to assist the demonstration sites in their efforts to recruit patients and encourage referrals from community physicians. Evaluating the impact of these marketing campaigns on their target audience (consumers) is an important component of determining the success of the demonstration.
As detailed in the Medicare Acute Care Episode Demonstration: Design, Implementation and Management Design Report, CMS has previously conducted two successful demonstrations that focused on using a bundled payment strategy. The first, the Medicare Heart Bypass Center Demonstration in the early 1990s, resulted in both financial savings and improved patient outcomes. This success was followed by the Medicare Cardiovascular & Orthopedics Centers of Excellence Demonstration, which was larger in size and scope of procedures. However, because of some administrative and fiscal issues, this demonstration was canceled in late 1999.
The current demonstration builds on the experience of these prior studies and considers bundling hospital and physician payment for the acute phase of specific episodes of care for selected inpatient-based cardiac and orthopedic procedures. Specifically, CMS will obtain discounts for care by using incentives that align the interests of hospitals and physicians, and focus on improvements in patient outcomes. Through a competitive bidding process, institutions were selected that met critical threshold volumes shown to enhance positive patient outcomes.
The focus group and interview protocols are submitted with this statement. Other accompanying documents include the Social Security Act Title XXI Section 1866C, Medicare Prescription Drug, Improvement, and Modernization Act of 2003 Section 646 (PL 108-173), and Site Visit Correspondence samples.
The qualitative data collection will consist of new data, never previously collected. The IMPAQ team will use the collected information to evaluate the effects of the demonstration’s key features (incentives to providers/hospitals and beneficiaries, targeted marketing, anticipated cost savings, and improved quality of care). Long-term success of the bundled payment approach may be dependent on end-user satisfaction and willingness to participate.
Stakeholders will be queried directly through a series of focus groups and semi-structured interviews. Researchers from the IMPAQ team will conduct focus groups for stakeholders at each demonstration site specified in the technical proposal for the evaluation.
Focus groups and interviews will be digitally recorded for later transcription and uploading into Atlas.ti, a qualitative analytic software. Audio and/or video recording is a routine technique utilized for narrative data collections. In the case of this evaluation, there will be a large volume of data collected across 11 hospitals (15 demonstrations due to concurrent orthopedic and cardiac demonstrations at 4 hospitals), during two site visits (in the first year and third year of the demonstration), resulting in an estimated 312 hours of recorded responses detailed in more than 5,000 pages.
It is necessary to collect information from the particular participants in this demonstration. While this effort builds on previous CMS demonstrations, this project has unique features that require its participants to be queried about its impact on them. No other information would be similar enough for this purpose, even if the questions were exactly alike. This information collection does not duplicate any other effort, and the information cannot be obtained from any other source.
No small business is requested to submit information as part of this information collection.
In order to identify changes in the program and in the experiences of participants during the early and mature phases of the demonstration, information collection will occur at two points in time: at the end of the first year of the demonstration and, again, in year three.
Failure to gather qualitative information would yield a less robust analysis of the outcomes that reflect the ”human experience” of the demonstration and of factors that might impact long-term success, if larger scale implementation is desired later.
In all respects, the data will be collected in a manner consistent with federal guidelines. Information will not be collected more often than quarterly, will not require a less than 30-day written response, will not require more than one original or two copies of any document, and will not require record retention by respondents for more than three years. This data collection does not involve a statistical survey or statistical data classification not approved by the OMB.
Confidentiality requirements will be imposed by the demonstration sites’ own human subjects protection entities. In key stakeholder interviews of hospital and physician group administrators, the evaluation team will query site-specific implementation approaches and experiences, which might be proprietary in nature, but which will be protected and held confidentially.
Federal Register Notice and Comments
A 60-day Federal Register Notice was published in the Federal Register on June 18, 2010 (Vol. 75, No. 117, p. 34741). No comments were received in response to the publication.
Consultation Outside of the Agency
The following individuals were consulted in designing the data collection plan and developing the questionnaire:
Name |
Affiliation |
Telephone Number |
Oswaldo Urdapilleta |
IMPAQ International |
(443) 539 1394 |
Donald Nichols |
IMPAQ International |
(443) 539 0218 |
Jasmine Ainetchian |
IMPAQ International |
(443) 539 0216 |
Annette Snyder |
Hilltop Institute |
(410) 455-6386 |
Cynthia Boddie-Willis |
Hilltop Institute |
(410) 455-6518 |
Only Medicare beneficiaries will receive a monetary incentive for participating in the focus groups. Physician group representatives or hospital administrators and other employees will participate in interviews or focus groups under the auspices and according to arrangements made by their employer. Their participation will be deemed a function of their work responsibilities and remuneration. Beneficiaries will receive a modest incentive of $25 to partially compensate them for the time spent in the focus group and transportation costs that may have been incurred in their participation. The beneficiary focus groups are planned for 90 minutes. The informed consent form for participation in the focus groups will explain the direct monetary incentive and the indirect non-monetary benefits.
Demonstration sites are responsible for adhering to all Health Insurance Accountability and Portability and Privacy Act (HIPAA) requirements, as governed by their local human subjects protection processes and as covered entities under HIPAA. A HIPAA Waiver will be requested since the information to be collected is for the purpose of program operations/evaluation and thereby exempted from the statute. Even though participants will be queried about their health care delivery experience during the demonstration and not about their personal diagnosis(es), some confidential health information may be inadvertently shared by participants.
IMPAQ International will follow procedures for assuring and maintaining confidentiality consistent with provisions of the Privacy Act. Respondents will receive information about confidentiality protection in an advance letter describing the focus group or interview, as appropriate, (letters submitted with package) and again at the outset of the focus group or interview as part of the facilitator's introductory comments. Respondents will be informed that all information they provide will be treated confidentially. Facilitators will be trained in confidentiality procedures and will be prepared to describe these procedures in full detail, if needed, or to answer any related questions from the respondents.
All data items that identify respondents will be kept only by the contractor, IMPAQ International, for use in assembling records data and conducting the interviews. Any data received by CMS will not contain personal identifiers thus precluding individual identification. The evaluator will provide the appropriate safeguards during data collection, storage, and analysis to ensure that a minimal number of appropriate persons have access to the data and that the data is de-identified as quickly as possible should inadvertent identifiers be included. It should be emphasized that the use of identifiers is not part of the study design.
The planned protocol does not include questions of a sensitive nature. As described in item 10, all respondents will be assured of confidentiality at the outset of the focus group or interview. All responses will be held in strict confidence without the use of individual identification. IMPAQ International will comply with the requirements of the Privacy Act of 1974 in collecting all information.
The information collection for the qualitative portion of the demonstration evaluation will consist of one-on-one interviews and focus groups at each of the 11 hospitals involved (15 demonstrations, due to concurrent orthopedic and cardiac demonstrations at 4 hospitals). These interviews and focus groups will take place during site visits planned for year 1 and then again in year 3 of the demonstration. See the table below for a breakdown of interviews and focus groups by hospital and demonstration type (cardiac and orthopedic).
Data Collection Design
U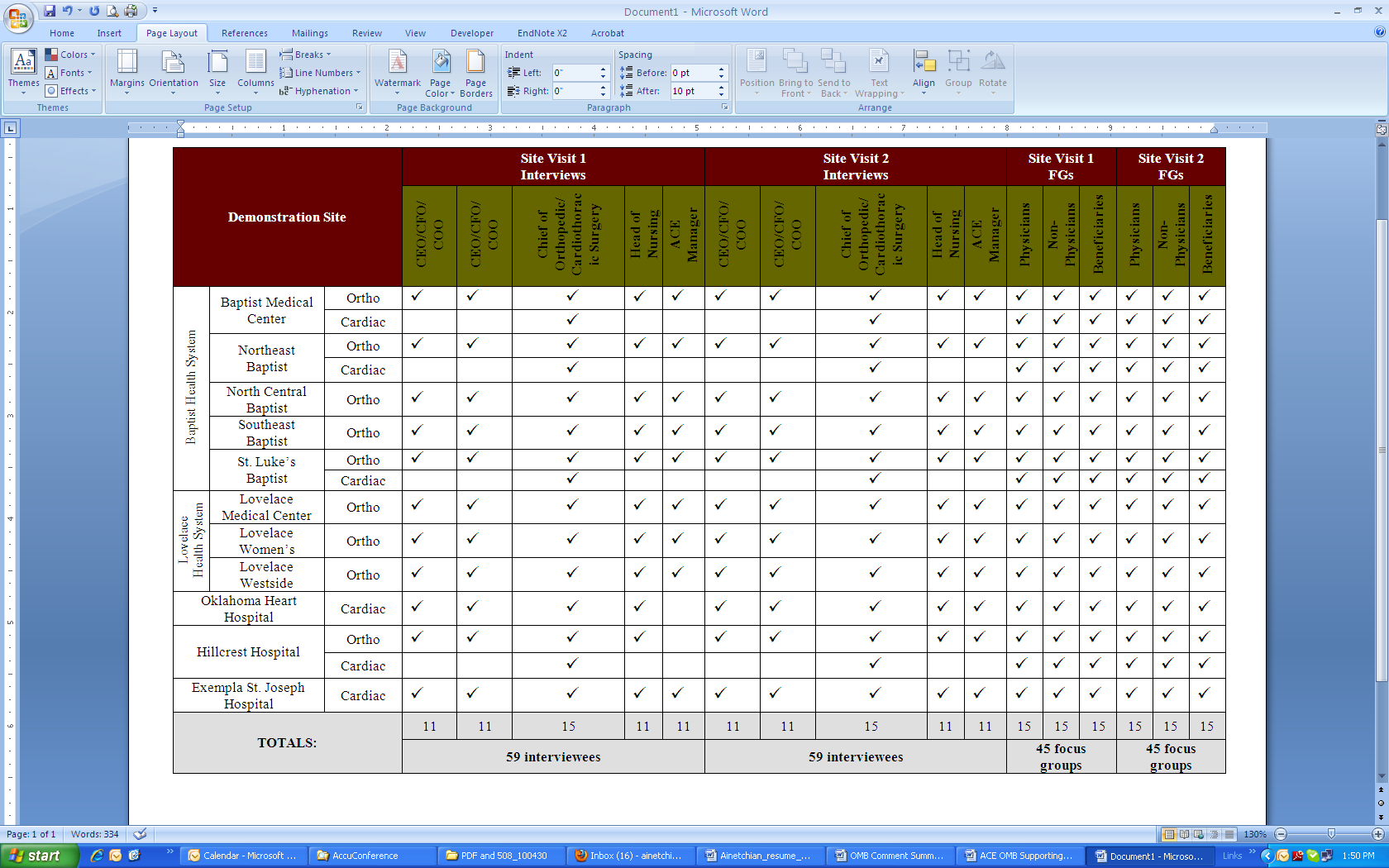 p
to six administrators per hospital will be interviewed for a total of
up to 59 interviews per site visit year (year 1 and year 3). These
interviews will last approximately 90 minutes each and will include
discussions with two hospital executives (CEO/COO/CFO or his/her
designee), managers/department heads from targeted areas involved in
the demonstration (e.g. orthopedics, cardiothoracic medicine,
nursing, etc.), and the ACE manager. The same participants from year
1 will be interviewed in year 3, as feasible.
p
to six administrators per hospital will be interviewed for a total of
up to 59 interviews per site visit year (year 1 and year 3). These
interviews will last approximately 90 minutes each and will include
discussions with two hospital executives (CEO/COO/CFO or his/her
designee), managers/department heads from targeted areas involved in
the demonstration (e.g. orthopedics, cardiothoracic medicine,
nursing, etc.), and the ACE manager. The same participants from year
1 will be interviewed in year 3, as feasible.
We will conduct focus groups with the following audiences: physicians, non-physicians (nurses, physical therapists, and other support staff), and Medicare beneficiaries. The physician and non-physician groups will be drawn separately from the cardiac and/or orthopedic demonstration(s), depending on whether the site has included one or both sets of procedures. During each site visit, we will conduct one focus group per audience for a total of 3 focus groups per demonstration. Each will consist of 10 participants and will last no longer than 90 minutes. Thus, 45 focus groups will be conducted per site visit year (year 1 and year 3) and will involve 450 participants.
Interim conference calls will be conducted with four or five key persons at each hospital (to the CEO/CFO/COO, Chief of Orthopedic/Cardiothoracic Surgery, Head of Nursing, and the ACE Manager). Five persons will be contacted at hospitals that are participating in both the cardiac and ortho demonstrations. These calls will take place during year 2 and will last, on average, for 45 minutes. The purpose of the calls will be qualitative data collection to ascertain critical developments that might occur during the demonstration.
Table 1: Reporting and Recordkeeping Hour Burden (Year 1)
Activity Name |
Number of Respondents |
Number of Responses per Respondent |
Hours per Response |
Total Burden Hours |
Key Informant Interviews |
59 |
1 |
1.5 |
88.5 |
Focus Groups - Physicians |
150 |
1 |
1.5 |
225 |
Focus Groups – Non-physicians |
150 |
1 |
1.5 |
225 |
Focus Groups -Beneficiaries |
150 |
1 |
1.5 |
225 |
TOTAL: |
763.5 |
|||
Table 2: Reporting and Recordkeeping Hour Burden (Year 2)
Activity Name |
Number of Respondents |
Number of Responses per Respondent |
Hours per Response |
Total Burden Hours |
Interim Conference Calls |
48 |
1 |
0.75 |
36 |
TOTAL: |
36 |
|||
Table 3: Reporting and Recordkeeping Hour Burden (Year 3)
Activity Name |
Number of Respondents |
Number of Responses per Respondent |
Hours per Response |
Total Burden Hours |
Key Informant Interviews |
59 |
1 |
1.5 |
88.5 |
Focus Group - Physicians |
150 |
1 |
1.5 |
225 |
Focus Group – Non-physicians |
150 |
1 |
1.5 |
225 |
Focus Groups - Beneficiaries |
150 |
1 |
1.5 |
225 |
TOTAL: |
763.5 |
|||
Table 4: Annualized Cost Burden |
||||
|
|
|
|
|
Activity Name |
Number of Respondents |
Total Burden Hours |
Average Hourly Rate* |
Total
Cost Burden |
Key Informant Interviews |
59 |
1.5 |
$76.23 |
6.75 |
Focus Group - Physicians |
150 |
1.5 |
$86.63 |
19.49 |
Focus Group – Non-Physicians |
150 |
1.5 |
$30.80 |
6.93 |
Focus Group - Beneficiaries |
150 |
1.5 |
$25.00** |
3.75 |
Interim Conference Calls |
48 |
.75 |
$76.23 |
2.74 |
|
Total |
39.66 |
||
*Source: The Bureau of Labor Statistics 2007 National Compensation Survey.
**The average hourly wage of beneficiaries represents the monetary incentive each will receive by participating in the focus groups.
The total annual number of respondents is 509, as indicated in item #10 of form 83-II. Throughout the three year demonstration, the same participants will be contacted for Key Informant Interviews as well as Interim Conference Calls (59 total) and the same is true for the physicians and non-physicians in the year 1 and year 3 focus groups (150 total physicians and 150 total non-physicians). However, two groups of beneficiaries will participate in the focus groups (150 beneficiaries in year 1 and 150 different beneficiaries in year 3). That is, 59 key informants, 150 physicians, 150 non-physicians, and 150 beneficiaries for the Demonstration. In item #10 of form 83-II, the frequency of data collection has been selected as two responses per respondent biennially in order to represent the two main data collections (year 1 and year 3) which will take place over a three year period. Beneficiaries who participate in focus groups will only respond once over the duration of the demonstration, while all other respondents will respond at two points in time.
There is no cost burden anticipated for individuals participating in this demonstration. Professional respondents (administrators, physicians, nurses, and other institutional staff) will participate as a function of their employment during work hours. Beneficiary focus group respondents will generally be older adults, most of whom, it is anticipated, will be retired or otherwise away from work due to recuperation from the procedure creating the eligibility for the demonstration.
Cost to Federal Government
The cost to the federal government is $347,151, which is the total contractor cost of conducting the site visits.
Changes to Burden
This is a new data collection effort.
None at this time.
No request made.
There are no exceptions taken to item 19 of OMB Form 83-1.
IMPAQ International, LLC
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Supporting Statement Part A—Justification |
| Author | asnyder |
| File Modified | 0000-00-00 |
| File Created | 2021-02-02 |
© 2026 OMB.report | Privacy Policy