Supporting Statement B
Supporting Statement B.docx
Medicare Part C and Part D Data Validation (42 C.F.R. 422.516g and 423.514g)
OMB: 0938-1115
Supporting Statement – Part B
Collections of Information Employing Statistical Methods
Describe (including a numerical estimate) the potential respondent universe and any sampling or other respondent selection method to be used. Data on the number of entities (e.g., establishments, State and local government units, households, or persons) in the universe covered by the collection and in the corresponding sample are to be provided in tabular form for the universe as a whole and for each of the strata in the proposed sample. Indicate expected response rates for the collection as a whole. If the collection had been conducted previously, include the actual response rate achieved during the last collection.
All Part C organizations and Part D sponsors are required to undergo the data validation review process. Because 100% of organizations will undergo the data validation process, sampling is not applied for respondent selection.
Within the data validation process, data validation contractors will be encouraged to collect the entire data set (the census) relied on by organizations to meet Part C and D reporting requirements. If collecting the census proves impractical due to an unusual time burden placed on organizations during data extraction, there is a sampling task that each organization will be required to perform in collaboration with the data validation review contractor. Each organization will draw an initial sample, at a minimum, of either 205 or 150 administrative records, depending on the Part C or Part D measure. Sample sizes may be larger and will be determined by the data validation contractor using standard statistical methodologies. All relevant records associated with these samples will then be selected for review (for example, all claims for a random sample of 205 members). In cases where the population is smaller than the required sample size, records for the entire population should be provided for evaluation.
This sampling process is described in detail in the document titled “Data Extraction and Sampling Instructions for Data Validation Contractors.”
Describe the procedures for the collection of information including:
Statistical methodology for stratification and sample selection,
Estimation procedure,
Degree of accuracy needed for the purpose described in the justification,
Unusual problems requiring specialized sampling procedures, and
Any use of periodic (less frequent than annual) data collection cycles to reduce burden.
For measures requiring sampling, simple random samples are used in the data validation review. The underlying standard is a quantifiable error rate in key fields. This is assumed to have a binomial distribution. The sample sizes are designed to detect error rates of 15% or more, with a one-tailed Type I error rate (α)=.05, except for samples based on eligibility. In those cases, because more confidence is needed, α is set at .025. A standard normal approximation to the binomial distribution is used to establish critical values. A finite population correction (FPC) factor has been included in sample size calculations. The variation formula below is solved for n to obtain sample size.
 ,
,
where
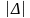 is the desired precision (5%), N is the number in the population, p
is the assumed proportion (.15), Z is the appropriate critical value
from the normal curve (either 1.645 or 1.96, depending on α and
n is the sample size.
is the desired precision (5%), N is the number in the population, p
is the assumed proportion (.15), Z is the appropriate critical value
from the normal curve (either 1.645 or 1.96, depending on α and
n is the sample size.
Describe methods to maximize response rates and to deal with issues of non-response. The accuracy and reliability of information collected must be shown to be adequate for intended uses. For collections based on sampling, a special justification must be provided for any collection that will not yield 'reliable' data that can be generalized to the universe studied.
Since extraction of the full census or use of the sampling process is required for all organizations, survey-related issues such as non-response bias are not applicable.
Describe any tests of procedures or methods to be undertaken. Testing is encouraged as an effective means of refining collections of information to minimize burden and improve utility. Tests must be approved if they call for answers to identical questions from 10 or more respondents. A proposed test or set of tests may be submitted for approval separately or in combination with the main collection of information.
Pilot tests of all methodology, including sampling and all supporting documents, have been conducted with one Part C organization and one Part D sponsor.
Provide the name and telephone number of individuals consulted on statistical aspects of the design and the name of the agency unit, contractor(s), grantee(s), or other person(s) who will actually collect and/or analyze the information for the agency.
Terry Lied, Ph.D.
Centers for Medicare & Medicaid Services
Medicare Drug Benefit and Part C and D Data Group
Division of Drug Plan Policy and Quality
410-786-8973
INFORMATION NOT RELEASABLE TO THE PUBLIC UNLESS AUTHORIZED BY LAW:
This information has not been publicly disclosed and may be privileged and confidential. It is for internal government use only and must not be disseminated, distributed, or copied to persons not authorized to receive the information. Unauthorized disclosure may result in prosecution to the full extent of the law.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | CMS |
| File Modified | 0000-00-00 |
| File Created | 2021-02-02 |
© 2026 OMB.report | Privacy Policy