Attachment H
attach - H.doc
Submission of Unreasonable Adverse Effects Information Under FIFRA Section 6(a)(2)
Attachment H
OMB: 2070-0039
Attachment H
Industry’s
Voluntary 6(a)(2) Incident
Reporting Forms
&
Guidance Documents
August 4, 1998
Table of Contents
Page
Introduction 3
General Considerations 4
Reporting categories and Time Frames (TABLE) 5
Incident Reporting Process (SCHEMATIC) 6
Trade &
Professional Association contact list 6
Key to Form Data Fields 7
Administrative/ Pesticide/Incident Data 7
Human Incident Information Data 8
Fish, Wildlife or Other Non-Target Organisms Data 9
Domestic Animal Data 10
Detections of Pesticides in Surface Water Data 11
Detections of Pesticides in Groundwater Data 12
Property Damage with Risk of Human Injury Data 13
Unauthorized Residue in Food & Feed 14
Individual Incident Forms 15
Administrative/ Pesticide/Incident Form 15
Human Incident Information Form 16
Fish, Wildlife or Other Non-Target Organisms Form 17
Domestic Animal Form 18
Detections of Pesticides in Surface Water Form 19
Detections of Pesticides in Groundwater Form 20
Property Damage with Risk of Human Injury Form 21
Unauthorized Residue in Food & Feed Form 22
Aggregate Reporting Key 23
Summary of Exposure Types & Severity Categories Included in Aggregate Reporting (Table) 24
Aggregate Form Example I 25
Aggregate Form Example II 26
Information Regarding the Use of the
Voluntary 6(a)(2) Incident Reporting Forms
Introduction
A number of industry trade organizations, registrants, professional groups and other interested parties have worked together in cooperation with the EPA to develop a set of reporting forms that can be used by Registrants to report 6(a)(2) related incidents to the EPA. These forms and their corresponding guidance documents provide useful tips and suggestions for meeting reporting requirements under 6(a)(2). Use of these specific forms for required reporting is voluntary. These forms have been developed to meet incident reporting requirements. EPA supports their use in 6(a)(2) compliance efforts as well as other methods of reporting to achieve the same purpose.
There are two sets of forms to reflect the two methods of reporting incidents to the Agency. Incidents that are of a more significant nature must be reported individually. Incidents that are either minor or more commonly encountered must be reported in aggregate. Severity categories and their respective reporting requirement are noted in the guidance instructions.
Typically, the single incident forms can be used to collect data regarding any incident. After the severity category of any given incident is determined the appropriate method of reporting that incident to the EPA can then be determined.
The single incident forms are divided into two specific areas. The first area describes “administrative data” or general demographic and product information that is gathered for any incident. There are seven supplemental addenda relating to the seven incident types i.e., human, domestic animal, fish/wildlife/plant/other non-target organisms, groundwater, surface water, food and feed, and property damage with risk of human injury. After collecting the information and the severity classification is determined, the Registrant can then ascertain whether the incident must be reported individually or in aggregate. If the incident must be reported individually the single incident forms can be used to submit the information directly. If the incident must be reported in aggregate certain information must be transferred to the aggregate report form and submitted in that manner.
General guidance information is provided in this document to aid in the completion of these forms. For further, or more specific guidance, consult 40 CFR Part 159.184 for full text and definitions. Some additional information is provided in Pesticide Registration Notice (PR Notice) 98-3.
These forms contain fields for reporting of information which may or may not be required to be reported to EPA under FIFRA 6(a)(2) and the regulations at 40 CFR 159.152 et.seq.. Use of this form is voluntary and is not intended to infer that any designated fields should be submitted to EPA or to mandate reporting of any specific information to EPA. Registrants/or applicants should consult their own legal counsel or FIFRA 6(a)(2) Reporting Officers before responding.
General Considerations:
Due to the nature of incidents and how they are typically reported through the 6(a)(2) process, Registrants do not guarantee the authenticity or accuracy of information contained in the reports required to be submitted to the Agency.
The forms contain some fields of data that are optional. These fields are used to aid the Registrant in general data collection purposes. In some cases these fields represent data that are not required but can be submitted to document important information the Registrant believes is vital to a better understanding of the incident.
The information collected on the form for Domestic Animal data is not required for single incident reporting as none of the severity classifications related to domestic animals are reported individually. All of these incidents are submitted on the aggregate forms. Data collected on the domestic animal form may be required by the EPA in cases of incident follow-up.
The Registrant is only required to submit information that has been provided. No investigation or follow-up is required, but may be useful if further information helps qualify or clarify reported incidents.
A portion of the category of H–E (anticipated or “may suffer” clinical effects) has been dropped from the reporting requirement by the EPA until further notice (see PR notice 98-4). The category of H-E is now limited to adverse effects that are unspecified or unknown.
Exposure type and severity categories of humans and domestic animals take into account both duration and intensity of clinical effects. As noted in PR Notice 98-3 for human incidents:
“The
persistence of symptoms or the development of delayed symptoms should
be considered when classifying severity. For example, human cases
may report developing common symptoms like headaches, general
weakness, memory and concentration problems, depression,
irritability, muscular aches and pains, or shortness of breath. If
these symptoms last for just a few days and are minimally troublesome
(do not require treatment) then they would be classified as minor
(H-D). However, if symptoms persist for one month or longer they
would be classified as moderate (H-C). Symptoms persisting for two
or more months that significantly alter daily activities would be
classified as major (H-B).”
Generally, major
effects would
include “life threatening” or effects resulting in
“residual disability”. They could also include “adverse
reproductive” effects.
Moderate
effects are
typically “more pronounced, more prolonged, or of a more
systemic nature than minor effects”. Examples include,
“isolated brief seizures”, “gastro-intestinal
systems leading to dehydration” and “corneal
abrasions.”
Minor
effects would
include effects such as “skin rash, itching, conjuctivitis
(red, tearing eyes), drowsiness, transient cough, headache, joint
pain, agitation, restlessness, or mild gastro-intestinal symptoms
such as self-limited diarrhea, stomach cramps, or nausea. These
effects are reported to have lasted less than one month.”
Note:
See PR Notice 98-3 for a more detailed description of severity
categorization for humans and domestic animals.
The following table provides a quick reference guide to reporting timeframes and content:
SEVERITY CATEGORIES and/or Other Reporting Categories |
REPORTING TIMEFRAMES |
Human Deaths (H-A) |
ASAP-No Later than 15 days
Submission Format: Individual Report
Provide detailed information for each incident
|
Scientific Studies described in (159.165) Information about discontinued studies (159.167) Human epidemiology and exposure studies (159.167) Detection of a unauthorized pesticide in or on food or feed (159.178) Detection of metabolites, degradates, contaminates, impurities (159.179) Failure of performance studies related to public health products (159.188 (a)(2), (b)(2)) Substantiated incidents of pest resistance (159.188 (c)) Other information described in (159.195) Property Damage with risk to human health (PD-A) and other information (159.195)
|
Submit within 30 calendar days
Submission Format: - Individual Report (Food/Feed, Property Damage with risk of injury to humans)
- For all other submissions refer to 159.156 |
Human – Major (H-B) Human – Moderate (H-C)
Major – Wildlife (W-A) Plant (P-A)
Detection
of pesticide in water at levels greater than
Efficacy failure incidents regarding public health products(159.188 (a)(1) & (b)(1))
|
Accumulate 1 Month
Submit by the end of the month following the accumulation period.
Submission Format: Individual Report
Provide detailed information for each incident as required in section 159.184(c) |
Human – Minor (H-D) Unspecified or Unknown effects (H-E)
All Domestic Animal (D-A,B,C,D,E) All Other Categories for Wildlife (W-B) Plant
(P-B)
|
Accumulate 3 Months
Submit by the end of the 2nd month following the accumulation period
Submission Format: Aggregate
Aggregate and submit count of incidents and effects for each product or AI as required in section 159.184 (e)
|
Incomplete toxicological and ecological studies (159.165 (d)) |
Consult sec. 159.165 (d) when testing is completed but study not finalized
|
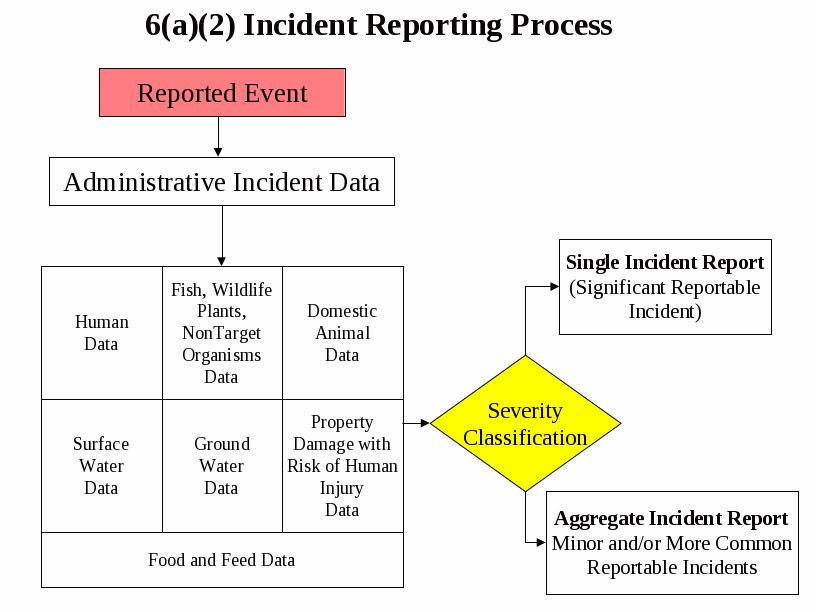
The overall flow of the reporting process is depicted in the following diagram:
Original and updated versions of these forms and guidance tools are available from the following organizations. These organizations have been closely involved in the form development process and serve as a valuable resource to their constituents and others interested in 6(a)(2) related efforts.
American Crop Protections Association Chemical Specialties Manufacturers Association
1156 Fifteenth St., NW, Suite 400 1913 Eye St. NW
Washington, DC 20005 Washington, DC 20006
Phone: (202) 296-1585 Phone: (202) 872-8110
Fax: (202) 463-0474 Fax: (202) 872-8114
Staff contact: Ray McAllister Staff contact: Steve Kellner
Web site: http://www.acpa.org Web site: http://www.csma.org
Chemical Manufacturers Association International Sanitary Supply Association
1300 Wilson Blvd. 7373 North Lincoln Ave.
Arlington, VA 22209 Lincolnwood, IL 60646-1799
Phone: (703) 741-5637 Phone: (847) 982-0800
Fax: (703) 741-6091 Fax: (847) 982-1012
Staff contact: Has Shah Staff contact: Bill Balek
Web site: http://www.cmahq.com Web site: http://www.issa.com
Chemical Producers and Distributors Association PROSAR Product Safety Call Center
1430 Duke Street 1295 Bandana Blvd. Suite 335
Alexandria, VA 22314 St. Paul, Minnesota 55108
Phone: (703) 548-7700 Phone: (651) 917-6100
Fax: (703) 548-3149 Fax: (651) 641-0341
Staff contact: Warren Stickle Staff contact: Joele Richardson
Web site: http://www.cpda.com Web site: http://www.prosarcorp.com
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Administrative, Pesticide and Incident Circumstance Data Section
(See attached form)
Row 1 Administrative Data
Field |
Comments/Description |
Reporter name, Address, Phone #
|
This field refers to the individual that is reporting the incident to the Registrant or Registrants Agent. Anonymous reports where an individual declines to provide a name and other identifying information are not reportable to the EPA.
|
Submission date
|
This field refers to the date the report is submitted to the agency. |
Contact person (If different than reporter), Address, Phone #
|
This field can be used to identify an individual, other than the reporter, to be contacted for further information related to the incident. Examples could include; parent, physician, lawyer, etc. |
Internal ID
|
This optional field may be used by the registrant for internal purposes. |
Incident status: New, Update
|
Use this field to identify if this is a new report or an update regarding a previous report. |
Location and date of incident
|
Self-explanatory
|
Date registrant became aware of incident
|
This date refers to when the registrant, or the registrants agent, was advised of the incident. |
Was incident part of larger study |
An example would include ongoing monitoring of detections of pesticides in ground or surface water. |
Row 2 Pesticide Data
Field |
Comments/Description |
EPA Registration # (for up to 3 product identifications)
|
In order for the incident to be a reportable event the product must be identified in at least one of two ways. In order of preference by the agency, they are: 1) EPA Product Registration Number or, 2) Active Ingredient. The product name must also be included, if known, but must be accompanied by either EPA Product Registration Number or Active Ingredient.
|
AI (s)
|
Identify the active ingredient here. (see above) |
Product name
|
If known, identify the Product Name here. (see above) |
Exposed to concentrate prior to dilution
|
If product is sold in a concentrated form intended for dilution and the incident involves the concentrate prior to being diluted, indicate so here.
|
Formulation
|
Identify the formulation if known. Examples could include wettable powder, liquid, granules, etc. |
Row 3 Incident Circumstances
Field |
Comments/Description |
Evidence label directions were not followed
|
If this can be determined from the history and circumstances of the incident indicate so here. |
Applicator certified PCO
|
If the individual applying the pesticide is a certified Pest Control Operator, indicate so here.
|
Incident site
|
Indicate where incident occurred in this area. Examples of incident sites could include home, yard, school, industrial, nursery/greenhouse, surface water, commercial turf, building/office, forest/ woods, agricultural (specify crop), right-of-way (rail, utility, highway)). Use descriptors that best describe the information reported. |
Situation
|
Describe how the product was being used at the time of the reported incident or what the exposed individual was doing when the exposure occurred. Examples could include mixing/loading, reentry, application, transportation, repair/ maintenance of application equipment, manufacturing/ formulating). |
How Exposed
|
Indicate how the individual came in contact with the substance/product. Examples could include direct contact with treated surface, ingestion, spill, drift, runoff, etc.
|
Incident circumstances
|
Provide a brief description of what happened. |
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Human Incident Information Addendum
(See attached form)
Field |
Comments/Description |
Demographic (Age, Sex, Occupation)
|
Provide the age and sex of the individual exposed. If the incident was occupationally related state the occupation of the individual involved.
|
Exposure route
|
Exposure route refers to how the person came in contact with the substance or product. Examples could include skin, respiratory, ingestion etc.
|
Suicide/homicide related event
|
Indicate here if the incident was the result of a suicide or homicide. |
Protective clothing
|
Indicate what type of protective clothing was being used by the individual at the time of the incident.
|
Pregnancy Status
|
If the individual is a female and her pregnancy status is known, indicate here.
|
Occupational exposure status and workdays lost
|
For those incidents occurring in the workplace, and where the number of workdays lost is known, indicate so here. |
Time from exposure to development of symptoms
|
Indicate how long after the incident occurred that the first signs and symptoms were noted. |
Type of medical care sought
|
If the individual sought medical care indicate the type of medical care sought. Examples include none, clinic, hospital emergency department, private physician, PCC (Poison Control Center), hospital inpatient.
|
Signs/symptoms
|
Provide a description of the reported signs and symptoms. |
Lab tests
|
If laboratory tests related to the exposure were performed indicate the results.
|
Exposure Data: (amount, duration, Patient Weight)
|
If amount of product involved and the duration of contact with the product can be determined indicate so here. |
Severity category
|
See information provided in the guidance attachment. |
Qualifying information
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
Internal ID
|
(Optional field) This field is for internal use by Registrant. |
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Fish, Wildlife, Plant or Other Non-Target Organism Incidents Addendum
(See attached form)
Field |
Comments/Description |
List species affected and number of individuals per species
|
Record as noted. |
List symptoms or adverse effects
|
Record as noted. |
Magnitude of the effect
|
Record as noted. |
Pesticide application rate, intended use site, application method
|
Record as noted. |
If Plant, plant type
|
Record as noted. |
If lab test(s) performed, list name of tests and results (submit laboratory report(s) if available)
|
Record as noted. |
Description of the habitat and the circumstances under which the incident occurred.
|
Record as noted. |
Distance from treatment site
|
This
is defined as the distance generally reported in feet or yards
that a species, generally a bird or fish has been found (usually
dead) adjacent to the treated field or site. For instance, a fish
kill could have been reported to have occurred 100 yard from the
treatment site. |
Fish, wildlife, plant, other non-target organism severity category
|
Record as noted. |
Other severity categories reported:
|
Record as noted. |
Additional space for answers or explanatory information in this box.
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
Internal ID#
|
(Optional field) This field is for internal use by Registrant. |
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Domestic Animals
(See attached form)
Field |
Comments/Description |
Type of animal
|
Record as noted. |
Breed/species (name, no./adv effect)
|
Identify the breed and species as well as number of animals displaying each category of adverse effect. |
Exposure route
|
Exposure route refers to how the animal came in contact with the substance or product. Examples could include skin, respiratory, ingestion etc.
|
Time between exposure and onset of symptoms
|
Indicate how long after the incident occurred that the first signs and symptoms were noted. |
Signs/symptoms/adverse effects
|
Provide a description of the reported signs and symptoms. |
If lab test(s) performed, list name of tests and results (submit laboratory report(s) if available)
|
Record as noted. |
Additional space for answers or explanatory information
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Detections of Pesticides in Surface Water Information
(See attached form)
Field |
Comments/Description |
Pesticide/degradates analyzed for, methods of analysis, corresponding detection limits and amount detected
|
Record as noted. |
If raw water samples, water bodies sampled and approximate locations in each water body
|
Record as noted. |
If raw water samples, proximity of sampling locations to drinking water supply intakes and identities of systems supplied
|
Record as noted. |
If finished water samples, water supply systems sampled
|
Record as noted. |
If finished water samples, percent surface water source by specific surface water sources to water supply system(s)
|
Record as noted. |
Amount of pesticide detected |
Record as noted.
|
Sampling times/frequency
|
Record as noted. |
Sample type: (Grab, composite, Other)
|
Record as noted. |
Water severity category
|
Record as noted. |
Additional space for answers or explanatory information in this box
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Detections of Pesticides in Groundwater Incident
(See attached form)
Field |
Comments/Description |
Pesticide/degradates analyzed for, methods of analysis, corresponding detection limits and amount detected |
Record as noted. |
Sample dates (s)
|
Record as noted. |
Depth to Water
|
Record as noted. |
Well use/well identifier
|
Record as noted. |
Screened interval
|
Record as noted. |
Soil series/texture: (Sand/Clay/Silt/Other)
|
Record as noted. |
Latitude/longitude
|
Record as noted. |
Aquifer description: Confined/Unconfined
|
Record as noted. |
Hydrologic group
|
Record as noted. |
Hydraulic conductivity
|
Record as noted. |
pH of water
|
Record as noted. |
Organic matter/organic carbon (percent)
|
Record as noted. |
Maximum rainfall/date
|
Record as noted. |
Annual cumulative rainfall (inches)
|
Record as noted. |
Cumulative irrigation (inches)
|
Record as noted. |
Years pesticide used
|
Record as noted. |
Application frequency/yr.
|
Record as noted. |
Application method
|
Record as noted. |
Date of last application
|
Record as noted. |
Water severity category
|
Record as noted. |
Additional space for answers or explanatory information in this box
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Property Damage with Risk of Human Injury Information
(See attached form)
Field |
Comments/Description |
Describe Property damage
|
Record as noted. |
Property severity category
|
All reportable property damage should be labeled “PD-A” |
Additional space for answers or explanatory information in this box
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
KEY TO DATA FIELDS ON INDIVIDUAL INCIDENT FORMS
Unauthorized Residue in Food & Feed
(See attached form)
Field |
Comments/Description |
Pesticide/degradates analyzed for and corresponding detection limits
|
Record as noted. |
Amount of Pesticide detected
|
Record as noted |
Sample type
|
Record as noted
|
Method of analysis
|
Record as noted |
Tolerance level
|
Record as noted |
Additional space for answers or explanatory information
|
(Optional field) The rule allows Registrants to provide any clarifying or qualifying information related to the incident or their evaluation of the incident. Registrant may use this space to record this information and, attach additional pages if necessary.
|
Voluntary Industry Reporting Form for 6(a)(2) Adverse Effects Incident Information
Provide all known, required information. If required data field information is unknown, designate as such in appropriate area. Page# of
Row 1
Administrative Data |
Reporter Name
|
Submission date.
|
Contact person (if different than reporter)
|
Internal ID
|
||||||
|
Address
|
Address
|
||||||||
|
Phone #
|
Phone #
|
||||||||
|
Incident Status: New__ Update__ If update, include date of original submission. |
Location and date of incident. (City, County, State)
|
Date registrant became aware of incident.
|
Was incident part of larger study? Y___N___U___ |
||||||
Row 2
Pesticide(s) Involved |
EPA Registration # (Product 1)
|
EPA Registration # (Product 2)
|
EPA Registration # (Product 3
|
|||||||
|
A.I. (s)
|
A.I. (s)
|
A.I. (s)
|
|||||||
|
Product 1 name
|
Product 2 Name
|
Product 3 Name
|
|||||||
|
Exposed to concentrate prior to dilution? Y___N___U___NA___ |
Exposed to concentrate prior to dilution? Y___N___U___NA___ |
Exposed to concentrate prior to dilution? Y___N___U___NA___ |
|||||||
|
Formulation
|
Formulation
|
Formulation
|
|||||||
Row 3
Incident Circumstances |
Evidence label directions were not followed? Yes___No___U___ Intentional misuse___
|
Incident site: (examples include home, yard, school, industrial, nursery/greenhouse, surface water, commercial turf, building/office, forest/ woods, agricultural (specify crop) right-of-way (rail, utility, highway)).
|
Situation (act of using product): (examples include mixing/loading, reentry, application, transportation, repair/ maintenance of application equipment, manufacturing/ formulating).
|
|||||||
Applicator certified PCO? Yes__No__U__ |
||||||||||
|
How exposed: (examples include direct contact with treated surface, ingestion, spill, drift, runoff)
|
Brief description of incident circumstances.
|
||||||||
Voluntary Industry Reporting Form for 6(a)(2) Incident Information Involving Humans
Provide all known, required information. If required data field information is unknown, designate as such in appropriate area. Page# of
Demographic information: Age_____ Sex_______ Occupation (if relevant)
|
Exposure route: Skin___ Eye___ Oral___ Respiratory___ Unknown___ Other:
|
Was adverse effect result of suicide/homicide or attempted suicide/homicide?
|
Was protective clothing worn (specify)?
|
|
If female, pregnant? Yes___ No___ Unknown___ |
Was exposure occupational? Yes___ No___ Unknown___ If yes, days lost due to illness:
|
Time between exposure and onset of symptoms:
|
||
Type of medical care sought: (examples include none, clinic, hospital emergency department, private physician, PCC, hospital inpatient).
|
List signs/symptoms/adverse effects
|
If lab tests were performed, list test names and results (If available, submit reports)
|
||
Exposure data: Amount of pesticide:
Exposure duration:
Victim weight: ____lb ____kg ____unknown
|
||||
Human severity category___ |
||||
This box can be used to provide any explanatory or qualifying information surrounding the incident. (add additional pages if necessary) |
||||
|
Internal ID # |
|||
Voluntary Industry Reporting Form for 6(a)(2) Incident Information Involving Fish, Wildlife, Plants or Other Non-Target Org.
Provide all known, required information. If required data field information is unknown, designate as such in appropriate area. Page# of
List species affected and number of individuals per species.
|
|||
List symptoms or adverse effects.
|
|||
Magnitude of the effect: (Examples include miles of streams, square area of terrestrial habitat).
|
Pesticide application rate, intended use site (examples: corn, turf), and application method
|
||
If plant, plant type: (Examples include crop, forest, forage, orchard, home garden, ornamental).
|
|||
If lab test(s) performed, list name of tests and results (submit laboratory report(s) if available).
|
|||
Description of the habitat and the circumstances under which the incident occurred.
|
|||
Distance from treatment site.
|
Fish, wildlife, plant, other non-target organism severity categories:______:______:______ Include all categories that apply, ex. W, P, ONT |
||
This box can be used to provide any explanatory or qualifying information surrounding the incident (add additional pages if necessary).
|
|||
|
Internal ID# |
||
Voluntary Industry reporting form for 6(a)(2) Incident Information
If incident involves domestic animals use this form to collect information to be reported on the aggregate form. Page# of
Type of animal: (Examples include livestock, bird, fish, poultry, pet (specify)).
|
Breed/species (name, no./adv.Effect)
|
Exposure route: (Examples include skin, eye, oral, respiratory, unknown). |
|
Domestic animal severity category___
|
Time between exposure and onset of symptoms:
|
||
List sign/symptoms/adverse effects. Was animal treated (optional)?
|
|||
If lab test(s) performed, list name of tests and results (submit laboratory report(s) if available)
|
|||
This box can be used to provide any explanatory or qualifying information surrounding the incident (add additional pages if necessary).
|
|||
|
Internal ID# |
||
Voluntary Industry Reporting Form for 6(a)(2) Incident Information
Detections of Pesticides in Surface Water
Provide all known information. If required data field information is unknown, designate as such in appropriate area. Page# of
Pesticide/degradates analyzed for, methods of analysis, corresponding detection limits and amount detected:
Pesticides Degradates Method of analysis Detection limit Amount detected |
||
Sampling times/frequency
|
Sample type: (Grab, composite, other)
|
|
If raw water samples, water bodies sampled and approximate locations in each water body. |
If raw water samples, proximity of sampling locations to drinking water supply intakes and identities of systems supplied |
|
If finished water samples, water supply systems sampled
|
If finished water samples, percent surface water source by specific surface water sources to water supply system(s) |
|
Water severity category |
||
Additional space for answers or explanatory information in this box.
|
||
|
Internal ID# |
|
Voluntary Industry Reporting Form for 6(a)(2) Incident Information
Detections of Pesticides in Groundwater
Provide all known information. If required data field information is unknown, designate as such in appropriate area. Page # of
Pesticide/degradates analyzed for, methods of analysis, corresponding detection limits and amount detected:
Pesticides Degradates Method of analysis Detection limit Amount detected |
||||||
Date sample collected |
Depth to groundwater |
Well use/well identifier |
||||
Screened interval |
Soil series and texture: (sand, clay, silt, other) |
Latitude/longitude |
||||
Aquifer description: Confined ____ Unconfined ____ |
Hydrologic group. |
Hydraulic conductivity. |
||||
pH of water. |
Organic matter/organic carbon (percent). |
Maximum rainfall and date |
||||
Annual cumulative rainfall |
Cumulative irrigation (inches). |
Years Pesticide used. |
||||
Application frequency per year. |
Application method |
|||||
Date of last application |
Water severity category |
|||||
Additional space for answers or explanatory information in this box.
|
||||||
|
Internal ID# |
|||||
Voluntary Industry Reporting Form for 6(a)(2) Incident Information
Incident involving property damage with risk of human injury. Page# of
Describe property damage (if any). |
PD - A |
|
|
||
Additional space for answers or explanatory information in this box
|
||
|
Internal ID# |
|
Voluntary Industry Reporting Form for 6(a)(2) Incident Information
Unauthorized Residue in Food and Feed
Provide all known information. If required data field information is unknown, designate as such in appropriate area. Page# of
Pesticides/degradates analyzed for and corresponding detection limits |
||
Amount of Pesticide detected
|
||
Sample type
|
Sampling times/frequency
|
|
Method of analysis |
Tolerance Level |
|
This box can be used to provide any explanatory or qualifying information surrounding the incident (add additional pages if necessary).
|
||
|
Internal ID# |
|
FIFRA 6(a)(2) Aggregate Incident and Effect Information
Submission Form Instructions
Under 6(a)(2) aggregate reporting, the registrant is required to provide an aggregate summary of adverse incidents reported to the registrant that are outside of serious incidents which require individual reports. The attached form is a reporting template developed by industry representatives, in cooperation with the EPA, to facilitate this aspect of 6(a)(2) reporting. Use of this specific form is voluntary and other methods of reporting to achieve the same goal are also acceptable. The instructions for filling in the fields on this form are as follows:
Product Identification: In order for the incident to be a reportable event the product must be identified in at least one of two ways. In order of preference by the agency, they are: 1) EPA Product Registration Number or, 2) Active Ingredient. The product name must also be included, if known, but must be accompanied by either EPA Product Registration Number or Active Ingredient.
Internal ID #: The Internal ID # is an optional field reserved for use by the registrant to reference that particular summary.
Submission Date: The Submission Date refers to the registrant’s date of submission for this report.
Time Period Covered: Although some registrants may elect to report more frequently, data may be accumulated for a maximum of 90 days (interpreted by EPA as 3 months) then reported within 60 days (interpreted by EPA as 2 months). The registrant should state the time period this aggregate report covers. Considering maximums, an example would be accumulating data for the months of July, August and September, then submitting the aggregate report to the Agency on or before the last day of November.
Total Incidents: This field represents the total number of incidents which resulted in one or more of the “Exposure types and Category Designations.”
Exposure
Types and Severity Category Designations: There are a total of
12 exposure types and severity category designations included in
aggregate reporting to describe a given type of exposure and reported
effects (see definitions on attached page). Each incident will
involve a minimum of one exposure type and severity category
designation but could involve multiple designations. When an exposure
type and severity category designation is reported it is counted only
once per incident, regardless of the number occurring in that
incident. As an example, one incident involving 5 humans each having
effects that would be categorized as H-D would result in category H-D
being counted just once in the aggregate report. If that same
incident also included 3 occurrences of a B effect in domestic
animals then the “D-B” category designation would have a
1 placed in that box also. It should be noted that in the exposure
types involving wildlife or plants the number of organisms affected
is reflected in the level of the category.
Additional Information: Registrant may use this optional area to provide supplemental information that may explain, qualify, or otherwise aid in the interpretation of information provided in the aggregate summary. There is no limit as to the amount of information that can be provided in this area. Please note that this information will not appear in the EPA database but will be made available to the scientific review committees.
These forms contain fields for reporting of information which may or may not be required to be reported to EPA under FIFRA 6(a)(2) and the regulations at 40 CFR 159.152 et.seq.. Use of this form is voluntary and is not intended to infer that any designated fields should be submitted to EPA or to mandate reporting of any specific information to EPA. Registrants/or applicants should consult their own legal counsel or FIFRA 6(a)(2) Reporting Officers before responding.
SUMMARY OF EXPOSURE TYPES & SEVERITY CATEGORIES INCLUDED IN AGGREGATE REPORTING*
H-D (Human) |
If the person alleged or exhibited some symptoms, but they were minimally traumatic. The symptoms resolved rapidly and usually involve skin, eye or respiratory irritation. |
H-E (Human) |
If symptoms are unknown, unspecified. |
D-A (Domestic Animal) |
If the domestic animal died or was euthanized. |
D-B (Domestic Animal) |
If the domestic animal exhibited or was alleged to have exhibited symptoms which may have been life-threatening or resulted in residual disability. |
D-C,D,E (Domestic Animal) |
D-C: If the domestic animal exhibited or was alleged to have exhibited symptoms which are more pronounced, more prolonged or of a more systemic nature than minor symptoms. Usually some form of treatment would have been indicated to treat the animal. Symptoms were not life threatening and the animal has returned to its pre-exposure state of health with no additional residual disability. D-D: If the domestic animal was alleged to have exhibited symptoms, but they were minimally bothersome. The symptoms resolved rapidly and usually involve skin, eye or respiratory irritation. D-E: If symptoms are unknown or not specified.
|
W-B (Wildlife) |
Use W-B if none of the following criteria are met: (A) Involves any incident caused by a pesticide currently in Formal Review for ecological concerns. (B) Fish: Affected 1,000 or more individuals of a schooling species or 50 or more individuals of a non-schooling species. (C) Birds: Affected 200 or more individuals of a flocking species, or 50 or more individuals of a songbird species, or 5 or more individuals of a predatory species. (D) Mammals, reptiles, amphibians: Affected 50 or more individuals of a relatively common or herding species or 5 or more individuals of a rare or solitary species. (E) Involves effects to, or illegal pesticide treatment (misuse) of a substantial tract of habitat (greater than or equal to 10 acres, terrestrial or aquatic). (F) Involves a major spill or discharge (greater than or equal to 5,000 gallons) of a pesticide. (G) Involves adverse effects caused by a pesticide, to federally listed endangered or threatened species.
|
P-B (Plant) |
If an alleged effect involves damage to plants, label the incident P-A if the single criterion listed in (A) of this section is met, or P-B if the criterion is not met: (A) The effect is alleged to have occurred on more than 45 percent of the acreage exposed to the pesticide.
|
ONT (Other non Target Organism) |
If an alleged effect involves damage to non-target organisms other than fish, wildlife or plants (for example, beneficial insects), label the incident ONT. |
G-B (Ground-water) |
If a pesticide is alleged to have been detected in groundwater, surface water or finished drinking water, label the incident in accordance with the following criteria: G-B: If the pesticide was detected at levels greater than 10 percent of the MCL, HAL or a criterion for ambient water quality but does not exceed the MCL or other applicable level. |
G-C (Ground-water) |
If the pesticide was detected at levels less than 10 percent of the MCL, HAL, or other applicable level, or there is no established level of concern. |
*See 40 CFR Part 159.184 for full text and definitions.
FIFRA 6(a)(2) Aggregate Incident and Effect Information Submission Form (Suggested Format) |
Submission Date
|
page # _____ of _____
|
||||||||||||
Product Registration #
|
Time Period Covered:
|
Total Incidents = |
||||||||||||
Active Ingredient(s)
|
Product Name (if known)
|
|||||||||||||
Internal ID |
Exposure Types and Severity Category Designations
|
|||||||||||||
H-D |
H-E |
D-A |
D-B |
D-C,D,E |
W-B |
P-B |
ONT |
G-B |
G-C |
|||||
|
|
|
|
|
|
|
|
|
|
|||||
Additional Information:
|
||||||||||||||
FIFRA 6(a)(2) Aggregate Incident and Effect Information Submission Form (Suggested Format) |
Submission Date |
Page # _____ of _____ |
|||||||||||||
Product Registration #
|
Time Period Covered |
Total Incidents = |
|||||||||||||
Active Ingredient(s)
|
Product Name (if known) |
||||||||||||||
Internal ID
|
Exposure Types and Severity Category Designations |
||||||||||||||
H-D |
H-E |
D-A |
D-B |
D-C,D,E |
W-B |
P-B |
ONT |
G-B |
G-C |
||||||
|
|
|
|
|
|
|
|
|
|
||||||
Additional information:
|
|||||||||||||||
Product Registration #
|
Time Period Covered |
Total Incidents = |
|||||||||||
Active Ingredient(s)
|
Product Name (if known) |
||||||||||||
Internal ID
|
Exposure Types and Severity Category Designations |
||||||||||||
H-D |
H-E |
D-A |
D-B |
D-C,D,E |
W-B |
P-B |
ONT |
G-B |
G-C |
||||
|
|
|
|
|
|
|
|
|
|
||||
Additional information:
|
|||||||||||||
Product Registration #
|
Time Period Covered |
Total Incidents = |
|||||||||||
Active Ingredient(s)
|
Product Name (if known) |
||||||||||||
Internal ID
|
Exposure Types and Severity Category Designations |
||||||||||||
H-D |
H-E |
D-A |
D-B |
D-C,D,E |
W-B |
P-B |
ONT |
G-B |
G-C |
||||
|
|
|
|
|
|
|
|
|
|
||||
Additional information:
|
|||||||||||||
Product Registration #
|
Time Period Covered |
Total Incidents = |
|||||||||||
Active Ingredient(s)
|
Product Name (if known) |
||||||||||||
Internal ID
|
Exposure Types and Severity Category Designations |
||||||||||||
H-D |
H-E |
D-A |
D-B |
D-C,D,E |
W-B |
P-B |
ONT |
G-B |
G-C |
||||
|
|
|
|
|
|
|
|
|
|
||||
Additional information:
|
|||||||||||||
| File Type | application/msword |
| File Title | Row 1 Administrative Data |
| Author | Rick Kingston |
| Last Modified By | Cameo smoot |
| File Modified | 2010-07-02 |
| File Created | 2010-07-02 |
© 2026 OMB.report | Privacy Policy