Oral Health Dental Fluorosis Imaging
National Health and Nutrition Examination Survey (NHANES)
NHANES Att B Mar 2011
Feasibility Study to Identify Physical Activity and Oral Health Study and 24 Hour Urine Study and Birth Cert Linkage Study
OMB: 0920-0237
Attachment B.
Protocol for Oral Health – Dental Fluorosis Imaging/Examination Feasibility Study (ages 12-16)
National Health and Nutrition Examination Survey (NHANES)
Oral Health – Dental Fluorosis Imaging/Examination Feasibility Study
OMB no. 0920-0237
Expires: 11/30/2012
Notice - Information contained on this form which would permit identification of any individual or establishment has been collected with a guarantee that it will be held in strict confidence, will be used only for purposes stated for this study, and will not be disclosed or released to others without the consent of the individual or establishment in accordance with section 308(d) of the Public Health Service Act (42 USC 242m). Public reporting burden of this collection of information is estimated to average 30 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road, MS D-74, Atlanta, GA 30333. ATTN: PRA (0920-0214).
Oral Health – Dental Fluorosis Imaging/Examination Feasibility Study:
Age Eligibility:
A sample of 75 adolescents’ age 12-16 years will be recruited for this study.
Informed Consent:
Informed consent forms signed by a parent or legal guardian will be obtained. An additional informational sheet will be provided to the volunteer child that shows what will happen if they participate in this study. The consent form is shown on page 8 and the informational sheet is shown on page 9.
Study Design:
This is a cross-sectional, observational epidemiology study. This study will be directed by NHANES staff in coordination with Westat staff.
Data collection for the Fluorosis Study will occur over 2 different periods. The first period will consist of a dental fluorosis examination and the taking of 3 dental photographs per volunteer at a mobile examination center and will last for approximately 1 week (part 1); the second period will consist of grading the digital images captured at the time of the examination by subject matter experts and collecting that information electronically (part 2). Procedures are described in greater detail in forthcoming sections. As a synopsis, the following outline describes the steps of the volunteer’s experience from start to finish (part 1) and the flow of information regarding grading the dental photographs (part 2).
Key Study Steps (part 1)
1. Recruitment and screening of volunteers
2. Scheduling appointments and follow-up contact for the volunteers
3. Obtaining informed consent
4. Taking the dental photographs
5. Conducting the tooth count and dental fluorosis examination
6. Providing general feedback to the volunteer regarding the clinical exam
Key Study Steps (part 2)
1. Electronically capturing the dental images
2. Electronically distributing the images to pre-determined subject matter experts for image reading
3. Electronically receiving the experts’ fluorosis scores
4. Implementing an algorithm to automatically read a standard aspect of the QLF image to assign a machine-read fluorosis score.
Study Protocols:
Recruitment, Screening, Appointing, and Consenting
A convenience sample of 75 participants will be selected from among adolescents living in and around the Washington DC area who respond to advertisements. This study will be limited to adolescents because persons who are this age will typically have most if not all of their 28 permanent teeth erupted. Additionally, all six upper anteriors should be fully erupted, which is important in obtaining a useful photograph image. Advertisements will be placed by Westat (NCHS contractor for this study) in media and at venues that have proven successful for recruitment of other convenience samples in the Washington DC area. Advertisements will be published in English. All participants will be recruited from the Washington, DC metropolitan area, primarily from Montgomery County, Maryland. Advertisements will be placed in the following media outlets WesInfo, Montgomery County Gazette, and outreach will be conducted with local organizations such as churches, etc. to maximize the study’s selection criteria. These organizations have worked with Westat in previous trainings and pilot studies. Westat will contact the representative from the local organization and ask to post the recruitment ad. The local organizations role will be a passive one, they will simply post the ad and refer interested volunteers to call the recruitment line provided on the advertisement. The reading level for the recruitment ad is 8.2. The Flesh-Kincaid readability method has been used to determine the reading level. NCHS uses this mechanism to determine the readability of other study forms such as consent forms.
The advertisement script to be used is:
“A national study is seeking teenagers to be part of a dental health study. The study will consist of a taking 3 dental photographs of your teeth and a dentist will do a brief exam. The exams will be held in Rockville, MD. The exams will be from Wednesday to Saturday in the mornings and/or afternoons. Volunteers will be asked for 30 minutes of their time and will receive $25. They must be 12-16 years of age or older, have all 6 upper front permanent teeth, and meet other exam criteria. If you are interested please call us at (TBD).”
The first introduction to the study for potential participants will be with a Westat telephone screener. In summary, the screener will:
1. Provide information about the study to callers
2. Answer questions about the study
3. Assess eligibility
4. Obtain contact information
5. Schedule examination appointment
When a parent/guardian contacts the study recruiter in response to a recruitment advertisement, the recruiter will initiate conversation and administer the screening questions as follows:
“Hello, thank you for calling the NHANES oral health study recruitment line. We are recruiting volunteers to participate in a dental study to help us improve the way dental health experts monitor changes in people’s tooth enamel as a results of too much exposure to fluoride, which is referred to as dental fluorosis. The study will consist of a short dental exam to assess for dental fluorosis conducted by a dentist and the taking of 3 digital photographs using a camera that is modified to take pictures of your front teeth. The dental examination and taking of pictures will be at a mobile examination center. The total time to participate in the examination will take about 15 minutes and you will be compensated $25 for your time.”
“Before we begin, I’d like you to know that participating in this study is voluntary. You may choose to stop the exam at any time or not answer any question you don’t want to. We are required by federal laws to keep your information strictly private. I can describe these laws if you wish. They guarantee that your answers will be used only for research. Your (child’s) name will not be used. I’d like to continue now unless you have any questions.”
READ IF NECESSARY: The Public Health Service Act is Volume 42 of the US Code, Section 242k. The collection of information in this survey is authorized by Section 306 of this Act. The confidentiality of your responses is assured by Section 308d of this Act and by the Confidential Information Protection and Statistical Efficiency Act.
A set of screening questions will be asked to determine eligibility. These questions include inquiries on age, the presence of teeth, including the presence or absence of white spots on teeth. These questions are:
1.
What is your child’s age?
[If
12-15 years of age, continue; if
<12
or >15 years, NOT eligible and END]
2. Is your child in good overall health?
[If yes, continue; if no, NOT eligible and END]
3. Does your child have all six of their upper front permanent teeth?
[[If yes, continue; if no, NOT eligible and END]
4. Does your child have fixed braces or other orthodontic items fastened to their front teeth?
[If yes, NOT eligible and END; if no, continue]
5. Does your child have “white spots” or “whitish streaks” on their front teeth that do not disappear after tooth brushing?
[If yes, volunteer is eligible; if no, may be eligible, but approximately 25 of the overall study sample of 75 must have responded “yes” to this question.
If the respondent is determined not to be eligible, the screener will read the following script:
“Based on your answers, you are not eligible to participate in this study. Thank you very much for your interest.”
If the volunteer is eligible, the screener will proceed by stating the following:
“I would like to now schedule an appointment for the dental exam and the taking of the dental photographs at our mobile examination center. At the exam center we will review the study with you and seek your written consent to permit your child to participate in this study. For this part of the study, the discussion, exam and photographs should take about 30 minutes or less.” We have the following dates and times available (TBD). When would you like to schedule your appointment?
The exam will take place in (address—directions—TBD). We ask you to arrive on time for your appointment. We will call you before your appointment time to remind you, can you provide us with a number? If you need us to reschedule your appointment, please call us at (TBD).
I’d like to thank you on behalf of the National Health and Nutrition Examination Survey for agreeing to participate on this dental study. If you have any questions about this study, you may call our Medical Officer, Dr. Kathryn Porter toll-free at 1-800-452-6115. If you have questions about your rights as a study participant, you may call the chairman of the Research Ethics Review Board at 1-800-223-8118.
Thanks again for your participation.”
The Westat project officer will keep a list of study participants’ contact information. Participants, who do not show up for their scheduled appointment, will be given a courtesy call and offered an opportunity to re-schedule their appointment. This information will be retained for 90 days following the completion of data collection and then destroyed.
When the volunteer arrives to the MEC, study staff will greet the parent/guardian and volunteer, confirm that the volunteer is on the schedule, their name and age, and initiate the written consent process. Staff will provide a consent form for the volunteer to read and sign. An additional informational sheet will be provided to the volunteer child that shows what will happen if they participate in this study. The consent form is shown on page 8 and the informational sheet is shown in page 9. Following consent procedures, the study participant will then have the dental photographs taken and receive a dental fluorosis examination.
Dental Imaging
The dental camera to be used in this study is the property of the DHU and their staff will assist in the integration of the camera-related software with the NHANES data collection software and will train a NHANES staff person to operate the camera and related IT software. Following completion of the study, the camera and related equipment will be returned to the DHU.
The dental camera technician will introduce him/herself to the volunteer and will remind the volunteer that the dental photographs to be taken are for research purposes only; thus, cannot be read today to provide any type of a diagnostic assessment for the volunteer. The camera technician will assist in positioning the volunteer into the camera frame to ensure a complete set of standardized images. The technician will inform the volunteer that the frame will help hold their head in the right place and that 3 digital images of their front teeth will be taken.
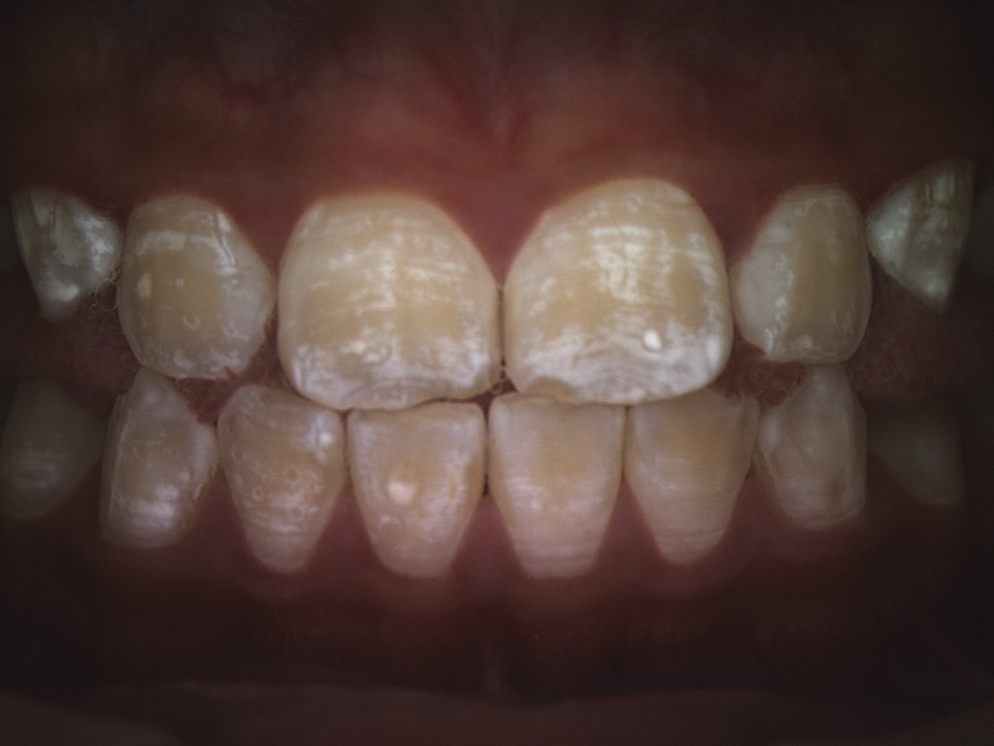
The first image to be taken is a standard white light digital photograph, followed by a polarized white light digital photograph, and finally a fluorescent light digital image will be made. None of the images will have any identifying aspects of the child’s face visible. These images will be stored on a secure PC using a unique number identifier. Figure 1 presents the standard image to be taken.
The captured standard white light and polarized white light images will be read remotely. Two images from each volunteer will be made electronically available to 3 readers. Readers will score each of the upper four anterior incisors using the same guidelines as prescribed for the clinical assessment. Readers will receive detailed criteria on how to apply the modified Dean scoring system, and how to enter and return their assessments electronically. Readers will not be able to electronically download the digital images. When there is discordance among the three readers, a set of a priori rules will be followed to apply a consensus reading. Once the reader scores are ready and the consensus reading is established, analyses will be conducted to evaluate the consensus reading results to the QLF algorithm findings.
Dental Exam
Once the images are taken, the volunteer will have a brief dental examination administered to count the number and type of teeth present and to evaluate for the presence of dental fluorosis.
The tooth count ascertains the number of primary and permanent teeth, the number of missing teeth, and the number of dental implants. The dental fluorosis assessment evaluates for visible changes in the tooth enamel as a result of early fluorosis activity using the standard Deans Index method. A visual dental exam will be conducted for both tooth count and dental fluorosis, which requires only the use of a dental mirror. Both assessments proposed for this fluorosis study are currently being used on NHANES 2011-12.
Figure 1. Mild Dental Fluorosis (Polarized white light technique)
Risks to Subjects:
Minimal risks. There will be no exposure to radiation (no x-rays), and no use of anesthetic agents or potentially hazardous materials.
Safety Precautions:
Non-latex protective wear, including gloves, will be worn by the examiners. CDC infection control guidelines for dental practice will be observed during data collection. The examining dentists have the skills and training to implement any procedures that will be taken in response to potential adverse events (e.g., fainting).
Respondent Burden
It is estimated that this fluorosis study will require 15 minutes to complete the dental exam and to conduct the digital imaging. Volunteers will be notified that the process of registering, examining, and taking the dental photographs will require less than 30 minutes of their time.
Justification for using vulnerable populations
• Minors are included in this component because they are an important target population group for dental fluorosis.
• There is no reason to exclude mentally impaired or handicapped individuals because there is no contraindication.
Report of Findings:
Tests and procedures conducted in this study are not considered diagnostic exams and are not a substitute for an evaluation by a health care professional. No clinical treatments or health interventions of any type are performed as part of this study. If a health problem is discovered during the course of the dental exam, the dental examiner will inform the parent/guardian. If a participant is found to have a serious condition requiring immediate attention, the local emergency responders may be summoned or the participant will be advised to seek immediate medical treatment.
Each participant will receive some general results about the dental examination he/she received in this study at the conclusion of the exam. Each participant will receive some general comments regarding the overall condition of their teeth using a form approved for earlier studies at this study site (see page 10 ).
Key Study Team Members:
Principal Investigator: Dr. Bruce Dye
CDC/NCHS
Hyattsville, MD
Co-PI: Dr. Iain Pretty
University of Manchester Dental Health Unit
Manchester, United Kingdom
Co-PI: Dr. Tim Iafolla
NIH
/ NIDCR
Bethesda, MD 20892
Consultant: Dr. Angeles Martinez Mier
Oral Health Research Institute
Indiana University School of Dentistry
Indianapolis, IN
Project Officer (Contractor): Ms. Mita Patel
Westat
Rockville, MD
Project Officer: Ms. Brenda Lewis
CDC/NCHS
Hyattsville, MD
NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY (NHANES)
CONSENT FORM
Please read the following information.
If you agree to participate, sign your name at the bottom
The Centers for Disease Control (CDC) is asking you to participate in health research. Your participation will help improve oral health measures in the National Health and Nutrition Examination Survey. You will have an exam by a dentist and have 3 photographs taken of your front teeth. All procedures are done by trained staff, who will explain procedures in more detail as you go along. If the dentist discovers a problem, he will talk to you about it and suggest that you follow up with a dentist or doctor. This exam does not take the place of a general exam by your own dentist. The CDC cannot provide treatment or medication, nor pay for follow-up you may require.
You are asked to participate in the oral health component. This will take about 30 minutes or less and you will be paid $25.00 for your time and effort.
You may refuse to answer a question at any time. You may also choose to end your participation. There is no penalty for withdrawing from the study. You will still be paid for your time. All data collected will be kept strictly confidential and will be used only in NHANES planning. By law, the information you provide cannot be used for any other purpose without your permission.
If you have any questions, feel free to speak to any member of the staff. You may also contact Dr. Kathryn Porter of the U.S. Public Health Service at 1-800-452-6115.
I HAVE READ THE INFORMATION PRESENTED ABOVE, AND CONSENT TO PARTICIPATE IN THE EXAMINATION.
__________________________________________
Print first, middle, last name of volunteer
___________________________________________________________
Signature of adult volunteer Date
(Flesch-Kincaid Reading level 8.0)
We would like to give you an idea of what to expect if you participate in our dental health study. First, we need to make sure that your parent or guardian has said it is OK for you to take part in this study. Then we will take 3 pictures of your front teeth and examine your teeth as shown below:
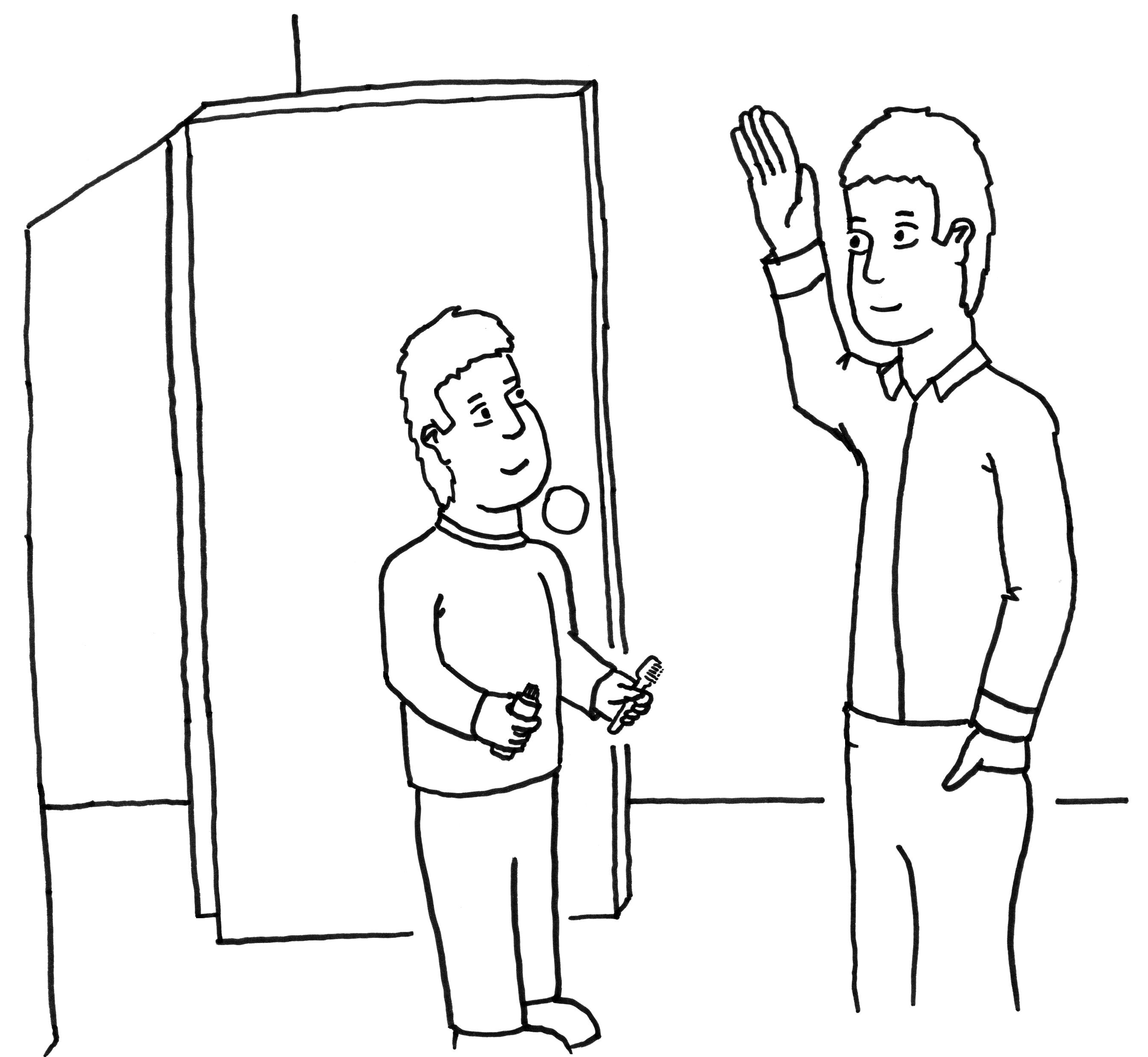
We
would like you to help us see if experts looking at pictures of
teeth can come up with a similar diagnosis to what a dentist does by
looking directly at your teeth regarding color changes in your front
teeth.
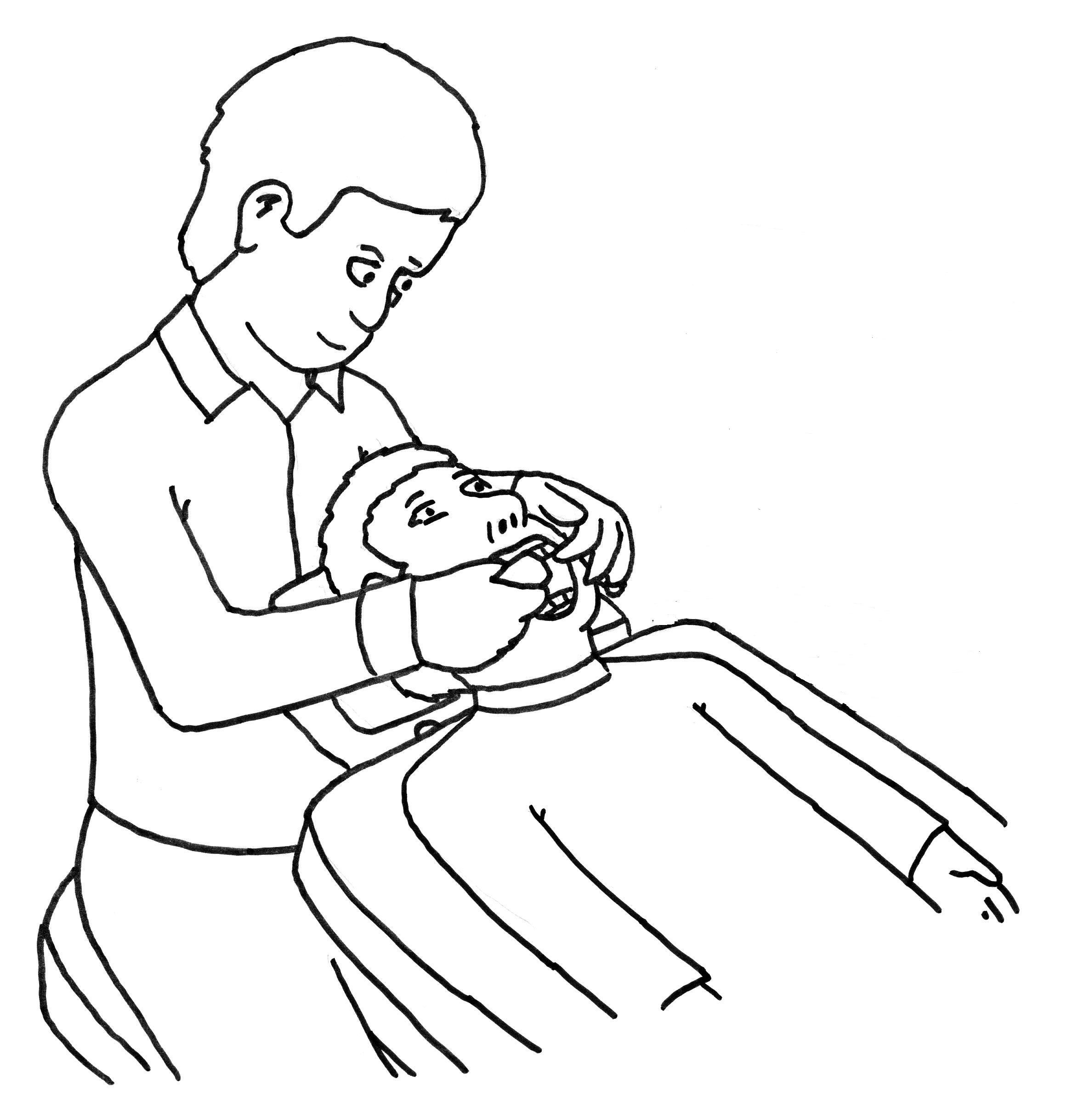
We
will look in your mouth like a normal check-up at the dentist. We
will look to see if there are white patches on your teeth.
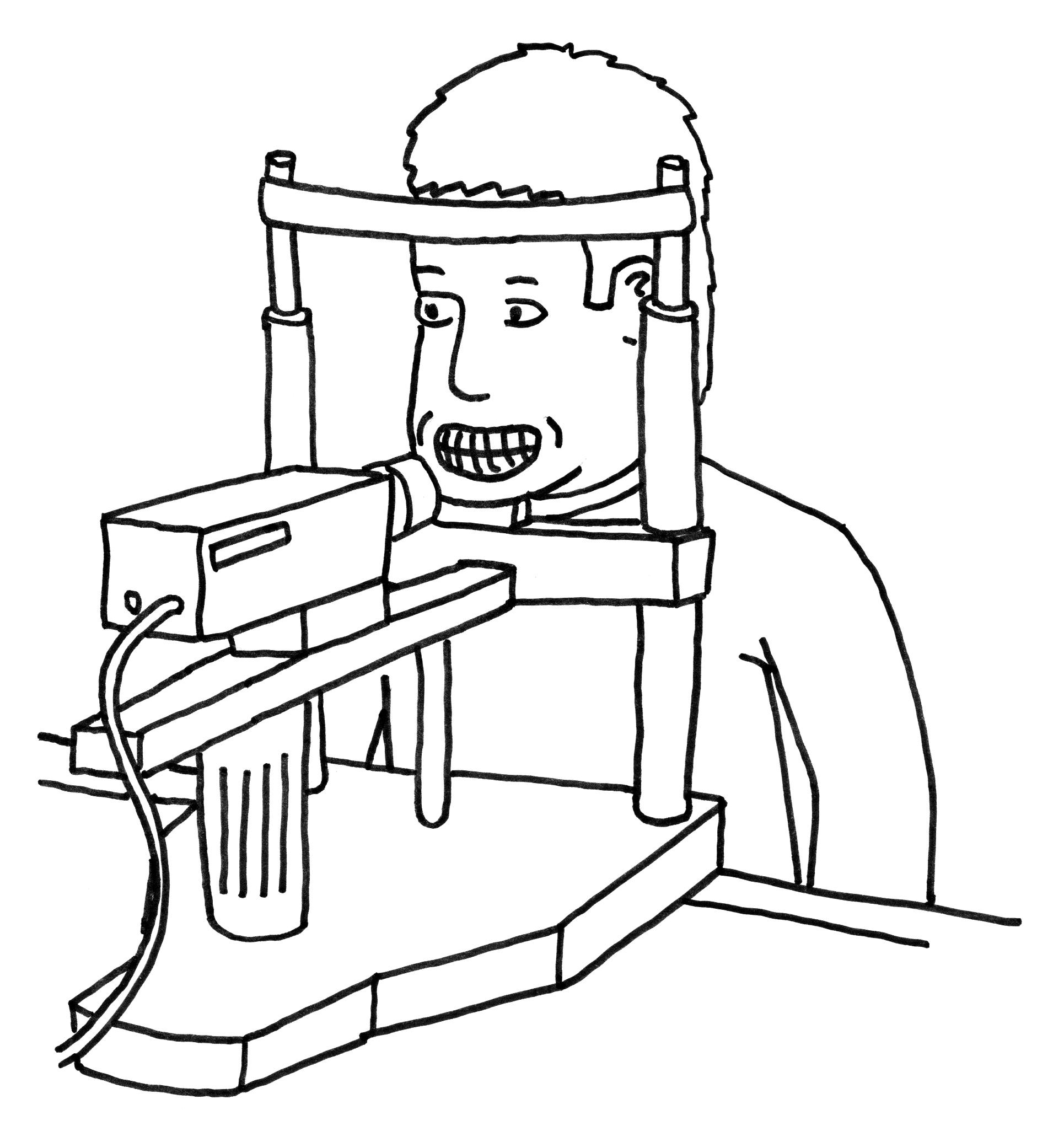

We
will take pictures of your front teeth. These pictures will be of
only your front teeth using a special camera with a frame to hold
your head in the right place.
After
taking the pictures and doing the exam, there is nothing else to do
except answer any of your questions.
NHANES-OH 2011 Dental Fluorosis Imaging Feasibility Study
Report of Findings
Date of Examination:
Volunteer ID:
The dental examination you received today is not, and is not intended to be, a substitute for the examination usually given to persons seeking care from their own dentists. Neither a dental history nor x-rays are taken, and therefore the findings are solely the result of what can be seen at the time of the examination. The examining dentist recommends that you:
Should see a dentist immediately……………………….………..1
Should see a dentist within the next 2 weeks…………….………2
Should see a dentist at your earliest convenience………………..3
Should continue with your regular routine dental care…………..4
The examining dentist observed the following conditions:
|
Decayed Teeth |
Periodontal Disease/Problem |
Oral Hygiene |
Soft Tissue Problem |
Denture/RPD Problem |
No Significant Findings |
Other Finding |
Condition |
A |
B |
C |
D |
E |
F |
G |
If “Other Finding” (G), specify:
| File Type | application/msword |
| File Title | Attachment A |
| Author | vlb2 |
| Last Modified By | nef1 |
| File Modified | 2011-03-17 |
| File Created | 2008-05-14 |
© 2026 OMB.report | Privacy Policy