Attach8
attach8.doc
National Epidemiologic Survey on Alcohol and Related Conditions-III (NIAAA)
Attach8
OMB: 0925-0628
Attachment 8
OVERVIEW OF NESARC-III INTERVIEW FLOW
(Including All Consent Documents and Procedures, Advance Letter, DNA Collection Procedures and Associated Script and Screenshots)
Advance
Letter
(1)
Case
Folder Introduction
(2)
Screener
(3)
Screener
Incentive
(4)
Study
Brochure and Consent
(5)
Incentive
#1
(6)
AUDADIS
-V
(7)
Incentive
#2
(8)
Consent
for Follow-Up Interview and
(9)
Saliva
Collection
(10)
Consent
to Record Validity Study Interview
(11)
Recontact information
Overview
of the NESARC-III Interview Flow
Pilot
Study Consent
(12)
Advance Letter (1)
OVERVIEW
Hardcopy letter sent to selected addresses in advance of
interviewer visit
USPHS letterhead and envelope
English with Spanish on back
Dear Resident:
To better serve all people across the nation, the United States Public Health Service (USPHS) is conducting a national study on alcohol and health-related issues. The goal of the National Health and Alcohol Study (NHAS) is to improve the health of all Americans. Your address was chosen at random along with more than 60,000 others.
The major purpose of the study is to collect national health information. The study has two parts. For the first part, one adult household member will be randomly chosen to participate in an interview which will take about one hour. In a small number of households two adult members may be selected to participate. The interview collects information on background and lifestyle, like age and education. It also asks about drinking practices and related mood, anxiety, behavior and medical conditions. The purpose of this part is to decide how to best use money and staff to solve health problems. After the interview, the respondent will be asked to give a saliva sample for research purposes. Genetic material will be obtained from the saliva sample and studied in the laboratory.This information will be used to understand how background and lifestyle factors work together with genes to affect our health.
Westat is a company working with the USPHS to carry out this study. Within a few weeks, a Westat interviewer will be in your neighborhood. When the interviewer arrives, please ask to see his/her personal ID card as shown below. The interviewer will then ask a few questions to see if anyone in your household is eligible for the interview. The person who answers these few questions will get $10 in cash. The person selected for an interview (who may be the same person who answered the selection questions) will receive a $45 check before the interview and another $45 check at the end of the interview. We are conducting this study under the authority of Title 42 United States Code (USC), Section 285n, 42 USC 241(d), and 18 USC 1905. Participation is voluntary. It is also okay to skip questions that the respondent does not want to answer for any reason.
All people involved in this study will treat the information collected as private and all the information will be used for research purposes only. Information and saliva samples collected will be identified only by a random number. We will remove and destroy all personal information that could identify the person(s) such as name, address and phone number, thereby making the information anonymous.
Your help is very important to this study’s success. If you have any questions, the interviewer will be glad to answer them. You may also call NAME (Title) at toll-free NUMBER. Thank you in advance for your help.
Sincerely,
National Study Director “Image of Identification Badge”
Case Folder Introduction (2)
OVERVIEW
Hardcopy folder produced for each assigned case
Study introduction on front
Also contains: case label, address verification, hidden DU
procedures, record of calls, status codes, etc.
Hello, my name is (NAME). Here is my identification card. Is this (ADDRESS)?
[HAVE LETTER VISIBLE.] You should have received this letter from the United States Public Health Service. Did you receive the letter?
YES, RECEIVED MAILING
Have you had a chance to look over the letter?
YES: Wonderful! Then you know this is a study for the United States Public Health Service to obtain information on alcohol and health-related issues.
Our computer will randomly select one or possibly two adults in certain households to complete the interview. The person selected will receive $90 for completing the interview. Let’s see if someone is selected. For answering the selection questions, you will receive $10. First, tell me only the first names of those who live here, their ages, and I’ll need to ask a few other questions before we’ll know if someone is selected. All of your responses will be kept private.
NO, DID NOT RECEIVE OR DID NOT READ LETTER
We feel this is an important project. I want you to know all about it. Let’s look at this letter together.
[POINT TO USPHS LETTERHEAD]
[READ ADVANCE LETTER WITH RESPONDENT]
Screener (3)
OVERVIEW
CAPI screening instrument
Household roster and enumeration
Sample Person (SP) selection
Telephone collection for quality control purposes and setting
AUDADIS appointments
Mailing address confirmation for nonresponse follow-up
SEE ATTACHED SCREENER CAPI SPECIFICATIONS
Screener Incentive (4)
OVERVIEW
CAPI instrument
Collects whether screener respondent accepts/declines payment
I would like to give you $10 to thank you for your time today.
DID THE RESPONDENT ACCEPT OR DECLINE THE INCENTIVE?
ACCEPT INCENTIVE
DECLINE INCENTIVE
Thank you.
IF SP AVAILABLE, ATTEMPT TO ADMINISTER AUDADIS-V.
IF SP NOT AVAILABLE, SCHEDULE APPOINTMENT FOR RETURN VISIT.
Study Brochure and Consent (5)
OVERVIEW
Used for initial contact with SP (at the door)
Brochure handed and read to SP
Collects SP consent for study
Hello, my name is (NAME). Here is my identification card. We are conducting a study for the United States Public Health Service on alcohol use, health and related conditions, as well as the demographic data used in analyzing health data. Information collected in this study will be used to direct health care services where needs are greatest for the American people. All of your responses will be kept private. Did you receive our letter?
PROVIDE STUDY BROCHURE (AND LETTER, IF NEEDED).
Here is a study brochure. I will read it to you at this time.
READ BROCHURE TO SP. DISCUSS BROCHURE CONTENTS WITH SP, AS NEEDED.
Do you have any questions?
Study Brochure and Consent (5)
(Continued)
National
Health and
Alcohol
Study
The goal of the National Health and Alcohol Study (NHAS) is to improve the health of all Americans.
What is the National Health and Alcohol Study?
The National Health and Alcohol Study, conducted by the United States Public Health Service, is a nationwide study that is collecting national health statistics for adults in America aged 18 and older. Your address was chosen at random along with 60,000 others.
The interview will be conducted in your home by a trained interviewer. The study has two parts. The first part consists of an approximately one-hour interview in which background information, like age, sex and education, and health information is collected on drinking, medicine and drug use, mood, anxiety, behavior, and medical conditions.
Researchers and policy makers will use information from this study to determine how best to use money and staff to solve alcohol related health problems, and to direct health care services where needs are greatest for the American people.
The second part comes after the interview. You will be asked to provide a saliva sample for genetic research purposes.
Your saliva will be collected by spitting into a tube, with a kit that is easy to use. Your saliva sample contains genes which are made up of DNA that serves as an “instruction book” for the cells that make up our bodies. Researchers will use your saliva sample to learn more about which genes are most important for drinking, medicine and drug use and those physical and emotional conditions that are related to them. We also want to know how background and lifestyle factors like smoking, stress, social support and physical disabilities work together with genes to produce these conditions.
The genetic analysis to be done as part of this study is for research purposes only. Your results will be combined with those of many other people. The analysis will not give any information on your own health and you will not receive the results of any analyses done with your sample.
The information and DNA sample we collect from you will be stripped of any way to identify you.
Do I have to participate?
Your decision to participate in this survey is voluntary. You may choose to do one part and not the other. It is all right to skip any question that you do not want to answer for any reason.
How was our household chosen?
Because interviewing every adult in America is far too expensive, we use methods to randomly select household addresses to represent the total population. As a result, we do not know who lives in a selected household until we speak with a household member. In most households, one person is randomly chosen to be interviewed.
Why should I do this?
Because your household represents many others throughout the country, your participation is very important. Participation is voluntary; however, because of the methods used to select your address, we cannot substitute another address for yours if you do not participate.
Think of America as a jigsaw puzzle. Many pieces are needed to complete this puzzle. Each particular puzzle piece has unique characteristics and no other puzzle piece could replace it. You are a vital piece of this puzzle and your participation will ensure that all households like yours are represented.
Your participation in this study is truly a service to your community, and the nation as a whole, as it will ensure that our nation’s policymakers will be using the most complete information possible when making decisions that will affect all of us.
How will the information about me be kept private?
We will treat all of the information in this study as private, and it will be used for research purposes only. Information and saliva samples will be identified only by a random number. We will remove and destroy personal information that could identify an individual, such as name, address and phone number, making your information anonymous. We will destroy all identifying information once your participation in this study is over and we have verified the information and made sure that we have accurately matched your survey information with your saliva sample.
To help us keep the information you provide private, we have obtained a Certificate of Confidentiality from the Federal Government. This document will help protect your information from people who are not part of the study. This Certificate is designed to protect researchers from disclosing any information that might identify you, even by a court subpoena, in any Federal, State or local civil or criminal, administrative, legislative or other proceeding.
[However, if we learn that you or someone else is in serious danger of harm, disclosures may be made to protect you and/or other persons. (INCLUDE STATEMENT IN Any instances where such STATE laws exist)]
Where is my information kept?
The information collected in this study will be kept in limited access databases and available only to researchers who are approved by an NIH Data Access Committee. Your saliva samples will be stored in a secure NIH-sponsored storage facility and sent to a laboratory for analysis. Any part of your saliva sample remaining after this analysis will be destroyed.
What are the risks of participating in this survey?
Privacy of your information is of central concern in any study. Any risk of disclosure of your information is greatly minimized by removing all personally identifying information from your responses and from your saliva samples, by obtaining a Certificate of Confidentiality and by an additional law (18 USC 1905) that prohibits Federal employees from disclosing your personal identifying information.
People may have many reasons for deciding not to answer specific questions. You do not have to answer any question that makes you feel uncomfortable, or that you decide not to answer for any reason.
What are the benefits of participating in this study?
You will not personally benefit from participating in this study. We will not be able to identify which study and genetic information are yours. However, your participation will help researchers learn more about how background and lifestyle factors and genes are? related to health conditions which, in turn, may help to prevent them in the future.
Can I get the study results?
You cannot get individual results or information from this study since we are destroying the identifiable information and we will not know what information belongs to you.
Do I get anything for participating?
The person selected for the interview will receive $90 for completing the interview. This will be divided into two $45 checks – one provided prior to the beginning of the interview and the other immediately after the interview.
Who do I contact if I have any questions about this study?
The interviewer will be able to answer any questions you may have about this study.
If you have any questions regarding this study, you may call (NAME,TITLE) at 1-888-555-5555. Or call 1-888-555-5555 with questions about your rights as a participant in this study.
Do you have any questions at this time? Having read this document, I would like to know if you agree to participate in the interview and to provide a saliva sample, to participate in the interview but prefer not to provide a saliva sample or do you prefer not to participate at all?
[THE DATA COLLECTOR WILL RECORD A “1” FOR AGREEMENT TO PARTICIPATE IN BOTH PARTS, A “2” TO PARTICIPATE IN INTERVIEW ONLY AND A “3” FOR PREFER NOT TO PARTICIPATE AT ALL IN THE STUDY AND THANK THE RESPONDENT FOR THEIR TIME.]
[These electronic certifications are permanently retained.]
Incentive #1 (6)
OVERVIEW
CAPI instrument
Collects whether SP accepts/declines payment
Collects check number for quality control purposes
As mentioned in some of the materials, you will receive $90 for participating in this interview. Half of the money, or $45, will be given to you now, before the interview. The other $45 will be given to you after the interview is over. TAKE OUT $45 CHECK.
DID THE SP ACCEPT OR DECLINE THE INCENTIVE?
ACCEPT INCENTIVE
DECLINE INCENTIVE (END)
ENTER THE CHECK NUMBER.
ENTER THE CHECK NUMBER AGAIN.
Thank you. Now let’s begin the interview.
LAUNCH AUDADIS TASK.
AUDADIS-V (7)
OVERVIEW
CAPI instrument
Public Burden Statement
OMB #: 0925-xxxx
Expiration Date: xx/xxxx
Public reporting burden for this collection of information is estimated to average 60 minutes per response including time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to NIH, Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-xxxx) (includes expiration date).
SEE SEPARATE AUDADIS-V CAPI SPECIFICATIONS.
Incentive #2 (8)
OVERVIEW
CAPI instrument
Collects whether SP accepts/declines payment
Thank you for completing the interview. As I said earlier, you will now receive another $45 to thank you for your time. TAKE OUT $45 CHECK.
DID THE SP ACCEPT OR DECLINE THE INCENTIVE?
ACCEPT INCENTIVE
DECLINE INCENTIVE (END)
ENTER THE CHECK NUMBER.
ENTER THE CHECK NUMBER AGAIN.
Thank you.
TRANSITION TO CONSENT FOR FOLLOW-UP.
Consent for Follow-Up Interview and Recontact Information (9)
OVERVIEW
CAPI instrument
Collects phone number for quality control purposes
Collects consent for recontact for Reliability/Validity
SP given copy to read along
Interviewer records consent to recontact in CAPI to certify SP’s
decision
If consent granted, collects additional contact information
SEE SEPARATE RECONTACT MODULE CAPI SPECIFICATIONS.
[Consent for Follow-up Interview]
[Procedure for consent for re-interview. This will be used for the reliability and validity study.]
[The Interviewer reads the following verbatim from the computer screen after the AUDADIS-V interview and provides a copy to the respondent to read.]
Purpose
In large studies like this, it is very important to verify the quality of the survey questions. We do this by asking the same or similar questions for a second time. We would like to ask you at this time if you would agree to participate in this second interview. This interview would either take place on the telephone or in your home in a few weeks and will last between one-half to one hour. You will receive a $100.00 check at the end of the interview if it is conducted in your home. For interviews conducted on the telephone, the check will be mailed to you immediately upon completion of the interview.
Do I Have to Participate?
Participation in this second interview is voluntary. Even if you do participate, it is okay to skip any questions that you do not want to answer for any reason.
Privacy of Your Information
The information from this second interview will be private in the same way as the information from the first interview. We will remove and destroy all personally identifying information after the second interview once we have verified that the random number from your first interview matches your second interview.
Where is This Information Kept?
The information will be kept in a limited access data base and available only to researchers involved directly in the conduct of the study.
Can You Get the Results of This Study?
Again, you cannot get individual results or information from this second interview since we are destroying the identifiable information and will not know what information belongs to you.
Risks
Privacy of your information is of central concern in any study. Any risk of disclosure of your information is greatly minimized by removing all personally identifying information, obtaining a Certificate of Confidentiality, and an additional law (18 USC 11905) that prohibits Federal employees from disclosing your personal identifying information.
People may have many reasons for deciding not to answer specific questions. You do not have to answer any question that makes you feel uncomfortable, or that you decide you do not want to answer for any reason.
Benefits
You will not personally benefit from this second interview. The information will only be used as a quality check on the questions we use in the study.
Contact Information
If you have any questions about the second study or your participation in it, please contact (NAME, TITLE) toll free, at (NUMBER)
Do you have any questions at this time? Having read and understood this document, I would like to know if you agree at this time to participate in this second interview.
[The interviewer records a “1” (for yes) or a “2” (for no) on the computer screen to certify the respondent’s decision. The electronic confirmation of the respondent’s decision will be permanently retained.]
Saliva Collection (10)
OVERVIEW
CAPI instrument
Incorporate DNA Genotek’s “Video User Instructions”
for SPs to view
Interviewer can also read text to explain procedures
Barcode scan/entry directly into CAPI
Here is the kit that we will use to collect the saliva. [HAND KIT TO SP]
I would now like to show you a brief video that explains how you will provide the saliva sample.
PLAY VIDEO.
TRANSCRIPT OF VIDEO:
The ORAgene DNA self collection kit is designed to collect saliva samples for DNA analysis. ORAgene is easy to use and completely noninvasive, making it ideal for safe collection of samples.
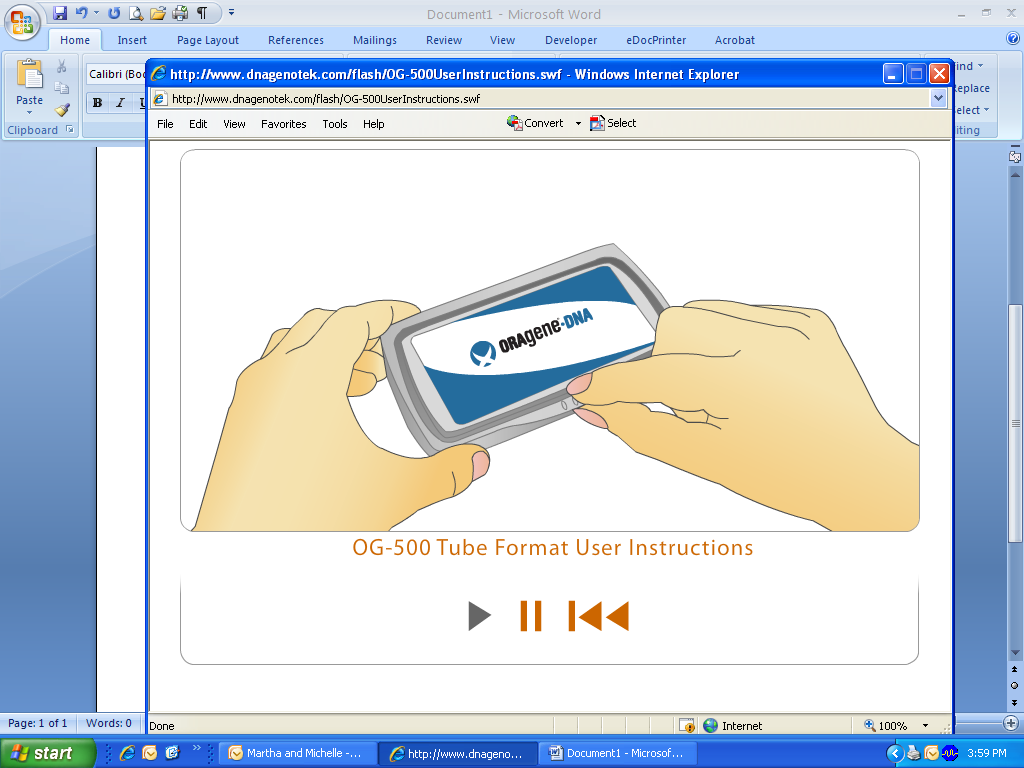
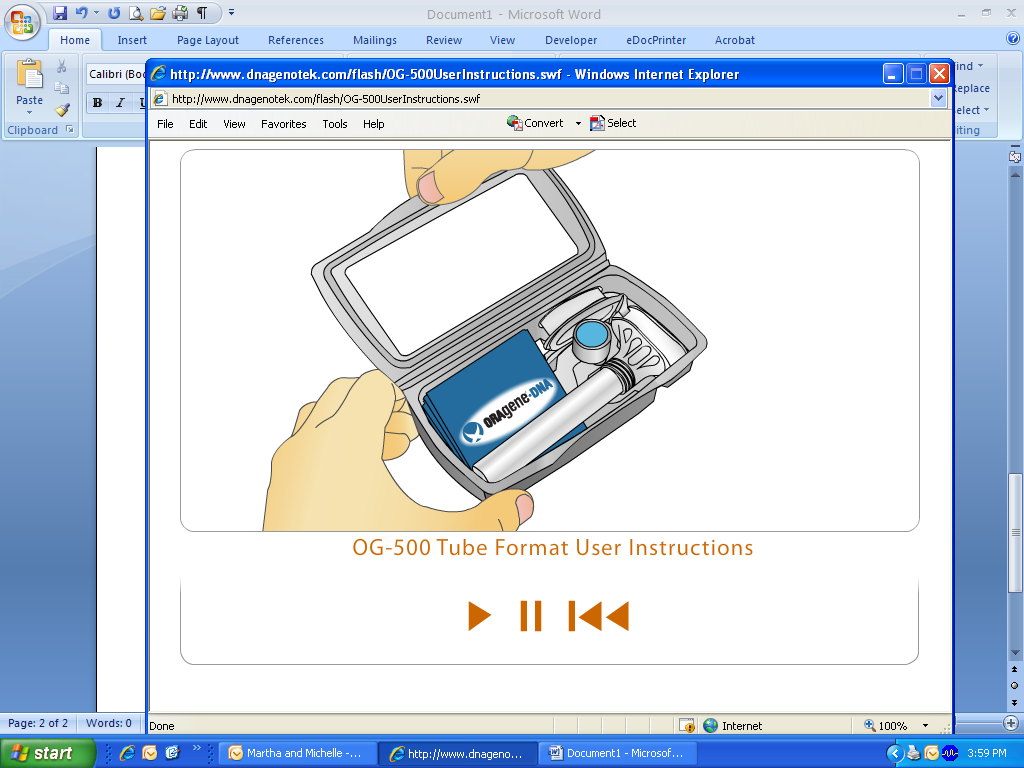
To use the ORAgene kit, open the packaging.
Remove the tube with the attached funnel and small cap.
The flip-top funnel lid contains a clear solution that will be mixed with the saliva sample when the kit is closed.
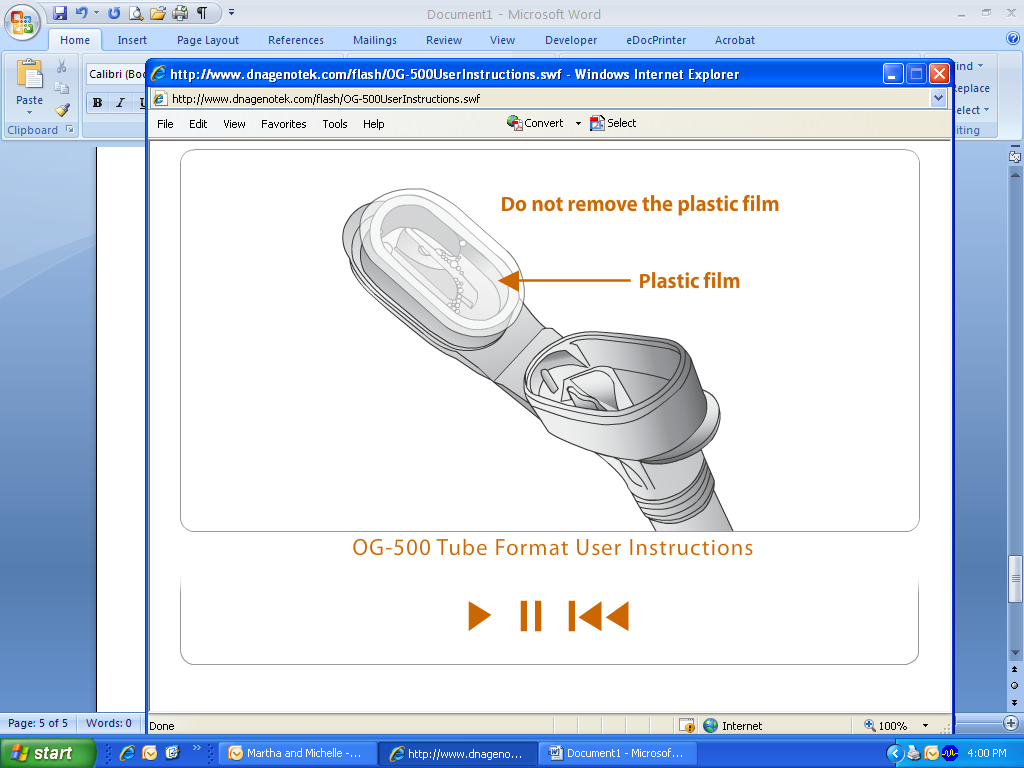
DO NOT remove the plastic film!
Most people take between two and five minutes to deliver a saliva sample.
Before spitting, relax and rub your cheeks gently for thirty seconds to create saliva.
Place the top of the funnel close to your bottom lip and start delivering your saliva sample by spitting into the funnel.
Some people will have bubbles or foam in their saliva. Be sure you that you spit enough liquid saliva, not including bubbles, to reach the fill line.
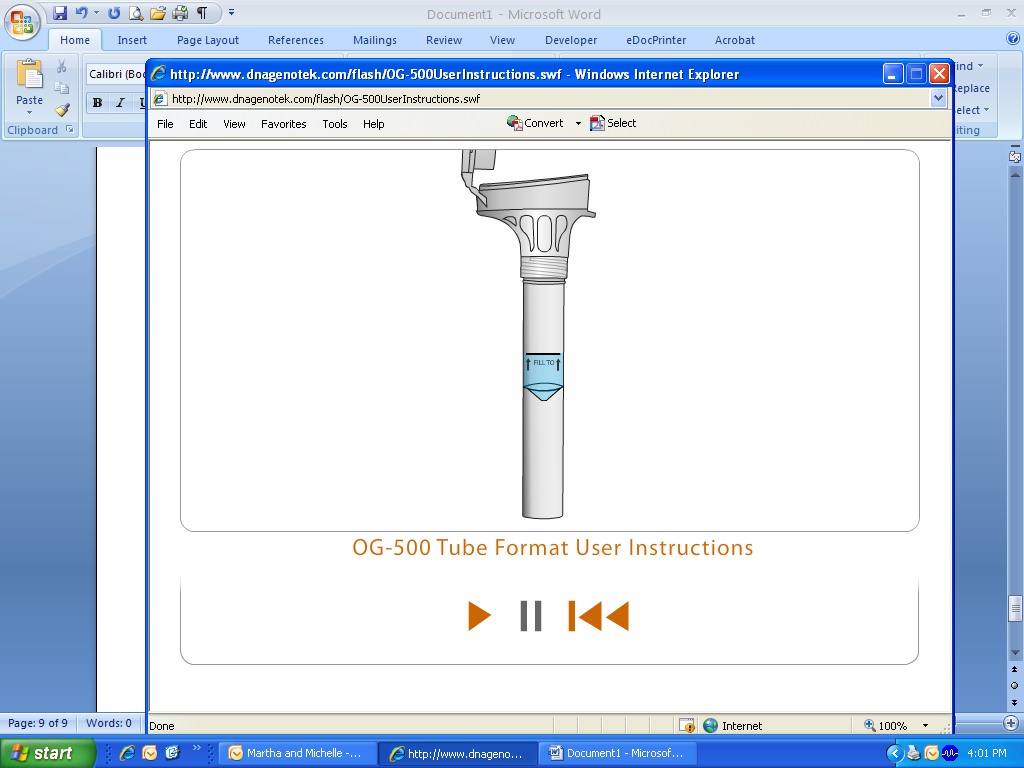
You will find the fill line on the label on the side of the tube.
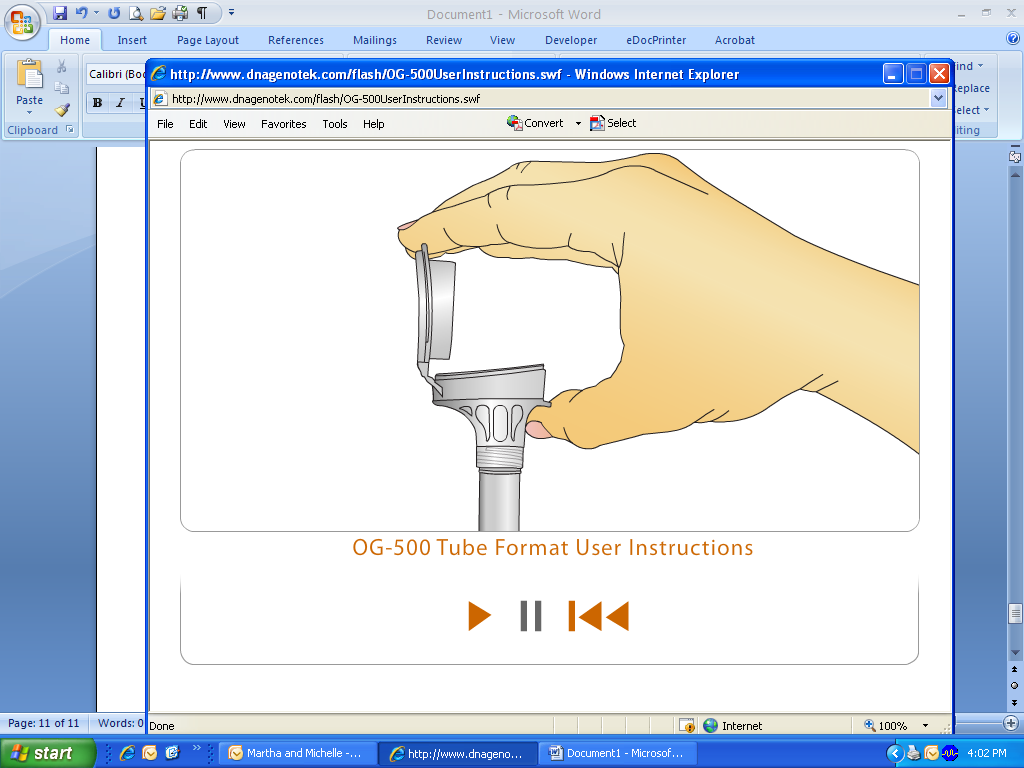
Once your saliva reaches the fill line, close the lid by firmly pushing the lid until you hear a loud CLICK.
When the lid is closed, you will notice that the solution from the cap will mix with the saliva in the tube.
This solution stabilizes and protects your sample until it is analyzed at the lab.
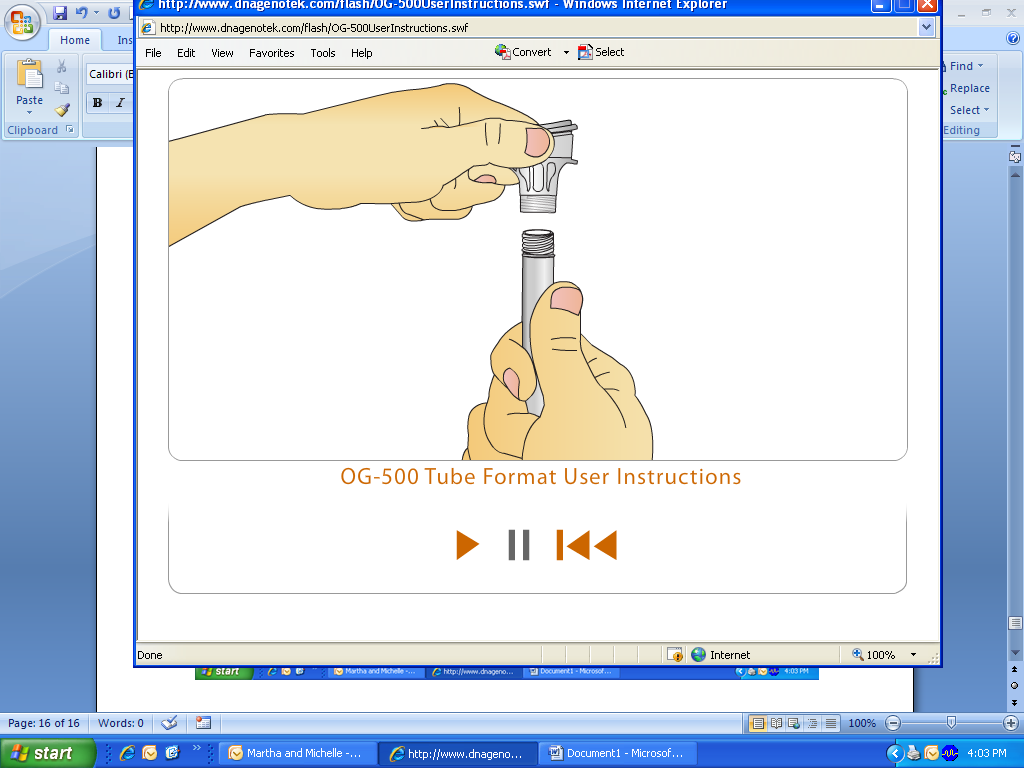
Hold the tube upright and screw the tube from the funnel. While keeping the tube completely upright, pick up the small cap and use it to close the tube containing your sample.
Shake the capped tube for five seconds.
SCREEN SHOTS FROM VIDEO:













TAKE SAMPLE FROM SP. DISCARD FUNNEL AND OTHER PACKAGING MATERIALS.
SCAN THE BARCODE INTO THE CAPI SYSTEM.
IF SCAN DOES NOT WORK, ENTER THE BARCODE TWICE.
THANK SP. HAND THANK YOU LETTER AND HELP BROCHURE TO RESPONDENT.
“We would like to thank you for your participation. We have also included useful contact information should you or someone you know need help with alcohol, drug or mental problems in the future.”
IF 2ND SP IN HOUSEHOLD, ATTEMPT AUDADIS WITH OTHER SP OR ATTEMPT TO SET APPOINTMENT.
INTRODUCTORY STATEMENT FOR RELIABILITY AND VALIDITY STUDY/
CONSENT TO RECORD VALIDITY STUDY INTERVIEW (11)
[FOR ALL RESPONDENTS PARTICIPATING IN THE RELIABILITY AND VALIDITY STUDY, DATA COLLECTORS WILL READ THE FOLLOWING INTRODUCTORY STATEMENT.]
“You recently agreed to participate in a second interview for the National Health and Alcohol Study to help verify the quality of the survey questions. I’m (here/calling) to do that interview. It will last about (one half/one) hour and you will receive a $100 check (that will be mailed to you) immediately after the interview. One of the things that’s very important in understanding a survey like this is whether people give you the same answers or different answers when they are asked the same question a different time. People often give the same answers, but they might change their answers for many reasons. For example, they might remember different things a second time, or the second interviewer might ask the questions a different way. Interviewing some people a second time is one way for us to check how much this might be happening. You are helping the survey very much by agreeing to answer our questions again. I don’t know anything at all about what you told the first interviewer. That way, what you said in the other interview won’t influence how I ask you the questions. When I ask you these questions, please tell me whatever answer seems right to you today. Don’t try to make your answers the same as last time, or different-just give the answer that seems right to you now. Do you have any questions for me before we start?”
[FOR THE VALIDITY STUDY, CONSENT TO RECORD THE INTERVIEW WILL BE OBTAINED AS FOLLOWS AFTER THE INTRODUCTORY STATEMENT.]
“We would like to record this interview for quality assurance purposes. The recording will be identified only by a random number. We will destroy personal information that could identify an individual, such as name, address and phone number, making your recording anonymous. We will destroy all identifying information on the recording once the interview is over and we have made sure that we have accurately matched the recorded information with other survey information you provided.
We will keep the recording identified with a random number only long enough to assess the quality of the survey questions, at which time it will be destroyed.
Your decision to have your interview recorded is voluntary and it is okay to skip any questions that you do not want to answer for any reason.”
[THE DATA COLLECTOR WILL RECORD A “1” FOR AGREEMENT TO RECORD THE SECOND INTERVIEW OR RECORD A “2” FOR RESPONDENT’S PREFERENCE NOT TO RECORD THE SECOND INTERVIEW.]
Pilot Study Consent (12)
National
Health and
Alcohol
Study
The goal of the National Health and Alcohol Study (NHAS) is to improve the health of all Americans.
What is the National Health and Alcohol Study?
The National Health and Alcohol Study, which will be conducted by the United States Public Health Service, is a nationwide study that is collecting national health statistics for adults in America aged 18 and older. You are helping us very much today by testing the procedures for the study.
The interview will be conducted by a trained interviewer. The study has two parts. The first part consists of an approximately one-hour interview in which background information, like age, sex and education, and health information is collected on drinking, medicine and drug use, mood, anxiety, behavior, and medical conditions.
Researchers and policy makers will use information from the study to determine how best to use money and staff to solve alcohol related health problems, and to direct health care services where needs are greatest for the American people.
The second part comes after the interview. You will be asked to provide a saliva sample to test the saliva collection procedure. Your saliva sample will not be analyzed and will be destroyed immediately after it is collected.
Your saliva will be collected by spitting into a tube, with a kit that is easy to use. Your saliva sample contains genes which are made up of DNA that serves as an “instruction book” for the cells that make up our bodies. Researchers will use saliva samples collected during the study to learn more about which genes are most important for drinking, medicine and drug use and those physical and emotional conditions that are related to them. Researchers will also want to know how background and lifestyle factors like smoking, stress, social support and physical disabilities work together with genes to produce these conditions.
How will the information about me be kept private?
We will treat all of the information in this study as private. Information and saliva samples will be identified only by a random number. We will not identify any of your responses or saliva sample with your name or any other information that could identify you. We will destroy all of your responses and your saliva sample within one week after your participation.
What are the risks of participating in this pilot study?
There are no risks of participating in this pilot study.
What are the benefits of participating in this pilot study?
You will not personally benefit from participating in this pilot study.
Can I get the study results?
You cannot get individual results or information from this study since we are destroying all of the information and saliva samples in this pilot study.
Do I get anything for participating?
For your participation in this pilot study you will receive a $100 check after the completion of the interview and collection of the saliva sample.
The interviewer will be able to answer any questions you may have about this study now.
[DATA COLLECTOR BEGINS STUDY.]
| File Type | application/msword |
| Author | kaplank2 |
| Last Modified By | kaplank2 |
| File Modified | 2010-12-13 |
| File Created | 2010-12-06 |
© 2026 OMB.report | Privacy Policy