State_based_surv_SS_Part A_33111-final
State_based_surv_SS_Part A_33111-final.doc
Creation of State and Metropolitan Area Based Surveillance Projects for Amyotrophic Lateral Sclerosis (ALS)
OMB: 0923-0043
State and Metropolitan-Area Base Amyotrophic Lateral Sclerosis (ALS) Surveillance
Part A
October 21, 2010
Point of Contact:
Oleg Muravov, MD, PhD
Division of Health Studies
Agency for Toxic Substances and Disease Registry
4770 Buford Highway, MS F-57
Atlanta, GA 30341
Phone: 770-488-3817
Fax: 770-488-1537
Email: [email protected]
A. Justification
1. Circumstances Making the Collection of Information Necessary
ATDSR is authorized by the Public Health Law No: 110-373, ALS Registry Act (Attachment 1) to (1) develop a system to collect data on amyotrophic lateral sclerosis (ALS) and other motor neuron disorders that can be confused with ALS, misdiagnosed as ALS, or progress to ALS; and (2) establish a national registry for the collection and storage of such data to develop a population-based registry of cases.
This new data collection activity is a result of several meetings between the stakeholders including scientists, neurologists, advocacy groups, and ethicists and ATSDR. The objective of this project is to develop state-based and metropolitan area-based surveillance activities for ALS. The primary goal of the state-based and metropolitan area-based surveillance activities is to use these data to evaluate the completeness of the National ALS Registry.
The state and metropolitan areas will be selected to be over representative some racial and ethnicity minorities because of concerns related to possible difference in access to medical care and advocacy services.
ATSDR awarded two contracts to conduct this surveillance activity. The contractor is responsible for the identification of state and metropolitan areas. Because ALS is a rare disease with prevalence estimated at 4/100,000, states under consideration had to have a population of at least 4 million in order to have enough cases. The contractor attempted to contact each state with 4 million or greater population and tell them about the project. States interested in the project were asked to submit a protocol explaining how they would conduct the activities in their geographical area. The contractor evaluated each of the applications received and rated them on defined criteria such as ability to provide the data, soundness of methods, and experience with similar projects. Based on the funds available, the top three states, New Jersey, Texas, and Florida, were selected and funded by the contractor.
For the metropolitan area contract, ATSDR instructed the contractor to identify areas with significant populations of African- and Asia-Americans. Because ALS is a rare disease, metropolitan areas had to have a population of at least 1.5 million to be considered. The contractor identified metropolitan areas with high percentages of African- or Asian-Americans and 1.5 million or more population. The metropolitan areas of New York, Philadelphia, Atlanta, Detroit, Chicago, Los Angeles, and San Francisco were invited to participate. Currently, Detroit, Philadelphia, and Atlanta have agreed to participate.
A population-based surveillance project for ALS will be created by identifying persons with ALS from medical care providers. Medical care providers will provide information on or allow abstractors to complete the reporting form for cases of ALS under their care. Initially, neurologists and Electromyogram (EMG) laboratories not associated with a neurology practice will be targeted for reporting. Information collected as a part of the abstraction/reporting will include identifying information to be able to de-duplicate records and information on symptoms that will be used to verify the diagnosis. A bill to amend the Public Health Service Act to provide for the establishment of an Amyotrophic Lateral Sclerosis Registry, S. 1382: ALS Registry Act, was signed into law on October 10, 2008 by President Bush and became Public Law No: 110-373. The activities described are part of the effort to create the National ALS Registry. As a result, a clearance package is being submitted in order to allow data collection immediately following OMB/PRA approval.
PRIVACY IMPACT ASSESSMENT INFORMATION
Overview of the Data Collection System
Neurologists or their staff will complete an ALS Case Reporting Form (Attachment 3) on each of their ALS patients. This will be transmitted to the state or metropolitan health department via a secure method which could include secure fax, overnight mail, or pick-up by ALS Surveillance Specialist. An ALS Medical Record Verification Form (Attachment 4) will be collected on a subset of cases reported.
Items of Information to be Collected
Surveillance items to be collected include information to make sure that there are no duplicates: Information in Identifiable form (IIF) include: full name, address, last 5 digits of the social security number, sex, race, ethnicity, date of birth, and disease information including family history. Personal identifiers will be discussed in further detail, in Section A.10. ATSDR will ensure that several safeguards remain in effect throughout the duration of the surveillance project. These safeguards are also discussed in Section A.10. A copy of the ALS Case Reporting form can be found in Attachment 3.
Identification of Website and Website Content Directed at Children Under 13 Years of Age
There is no website involved in this information collection; therefore no content is directed to children under 13 years of age.
2. Purpose and Use of Information Collection
The purpose of this information collection is to gather specific data related to Amyotrophic Lateral Sclerosis (ALS). The objective of this project is to develop state-based and metropolitan area-based surveillance projects for ALS. The primary goal of the state-based and metropolitan area-based surveillance project is to use these data to evaluate the completeness of the National ALS Registry. The state and metropolitan areas will be selected to have larger percentages of racial and ethnic minorities compared with the United States population as a whole. There is concern that some groups may not be included in the National ALS Registry which relies on a combination of identification of persons with ALS from administrative data (Medicare, Medicaid, Veterans Health Administration, and Veterans Benefits Administration) and self-registration. Individuals who do not receive VA, Medicare, or Medicaid Benefits will not be identified in the administrative data. There is concern that access to care might impact identification which could be related to race and ethnicity. In addition, self-registration is likely to be more common among younger individuals and those associated with advocacy groups. Advocacy groups tend to reach a lower percentage of minorities. Therefore, it is important to be able to identify gaps in racial and ethnic groups if they exist. Two contracts have been awarded. The contractor has awarded subcontracts for three states .
Privacy Impact Assessment Information
The information in identifiable form (IIF) will be used for the purpose of recording and clarifying information and to avoid duplication of reporting of cases. There are no plans to share the IIF with anyone other than ATSDR staff and contractors working on the ALS surveillance system.
Information that might be considered sensitive by a portion of the general public (particularly full name along with address, and disease information, particularly whether patient has dementia) is being collected, so there would be a likely effect on the respondent’s privacy if there were a breach of confidentiality. Accordingly, very stringent safeguards have been put in place as described in Section A.10.
While an IRB waiver of consent has been obtained, participation by neurologists and other medical staff is voluntary.
3. Use of Improved Information Technology and Burden Reduction
All participation is voluntary. The questions have been held to the absolute minimum required for the intended use of the data.
4. Efforts to Identify Duplication and Use of Similar Information
Because ATSDR staff is in communication with The Council of State and Territorial Epidemiologists, advocacy groups, and ALS researchers, it is clear that there is only 1 state-based ALS surveillance system in existence. The literature describes a number of research studies on hospital or physician based cases, but there is no prior history of a state and metropolitan area-based surveillance system. Communications with experts in ALS did not bring to light any similar data collection efforts.
5. Impact on Small Businesses or Other Small Entities
The questions have been held to the absolute minimum required for the intended use of the data. Contract surveillance staff will be in frequent contact with physician’s offices and will be available to assist with all activities. To assist with the completing reporting forms, a token of appreciation will be available to medical care providers calculated on a per case basis. Physicians will receive $100 for each case reported to offset reporting costs. An additional $$50 will be available as necessary to offset costs related to medical records abstraction necessary to complete the Medical Record Verification Form.
6. Consequences of Collecting the Information Less Frequently
The average life expectancy for an individual after diagnosis with ALS is 2-3 years. Because of this, it is necessary to collect information as soon as possible after diagnosis. There is only one report for each diagnosed cases of ALS, however it is unknown how many cases each neurologist will need to report.
There are no legal obstacles to reduce the burden.
7. Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
Special circumstances do exist which require information collection to be conducted in a manner more often than quarterly. Traditionally surveillance projects are an ongoing process where cases are reported as they are diagnosed to allow health departments’ access to the most timely data from which to evaluate disease burden.
Other than those mentioned previously, there are no other special circumstances associated with this data collection.
8. Comments in Response to the Federal Register Notice and Efforts to Consult Outside the Agency
A. A 60-day Federal Register Notice was published in the Federal Register on May 21, 2010, vol. 75, No. 98, pp. 28621 (Attachment 2). No comments were received.
B. The following individuals were consulted to obtain their views on the availability of data, the clarity of instructions, disclosure, and on the data elements to be recorded and reported. A meeting was held in Atlanta, Georgia, January 20-21, 2010.
Natalie Archer, MS
Epidemiologist
Epidemiology Studies and Initiatives Branch
Texas Department of State Health Services
PO Box 149347, MC1964
Austin, TX 78714-9347
Phone: (512) 776-3723
Fax: (512) 458-7222
Email: [email protected]
Richard Conlon, MPA
Public Health Advisor
McKing Consulting Corporation
2900 Chamblee Tucker Road
Building 10, Suite 100
Atlanta, GA 30341
Phone: 404-483-2826
Fax: 678-999-4884
Email: [email protected]
Jerald Fagliano, PhD, MPH
Program Manager
Environmental & Occupational Health Surveillance Program
New Jersey Department of Health & Senior Services
P.O. Box 369
Trenton, NJ 08625
Phone: (609) 826-4945
Fax: (609) 826-4983
Email: [email protected]
Tammie Johnson, DrPH
Florida Department of Health
Division of Disease Control
Bureau of Epidemiology
Chronic Disease Epidemiology Section
4052 Bald Cypress Way, Bin A-12
Tallahassee, Florida 32399
Phone (850) 245-4405
Fax: (850) 922-9299
Email: [email protected]
Wendy Kaye, PhD
Senior Epidemiologist
McKing Consulting Corporation
4770 Buford Highway, MS F-57
Atlanta, GA 30341
Phone: 770-488-3699
Email: [email protected]
Daniel Lefkowitz, PhD, MS
Research Scientist II
Environmental & Occupational Health Surveillance Program
New Jersey Department of Health & Senior Services
135 East State St., P.O. Box 369
Trenton, NJ 08625
Phone: (609) 292-0274
Fax: (609) 826-4983
Email: [email protected]
Maggie Ritsick, MPH
Vice President/ Project Manager
McKing Consulting Corporation
2900 Chamblee Tucker Road
Building 10, Suite 100
Atlanta, GA 30341
Phone: 770-220-0608
Fax:770-220-0670
Email: [email protected]
Julie Royer, MSPH
Statistician, Office of Research and Statistics
South Carolina Budget and Control Board
1919 Blanding Street
Columbia, SC 29201
Phone: 803-898-9701
Fax: 803-898-9972
Email: [email protected]
Tammy Sajak, MPH
Manager
Epidemiology Studies & Initiatives Branch
Texas Department of State Health Services
PO Box 149347, MC 1964
Austin, TX 78714-9347
Phone: (512) 458-7220
Fax: (512) 458-7222
Email: [email protected]
Jerry Shirah
Public Health Advisor
McKing Consulting Corporation
2900 Chamblee Tucker Road
Building 10, Suite 100
Atlanta, GA 30341
Phone: 678-523-3673
Fax: 678-999-4884
Email: [email protected]
Eric Sorenson, MD
Neurologist
Mayo Clinic
200 First Street SW, Plummer 6-56
Rochester, MN 55905
Phone: 507-284-8729
Fax: 507-284-1013
Email: [email protected]
David Thurman, MD, MPH
Centers for Disease Control and Prevention
4770 Buford Highway, NE MS K-51
Atlanta, GA 30341
Phone: 770-488-6090
Fax: 770-488-5486
Email: [email protected]
Laurie Wagner
Epidemiologist
Environmental Epidemiology and Toxicology
Division
Texas Department of State Health Services
PO Box 149347, MC 1964
Austin, TX 78714-9347
Phone: 512-776-6641
Fax: 512-458-7111
Email: [email protected]
9. Explanation of Any Payment or Gift to Respondents
Physicians will receive $100 for each case reported to offset reporting costs. Although the demographic information on the Case Reporting Form can be completed by office staff, certain information must be completed by the neurologist. The El Escorial Criteria at the top of the reporting form must be applied for each case. These criteria were developed by and agreed to by an international group of neurologists for use in clinical trials. Use of these criteria allow the comparison across studies. Even in large ALS referral centers this information will likely not be recorded in the medical record and the neurologist will need to review and assign it for each case. To access the accuracy of diagnosis, neurologists will be asked to complete a Medical Record Verification form for a subset of cases. An additional $50 will be available as necessary to offset costs related to completing the Medical Record Verification Form.
Assurance of Confidentiality Provided to Respondents
Case reporting information includes full name, address, date of birth, last five digits of the Social security number, sex, race, ethnicity, and disease information. This information is necessary because case information will be collected from a number of sources and it is imperative that duplicates be identified. The primary goal of this surveillance project is to access the accuracy of the National ALS Registry which cannot be done without identifiers.
PRIVACY IMPACT ASSESSMENT INFORMATION
This submission has been reviewed by the NCEH/ATSDR Privacy and Confidentiality Officer who determined that the Privacy Act does apply. The applicable Systems of Records Notice is 09-19-0001, “Records of Persons Exposed or Potentially Exposed to Hazardous or Toxic Substances.”
Data security is of paramount importance and technical, physical, and administrative safeguards are outlined below. A detailed description of the security in each state can be found in the protocol (Attachment 5).
Minimize collection of identifiable information
The information required for reporting a case of ALS to the surveillance project has been limited to only that needed to describe the basic demographics of the cases being reported and to make sure that an individual truly has ALS and is not already been submitted. To truly de-duplicate case reports, SSN is needed, however only the last five digits SSN will be collected.
ATSDR data management
ATSDR will maintain the National ALS Registry on a secure server or stand-alone hard-drive. Data will be password protected and access to the data will be limited to approved study personnel. Data from the states and metropolitan areas will be compared with that in the National ALS Registry to evaluate completeness. De-identified data sets will be used for data analysis.
Data management
Health departments will collect and enter the data and transmit the data to ATSDR using a secure file transfer method. Health departments are accustomed to dealing with confidential data collected for surveillance of a variety of diseases including, but not limited to, cancer, HIV, TB, and elevated blood lead levels.
CDC/ATSDR IRB approval for the ALS registry protocol was obtained on May 18, 2010. (Attachment 6) A request to waive informed consent was granted by the IRB. An amendment to address minor changes was approved on August 6, 2010 (Attachment 7).
HIPAA considerations
Under 45 CFR 164.512 (b)(1)(i) HIPAA permits disclosure of PHI by a covered entity to a public health authority without authorization for the purpose of public health surveillance. State regulations concerning the collection and disclosure of PHI by states for surveillance varies and not all states can use PHI in the way required by this project. The states and metropolitan areas that applied for participation in this project have all determined that collection and use of PHI for this project is allowed under their laws and regulations as well as under HIPAA. The CDC Office of General Counsel has agreed that the contractor as an agent of ATSDR is covered as a public health authority under HIPAA for release of PHI.
Justification for Sensitive Questions
Questions that might be considered sensitive by a portion of the general population include full name, date of birth, last five digits of the Social security number, disease information, including whether patient has dementia. This information is necessary because case information will be collected from a number of sources and it is imperative that duplicates be identified. Information is collected on dementia because it may have in impact on the diagnosis of ALS. Because the data will be abstracted from existing records, “unknown” is allowed for race and date of death.
The National ALS Registry is a combination of individuals identified through existing datasets and self-registration. The administrative data sets, Medicare, Medicaid, VHA, and VBA use SSN as a unique identifier. ATSDR received OMB approval to collect the last 5 digits of the SSN as part of the National ALS Registry (OMB No. 0923-0041).
The primary goal of the surveillance project is to access the completeness of the National ALS Registry which cannot be done without being able to identify matches. Because the National ALS Registry is relying on the last 5 digits of the SSN, it is necessary for this project as well. Epidemiologic characteristics such as sex and geographic location are routinely collected because of their significance in describing effected populations and evaluating resource allocation.
Estimates of Annualized Burden Hours and Costs
A. Burden hours are included in Table 1. Although not all the metropolitan areas have been identified, the burden table includes the expected cases from New Jersey, Texas, Florida and all six metropolitan areas. Approximately 6,750 individuals will be reported to the surveillance projects during a 3 year period. Contractor surveillance specialists will train medical personnel and neurologists to complete the forms. The case reporting form takes 5 minutes to complete. For quality assurance purposes a case verification form will be completed on 20% of the cases. This form takes 20 minutes to complete based on a small pilot test. The number of medical personnel trained is based on the number of neurologists that treat or diagnosis ALS patients divided by 3. Training will give by contract surveillance specialists in person. Each medical practice seeing ALS patients will keep a listing of cases reported. The line listing will include name individuals diagnosed with or thought to have ALS along with information on whether or not the case was reported and if not, the reason.
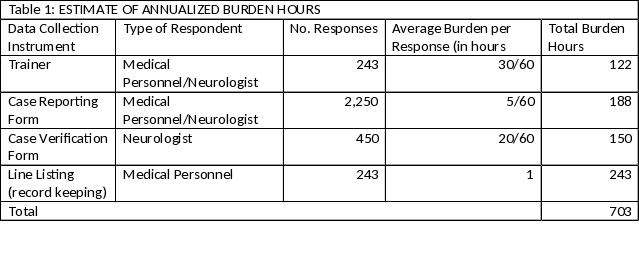 B.
Burden costs are included in Table 2. The ALS cases will be members
of the general public. The hourly wage rate of $100 for
neurologists is based on PayScale.com for neurologists and the US
Department of Labor, Bureau of Labor Statistics 2008 National
Occupation Employment and Wage Estimates using category 29-1069
Physicians and Surgeons, All Others practicing in offices or
outpatient care centers, because neurologists are not listed
separately. (http://www.bls.gov/oes/2008/may/oes291069.htm#nat).
The hourly rate is based on the US Department of Labor, Bureau of
Labor Statistics 2008 National Occupation Employment and Wage
Estimates using category 29-1111 Registered Nurses, Offices of
Physicians. (http://www.bls.gov/oes/2009/may/oes291111.htm)
B.
Burden costs are included in Table 2. The ALS cases will be members
of the general public. The hourly wage rate of $100 for
neurologists is based on PayScale.com for neurologists and the US
Department of Labor, Bureau of Labor Statistics 2008 National
Occupation Employment and Wage Estimates using category 29-1069
Physicians and Surgeons, All Others practicing in offices or
outpatient care centers, because neurologists are not listed
separately. (http://www.bls.gov/oes/2008/may/oes291069.htm#nat).
The hourly rate is based on the US Department of Labor, Bureau of
Labor Statistics 2008 National Occupation Employment and Wage
Estimates using category 29-1111 Registered Nurses, Offices of
Physicians. (http://www.bls.gov/oes/2009/may/oes291111.htm)
-
Table 2: Estimate of Annualized Burden Costs
Type of
Respondents
Total Burden Hours
Hourly Wage Rate
Total Burden Costs ($)
Neurologists
338
$100
$33,800
Medical Personnel – reporting
122
$32
$3,904
Medical Personnel – record keeping
243
$32
$7,776
Total
$45,480
13. Estimates of Other Total Annual Cost Burden to Respondents or Record Keepers
There are no capital or maintenance costs incurred by respondents because the information will be collected on paper reporting forms. The record keeping costs are minimal and reflected in Table 1 and Table 2.
14. Annualized Cost to the Government
Data analysis by ATSDR may result in action taken by the Division of Health Studies in response to the required CDC mandate in maintaining preventive health activities and surveillance projects. The action taken will vary, depending on the analysis.
The total cost to the federal government for the collection of this information for the three year ongoing project is $8,295,000 as itemized below.
Annual ATSDR personnel costs $220,000.
Additional expenses will be incurred by ATSDR in order to operate a successful surveillance program. Contract staff will contribute to this program: a program analyst (25% contribution = $35,000/year). A contractor will be used to operate, maintain, and provide support for surveillance projects in 2-3 state health departments and 4-6 metropolitan area health departments ($7,500,000). Lesser expenses may include computer resources, telephone calls, and training materials (approximately $10,000/year).
The estimated annual cost to the government is $2,765,000.
15. Explanation for Program Changes or Adjustments
This is a new surveillance data collection.
16. Plans for Tabulation and Publication and Project Time Schedule
Statistical Analysis Plan:
CDC will aggregate the data provided by the states and metropolitan areas.
-
A. 16-1
Activity
Time Schedule
Activation
1 - 2 months after OMB approval
Surveillance Activity
Ongoing data collection
Summary Reports
Every year after OMB approval
Yearly Evaluation
Each year after OMB approval
We also plan to publish selected summary reports on CDC’s website during the third year of this project.
17. Reason(s) Display of OMB Expiration Date is Inappropriate
Exemption from displaying the expiration date for the OMB approval of forms is not being requested.
18. Exceptions to Certification for Paperwork Reduction Act Submissions
There are no exceptions to the certification.
List of Attachments
Attachment 1 Authorizing Legislation: Public Law No: 110-373, amendment to the Public Health Service Act to provide for the establishment of an Amyotrophic Lateral Sclerosis Registry
Attachment 2 Federal Register Notice
Attachment 3 ALS Case Reporting Form
Attachment 4 ALS Medical Record Verification Form
Attachment 5 IRB Approved Protocol
Attachment 6 IRB Approval
Attachment 7 IRB Amendment Approval
| File Type | application/msword |
| File Title | Request for Emergency OMB Approval for Information Collection |
| Author | Rebecca LePrell |
| Last Modified By | wek1 |
| File Modified | 2011-03-31 |
| File Created | 2011-03-31 |
© 2026 OMB.report | Privacy Policy