Form 1 Screener
Online Skills Training for PCPs on Substance Abuse (NIDA)
N44DA-9-2214Attachment3ScreeningTool_March2011
Physicians
OMB: 0925-0632
Attachment 3: Screening Instrument
Online Skills Training for PCPs on Substance Abuse (NIDA)
January 2011
SCREENING INSTRUMENT
(This instrument is online at: http://www.sbirttraining.com/screeningsurvey)
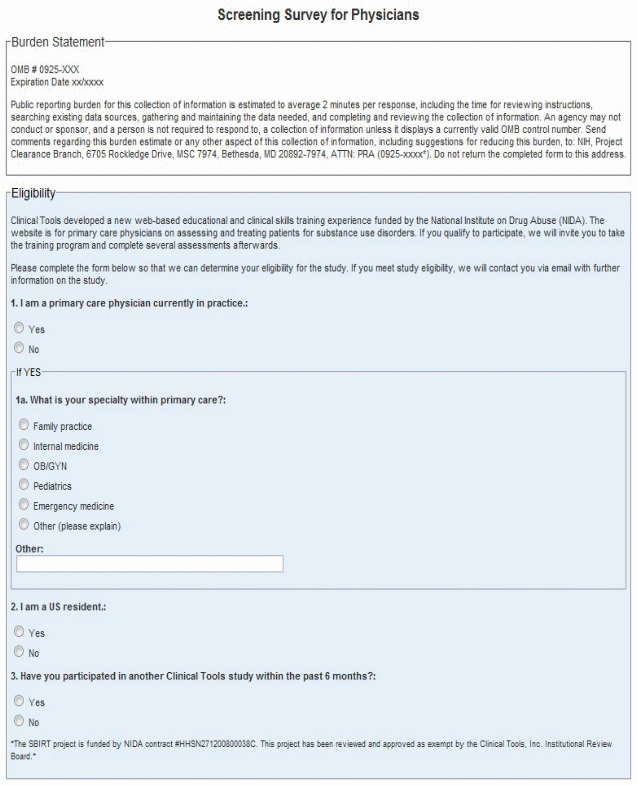
OMB # 0925-XXX
Expiration Date xx/xxxx
BURDEN STATEMENT
Public reporting burden for this collection of information is estimated to average 2 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden, to: NIH, Project Clearance Branch, 6705 Rockledge Drive, MSC 7974, Bethesda, MD 20892-7974, ATTN: PRA (0925-xxxx*). Do not return the completed form to this address.
Screening Survey for Physicians
Clinical
Tools developed a new web-based educational and clinical skills
training experience funded by the National Institute on Drug Abuse
(NIDA). The website is for primary care physicians on assessing and
treating patients for substance use disorders. If you qualify to
participate, we will invite you to take the training program and
complete several assessments afterwards.
Please complete
the form below so that we can determine your eligibility for the
study. If you meet study eligibility, we will contact you via email
with further information on the study.
Eligibility
1. I am a primary care physician currently in practice.
Yes
No
(If they answer “No”, they are not eligible and will receive a message explaining why they are not eligible, see below)
1a. What is your specialty within primary care?
Family practice
Internal medicine
OB/GYN
Pediatrics
Emergency
medicine
Other (please explain)
2. I am a US resident.
Yes
No
(If they answer “No”, they are not eligible and will receive a message explaining why they are not eligible, see below)
3. Have you participated in another Clinical Tools study within the past 6 months?
Yes
No
(If they answer “Yes”, they are not eligible and will receive a message explaining why they are not eligible, see below)
*The SBIRT project is funded by NIDA contract #HHSN271200800038C. This project has been reviewed and approved as exempt by the Clinical Tools, Inc. Institutional Review Board.*
Please enter your name and email address so that we can contact you if you are eligible.
Name:
Email Address:
Phone Number:
Messages for Ineligible Individuals:
Non-Primary Care Physicians:
This study includes only practicing primary care physicians, so we regret that you can not participate.
Non-US
Residents:
If you do not reside in the United States, we regret
that you cannot participate due to funding restrictions.
Recent
Participant:
Due to funding restrictions, you may participate in
only one survey per project.
(This instrument is online at: http://www.sbirttraining.com/screeningsurvey)
| File Type | application/msword |
| Last Modified By | Susan Wilhelm |
| File Modified | 2011-03-28 |
| File Created | 0000-00-00 |
© 2026 OMB.report | Privacy Policy