#4_SC_Attach1_betatest_study design_consent_procedures
#4_SC_Attach1_betatest_study design_consent_procedures.doc
Generic Clearance for the Collection of Qualitative Feedback on Agency Service Delivery
#4_SC_Attach1_betatest_study design_consent_procedures
OMB: 0925-0642
Attachment #1:
Part 1: Beta Testing
Study Diagram
Consent Form
Procedures
Thank you letter
Email/Letter Invitation
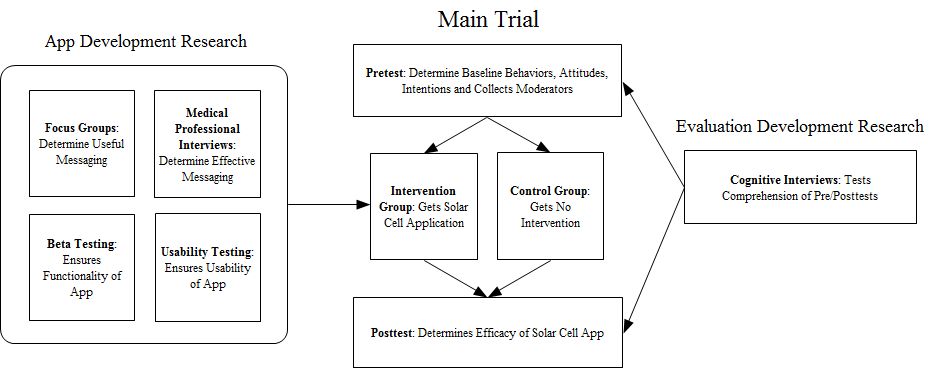
Study diagram:
Outlines the components of the sub-study (0925-0642-04) which include:
Focus Groups
Medical/Professional Interviews
Beta Testing
Usability Testing
Cognitive Testing
The full, randomized trial will be submitted as a separate submission and OMB should expect to receive it in, April, 2012. The 60-day Federal Register Notice will be published January 27, 2012.
APPROVED
Oct 17, 2011
WIRB
National Cancer Institute (NCI), Bethesda, Maryland, United States
20111501
Research subject information and Consent form |
|
TITLE: |
Solar Cell: A Mobile UV Manager for Smart Phones Beta Testing |
This consent form contains important information to help you decide whether to participate in a research study.
The study staff will explain this study to you. Ask questions about anything that is not clear at any time. You may take home an unsigned copy of this consent form to think about and discuss with family or friends.
Being in a study is voluntary – your choice.
If you join this study, you can still stop at any time.
No one can promise that a study will help you.
Do not join this study unless all of your questions are answered.
After reading and discussing the information in this consent form you should know:
Why this research study is being done;
What will happen during the study;
Any possible benefits to you;
The possible risks to you;
Other options you could choose instead of being in this study;
How your research information will be treated during the study and after the study is over;
Whether being in this study could involve any cost to you; and
What to do if you have problems or questions about this study.
Please read this consent form carefully.
RESEARCH SUBJECT INFORMATION AND CONSENT FORM
Beta Testing
Title: Solar Cell: A Mobile UV Manager for Smart Phones
Protocol No.: None
WIRB® Protocol #20111501
Sponsor: National Cancer Institute (NCI)
Bethesda, Maryland
United States
Investigator: David Buller, Ph.D.
Suite 225
1667 Cole Blvd
Golden, Colorado 80401
United States
Site(s): Klein Buendel, Inc.
Suite 225
1667 Cole Blvd
Golden, Colorado 80401
United States
STUDY-RELATED
PHONE NUMBER(S): David Buller, Ph.D.
303-565-4340
This consent form may contain words that you do not understand. Please ask the principal investigator or the study staff to explain any words or information that you do not clearly understand. You will have time to ask questions and if you agree will be asked to sign this consent form.
SUMMARY
You are being asked to be in a research study. The purpose of this consent form is to help you decide if you want to be in the research study. Please read this form carefully. To be in a research study you must give your informed consent. “Informed consent” includes:
Reading this consent form,
Having the principal investigator or staff explain the research study to you, and
Asking questions about anything that is not clear.
You should not join this research study until all of your questions are answered. If you take part in this research study, you will be given a copy of this signed and dated consent form.
PURPOSE OF THE STUDY
The overall goal of the study is to design a smart phone application, Solar Cell, which uses smart phone technology to aid users in protecting their skin from damaging ultraviolet radiation (UV) in sunlight, a primary cause of skin cancer. The purpose of this part of the study is to test the Solar Cell mobile applications to see how easy it is to use and what errors there are in the program.
PROCEDURES
The Beta Testing Introduction Session will take place at Klein Buendel’s offices located in Lakewood, CO. It will take about 60 minutes. Your entire participation in the study will take place on the same day in the same offices.
We will ask that you use your Android or iPhone handset to download the Solar Cell mobile application. We will ask you to use the Solar Cell mobile application during the visit to test the algorithms and application features for platform stability and code errors. During the time that you are using the Solar Cell application, if you experience any troubles or errors with the application, we ask that you access to a website and complete an error reporting form that will allow you to explain the scenario in which you were using Solar Cell and experienced the problem. This data will be sent to KB to determine if it’s a bug that can be eliminated quickly or if the bug will need to be fixed on a later release.
If the bug is fixed quickly, you will be asked to reinstall the application on your phone and continue using Solar Cell.
Once the Beta Testing is over, we will ask you to provide feedback and complete a brief questionnaire related to your experience with using Solar Cell. The feedback will be documented and used to make slight adjustments to the program for more testing. This Beta Testing will also uncover bugs that may have been overlooked during the in-house testing phase.
RISKS AND DISCOMFORTS
There are no known risks for participating in this research.
NEW INFORMATION
You will be told about any new information that might change your decision to be in this study.
BENEFITS
There are no direct benefits to you for participating in this study. However, you may benefit from the knowledge that you have helped evaluate the effectiveness of a state-of-the-art mobile software application, Solar Cell, to support decision-making related to sun protection and exposure by Americans to reduce the risk of developing skin cancer attributable to chronic and severe UV exposure and developing other cancers attributable to vitamin D deficiency.
COSTS
There are no costs associated with this study, except for your time.
INCENTIVE FOR PARTICIPATION
Subjects who participate in the beta testing session will receive $40 as a thank you for your time and input.
ALTERNATIVES
Your alternative is not to participate in this study.
Confidentiality
Study information collected about you will be given to the sponsor. “Sponsor” means any persons or companies that are working for or with the sponsor, or owned by the sponsor.
The consent form signed by you will be looked at and/or copied for research or regulatory purposes by:
Department of Health and Human Services (DHHS) agencies,
the sponsor;
Western Institutional Review Board® (WIRB®).
Total confidentiality cannot be guaranteed because of the need to give information to these parties. The results of this research study may be presented at meetings or in publications. Your identity will not be given out during those presentations. The information you provide us will be kept private under the Privacy Act.
VOLUNTARY PARTICIPATION AND WITHDRAWAL
Your participation in this study is voluntary. You may decide not to participate or you may leave the study at any time. Your decision will not result in any penalty or loss of benefits to which you are entitled.
Your participation in this study may be stopped at any time by the study investigator or the sponsor without your consent for any reason.
SOURCE OF FUNDING FOR THE STUDY
Funding for this research study is provided by the National Center on Minority Health and Health Disparities at the National Institutes of Health.
QUESTIONS
Contact David Buller, Ph.D. at 303-565-4340 for any of the following reasons:
if you have any questions about this study or your part in it,
if you feel you have had a research-related problem, or
if you have questions, concerns or complaints about the research.
If you have questions about your rights as a research subject or if you have questions, concerns or complaints about the research, you may contact:
Western Institutional Review Board® (WIRB®)
3535 Seventh Avenue, SW
Olympia, Washington 98502
Telephone: 1-800-562-4789 or 360-252-2500
E-mail: [email protected].
WIRB is a group of people who independently review research.
WIRB will not be able to answer some study-specific questions, such as questions about appointment times. However, you may contact WIRB if the research staff cannot be reached or if you wish to talk to someone other than the research staff.
Do not sign this consent form unless you have had a chance to ask questions and have gotten satisfactory answers.
If you agree to be in this study, you will receive a signed and dated copy of this consent form for your records.
CONSENT
I have read this consent form. All my questions about the study and my part in it have been answered. I freely consent to be in this research study.
By signing this consent form, I have not given up any of my legal rights.
I authorize the release of my research records for research or regulatory purposes to the sponsor, DHHS agencies, and WIRB®.
I have not given up any of my legal rights by agreeing to take part in this research study.
__________________________________________
Subject Name (printed)
CONSENT SIGNATURE:
________________________________________ __________________
Signature of Subject (18 years and older) Date
I confirm that the research study was thoroughly explained to the subject. I reviewed the consent form with the subject and answered the subject’s questions. The subject appeared to have understood the information and was able to answer the following questions correctly:
What is the purpose of this study?
If you decide to be in the study, what will you be asked to do?
What is the possible benefit of participating in this study?
What are the possible risks of participating in this study?
If you decide not to participate in this study, what options do you have?
Will participating in this study cost you anything? If so, what will you have to pay for?
Do you have to be in this study?
If you decide to be in the study, can you leave the study when you want to?
________________________________________ __________________
Printed Name of Person Conducting the Position
Informed Consent Discussion
________________________________________ __________________
Signature of Person Conducting the Date
Informed Consent Discussion
Beta Testing
Procedures
This cohort will consist of 10 individuals who are 18 or older, proficient in English, and own a smartphone that is compatible with Solar Cell.
Recruitment
Beta test participants will be recruited in Colorado via word-of-mouth.
Screening
When potential participants contact the project coordinator, he/she will use the screening questions (see Attachment 2) to determine the potential participant’s eligibility. Ineligible people will be thanked for their time and told that their information will be destroyed. Eligible participants will be asked to provide their availability so that they can be scheduled for a time to be introduced to the Solar Cell application and to be consented.
Scheduling
Eligible participants will be appropriately scheduled. The project coordinator will call each participant to verify that the schedule works for them. Participants who agree to the schedule will be sent directions to KB’s offices.
Confirmation
Two days prior to the appointment, a study staff member will call participants to confirm their attendance, ensure participants know how to get to KB and answer any questions participants may have. If participants indicate that they can no longer attend, the study staff will attempt to reschedule the appointment or find a replacement. Additionally, confirmed participants will be contacted two hours prior to their scheduled time to remind them of the time and ask if they need additional directions.
Participant Incentive
When the Beta Testing is complete, the participant will fill out the receipt form (see below) and have their check mailed to them in approximately two weeks. All forms should be given to the research assistant, who will then turn all receipts to KB’s Office Manager to process a check request. The Office Manager will also attach a thank you letter (see below) to the receipt form to send along with the check when it is processed.
Conducting the Beta Test
Welcome & Consenting
If the appointment is after office hours, one staff person will wait outside or in the lobby to make sure participants can get into the building. When participants arrive, the Research Assistant will welcome them and ask them to take a seat and enjoy refreshments. If the scheduled participant has not arrived 10 minutes after the scheduled time, the Research Assistant will call them to see if they are coming or need additional directions.
Once the participant is settled, the Research Assistant will go over the consent form (see Attachment 1) and ask if they have any questions, making sure to let the participant know that they are happy to answer questions. The Research Assistant will check to make sure the participant has completely read the consent form and fully understands it, then ask the participant to sign the consent form. Once the consent form is signed, the Research Assistant will make copies and provide the participant with a copy of their signed consent form (keeping the original for KB’s records).
The Research Assistant will explain to the participant that they will be using their smartphone to test a new smartphone application that KB is developing.
The Research Assistant will help the participant download the application to their smartphone and show them how to use the application. They will also be instructed on what to do if they find a problem with the application and answer any questions the participant may have.
Participants will be given a copy of the application that will have some tracking that tells KB when the user is using Solar Cell on their phone.
If during the test the user runs into a problem, they will be asked to access to a website and complete an error reporting form that will allow them to explain the scenario in which they were using Solar Cell and the problem they had. This data will be sent to KB to determine if it’s a bug that can be eliminated quickly or if the bug will need to be fixed on a later release.
If the bug is fixed quickly, the tester will be asked to reinstall the application on their phone and continue using Solar Cell.
Once the beta test is over the tester will be asked to provide final feedback by filling out a brief questionnaire related to their experience with using Solar Cell.
Wrap-up
At the end of the introductory session, the participant will be asked if they have any questions and reminded that they can always contact study staff or the Western Institutional Review Board with any questions or concerns they might have about their participation in the study.
THANK YOU LETTER
Letter to accompany incentive (printed on KB letterhead)
Date
Name
Address
City, State Zip Code
Dear Name,
Thank you for your recent participation in the Solar Cell Beta Testing. We appreciate you taking the time to assist us and value your input regarding this new application.
Enclosed
you will find your check for $40 as a thank you for your time and
input in the program.
Sincerely,
Name
Project Manager
Beta Testing
Invitation Letter
To Whom It May Concern,
Klein Buendel, Inc. is developing a smart phone application to help users protect their skin from damaging ultraviolet radiation (UV) in sunlight, a primary cause of skin cancer. We are currently seeking temporary beta testers to help test the application on their own Android smartphones. Testing will take approximately one hour and testers will be given an incentive of $40 for their time and input. For more information please contact Marissa Glatter at 303-565-4355 or [email protected].
Sincerely,
David Buller, PhD
| File Type | application/msword |
| File Title | RESEARCH SUBJECT INFORMATION AND CONSENT FORM |
| Author | dgogue |
| Last Modified By | Vivian Horovitch-Kelley |
| File Modified | 2012-01-25 |
| File Created | 2012-01-24 |
© 2026 OMB.report | Privacy Policy