FINAL_HCS_OMB_Supporting_Statement_A _September 2011[1]
FINAL_HCS_OMB_Supporting_Statement_A _September 2011[1].docx
The Healthy Communities Study: How Communities Shape Children?s Health (NHLBI)
OMB: 0925-0649
Supporting Statement A
for
The Healthy Communities Study:
How Communities Shape Children’s Health (NHLBI)
September 14, 2011
Name: Sonia Arteaga
Address: 6701 Rockledge Drive, Suite 10018, Bethesda, Maryland 20892
Telephone: (301) 435-6677
FAX: (301) 480-5158
Email: [email protected]
TABLE OF CONTENTS
Summary of the Healthy Communities Study (HCS) 1
A. JUSTIFICATION 4
A.1 Circumstances Making the Collection of Information Necessary 4
A.2 Purpose and Use of the Information Collection 6
A.3 Use of Information Technology and Burden Reduction 11
A.4 Efforts to Identify Duplication and Use of Similar Information 15
A.5 Impact on Small Businesses or Other Small Entities 16
A.6 Consequence of Collecting the Information Less Frequently 16
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5 17
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agency 17
A.9 Explanation of Any Payment of Gift to Respondents 20
A.10 Assurance of Confidentiality Provided to Respondents 22
A.11 Justification for Sensitive Questions 24
A.12 Estimates of Annualized Burden Hours and Costs 25
A.13 Estimate of Other Total Annual Cost Burden to Respondents and Recordkeepers 33
A.14 Annualized Cost to the Federal Government 33
A.15 Explanation for Program Changes or Adjustments 33
A.16 Plans for Tabulation and Publication and Project Time Schedule 33
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate 35
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions 35
LIST OF TABLES
Table A.12.1 Estimates for Annualized Hour Burden for Years 1-3 of Data Collection 29
Table A.12.2 Annualized Cost to Respondents for Years 1-3 of Data Collection………………. 298
Table A.12.3 Communities Visited During the First Three Years of Data Collection ….………..29
Table A.12.4 Variation in Child and Parent/Caregiver Involvement in Data Collection Activities
(in minutes) by Protocol Type and Age of Child
Table A.12.5 Estimated Duration of Each Standard Protocol Component for the Baseline Visit 32
Table A.12.6 Estimated Duration of Each Enhanced Protocol Component for the Baseline Visit 25
Table A.12.7 Annualized Cost to Respondents for Years 1-3 of Data Collection for the HCS 28
Table A.14.1 Estimate of Annualized Cost to the Government (in Thousands) 33
LIST OF FIGURES
LIST OF ATTACHMENTS
attachment 1. Healthy Communities Study Glossary of Terms
Attachment 2. Board of External Experts Roster
Attachment 3. NHLBI Advisory Council Meeting Minutes and Roster
Attachment 4. Informational Letter Sent to Families
Attachment 5. Study Brochure Sent to Families
Attachment 6. Family Household Visit Screening Overview
Attachment 7. Family Household Visit Protocol Overview for Parents/Caregiver Participants
Attachment 8. Family Household Visit Protocol Overview for Second Parent/Caregiver Participants
Attachment 9. Data Collection Protocol for Parents who Refuse to Participate in the Study
Attachment 10. Family Household Visit Protocol Overview for Child Participants
Attachment 11. Informational Letter Sent to Key Informants
Attachment 12. Study Brochure Sent to Key Informants
Attachment 13. Key Informant Screening Protocol Overview
Attachment 14. Key Informant Interview Protocol
Attachment 15. Community and School Observations Protocol (Modified and Full Windshield Surveys, Physical Activity Resource Assessment (PARA), and Ground Truthing of GIS mapping)
Attachment 16. School Observations – Protocol for Lunch Personnel Participants
Attachment 17. School Observations – Protocol for Physical Education Instructors
Attachment 18. State Health Department Interview Script
Attachment 19. Medical Record Retrieval – Protocol for Physicians/Medical Secretaries
Attachment 20. NCCOR Letter of Support
Attachment 21. Steering Committee Membership By Subcommittee
Attachment 22. Battelle Institutional Review Board Notice of Approval
Attachment 23. Applicability of the Privacy Act Memo
Attachment 24. Calculation of Total Parent/Caregiver and Child Participants Visited in First Three Years of Data Collection
Attachment 25. Detailed Estimation of Parent /Caregiver and Child Participant Average Burden by Protocol Components and Child Age in First Three Years of Data Collection
Attachment 26. Estimation of Parent/Caregiver and Child Participant Average Burden by Protocol Type and Child Age in First Three Years of Data Collection
Attachment 27. Estimation of Key Informant Average Burden by Protocol Type in First Three Years of Data Collection
Information Collection Request for OMB Review
Supporting Statement for Paperwork Reduction Act Submissions:
“The Healthy Communities Study: How Communities Shape Children’s Health”
Summary of the Healthy Communities Study (HCS)
The following text provides information on the National Heart, Lung, and Blood Institute’s (NHLBI) planned “Healthy Communities Study: How Communities Shape Children’s Health.” The information is organized to respond directly to the 18 itemized subsections of Section A (Justification) of the Supporting Statement for Paperwork Reduction Act Submissions. A general description of the scope of work for the study is included below, as well as specific items in the Supporting Statement for Paperwork Reduction Act Submissions. Please refer to Attachment 1 for a list of study glossary of terms.
Rationale: Community programs and policies targeting childhood obesity are being implemented across the country, but their approaches not been systematically studied. There is natural variation in many aspects of these programs and policies, including intensity level, duration, funding, target population, and how they are implemented. However, no previous studies have examined these variations and how such aspects of community programs and policies are related to childhood obesity outcomes. Moreover, no studies have examined factors across a wide range of communities that may modify or mediate the associations between childhood obesity and programs and policies, such as community and family socio-demographic characteristics. The Healthy Communities Study (HCS) will address the need for a cross-cutting national study of community programs and policies and their relationship with childhood obesity.
The HCS is an observational study of communities conducted over five years that aims to
(1) determine the associations between community programs/policies and body mass index (BMI), diet, and physical activity in children; and (2) identify the community, family, child factors that modify or mediate the associations between community programs/policies and BMI, diet, and physical activity in children. A total of 279 communities and over 23,000 children and their parents will be part of the HCS. A HCS community is defined as a high school catchment area and the age range of children is 3-15 years upon entry into the study. Data will be collected on at least 78 children within each community (three boys and three girls for each yearly age interval). The study will examine quantitative and qualitative information obtained from community-based initiatives, community characteristics (e.g., school environment), and from child and parent assessments and measurements of physical activity levels and dietary practices of children, and children’s and parent’s BMI.
Design: The HCS employs a complex study design that includes a nationally representative sample of communities that will both (1) maximize the opportunity to identify “best practices” for reducing childhood obesity, and (2) yield results that are generalizable to the United States population and important subpopulations. The study design combines current/prospective and retrospective data in four communities in Year 1 (Wave 1) and 275 communities in Years 2-4 (Wave 2). The focus of Wave 1 is protocol refinement to inform the Wave 2 protocol. Wave 1 will include the same data collection components as in Wave 2 but no remote or in-person follow-up data will be collected (remotely or in-person) from children in these communities.
Of the 275 Wave 2 communities, 195 communities will be selected using a stratified National Probability Based Sample (NPBS) and 80 communities will be selected with “certainty” to represent communities with the most promising programs/polices in child obesity prevention. The purpose of the 195 NPBS is to ensure that the HCS can yield estimates that can be generalized to the entire U.S. when conducting weighted analyses of the study data. The purpose of the 80 certainty communities is to ensure the inclusion of communities with promising programs and policies aimed at reducing childhood obesity.
Data collection: In each community, retrospective and prospective data will be collected. The retrospective data will include children’s height and weight extracted from medical charts and details of community programs/policies dating back ten years. Prospective data will include in-home assessment of children’s height and weight, diet, and physical activity. When available, the parents/caregivers height and weight will also be measured. Two hundred communities will also receive a remote follow-up assessment of children’s diet and physical activity behaviors. Three years after baseline, 40 of these 200 communities will receive a repeat in-person assessment (RIPA) of height and weight, diet and physical activity.
Data collection will consist of a two-staged sampling approach, with all study children receiving less detailed Standard Protocol measures (e.g., brief questionnaires). The Standard Protocol visit will take approximately 75 minutes to complete. A randomly selected subset of children (approximately 17%) will receive more detailed Enhanced Protocol measures (e.g., accelerometers, dietary recalls). The Enhanced Protocol will take approximately 180 minutes to complete, which includes two home visits, and use of an accelerometer for a one week period. Statistical modeling techniques will be employed to adjust measures from the Standard Protocol for bias and error using measures from the Enhanced Protocol. This two-step statistical design will improve the study’s power without increasing burden for all participants.
Program/policy and environmental data will be collected through interviews with community key informants, participant perceptions of the school and home environments, interviews with school personnel, document review, GIS data, and direct observations of communities and schools, and other sources. Interviews with key informants will take up to one hour to complete.
Timeline: The HCS is a five-year observational study.
Months 1 to 15 – Study design and protocol development will be finalized, the Office of Management and Budget Information Collection Request and Institutional Review Board approval packages were prepared and submitted, and additional activities related to field implementation (such as database development, development of the Manual of Operations, including training and quality control activities) will be prepared.
Months 16 to 17 –Wave 1 of Data Collection will be implemented, to include the assessment of the four communities.
Months 18 to 29 – The initial year of Wave 2 Data Collection will sample the first 100 of the 275 Wave 2 communities. The child/parent and community Standard and Enhanced Protocols will be conducted on these 100 communities. Of those 100 communities, 60 communities will have 78 children each. The other 40 communities are designated as RIPA communities, and will be revisited for an in-home assessment approximately three years after baseline to repeat the original data collection on the participants and the community. In the 40 RIPA communities, there will be 117 children per community. The purpose of having 50% more children per RIPA community is to ensure for adequate sample size due to loss to follow-up three years later.
Months 30 to 41 –The second year of Wave 2 Data Collection will be a baseline assessment of the second 100 communities of Wave 2. All 100 communities will have 78 children per community. The child/parent and community Standard and Enhanced Protocols will be conducted on these 100 communities.
Months 42 to 53 – The third year of Wave 2 Data Collection will be a: a) remote (via phone or internet) follow-up with the child/parent and key informants in the first 100 communities from Year 1 of data collection; b) Remote follow-up with children and key informants of the second 100 communities from Year 2 of data collection; and, c) an assessment of the remaining 75 communities. The child/parent and community Standard and Enhanced Protocols will be conducted on these 75 communities. The remote follow-up assessment will take approximately 35 minutes to complete.
Month 54 to 60 – The fourth year of Wave 2 Data Collection will be an in-home follow-up of the 40 RIPA communities initially sampled in the first year of data collection. Data analyses and preparation of manuscripts will continue and intensify during this last year of the study.
Figure A1, below, shows the timing of assessments for each year of the Wave 2 data collection.
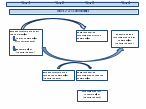
Figure A1: Healthy Communities Study Design by Wave 2 Data Collection Year
The study design maximizes the use of the data and resources, and allows both cross-sectional and longitudinal questions to be addressed.
The Research Team: The Research Coordinating Center leading the development and implementation of the HCS is Battelle Memorial Institute (Battelle). Battelle has formed a research team with key partners for each of the different but interrelated domains of the study. Investigators at the University of California at Berkeley are responsible for developing the tools and protocols for assessing dietary behaviors among child participants; investigators at the University of South Carolina are responsible for developing the tools and protocols for assessing physical activity and sedentary behavior among child participants; investigators at the University of Kansas are responsible for designing the tools and protocols for the characterization of community programs and policies; and Examination Management Services, Inc. (EMSI), a company specializing in conducting home data collection across the country, is responsible for the in-home data collection. Coordination of methods, instruments, training, data analysis, and dissemination will take place at Battelle.
The study is funded by several National Institutes of Health (NIH) institutes and centers including the National Heart Lung and Blood Institute (NHLBI), the National Cancer Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Eunice Kennedy Shriver National Institute of Child and Health and Human Development, and the Office of Behavioral and Social Sciences Research. In addition to the NIH scientific partners, the Centers for Disease Control and Prevention (CDC) and the Robert Wood Johnson Foundation (RWJF) are also non-funding partners in this study.
A.1 Circumstances Making the Collection of Information Necessary
Responding to a Legislative Mandate: The objective of this information collection is within the NHLBI mandate described in the PHS Act, Section 421 (42USC 285b-3) and specifies provision of "investigation into the epidemiology, etiology and prevention of all forms and aspects of heart, blood vessel, lung, and blood diseases, including investigations into the social, environmental, behavioral, nutritional, biological, and genetic determinants and influences involved in the epidemiology, etiology, and prevention of such diseases”.
The National Institutes of
Health (NIH), through the NHLBI, released a Request for Proposals
(RFP) titled “Studying Community Programs to Reduce Childhood
Obesity” in October of 2009. The RFP and subsequent project’s
study objectives are within the NHLBI’s mandate, and the
Institute has the unique capability to coordinate this study within
279 communities over an extended period of time as proposed.
The NHLBI Board of External Experts (BEE) (see Attachment 2) and the NHLBI Advisory Council (see Attachment 3) reviewed the research initiative used to develop the RFP and approved it.
Why the need to collect these study data? Previous studies have not systematically examined community programs and policies implemented across the country and their relationship to childhood obesity. There is natural variation in many aspects of programs and policies, including intensity level, duration, funding, target population, and how they are implemented. However, no previous studies have examined this variation and how such aspects of community programs and policies are related to childhood obesity outcomes. Moreover, no studies have examined factors across a wide range of communities that may modify or mediate the associations between childhood obesity and programs and policies, such as community and family socio-demographic characteristics. The HCS will address the need for a crosscutting national study of community programs and policies and their relationship with childhood obesity.
Numerous observational studies have demonstrated an increased risk of obesity in communities with greater access to unhealthy foods, less access to healthy foods, and fewer opportunities to be physically active. These community characteristics tend to be associated with low-socioeconomic status and help explain the reason for the significant health disparities1,2 that are associated with higher obesity prevalence in such communities. The need to identify the most promising community approaches that all communities can use to reduce the obesity epidemic is urgent3,4,5, 6, 7, 8, 9. The purpose of HCS is to assess the relationships between programs/policies targeting childhood obesity and children’s BMI, diet, and physical activity.
Why should the Federal Government sponsor this research? Children in the U.S. are at increased risk of developing obesity and consequently of developing chronic diseases earlier in life than previous generations. A comprehensive assessment of programs in communities to stop this epidemic, which affects all segments of children in the U. S. population, falls within the NIH mission to “seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability.” The development and implementation of a study that is national in scope with a large enough community sample to ensure generalizable data and that captures the full range of community programs and policies requires the support of a federal entity such as the NHLBI with the authority to support such work for the U.S. population.
What information and evaluation components that relate to this research already exist? The major dietary behaviors contributing to energy imbalance among children have been extensively reviewed and identified.10 Low levels of physical activity have clearly made the population susceptible to excess weight gain as calories have become ever more available and inexpensive. The social and environmental determinants of obesity are less well studied, but the evidence is mounting. The HCS study design incorporates this research and allows the simultaneous examination of dietary behaviors, physical activity, and environments, including those modified through community programs and policies. This research is reflected in the three areas which comprise the study’s core activities of: a) community and environmental assessments of health-related programs and policies impacting children 3-15 years of age; b) physical activity assessments; and c) dietary behavior assessments. The protocol and survey instruments build on the foundation of existing research in these three separate areas.
A.2 Purpose and Use of the Information Collection
The purpose of HCS is to assess the relationships between community programs/policies targeting childhood obesity and children’s BMI, diet, and physical activity. This study will include 279 communities and over 23,000 children and their parents. Below is a description of the types of data that will be collected and examples of research questions that can be answered with the data collected.
Prospective and Retrospective data: In each community, prospective and retrospective data will be collected. Prospective data will include in-person assessment of BMI, diet, and physical activity on a random sample of children in each of the 279 communities. Remote follow-up assessments in 200 communities will be conducted to collect data on changes in diet and physical activity behaviors one to two years after the baseline data collection. Additionally, 40 of these 200 communities will receive a repeat in-person assessment (RIPA) three years after the baseline assessment to repeat the data collection on community programs/policies and children’s height and weight, diet and physical activity. Retrospective data to be collected from all 279 communities will include the history of childhood obesity programs and policies and how they unfolded over the previous ten years in each community. Additionally, BMI trajectories for each child will be created by combining BMI measured at baseline with BMI calculated from height and weight data abstracted from the children’s medical records for a period of up to 10 years. Thus, data available for analysis from all communities includes information about community programs/policies targeting childhood obesity over the previous 10 years and children’s height and weight for a ten-year time period.
Data collection protocols: There are two types of data collection protocols, Standard and Enhanced for both children/parents and communities.
1) Standard Protocol
a) Child/Parent Standard Protocol: Parents and children in all 279 communities will be assessed with the Standard Protocol during the baseline in-home visit. The Standard Protocol baseline visit includes height, weight and girth measurements of the child, height and weight measurements or reported measurements of the parents/caregivers when available, completion of general demographic and background questions, brief diet and physical activity behaviors questionnaires, and medical record abstraction to develop longitudinal children’s BMI trajectories. We estimate approximately 70% of parents in each community will give consent for the HCS to contact their children’s primary care or usual provider to review their child’s medical records. Please see Attachments 4 and 5 for the informational letter and brochure for the families; 6 for the household screening protocol; Attachment 7 for the household visit protocol for parents/caregivers (including the recruitment script, consent and the medical record release authorization forms, anthropometrics form, and the home interview); Attachment 8 for the household visit protocol for the second parent/caregiver (including the consent and anthropometrics forms); Attachment 9 for data collection protocol for parents/caregivers who refuse to participate in the study; and Attachment 10 for the household visit protocol for children (including the assent and anthropometrics forms, and the home interview).
b) Community Standard Protocol: Within each community, 10-15 key informants will be interviewed to assess and document community programs and policies targeting childhood obesity and how they have evolved over the previous ten years. Key informants will consist of individuals from several key settings/sectors, including schools, healthcare organizations/coalitions, government, and non-profit/community organizations/service agencies. Please see Attachments 11 and 12 for the informational letter and brochure for key informants, and Attachment 13 for the key informant screening protocol and Attachment 14 for the key informant interview protocol.
EMSI field interviewers will conduct a condensed five-item windshield survey (derived from the Neighborhood Attributes Inventory [NAI],) in the street segment immediately outside each participant’s house during the baseline home assessment (please see Attachment 15 for the modified windshield survey). The Battelle community liaison will also conduct limited assessments of the physical activity and nutritional environment within each community during the baseline visit. These assessments will be conducted in up to four schools (two elementary and two middle) per community including: (1) completing an observation form on the school’s lunch period; (2) requesting food service personnel to complete a self-administered questionnaire; (3) conducting the Physical Activity Resource Assessment (PARA); and (4) a brief interview with a Physical Education (PE) instructor. Please refer to Attachments 15, 16 and 17 for the community and school observations protocol, the school lunch personnel protocol and the physical education instructor protocol respectively.
2) Enhanced Protocol
a) Child/Parent Enhanced Protocol: Approximately 17% of children within each community (i.e., one in six children, one child for every age from 3-15) will receive an Enhanced Protocol that includes all the Standard Protocol measures plus more detailed measures of diet (i.e., two 24-hour dietary recalls) and physical activity (i.e., wearing an accelerometer during waking hours for one week and completing the Physical Activity Behavior Recall instrument).
b) Community Enhanced Protocol: EMSI field interviewers will conduct the full NAI for these participants (please see Attachment 15 for the full windshield survey).
Research Questions: We designed the HCS to address a variety of research questions that are both cross-sectional and longitudinal in nature. The main outcome variables of interest are BMI, diet, and physical activity behaviors in children. We expect to answer questions about how these variables are related to aspects of community programs and policies, which can be grouped into four broad areas: (a) intensity, (b) specific attributes, such as duration, funding, and target population, (c) combinations of programs and policies, and (d) factors that modify or mediate associations with the outcome variables of interest.
These research questions can be answered with both cross-sectional and longitudinal data. Cross-sectional analyses can examine the association of community programs and policies with BMI, diet, and physical activity at a single point in time on a large, nationally representative sample of children using measured height and weight to calculate BMI. In longitudinal analyses, BMI trajectories can be modeled as a function of the intensity of community programs and policies within each community over the same period. Analyses also can be conducted on two time points of diet and physical activity data to detect relationships between programs and policies and changes in diet and physical activity behaviors. Both cross-sectional and longitudinal analyses can explore which attributes or combinations of programs and policies are most strongly associated with BMI, diet, and physical activity among children, and if these associations are modified or mediated by community, family, or child factors.
Examples of the primary HCS research questions are provided below:
A. Research Questions Related to Community Programs/Policies
1. What intensity of community programs/policies is associated with BMI, diet, and physical activity behaviors among children? (Cross-sectional)
2. Are changes in intensity of community programs/policies associated with changes in BMI, diet, and physical activity behaviors among children? (Longitudinal)
Answers to these questions can lead to a better understanding of how the intensity of community programs and policies targeting childhood obesity is associated with lower BMI, as well as protective diet and physical activity behaviors among children.
B. Research Questions Related to Specific Attributes of Community Programs/Policies
What attributes of community programs/policies are most associated with BMI, diet, and physical activity among children? (Cross-sectional)
For example, which of the following community program/policy attributes has the strongest association with childhood BMI: community program/policy duration, funding, or target population (e.g., targeting at-risk youth versus the general population)?
What attributes of community programs/policies are most associated with changes in BMI, diet, and physical activity? (Longitudinal)
Answers to these questions can help provide insights into which specific attributes of community programs/policies are most essential in lowering childhood obesity.
C. Research Questions Related to Specific Combinations of Community Programs/Policies
What combinations of community programs/policies are associated with BMI, diet, and physical activity among children? (Cross-sectional)
What combinations of community programs/policies are associated with changes in BMI, diet, and physical activity among children? (Longitudinal)
Answers to these questions will help address whether combinations of programs/policies – such as enhanced school programs in conjunction with expanded parks and recreational opportunities – have a stronger association with BMI, diet, and physical activity than one particular program.
D. Research questions related to Factors that Modify or Mediate Associations
What factors modify or mediate associations between community programs/policies and BMI, diet, and physical activity among children? (Cross-sectional)
For example, do community and family socio-demographic characteristics modify the associations between community programs/polices and BMI, diet, and physical activity?
Are community programs/policies that are associated with a lower BMI and protective diet and physical activity behaviors in children mediated through parent support for healthy eating and physical activity?
What factors modify or mediate associations between community programs/policies and changes in BMI, diet, and physical activity among children? (Longitudinal)
Answers to these questions will help address whether factors such as greater availability of healthy foods and less availability of unhealthy foods at schools or the presence of parks and walking paths in a community modify the association between community programs/polices and BMI, diet, and physical activity among children.
Identifying Best Practices in Preventing Childhood Obesity Will Be A Major Benefit of the Healthy Communities Study Results. Previously funded community efforts to prevent childhood obesity include both single-component interventions, such as reducing the price of fruits and vegetables, and multi-component interventions, such as Shape Up Somerville, to affect changes in adiposity, dietary intake, and/or physical activity.11,12,13,14 The CDC and the Institute of Medicine (IOM) have published recommendations for communities (e.g., the IOM reports on Preventing Childhood Obesity15 and the CDC Recommended Community Strategies and Measurements to Prevent Obesity in the United States16).
These national organizations have acknowledged, however, that the evidence base is relatively weak, and more data are urgently needed to develop stronger evidence-based recommendations. Few studies with robust designs exist that have examined change in adiposity as an outcome, such as is planned for the HCS. Studies that examine dietary and/or physical activity behavior change as outcomes are more numerous, but many are methodologically inconsistent or weak, given the difficulties inherent in measuring these behaviors.17,18
While many programs/policies are funded through national, state, or local initiatives, and have an evaluation component, these evaluations are variable in design and rigor. Documentation of the range and extent of efforts in which communities are investing to prevent childhood obesity is limited, and large-scale systematic evaluation using objective data, such as measured height and weight, do not exist. Comprehensive multi-component programs have the potential to make the greatest impact, but these have not been systematically examined. In addition, no studies have conducted analyses to determine the relative contribution of the various intervention components to the measured outcomes. Further, very few studies have examined the extent of implementation of various program and policy models and components, contextual factors that influence long-term impact, program sustainability, feasibility, or potential for widespread dissemination.
The study of the relative impact of different programs and policies across research studies has been limited by methodological differences and by differences in the study populations and locations. Very little is known about the minimum program/policy intensity or combination of approaches needed for measurable impact. In addition, little is known about the community factors and processes that are important to enable effective implementation and sustainability of promising program/policy approaches. The HCS will begin to investigate these questions by examining common themes across community programs and policies and linking them to obesity-related outcomes using a study design that maximizes data collected retrospectively and prospectively for 279 communities across the country and more than 23,000 children 3-15 years of age (at baseline).
Government Agencies Will Use the Healthy Communities Study Results: Results from the HCS will address the associations between community programs/policies targeting childhood obesity and children’s BMI, diet, and physical activity. This information may influence decisions by federal, state, and local governments and organizations charged with improving children’s health (including the NIH, CDC, United States Department of Agriculture (USDA), and all state health departments across the U.S.), specifically how they develop and fund future policies and programs to reduce childhood obesity. Furthermore, HCS results will be published in scientific journals, meetings, and will be used for the development of future research initiatives targeting childhood obesity.
A.3 Use of Information Technology and Burden Reduction
Tracking System Software
The HCS will use a state-of-the-art system for data collection and management that maximizes data accuracy and minimizes participant burden. The tracking system software, PRECISE, has been developed for use in another large study (the NIH-funded National Children’s Study) and will be adapted for use by the HCS for its Information Management System (IMS). This IMS system will provide our team with the ability to recruit and track study participants throughout all years and waves of the study.
This software is designed to track and manage the recruiting activities that are part of the study, field visits and, follow-up visits and will facilitate data transmissions from the field. The software has been tested for efficient operations, accuracy in data collection, and compliance with the Federal Information Security Management Act (FISMA) of 2002. It has also been found to work well with the data collection software, DatStat Illume, that will be used to develop the survey instruments and data collection forms. Illume will work seamlessly with the IMS tracking software so that all recruitment, field management and data collection operations are accomplished efficiently.
Child/Parent Recruitment and Screening – One of the primary recruiting methods is through attempted telephone contact with the households using CATI (Computer Assisted Telephone Interview) screener. Contact information will be loaded into the database for a randomly selected sample of potentially eligible participants obtained from InfoUSA for households with addresses in the designated communities.
CATI screening calls to each household will be tracked using an automated tracking system. This system will provide the pre-loaded initial contact information from InfoUSA to the phone interviewer along with any additional information collected during the course of the phone call. The system will automatically track the number of calls to a specific household and records the outcome for each call attempt. The telephone interviewer will be able to see when the number of call attempts has reached a predetermined limit with no result, and can remove the number from the phone queue so that no household will be overburdened with phone calls and to contain recruiting costs. Screening information collected in an electronic CATI instrument will be stored in a central database that is located in the Battelle Information Security and Compliance (BISC) center. The screening process is designed to exclude ineligible households quickly without making unnecessary use of the respondent’s time. Once the household is determined to match eligibility criteria, the gender and date of birth of the children in the household are collected and matched with open cells for each gender and age. The system randomly selects a child for participation in the study if more than one child is eligible. Once a child is selected, if the parent agrees to participate, the telephone interviewer collects information about the household, including address, alternate phone numbers, parent’s name, and other information that can be used by the EMSI field interviewer at the home visit. In the event that a person is partially screened, the telephone interviewer will record a good time to call the household back to schedule the next call. The case will come up for the telephone interviewer to call at the agreed upon time so the respondent is prepared for the call and the call is not placed at inappropriate times.
A study website will be created to provide general information on the study, and allow the randomly-selected participants to indicate their interest in joining the study. This site will provide a lay-language study overview, a description of the roles for children and parents, a study timeline, answers to frequently asked questions, study contact information, and links to other relevant resources. A webpage will be available for potential participants who received the advance mailing to enter their contact information and answer broad screening questions (see Attachment 6 for a screenshot of the draft screening webpage). This webpage can only be accessed using a unique study code and password provided in the advance mailing to the household, ensuring that only invited participants can access the screening webpage. Furthermore, the use of a unique study code expedites the process of matching infoUSA data on the household with the screening information provided by the adult via the website. Information entered into these pages will be provided to the telephone interviewers for follow-up contact. This method of applying for the study will conserve recruiting costs by identifying and screening potential participants without the expense of personal contact, and entry of contact information by the telephone interviewers. Additionally, self-entering will ensure that contact information is accurate. All web pages will be §508 compliant for access by users with disabilities.
Scheduling Household Visits – Information about screened households is available to an EMSI staff supervisor, who then schedules household visits and assigns a field interviewer for that visit. The appointments are recorded in the central database, and then transmitted via secure web services to the laptop personal computer (PC) of the field interviewer, who is assigned to call the household prior to the visit to confirm the appointments and conduct the visit. By scheduling household visits using an automated system, overbooking, missed appointments and unbalanced case assignments are avoided. For the participant, this allows for on-time appointments and the convenience of scheduling at suitable times.
Child/Parent Interviews during Home Visit: EMSI field interviewers will arrive at the household visits equipped with laptop PCs that have Internet access via broadband cards. The IMS is accessible on the laptops via Internet access or locally using a remote data collection (RDC) component, in case there is no signal available to connect to the Internet. When that occurs, records are transmitted to the server and removed from the laptop as soon as Internet access is available. This eliminates the necessity of using paper forms when the Internet is not available. The EMSI field interviewer will use the field tracking system to view and record information about the household and prior household visits, which allows the visit to be conducted more efficiently. Redundant data collection will not occur because data collected to date is available to the interviewer. Information recorded at the visit is directly sent to the main database allowing real-time reporting and case management.
At the initial household visit the interviewer will administer the questionnaire from the laptop. None of the questionnaires will be available until the field interviewer has checked that the correct consent(s)/assent have been signed by all participants. Portions of the interview will be self-administered to parents and children and other sections will be conducted by the field interviewer. The IMS will only show the survey sections that are appropriate to the visit; for example, if the child is younger than 12 years old, self-administered child questionnaires that are only given to children aged 12 or older will not be shown. The survey sections will be provided in the IMS so that they can be administered in an order that is most convenient for the household. For example, if the required sections are complete and the child cannot stay for the full visit, the child interviews can be administered before the parent interviews. The anthropometric measurements will be recorded on a hard copy form by the interviewer and key-entered into the IMS prior to leaving the household; hard copy forms of the windshield surveys will also be completed, and will be entered into the IMS at a later time after the visit has been completed.
As previously noted, one in six respondents will be randomly selected to receive more detailed physical activity and nutrition measures (i.e., the Enhanced Protocol). The children will be asked to wear an accelerometer during waking hours for one week to record the child’s movement with minimal burden on the child or the parent. Estimates of dietary intake of the children will also be obtained, using the National Cancer Institute (NCI) Automated Self-Administered 24-hour Recall (ASA24 TM). Although the ASA 24 was intended for self-administration, the EMSI field interviewer will administer it because experience to date suggests that higher response and completion rates are obtained with children if the instrument is interviewer-administered (personal communication).19 The interviewer will use the laptop and broadband card to access the ASA 24 program website during the home visit. This mode of data recording will create fewer burdens on the respondent than by paper-based means because:
this will only require minimal instructions as the interviewer will be administering the instrument,
there are built-in visual images to select portion sizes,
participants will only be required to recall food and portion sizes consumed during a short time frame (the past 24 hours), and
the data recording will also occur faster and more conveniently.
The EMSI field interviewer will return after approximately one week to upload data recorded from the accelerometer into the IMS and administer the ASA24 for a second time. When the accelerometer records are transferred to the server, they are processed into aggregate data that can be analyzed.
At each visit, the respondents are offered an incentive. The respondent will sign a paper receipt for the gift, but the incentive record is also recorded into the IMS for accurate tracking and to reduce the risk of overpayments, non-payments, or theft.
Child/Parent Follow-up Data Collection: Respondents in the first 200 Wave 2 communities will be requested to participate in a remote questionnaire-based follow-up data collection that will occur via web or CATI telephone call. A respondent can complete the web-based questionnaire at a time most convenient for them and, if the respondent is not able to complete the questionnaire, data will be saved and the respondent can complete it at a later time. Likewise, the CATI follow-up can be completed across multiple sessions without risk of data loss.
Additionally, for the 40 RIPA communities, information regarding follow-up visits and data collected is recorded in the electronic tracking system so that EMSI field interviewers can keep track of work remaining for a particular case. In-person follow-up visits are recorded using the same scheduler software as the initial visits to provide flexibility for the EMSI field interviewer and the household.
Key Informant Recruitment and Screening: Potential key informants that are to be screened are added to the system in one of two ways. Prior to entering a community, a list of potential key informants for that community will be compiled and entered into the IMS. During the recruitment call and during the interview, the key informant will be asked to provide contact information about others who are knowledgeable about community programs and policies targeting childhood obesity. The Battelle community liaison will then enter these contacts into the IMS as candidate key informants. When candidate key informants are added into the IMS they will either be linked to a community program/policy that has already been entered into the system, or a new community program/policy will be added to the IMS and the candidate key informant linked to it. If the program or policy has not yet been entered, the program/policy is both entered into the IMS and linked to the key informant.
The IMS will show a list of candidate key informants that have yet to be screened and, if the Battelle community liaison scheduled a screening call with that person, the date and time of the scheduled call is displayed. During the call, the Battelle community liaison can access scripts in the IMS to facilitate the screening and entry screens to assist in recording contact information about other candidate key informants. The Battelle community liaison will enter the outcome of each screening call into the IMS.
Scheduling Key Informant Interview: Once a key informant is successfully screened, the Battelle community liaison will schedule a time for an in-person visit. The visit appointments are entered in a calendar in the IMS that shows all scheduled visits for that community. If it is not possible to conduct an in-person visit, a telephone interview will be scheduled instead. The Battelle community liaison will collect the preferred contact method for confirming the appointment (letter or email) and will verify that this information is correctly entered in the IMS. After the screening call has ended, the IMS will generate a reminder email if that is the preferred contact method, or a confirmation letter showing the scheduled visit date and time. During the screening call, the Battelle community liaison will request documents pertaining to the program or policy that the key informant represents and record the names of the documents that will be provided by the key informant in the IMS.
Key Informant Interview: Prior to the interview, if any documents provided by the key informant are received, the Battelle community liaison will locate the program/policy record that is linked to the key informant and pre-enter information about the program/policy in the questionnaire. During the key informant interview, the Battelle community liaison will launch a questionnaire from the IMS that gathers information about the key informant, their community, their organization, and related programs/policies.
The IMS will show known physical activity and/or nutrition programs/policies that are already linked to this key informant and, if other information about the program/policy was pre-entered from documents received, it will be displayed in the program/policy questionnaire. The key informant will be asked about other programs, policies, and environmental changes in the physical activity and nutrition areas. If the key informant reports a new program/policy, it will be added to the system and a program/policy questionnaire will be administered. The Battelle community liaison will enter the name of any document provided during the course of the interview. Throughout the interview, the Battelle community liaison will ask for other candidate key informants and will enter as much information as is provided into the IMS, including contact information and related programs/policies. That candidate will be later considered for future recruiting.
At the end of the interview, the Battelle community liaison will request consent for follow-up interviews and give the key informant the incentive gift. The outcome of the interview, the response to the consent request, and information about the incentive gift are recorded in the IMS.
Key Informant Remote Follow-up: At the designated time, the key informant will be contacted via the best method recorded at recruitment and asked to complete a follow-up questionnaire either via web or CATI. If the key informant does not respond to the request, a CATI reminder call will be placed. The interviewer will give the key informant the option to complete the questionnaire at that time over the phone. The IMS will record the outcome of the reminder calls and, if the survey is completed over the web, the IMS will record this so that reminder calls are stopped.
A.4 Efforts to Identify Duplication and Use of Similar Information
The primary objective of the HCS is to assess the relationships between community programs/policies targeting childhood obesity and children’s BMI, diet, and physical activity. Although many programs and policies are funded or supported by national, state, and local initiatives, the evaluation components of these programs vary in design and rigor. Documentation concerning the range and extent of efforts in which communities are investing to prevent childhood obesity is limited and a nation-wide assessment of community-based programs and policies aimed at battling childhood obesity is lacking.
Large observational studies such as NHANES and the National Children’s Study (NCS) do not collect the same type of data that will be collected within the HCS. NHANES is a cross-sectional survey focusing on individuals, and it does not collect longitudinal data on diet, physical activity, height, and weight. The NCS is a longitudinal observational study focused on the growth, development, and health of children across the United States, following them from before birth until age 21 years. While the NCS will collect data on BMI, the main objective is not on childhood obesity and the researchers will not collect the level of detail on factors that are related to childhood obesity as the HCS. Importantly, neither NHANES nor the NCS collects information on community programs and policies related to childhood obesity or detailed community characteristics, both of which are key components of the HCS. Similarly, the evaluation conducted by the CDC for its Communities Putting Prevention to Work (CPPW) study also does not collect the same type and breadth of data as collected in the HCS. In summary, current ongoing studies do not include all the data needed to examine the relation of community programs and policies designed to reduce childhood obesity and children’s BMI, diet, and physical activity.
A.5 Impact on Small Businesses or Other Small Entities
Physicians constitute the primary small business potentially burdened by the HCS. Physician’s offices are requested to provide medical records on selected patients identified by the study. To observe the impact of targeted community programs on childhood obesity rates over time, medical records for the most recent ten years will be requested from the primary care providers (PCP) of those participants that have consented to allow access to the child’s medical record as indicated by providing a signed medical release form. From the medical charts we will abstract height and weight information (to calculate BMI) as well as any nutritional or physical activity related information that may be included in the record, and information on chronic medical conditions that may be related to obesity (such as diabetes) and associated medical prescriptions. Medical charts will be obtained from approximately 70% of the participants as it is anticipated that some participants will refuse to allow access to the child’s records. This information is collected only once at baseline.
Participating parents will provide the study with contact information for their child’s PCP (see Attachment 7 for the Medical Record Release Authorization form). EMSI data abstractors will obtain the medical charts by submitting a request form to the identified PCP. Estimated time required by the physician’s office to comply with the chart request is 10 minutes, which covers reading the request, locating the medical chart, and providing the appropriate sections to EMSI. The study’s budget includes payment of standard fees charged by PCP offices to perform this service. Refer to Attachment 19 for the medical record retrieval protocol.
Key informants from different sectors of the community, some of which may be small business entities, will be interviewed in each community. The key informants are identified in the pre-interview phase to have knowledge about community programs/policies related to nutrition, physical activity, and healthy weight of children. These key informants may include individuals from schools, health organizations/coalitions, local government, and non-profit, community organizations and service agencies. The key informants will be asked to provide electronic and/or hard copy materials on programs and policies in their community promoting physical activity, nutrition, and healthy weight among children and youth. Additionally, key informants will be interviewed either in person or over the phone using a scripted questionnaire of no more than 60 minutes duration. As needed, telephone follow-up calls will clarify responses or seek further information. The study will provide an incentive worth $10 to participating key informants to compensate them for their time. Although the amount is relatively small, we also expect that compliance with the information requests should be consistent with their organization’s mission.
This information collection will not have a significant impact on any of these small entities.
A.6 Consequence of Collecting the Information Less Frequently
Data within the HCS cannot be collected less frequently because information on children’s diet behaviors, physical activity, and BMI must be collected at a minimum of two time points in order to assess the relationship between programs and policies targeting childhood obesity and changes in diet, physical activity, and BMI. The remote follow-up assessment in the 200 Wave 2 communities, either one year or two years after baseline, allows us to capture another data point of diet and physical activity behaviors and documentation of programs and policies through key informants. In these 200 communities, we will be able to assess the relationship between programs/policies and changes in diet and/or physical activity either one year or two years after baseline assessment. In the 40 RIPA communities, we will collect an additional third time point of diet, physical activity and a second time point of in-home assessment of height and weight. These data from the RIPA communities will allow us to adjust the Standard Protocol measures for bias and error. Due to budgetary and logistical (i.e., staff and equipment) constraints, all 275 communities in Wave 2 cannot be sampled within a one- or two-year period and thus need to be sampled across three and a half years.
Collecting information less frequently than proposed would seriously compromise the study’s ability to assess the relationship between programs and policies and changes in diet, physical activity, and BMI.
A.7 Special Circumstances Relating to the Guidelines of 5 CFR 1320.5
The HCS will comply with the guidelines of 5 CFR 1320.5. The current protocol designed for the HCS does not include any special circumstance that would cause information collection to be conducted in a manner outside of the guidelines of 5 CFR 1320.5.
A.8 Comments in Response to the Federal Register Notice and Efforts to Consult Outside Agency
On June 17, 2011, pages 35452-3, the Federal Register published NHLBI’s notice. The Project Officer received three comments from the public:
The first comment stated there was no need to collect data from the Healthy Communities Study. The Project Office acknowledged receipt of the comment.
The Project Office believes that the HCS is a very important study. Many children in the U.S. are at a high risk of developing obesity, and consequently of developing chronic disease earlier in life than previous generations. A comprehensive assessment of community programs and policies to address this significant public health problem, which affects all segments of children in the U. S. population, falls within the NIH mission to “seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability.”
The second comment was submitted by the Director of an Early Head Start program who requested copies of proposed tools from the HCS to use for outcomes assessment in a model nutrition program. The Project Office acknowledged receipt of the comment.
Since the HCS is in the process of finalizing several aspects of the study, the Project Office determined that it was better to provide information on other sources that may have tools that may be immediately useful to the requestor. Information on the Measures Registry produced by the National Collaborative on Childhood Obesity Research and the National Institutes of Health We Can! (Ways to Enhance Children’s Activity & Nutrition) program was provided to the requestor.
The Project Office was contacted twice by a representative of an Asian & Pacific Islander advocacy group. The initial request was for a copy of the data collection plans for the study. The Project Office acknowledged the request, and provided an overview of the study, as some of the study documents were being finalized.
The Project Office received a follow-up email with a letter from the advocacy group. The HCS was encouraged to 1) adopt proposed data collection standards implemented in Section 4302 of the Affordable Care Act (ACA), 2) oversample Asian Americans (AA) and Native Hawaiian and Pacific Islander (NHPI) subgroups, 3) assess the primary language of the participants, and 4) administer the HCS interview in the language of the participant.
The Project Office responded to the advocacy group by thanking them for their thoughtful comments and providing responses to their suggestions. The HCS project office responded that 1) it will collect granular data ethnic minority categories similar to those described in Section 4302 of the ACA, 2) ethnic minorities will be oversampled in the study, 3) the primary language spoken at home will be assessed, and 4) efforts will be made to conduct the interview in the language of the participant.
In 2008, members of the National Collaborative on Childhood Obesity (NCCOR) discussed the need to systematically study existing community efforts targeting childhood obesity. NCCOR is a public-private collaboration of four of the largest funders of childhood obesity research: the National Institutes of Health, Centers for Disease Control and Prevention, the Robert Wood Johnson Foundation, and the USDA. NCCOR’s mission is “to improve the efficiency, effectiveness, and application of childhood obesity research and to halt – and reverse – the childhood obesity trend through enhanced coordination and collaboration.” Members of NCCOR are part of the HCS Steering Committee and have contributed to the study design to ensure that it does not duplicate other ongoing efforts, but rather complements other childhood obesity efforts. NCCOR endorses this study and has written a letter of support (Attachment 20).
The scientific merit of the Healthy Communities Study was reviewed at many steps including the final review by the Advisory Council of the NHLBI. This study was approved by the NHLBI Advisory Council on October 21, 2008. The NHLBI Advisory Council is composed of non-government health professionals and provides final review of NHLBI Review (see Attachment 3).
A Healthy Communities Study Observational Study Monitoring Board (OSMB) will meet periodically to review the progress and to advise on study design, procedures, data analyses, and participant burden. The OSMB held its first meeting in August 2011, at which time reviewed the study protocol. OSMB members consist of six individuals with expertise in epidemiology, statistics, diet, physical activity, community measures, and childhood obesity.
The OSMB members are:
Shiriki Kumanyika, Ph.D., R.D., M.P.H. (OSMB Chair)
Associate Dean for Health Promotion & Disease Prevention
University of Pennsylvania School of Medicine
Center for Clinical Epidemiology & Biostatistics
Phone: 215-898-2629
Stephen R. Daniels, M.D., Ph.D.
Professor and Chairman
University of Colorado School of Medicine
Department of Pediatrics
The Children's Hospital
Phone: 720-777-2766
Henry A. Feldman, Ph.D.
Associate Professor of Pediatrics
Children's Hospital Boston
Clinical Research Program
Phone: 857-218-4713
Lawrence Green, Ph.D.
Adjunct Professor
University of California, San Francisco
Department of Epidemiology and Biostatistics
Phone: 415- 566-6178
Dianne Neumark-Sztainer, Ph.D., M.P.H., R.D.
Professor
University of Minnesota
Division of Epidemiology and Community Health
Phone: 612-624-0880
Gregory Welk, Ph.D.
Associate Professor, College of Human Sciences
Iowa State University
Department of Kinesiology
Phone: 515-294-3583
The HCS design and data collection components have been developed within several Committees and Subcommittees that began meeting regularly in the fall of 2010. The subcommittees include an Executive Committee; a Steering Committee; a Design and Data Analysis Subcommittee; a Nutrition Behaviors and Data Collection Subcommittee; a Physical Activity and Data Collection Subcommittee; a Community Measurement Subcommittee; a BMI and Medical Record Retrieval Subcommittee; a Public Image and Relations Subcommittee; a Publications, Presentations, and Ancillary Studies Subcommittee; and an Operations Committee. A listing of the subcommittee members is provided in Attachment 21.
A.9 Explanation of Any Payment of Gift to Respondents
Children and Family Members
Children and their family members will be provided an incentive for their participation in this study. It is anticipated that the Standard Protocol in-home visit will take on average 75 minutes to complete, while those participating in the Enhanced Protocol will require an additional 20 minutes during the first home visit, 35 minutes for the use of the accelerometer over a one week period, and another 50 minutes during the second home visit. The incentives will be explained to potential participants during recruitment over the telephone and as part of the informed consent process at the home visit. Proposed baseline incentives are based on both the age of the child and the level of participation (i.e., Standard Protocol or Enhanced Protocol). A family with a child aged three to 11 engaging only in the Standard Protocol will receive, at the completion of the assessment, an incentive worth $25 along with a small age-appropriate toy valued at $5. For Standard Protocol families with a child aged 12 and above, where the older child is expected to play a more active role in the interview, we will provide a $15 incentive for the family and a $15 incentive for the child. Families who agree to engage in the more involved Enhanced Protocol activity will receive an additional incentive in the form of a $50 money order at the time of the second home visit. For these families, at the time of consent, data collection staff will explain that the accelerometer has to be returned, and the PABR and ASA24 completed, to receive the additional incentive. These same incentives will be provided again to those families in the RIPA communities who complete a repeat of the baseline assessment three years later.
Family members who complete the remote data collection via web or telephone one or two years after the baseline assessment, estimated to take 35 minutes (average time allotted), will be mailed an incentive worth $10 once the data are provided.
Community Key Informants
Community key informants knowledgeable of community programs and polices targeting childhood obesity will be asked to provide information on programs/policies, such as supplying relevant program documentation and completing an in-person or telephone interview of not longer than 60 minutes. An incentive worth $10 will be given to the respondent as a token of appreciation after each data collection event (baseline, remote follow-up, and RIPA).
Medical Providers
Medical record abstraction from physician offices will be coordinated by EMSI. Though participating physicians will not be compensated directly, their offices may be paid per individual office policy regarding fees to cover costs associated with providing the requested information. EMSI, based on previous experience in this area, has developed estimates with respect to the proportion of medical offices that require a fee, in addition to the estimated range of fees. This amount has been built into the subcontract budget submitted by EMSI. No other form of compensation (financial or otherwise) will be provided to collect this type of data.
Compensation for participation in research studies, particularly of healthy subjects, is not new and is seen by most as fair and appropriate, even for participants of minor age. Monetary incentives for older children (who have a concept and appreciation for monetary compensation) and the provision of a small toy as an incentive for younger children (who lack this conceptual ability) have been found to be a common practice and appropriate in research studies involving children.20,21 In addition, an incentive has been found to encourage timely recruitment and continued participation by subjects (thus, improving response rates) in non-clinical studies.22
There is also clear and consistent evidence that monetary remuneration significantly increases response rates to mail, telephone, and face-to-face surveys, and experts on survey methods recommend their use .23,24 Church (1993)25 and Singer and colleagues (1999)26 have published meta analyses comparing the response rates of mail and interviewer-mediated surveys with and without monetary incentives. These studies have clearly shown that even a nominal gratuity increases response rates, and that the amount of the incentive is positively correlated with response rate .27,28,29,30 Previous research also suggests that monetary incentives may be especially effective in recruiting low-income and minority respondents. For example, analyses by Singer et al.31 indicate that a $5 incentive paid to a random half of households in a random digit dialed telephone survey brought a higher percentage of low-education respondents into the sample. For our national study, it will be important to include all sampled members of the selected communities, including low-income and minority households.
Finally, to serve as a comparison, several recent studies have provided a monetary incentive to respondents. For example, a CDC study entitled “Preventive Cardiac Health Care Knowledge, Beliefs, and Behaviors in Female Carriers of Duchenne/Becker Muscular Dystrophy” (OMB No. 0920-0718) provided $5 to each of 1,477 women who participated in a mail survey. Another study entitled “CDC’s Cervical Cancer Study (Cx3) – An Intervention Pilot Study of HPV in Illinois NBCCEDP” (OMB No. 0920-0814) provided a monthly incentive valued at $10 to clinic staff who completed a four-page survey each month for a year describing their clinic’s study participation. Additionally, a CDC study entitled the “Study to Explore Early Development (SEED)” (OMB No. 0920-0741) involved incentives to families with young children, many of which included children with autism or other developmental disabilities. In this longitudinal study, incentives ranged from $25 included in the enrollment packet, to $30 included in questionnaire packets, to $80 for clinic visits.
A.10 Assurance of Confidentiality Provided to Respondents
Confidentiality and Data Security: All HCS investigators and their institutions have agreed to comply with the Federal Privacy Act as part of their contractual agreement with NHLBI. The contract stipulates that research involving human subjects cannot be conducted until (1) the protocol has been approved by NHLBI; (2) written notice of such approval is provided by the Contracting Officer; and (3) completed Form HHS-596 certifying Institutional Review Board (IRB) review and approval of the protocol (Attachment 22).
All individuals participating in this study will be assured that the information they provide will not be released in a form that identifies individual respondents, unless required by law. No information will be reported by the contractor in any way that permits linkage to individual respondents. The study team is firmly committed to the principle that the confidentiality of participants’ data obtained from surveys and existing records must be protected. This principle is embedded throughout the process of gaining cooperation and obtaining approval. It holds whether or not any specific guarantee of confidentiality was given at the time of the data collection, or whether or not there are specific contractual obligations to the client. Confidentiality of participants’ data is an ethical responsibility of study staff and to ensure the confidentiality of participants’ data, ten specific confidentiality procedures are incorporated into each study. These procedures include:
All Battelle employees and subcontractors including office and data collection staff are required to sign an assurance of confidentiality. This assurance contains a listing of the organization's steps to maintain confidentiality and includes a pledge by employees and data collectors indicating that they will cooperate fully with these procedures. In addition, the data collectors' training manuals include a section on the ethics of data collection that stresses the importance of maintaining confidentiality. Additionally, EMSI’s corporate policy, which requires background checks of all its data collection staff, has recently been revised to include ongoing background checks every two years.
Unless specifically instructed otherwise for a particular project, employees are not allowed to abstract, collect or process data from a respondent whom they know personally.
Interviews are always to be conducted in the most private settings available. No individual other than the parent should be present in the room, or listening on the telephone, during an interview. While sensitive questions are being answered in the home interview by children twelve years and older onto a computer laptop, the EMSI field interviewer will be collecting other information from the parent/guardian, using this time, for example, to take the parents/caregivers anthropometric measures and/or distribute the incentive. As necessary, the EMSI field interviewer will engage the parent/guardian in conversation until the child completes this section.
Collected survey data, if gathered off-site, are mailed in separate envelopes from forms containing personal identifiers.
Survey data forms containing personal identifiers are kept in separate locked files or a locked room when not being used in routine survey activities. Forms with identifiers, such as face sheets, are kept separate from completed data collection forms or, if on data tape, from tapes with collected data.
Completed data collection forms are entered on the computer file without personal identifiers (that is, names, addresses, telephone numbers, social security numbers, etc.). On all data instruments, subjects are only identified by unique study identification numbers.
At the end of the survey performance period, the study manager arranges for proper storage or disposition of survey data depending on particular contractual requirements for storage or disposition.
The Battelle Institutional Review Board must approve all studies before any contact may be made with human subjects.
Reports and publications of collected data are presented in aggregate form only. The names or any other identifiers of participants are not made available to any person, group, or agency.
In addition, EMSI is committed to conducting business in compliance with all applicable laws, regulations, and customer requirements. Their written policies cover EMSI’s general approach to compliance with the security regulations used by the industry as best practices to (1) ensure the confidentiality, integrity and availability of all private and confidential information EMSI creates, receives, maintains or transmits; (2) protect against any reasonably anticipated threats or hazards to the security or integrity of such information; (3) protect against any reasonably anticipated uses or disclosures of such information that are not permitted or required; and (4) ensure compliance by its workforce. To enforce these policies, EMSI utilizes administrative, physical and technical safeguards.
Privacy Act: As stated above, in publications, the individual identities of participants are not disclosed, and data are reported only in the aggregate. Information obtained from the study will be included in the NIH Privacy Act Systems of Records Notice 09-25-0200, entitled, “Clinical, Basic and Population-based Research Studies of the National Institutes of Health (NIH), HHS/NIH/OD,” published in the Federal Register, Volume 67, No. 187, September 26, 2002 (Attachment 23).
Human Subjects Protection: The Battelle IRB has conducted a preliminary review and has approved this study for an expedited review. The initial IRB approval letter is included as Attachment 22.
A.11 Justification for Sensitive Questions
The HCS will collect sensitive information described briefly below along with a justification for inclusion in this study:
Maturity status of the child: The parent and child surveys collect information that may be considered sensitive by some respondents, including data concerning pubescent stage of the child. This information is required for the interpretation of changes in BMI, a critical component of the planned analyses.
Annual household income as an indicator of socioeconomic status (SES): Income has been related to obesity and, in most studies, children from families with a higher SES tend to have less obesity. Because family income may influence participation in childhood obesity programs, it is critical to collect.
Pregnancy status: Girls 12 and older will be asked about pregnancy status. Pregnancy-related weight gain needs to be accounted for in analyzing change in BMI.
As described in Section A.10 (Assurance of Confidentiality Provided to Respondents), appropriate measures to safeguard respondent privacy have been instituted. In addition, both child and adult respondents will be informed that they can decline to answer any question that they do not wish to answer.
A.12 Estimates of Annualized Burden Hours and Costs
The burden estimates shown in Table A.12.1 are for the first three years of data collection. Table A.12.1 is followed by a description of type of respondent, the annualized costs to respondents, and an explanation of how the time estimates in Table A.12.1 were determined.
Table A.12.1 Estimates for Annualized Hour Burden for Years 1-3 of Data Collection for the HCS*
Type of respondents |
Estimated number of respondents |
Estimated number of responses per respondent |
Average burden hours per response |
Estimated total annual burden hours requested |
Parents/Caregiver (screening) |
56,550 |
1 |
0.17 |
9,614.00 |
Parents/Caregivers |
9,908 |
1.46 |
1.14 |
11,295.00 |
Second Parents/Caregiver |
3,393 |
1 |
0.12 |
407.00 |
Parents/Caregiver who refuse to participate |
803 |
1 |
0.17 |
137.00 |
Children |
9,908 |
1.46 |
0.78 |
7,728.00 |
Key Informants (screening) |
1,607 |
1 |
0.08 |
129.00 |
Key Informants |
3,302 |
2.74 |
0.85 |
2,807.00 |
Food Service Personnel |
321 |
1 |
0.42 |
135.00 |
Physical Education Instructors |
321 |
1 |
0.25 |
80.0 |
State Health Department employees |
17 |
1 |
0.30 |
5.0 |
Physicians/medical secretaries |
4,750 |
1 |
0.17 |
808.00 |
TOTAL |
90,880 |
|
|
33,145 |
*The estimates in Table A.12.1 are based upon respondents in a sample of 241 communities for the first 3 years of data collection.
The following interview time estimates are based upon experience in prior studies using similar measures and pretests of the HCS questionnaires on fewer than nine individuals.
Parents/Caregivers screening time estimates: To meet recruitment goals, it is estimated that we will attempt to contact and screen 500-800 households in each non-RIPA community and 750-1200 in each RIPA community to find the eligible 78 and 117 study participants, respectively, who agree to participate. Therefore, on average 650 households will be screened for the 201 non-RIPA communities [(500+800)/2=650] and 975 households will be screened for the 40 RIPA communities [(750+1200)/2=975]. The total number of households screened in the first three years of data collection (169,650) is derived from the total for non-RIPA communities (650 households x 201 communities=130,650) plus the total for RIPA communities (975 households x 40 communities=39,000 households). Completion of the screening website and/or a brief telephone survey will take approximately ten minutes. Please refer to SSA Attachment 6.
Parents/Caregivers and Children time estimates: Of the 241 communities to be completed in the first three years, the four Wave 1 communities and 197 non-RIPA Wave 2 communities will have 78 child/parents pairs per community and the 40 RIPA Wave 2 communities will have 117 child/parents pairs per community. Thus, a total of 20,358 children and parents will be interviewed during the first three years of data collection. Of these 20,358 child/parent pairs, approximately 83% will be assigned to the Standard Protocol while 17% will be assigned to the Enhanced Protocol. We assumed that by the end of the first three years of data collection, 50% of the remote follow-up interviews (via telephone or web) will be completed, with an estimated 9,360 child/parent pairs contacted. Please refer to Attachment 7 for the parent/caregiver protocol. Please refer to Attachment 10 for the child protocol. Please see Attachment 24 for a table detailing these calculations.
Remote follow-up: Remote follow-up assessment will be completed in the first 200 Wave 2 communities; the duration of this phone or web survey will be approximately 35 minutes (average time allotted). The remote follow-up assessment will include the standard protocol questions with the exception of certain demographic questions (e.g. gender, language spoken at home) and height and weight will not be measured.
The frequency of response used to calculate burden (Table A.12.1) is an average based on the number of baseline visits and remote follow-up interviews that will occur during the first three years of data collection (i.e., some participants will have two assessments and others will only have one). Note that the second in-person visit in the RIPA communities will not occur within the first three years of data collection; thus, the burden associated with that visit is not included in the estimates above.
Second Parents/Caregivers time estimates: If the second parent/caregiver is present during the home visit, we will consent the second parent/caregiver and measure their height and weight. This component is anticipated to last an average of 7 minutes. Please refer to Attachment 8.
Parents/Caregivers ‘who refuse to participate’ time estimates: We will contact and interview parents whose children were eligible, but opted not to participate in the study. A ten-minute survey will be administered in ten randomly selected households among those that refused to participate in each community. Contact will be attempted with five "failed contact" and five "refusal" parents at the end of data collection in each community to assess potential bias due to non-response. The number of non-responders per community (n=10) was selected to provide sufficient data (pooled across all communities) to assess whether there are significant differences between responders and non-responders, and to make appropriate adjustments for such biases if they exist. Please refer to Attachment 9.
Key Informants screening time estimates: Approximately 10-15 key informants in each community will be selected to document the evolution of policies and programs aimed at reducing childhood obesity in their community. In each of the 241 communities, it is anticipated that approximately 20 potential key informants will need to be screened in order to identify 15 key informants that consent to take part in the study. Thus, a total of 4,820 (i.e., 20 x 241) key informants will be screened in the first three years of data collection. The screening call is anticipated to take approximately 5 minutes. Please refer to Attachment 13.
Key Informants time estimates: We anticipate that 3,615 key informants across the 241 communities (i.e., 15 x 241) will take part in the study. The key informants who consent to participate will complete a recruitment telephone call (estimated at 15 minutes), gather and provide documentation on their programs/policies (estimated at 30 minutes), and complete in-person or remote interviews (approximately 50 minutes). In addition, key informants in 100 communities will undergo a remote follow-up interview that is anticipated to take approximately 50 minutes. In the 40 RIPA communities, key informants will also be contacted on a quarterly basis to obtain updates or information on new programs and policies; these quarterly contacts (about eight during the first three years of data collection) are estimated at 15 minutes per contact (see Attachment 27 for details on the calculation of the key informant burden). Please refer to Attachment 14 for the key informant protocol.
Food Service Personnel time estimates: Food service personnel at two elementary and two middle schools in each of the 241 communities will be asked to complete a survey on the food served in the cafeteria and the lunch observation form. A total of 964 (i.e., 4 x 241) food service personnel will complete the self-administered survey and lunch observation form in the first three years, which, combined, are expected to take about 25 minutes. Please refer to Attachment 16.
Physical Education Instructors time estimates: The Physical Education (PE) instructor at two elementary and two middle schools in each of the 241 communities will be interviewed and may be asked to guide the community liaison on a brief walking tour of the school. The interview by the Battelle community liaison and brief walk is estimated to take a total of 15 minutes. A total of 964 (i.e., 4 x 241) PE instructors will complete survey questions. Please refer to Attachment 17.
State Health Department employees time estimates: One state health department employee in each State will be interviewed over the phone to identify 3-4 programs in their State that have the most promising programs and policies aimed at reducing childhood obesity. These experts lead chronic disease prevention programs or other programs related to reducing childhood obesity. The interviews are expected to last 18 minutes each. Please refer to Attachment 18.
Physicians (medical secretaries) time estimates: One medical primary care provider (PCP) per child will be contacted to request the child’s medical charts (for which consent was provided by the parent). Parents will provide the study with contact information for their child’s PCP. EMSI staff will obtain the medical charts by submitting a request form to the PCP. Estimated time required by the PCP’s office to comply with the chart request is 10 minutes, which covers reading the request, locating the medical chart, and providing the appropriate sections to EMSI. The study will reimburse standard fees charged by PCP offices to perform this service. Please refer to Attachment 19.
We anticipate that EMSI will be able to obtain the medical records for only 70% of the 20,358 children (n=14,251), due to parental refusal to consent to release the medical record or difficulty in locating the medical office or the child's medical record.
The estimated annualized costs to the respondents of the study are shown in Table A.12.2. There are no direct costs to the respondents other than their time to participate. We assumed an hourly rate for the participants equal to the 2009 U.S. median hourly rate across all job categories and states (http://www.bls.gov/oes/highlight_2009.htm). Mean hourly wages for federal, state, and local jobs in the overall category of community and social services occupations were averaged to
obtain a mean hourly wage for the key informant respondent. These wage estimates were taken from the Bureau of Labor and Statistics May 2009 National Industry-Specific Occupational Employment and Wage Estimates (http://www.bls.gov/oes/current/oessrci.htm#99). Using these estimates, the total annualized cost to all respondents for the study is $434,815.
Table A.12.2 Annualized Cost to Respondents for Years 1-3 of Data Collection for the HCS*
Type of respondents |
Number of Respondents |
Frequency of Response |
Average Time per Respondents |
Hourly Wage Rate |
Respondent Cost* |
Parents/Caregivers (screening) |
169,650 |
1 |
0.17 |
$15.95 |
$153,343 |
Parents/Caregivers |
20,358 |
1.46 |
1.14 |
$15.95 |
$180,155 |
Second Parents/Caregivers |
10,179 |
1 |
0.12 |
$15.95 |
$6,492 |
Parents/Caregivers who refuse to participate |
2,410 |
1 |
0.17 |
$15.95 |
$2,185 |
Children |
20,358 |
1.46 |
0.78 |
N/A |
|
Key Informants (screening) |
4,820 |
1 |
0.08 |
$25.96 |
$3,349 |
Key Informants |
3,615 |
2.74 |
0.85 |
$25.96 |
$72,844 |
Food Service Personnel |
964 |
1 |
0.42 |
$15.95 |
$2,153 |
Physical Education Instructors |
964 |
1 |
0.25 |
$15.95 |
$1,276 |
State Health Department employees |
50 |
1 |
0.30 |
$25.96 |
$130 |
Physicians/medical secretaries |
14,251 |
1 |
0.17 |
$15.95 |
$12,888 |
TOTAL |
247,619 |
|
|
|
$434,815 |
**The estimates in Table A.12.2 are based upon respondents in a sample of 241 communities for the first 3 years of data collection. The reported and calculated numbers differ slightly due to rounding.
Over the five-year study, a total of 279 communities will be sampled with 78 child/parent pairs recruited from each of 239 non-RIPA communities and 117 child/parent pairs recruited from 40 RIPA communities. During the first three years of data collection, it is estimated that baseline visits will be completed in 241 communities, including the four Wave 1 communities plus 237 Wave 2 communities (see Table A.12.3).
Table A.12.3 Communities Visited During the First Three Years of Data Collection
Community |
Year 1 |
Year 2 |
Year 3 |
Total in First 3 Years of Data Collection |
of Data Collection |
||||
Wave 1 Communities (N=4) |
||||
Non-RIPA (No Remote FU) |
4 |
--- |
--- |
4 |
Wave 2 Communities (N=275) |
||||
RIPA |
40 |
--- |
--- |
40 |
Non-RIPA (Remote FU 1 or 2 years after baseline) |
10 |
100 |
50 |
160 |
Non-RIPA (No Remote FU) |
0 |
0 |
37 |
37 |
TOTAL |
54 |
100 |
87 |
241 |
|
|
|
|
|
Remote Follow-up Communities* |
0 |
0 |
100 |
100 |
*Remote follow-up assessments will be completed for an estimated 100 Wave 2 communities in the third year of data collection, including 50 communities two years after their baseline visit and 50 communities one year after their baseline visit.
Given that older children will be able to complete more of the interview on their own than younger children, burden for parents and children will vary depending on the age of the child. Thus, the average time per response used to calculate burden (Table A.12.1) does not represent any one participant’s average response time, but rather, an average of response times over all ages. Table A.12.4, below, shows the variation in child and parent/caregiver involvement on average (in minutes) by protocol type (Standard and Enhanced) and age of the child. Attachment 25 provides the detail for the burden by protocol type, child age, and respondent (child and parent).
Table A.12.4 Variation in Child and Parent/Caregiver Involvement in Data Collection Activities (in minutes) by Protocol Type and Age of Child
PROTOCOL TYPE |
TOTAL TIME (minutes) |
||||
AGE OF CHILD |
AVERAGE TIME PER RESPONSE* |
||||
3-5 YEARS |
6-8 YEARS |
9-11 YEARS |
12-15 YEARS |
||
Standard Protocol, Parent/Caregiver: |
75.5 |
81.5 |
81.5 |
51.0 |
70.7 |
Standard Protocol, Child: |
9.0 |
42.8 |
49.5 |
54.5 |
40.2 |
Enhanced Protocol**, Parent/Caregiver: |
190.5 |
201.5 |
201.5 |
61.0 |
155.7 |
Enhanced Protocol**, Child: |
49.0 |
159.8 |
166.5 |
171.5 |
139.4 |
Remote Follow Up, Parent/Caregiver: |
38.0 |
44.0 |
44.0 |
13.5 |
33.2 |
Remote Follow Up, Child: |
0.0 |
30.5 |
30.5 |
35.5 |
25.0 |
*The average time per response is calculated using the weighted average based upon the proportion of children in each age range.
**Please note that the Enhanced Protocol calculation includes the burden for the first visit to the home, the use of the accelerometer over a 7-day period, and the second visit to the home.
Table A.12.5 and Table A.12.6 provide an overview of the components of the Standard and Enhanced Protocols, respectively, with the estimated duration of each component for the baseline visits. Note that some components will occur simultaneously during the home visit and others will occur less frequently (e.g., not all households will have both parents/caregivers available or willing to allow their height/weight to be measured). Therefore, the aggregated time for all protocol components is higher than the total average time allotted for the Standard and Enhanced baseline visits.
Standard Protocol time estimates for children/parents: The Standard Protocol, which includes an in-home assessment to measure height and weight and complete questions on physical activity and diet, is anticipated to take on average 75 minutes (Table A.12.5).
Table A.12.5 Estimated Duration of Each Standard Protocol Component for the Baseline Visit
Protocol Type |
Visit Component |
Sub-components |
Estimated Duration |
|
Minutes |
Hour |
|||
Standard Protocol |
Household Visit |
|||
Consent |
Parent/Caregiver Consent |
15 |
0.25 |
|
Medical Record Release |
||||
Child Assent (≥ 8) |
10 |
0.17 |
||
Survey: Socio-Demographic, Background, Exposure |
Community P/P Exposure |
15.5 |
0.26 |
|
Demographic/Socio-Economic |
||||
Child birth details |
||||
Medical Insurance/History |
||||
Child Behaviors |
||||
Survey: Physical Activity (PA) |
PA Parent Survey |
21 |
0.35 |
|
PA Behavior Recall (PABR) |
||||
PA Child Survey |
||||
Survey: Nutrition Behaviors |
Domains 1-10 |
18.5 |
0.31 |
|
Anthropometrics |
Parent/Caregiver 1 |
3.5 |
0.06 |
|
Parent/Caregiver 2 Consent & Measurements |
7 |
0.12 |
||
Child (Height/Weight/Girth) |
6 |
0.10 |
||
Distribute Incentive |
3 |
0.05 |
||
TOTAL ESTIMATED DURATION OF ALL PROTOCOL COMPONENTS: |
99.5 |
1.66 |
||
|
||||
AVERAGE TIME ALLOTTED FOR STANDARD PROTOCOL BASELINE**: |
75 |
1.25 |
||
**NOTE: Some protocol components will occur simultaneously, while others will occur less frequently (e.g., not all households will have both parents/caregivers available or willing to allow their height/weight to be taken); therefore the aggregated time for all individual protocol components (99.5 minutes) is higher than the total average time allotted for the standard protocol baseline (75 minutes). |
||||
Enhanced Protocol time estimates for children/parents: The Enhanced Protocol will include the following in addition to the Standard Protocol measures: (1) wearing accelerometers for one week to objectively assess physical activity; (2) two 24-hour dietary recalls (via the ASA24) to assess dietary intake in more detail; and, (3) the Physical Activity Behavior Recall (PABR) instrument.
Participants will wear the accelerometer during waking hours for seven consecutive days on the waist using a belt. The ASA24 will be administered to capture foods and beverages consumed during a 24-hour period. For children younger than six, the parent/caregiver will be asked to complete the recall; children aged between six and 11 years will complete the recall with the assistance of the parent/caregiver; and children aged 12 and over will complete the recall with input from the parent/caregiver if required. The ASA24 will take approximately 30 minutes to complete at each visit. The first dietary recall will be administered during the first home visit (when the accelerometer is distributed) and the second will be conducted approximately one week later during the second home visit (when the accelerometer is retrieved). At the second visit, a physical activity behavior recall (PABR) will also be completed to collect detailed information regarding a child’s participation in specific forms of activity on the previous day. For each of these activities, the PABR captures the intensity at which the child did the activity, the time spent in the activity, where he/she did the activity, who the child did the activity with, and the specific form or type of activity performed. Children aged six or older will complete the PABR either with the help of their parents/caregiver or unaided, while a modified version of the PABR instrument will be completed by parents/caregivers for children aged 3-5 years.
We anticipate the duration of the first and second home visit to be on average 145 minutes, with an additional 35 minutes for the use of the accelerometer during the week between home visits (Table A.12.6). The detailed child and parent burden calculations for the baseline and remote follow up assessments are provided in Attachment 26.
Table A.12.6 Estimated Duration of Each Enhanced Protocol Component for the Baseline Visit
Protocol Type |
Visit Component |
Sub-components |
Estimated Duration |
|
Minutes |
Hour |
|||
Enhanced Protocol (components included in Standard Protocol) |
Household Visit 1 |
|
|
|
Consent |
Parent/Caregiver Consent |
15 |
0.25 |
|
Medical Record Release |
||||
Child Assent (≥ 8) |
10 |
0.17 |
||
Survey: Socio-Demographic, Background, Exposure |
Community P/P Exposure |
15.5 |
0.26 |
|
Demographic/Socio-Economic |
||||
Child birth details |
||||
Medical Insurance/History |
||||
Child Behaviors |
||||
Survey: Physical Activity (PA) |
PA Parent Survey |
21 |
0.35 |
|
PA Behavior Recall (PABR) |
||||
PA Child Survey |
||||
Survey: Nutrition Behaviors |
Domains 1-10 |
18.5 |
0.31 |
|
Anthropometrics |
Parent/Caregiver 1 |
3.5 |
0.06 |
|
Parent/Caregiver 2 Consent & Measurements |
7 |
0.12 |
||
Child (Height/Weight/Girth) |
6 |
0.10 |
||
Distribute Incentive |
3 |
0.05 |
||
Enhanced Protocol (components not included in Standard Protocol) |
Accelerometer Initiation |
5 |
0.08 |
|
Dietary 24 hour Recall (ASA24) |
30 |
0.50 |
||
|
||||
Total Average Time Allotted for Enhanced Protocol Visit 1**: |
95 |
1.58 |
||
During Week in Between Visits |
|
|
||
Use of Accelerometer for 1 Week |
35 |
0.58 |
||
Household Visit 2 |
|
|
||
Distribute Second Incentive |
2 |
0.03 |
||
Collect Accelerometer |
3 |
0.05 |
||
Dietary 24 hour Recall (ASA24) |
30 |
0.50 |
||
Physical Activity Behavior Recall (PABR) |
15 |
0.25 |
||
|
||||
Total Average Time Allotted for Enhanced Protocol Visit 2**: |
50 |
0.83 |
||
TOTAL ESTIMATED DURATION OF ALL PROTOCOL COMPONENTS: |
219.5 |
3.66 |
||
|
||||
AVERAGE TIME ALLOTTED FOR ENHANCED PROTOCOL BASELINE**: |
180 |
3.00 |
||
**NOTE: Some protocol components will occur simultaneously, while others will occur less frequently (e.g., not all households will have both parents/caregivers available or willing to allow their height/weight to be taken); therefore the aggregated time for all individual protocol components (219.5 minutes) is higher than the total average time allotted for the enhanced protocol baseline (180 minutes). |
||||
A.13 Estimate of Other Total Annual Cost Burden to Respondents and Recordkeepers
There are no direct costs to record keepers or respondents other than their time to participate.
A.14 Annualized Cost to the Federal Government
The annualized cost of monitoring the project by NHLBI is estimated at $109,000. The average annualized cost (contracts and monitoring by NHLBI) to the U.S. Government for information collection is $6,377,000. This information is itemized in the Table A.14.1.
Table A.14.1 Estimate of Annualized Cost to the Government (in Thousands)
Type of Cost |
Contract |
Other |
Total |
Study Mgmt & Operations |
$5,948 |
$320 |
$6,268 |
Monitoring |
|
$109 |
$109 |
Total |
$5,948 |
$429 |
$6,377 |
A.15 Explanation for Program Changes or Adjustments
This study represents a new collection of information.
A.16 Plans for Tabulation and Publication and Project Time Schedule
The Healthy Communities Study will collect data after obtaining OMB approval. Battelle staff will analyze the data in a timely manner after the necessary data cleaning has been done and after data quality control procedures have been verified.
Overall Data Tabulation and Publication Plans: We have formed a Presentation, Publications, and Ancillary Studies (PPAS) Subcommittee that will oversee and direct the manner in which data will be tabulated and presented at lay and scientific sessions and submitted for publication in peer-reviewed journals. A series of publications are being planned to present and announce the plans, progress, and findings of the study. These publications will fall into three broad areas of results:
Publications documenting the hierarchical nature and key design elements of the study design;
Publications from the Wave 1 data collection for the study; data collected here will provide insights to the level of success implementing the various aspects of the study protocol in a selected number of communities; and
Publications reflecting on the overall goal of finding and reporting on the association between community programs/policies and BMI, diet, and physical activity outcomes in children and determining what is working at the community level that has an impact on reducing the prevalence of obesity among children.
General Statistical Analysis Plans: The general analysis approach will include production of various summary tabulations, as well as statistical modeling to evaluate program/policy characteristics most associated with reductions in childhood obesity rates. Cross-tabulations will summarize data by a number of different program/policy characteristics, including type of organization, years of funding, level of funding, community average household income, gender/race/ethnicity distribution, etc. We will design a core set of tabulations that will be updated on a regular basis throughout the study (e.g., on a quarterly basis). The content of this set of tabulations will be dynamic, with new summaries added as new analysis ideas arise and summaries dropped if they are not providing valuable insights. The statistical analysis plan also will specify the regression models planned to identify program and community characteristics associated with significant reduction in childhood obesity rates.
Our sampling approach builds on well-developed statistical methodology, and integrates the concepts of epidemiological study design and data analysis with missing covariates that our team developed for the National Children’s Study (NCS), and which are being pursued by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), CDC, and EPA. As stated earlier, our approach relies on nested stages of sampling, where each successive stage of sampling uses a subset of study participants selected in the previous stage.
Our design allows us to generate longitudinal BMI trajectories on a random sample of children within each community. These trajectories can be modeled as a function of the time-series of standardized community scores that we construct to rate the strength of obesity prevention/treatment programs and policies within each community. We will be able to capture a series of age-specific cohorts across the sample of communities involved in the study with sufficient sample size in each cohort to ascertain the association of different strategies to trends in childhood obesity.
Importance of Dissemination of Findings: Sharing of study objectives and plans allows potential and current participants to learn more about the project that they are either considering becoming involved in, or in which they have already enrolled. Providing this information should lead to higher participation rates. Likewise, sharing of results allows important findings to be transmitted to various stakeholder groups and programs around the country.
NHLBI Technical
Following approval of all plans and reports by NHLBI and any peer reviewers, we will post these approved materials on a public Website with communication to NHLBI and funding partners and all community programs that have won federal grants related to childhood obesity, and to local and state health departments. We will review the activity of users coming to the Website each month. Based on the reports of Website usage, Battelle will plan further ways to make the Website most effective in the distribution of study information and results. Further, results from the HCS will be published in appropriate scientific journals, presented at scientific meetings, and will be used for the development of future research initiatives and creation of opportunities targeting childhood obesity.
Major Timeline of Milestones for the Project:
Months 1 to 15 – Study design and protocol development, Manual of Operations, field work planning, database development, Institutional Review Board approval, Office of Management and Budget Information Collection Request clearance
Months 16 to 17 – Wave 1 of Data Collection (assessment of the four Wave 1 communities), evaluate results, prepare draft of Wave 1 results, and finalize study protocols
Months 18 to 29 – Year 1 of Data Collection (baseline assessment of 100 Wave 2 communities which includes the 40 RIPA communities); ongoing data management, data analysis, and reporting; Prepare Year 1 Publication of Results
Months 30 to 41 – Year 2 of Data Collection (baseline assessment of 100 Wave 2 communities); ongoing data management, data analysis, and reporting; Prepare Year 2 Publication of Results
Months 42 to 53 – Year 3 of Data Collection (assessment of 75 Wave 2 communities and remote follow-up assessment of first 200 Wave 2 communities); ongoing data management, data analysis, and reporting; Prepare Year 3 Publication of Results
Month 54 to 56 – Year 4 of Data Collection (repeat of baseline assessment at the 40 RIPA communities); Prepare Year 4 Publication of Results
Month 57 to 60 – Finalize study database, conduct final data analyses and prepare final report, prepare Overall Summary Publication of Findings for the Study
A.17 Reason(s) Display of OMB Expiration Date is Inappropriate
There is no need to not display the expiration date for OMB approval of the information collection.
A.18 Exceptions to Certification for Paperwork Reduction Act Submissions
No exceptions to the Certification for Paperwork Reduction Act Submissions are sought.
1 Committee on Environmental Health. Policy Statement: The Built Environment: Designing Communities to Promote Physical Activity in Children. Pediatrics. 2009; 123(6):1591-1598.
2 Beaulac, J., Kristjansson, E., Cummins, S. A systematic review of food deserts, 1966-2007. Prev Chronic Dis. Epub2009; 6(3):A105. Available from: http://www.cdc.gov/pcd/issues/2009/jul/08_0163.htm.
3 Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004; 7(1A):123-146.
4 French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annual Rev Public Health. 2001; 22:309-35.
5 Ritchie LD, Hoelscher M, Sothern M, Crawford PB. Position of the American Dietetic Association: Individual-, Family-, School-, and Community-Based Interventions for Pediatric Overweight. J Am Diet Assoc. 2006; 106:925-945.
6 Hills AP, King NA, Armstrong TP. The Contribution of Physical Activity and Sedentary Behaviours to the Growth and Development of Children and Adolescents: Implications for Overweight and Obesity. Sports Medicine, 2007, 37(6):533-545(13).
7 Must A & Tybor DJ. Physical Activity and Sedentary Behavior: A Review of Longitudinal Studies of Weight and Adiposity in Youth. Int J Obes 2005; 29: S84-S96.
8 Fulton JE, Dai S, Steffen LM, Grunbaum JA, Shah SM, Labarthe DR. Physical Activity, Energy Intake, Sedentary Behavior, and Adiposity in Youth. Am J Prev Med 2009; 37(1S): S40-S49.
9 Ortega FB, Ruiz JR, Sjöström M. Physical Activity, Overweight and Central Adiposity in Swedish Children and Adolescents: the European Youth Heart Study. Int J Behav Nutr Phys Act. 2007 Nov 19; 4:61.
10 Woodward-Lopez G, Ritchie L, Gerstein D, Crawford P, editors. Obesity: Dietary and Developmental Influences. Boca Raton: CRC Press; 2006.
11 French SA. Pricing effects on food choices. J Nutr. 2003; 133:841S-843S.
12 French SA. Public Health strategies for dietary change: schools and workplaces. J Nutr. 2005; 135:910-912.
13 James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: Cluster randomized controlled trial. BMJ. 2004; 328(7450):1237.
14 Laurence S, Peterken R, Burns C. Fresh Kids: the efficacy of a Health Promoting Schools approach to increasing consumption of fruit and water in Australia. Health Promotion International. 2007; 22(3):218-226.
15 Institute of Medicine. Preventing Childhood Obesity-Health in the Balance. Washington, DC: The National Academies Press; 2005.
16 Keener D, Goodman K, Lowry A, Zaro S, Kettel Khan L. Recommended Community Strategies and Measurements to Prevent Obesity in the United States: Implementation and Measurement Guide. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009.
17 Livingstone MBE, Robson PJ. Measurement of dietary intake in children. Proceedings of the Nutrition Society. 2000; 59:279-293.
18 Trost SG. State of the Art Reviews: Measurement of Physical Activity in Children and Adolescents. Am J Lifestyle Medicine. 2007; 1(4):299-314.
19 The ASA24 is currently being used in over 90 studies, some with children. To date, however, the ASA24 has not been validity tested as a self-administered tool, although two such NCI studies are currently underway (OMB Clearance #0925-0605) and are expected to report in 2011.
20 Weise KL, Smith ML, Maschke KJ, Copeland HL. National practices regarding payment to research subjects for participating in pediatric research. Pediatrics. 2002; 110(3):577-582.
21 Bagley SJ, Reynolds WW, Nelson RM. Is a “wage-payment” model for research participation appropriate for children? Pediatrics. 2007; 119; 46-51.
22 Grady C. Payment of clinical research subjects. Journal of Clinical Investigation. 2005; 115(7)1681-1687.
23 Dillman, DA. 2000. Mail and Telephone Surveys. New York, NY: John Wiley & Sons.
24 Sudman S. Mail surveys of reluctant professionals. Evaluation Review 1985; 9(3):349-360.
25 Church, AH. 1993. Estimating the effect of incentives on mail survey response rates: a meta-analysis. Public Opinion Quarterly, 57:62-79.
26 Singer, E, Gebler, N, Raghunathan, T, Van Hoewyk, J, McGonagle, K. 1999. The effect of incentives on response rates in interviewer-mediated surveys. Journal of Official Statistics, 15: 217-230.
27 Kropf, ME, Scheib, J, Blair, J. 1999. The effect of alternative incentives on cooperation and refusal conversion in a telephone survey. Presented at the 54th Annual Meeting of the American Association for Public Opinion Research, St. Petersburg, Florida, May 13-16, 1999.
28 Hopkins KD, Gullickson AR. Response rates in survey research: a meta-analysis of the effects of monetary gratuities. J Experimental Education 1992; 61(1):52-62.
29 Fox, RJ, Crask, MR, Kim, J. 1988. Mail survey response rate: A meta-analysis of selected techniques for inducing response. Public Opinion Quarterly, 52:467-491.
30 Harvey, L. Factors affecting response rates to mailed questionnaires: A comprehensive literature review. 1987. Journal of Marketing Research, 29(3):341-353.
31 Singer, E, Van Hoewyk, J, Maher, MP. 2000. Experiments with incentives in telephone surveys. Public Opinion Quarterly, 64(2):171-88.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Owner |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy