EHDI- Attachment A- EHDI-IS Cooperative Agreement
EHDI- Attachment A- EHDI-IS Cooperative Agreement.docx
Surveys of State, Tribal, Local and Territorial (STLT) Governmental Health Agencies
EHDI- Attachment A- EHDI-IS Cooperative Agreement
OMB: 0920-0879
Attachment A. EHDI-IS Cooperative Agreement
Amendments have been incorporated into FOA CDC-RFA-DD11-1101 – Development, Maintenance and Enhancement of Early Hearing Detection and Intervention on February 3, 2011 to the following sections:
I. Authorization and Intent Pre application support
V. Application Content
VI. Evaluation Criteria
Appendix A
Appendix D
Appendix E Replace 2007 EHDI Hearing Screening and Follow-up Survey (HSFS) with 2009 CDC Hearing Screening and Follow-up Survey (HSFS)
ALL AMENDMENTS ARE NOTED IN RED TYPE
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Disease Control and Prevention (CDC)
Development, Maintenance and Enhancement of Early Hearing Detection and Intervention Information System (EHDI-IS) Surveillance Programs
I. AUTHORIZATION AND INTENT
Announcement Type: New
Funding Opportunity Number: CDC- RFA- DD11-1101
Catalog of Federal Domestic Assistance Number: 93.283 Centers for Disease Control and Prevention Investigations and Technical Assistance
Key Dates:
Letter of Intent Deadline Date: February 17, 2011
Application Deadline Date: March 21, 2011, 5:00pm Eastern Standard Time
Authority: This program is authorized under Sections 311, 317(k)(2), 317(C), and 399M(b) of the Public Health Service Act, [42U.S.C. Sections 243, 247b(k)(2), 247b-4 and 280g-1(b)].
Pre-Application Support:
Pre-Application Conference Calls:
Funding Opportunity Announcement (FOA) information will be available for potential applicants on two separate conference calls, conducted by the Centers for Disease Control and Prevention Early Hearing Detection and Intervention (CDC EHDI) Team, as follows:
The first call is scheduled for Monday, February 7, 2011 from 1-2pm, EST. The second call is scheduled for Monday, February 7, 2011 from 7-8pm, EST.
Participant Instructions
How to participate in both video and audio portions of the program
To see the Video portion of the teleconference, click on this link: Join the meeting.
For the Audio portion, use the following toll-free number and passcode:
Toll-free: +1 (866) 842-6975
Participant code: 218840#
First
Time Users:
To
save time before the meeting, check
your system to make sure it is ready to use Microsoft Office Live
Meeting. (This is a test site only. The actual link for this
specific session is the Join
the Meeting
link above.)
Captioning service is available. If you would like the captioning service, please contact Steve Richardson at [email protected] promptly to allow time for testing system compatibility. This service is provided at no charge.
The purpose of the conference calls is to 1) help potential applicants understand the scope and intent of the FOA for the Development, Maintenance, and Enhancement of Early Hearing Detection and Intervention Information System (EHDI-IS) Surveillance Programs and 2) become familiar with the Public Health Services funding policies and application and review procedures. Since this is a competitive selection process, applicants should follow the requirements as laid out in the FOA and any related amendments. If potential applicants have questions or need clarification prior to this call please see section VII Agency Contacts.
Background:
Hearing loss identified in the newborn period has been referred to as a neuro-developmental emergency. Congenital hearing loss affects two to three infants per 1,000 live births. Undetected hearing loss can delay speech and language development. National goals have been established to ensure hearing screening for all newborns no later than one month of age, diagnostic audiological evaluation as early as possible but no later than three months of age for those who do not pass the screening and enrollment in early intervention services, as early as possible but no later than six (6) months of age for those identified with hearing loss. The three goals are frequently referred to as the “1-3-6” Early Hearing Detection and Intervention (EHDI) plan and reflect recommendation and endorsements of several federal agencies and national organizations.
Since the organized collection of newborn hearing screening data started in 2000 (for year 1999) demonstrated progress has been made in identifying and providing early intervention services to infants with hearing loss. Now more than 95% of U.S. infants can be documented as having their hearing screened, however remaining challenges include ensuring timely diagnostic evaluation for those who do not pass the screening and enrollment in early intervention for those with diagnosed hearing loss. Some of those infants might have received follow-up services, but the results were not reported to the EHDI program and their status could not be determined from available data.
Over the last decade the Health Resources and Services Administration (HRSA) has made awards of grants to develop statewide newborn and infant hearing screening, evaluation and intervention programs. As part of its Congressional authority, the Centers for Disease Control and Prevention (CDC) has provided technical assistance on data collection and funded cooperative agreements to State agencies to develop standardized procedures for data management and program effectiveness.
To ensure valid and verifiable information for quality monitoring, state/territorial EHDI programs should be able to identify, match, collect, and report data on all occurrent births within their jurisdiction that is unduplicated and individually identifiable throughout the three stages of the EHDI process (screening, diagnosis, and early intervention). These data are important in determining the impact of hearing loss on children and their families and the quality of the services they receive. Specifically, the data enable programs to:
• Assess progress towards meeting national EHDI objectives and goals;
• Accurately assess the number of infants who receive appropriate, timely follow-up services and document improvements in infant/family outcomes;
• Identify trends of the EHDI process that effect changes over time due to programmatic changes in policy and targeted interventions;
• Investigate service gaps within and among different socioeconomic, race/ethnicity, and gender groups; and
• Accurately compare progress with other jurisdictions and national trends.
Purpose:
The purpose of the program is to:
(1) Assist EHDI programs in developing and maintaining a sustainable, centralized newborn hearing screening tracking and surveillance system capable of accurately identifying, matching, collecting, and reporting data on all occurrent births that is unduplicated and individually identifiable through the three components of the EHDI process (screening, diagnosis, and early intervention).
(2) For those programs with fully developed* EHDI Information Systems (EHDI-IS), enhance electronic system capacity to collect data, ensure children receive recommended screening and follow-up services, and exchange data accurately, effectively, securely, and consistently between the EHDI-IS and Electronic Health Record Systems (EHR-S) with a specific focus on reducing the duplicate data entry burden and a reduction in loss to recommended follow-up services (screening, diagnosis, and intervention).
*A fully developed EHDI-IS can be considered as one that routinely collects and reports valid and verifiable unduplicated, individually identifiable data on every occurrent birth including demographic and age specific data throughout the EHDI process.
This program addresses the “Healthy People 2020” focus area(s) of Hearing and other Sensory or Communication Disorders.
Measurable outcomes of the program will be in alignment with one (or more) of the following performance goal(s) for the National Center on Birth Defects and Developmental Disabilities: Improve the Health and Quality of Life of Americans with Disabilities.
This announcement is only for non-research activities supported by CDC. If research is proposed, the application will not be reviewed. For the definition of research, please see the CDC Web site at the following Internet address: http://www.cdc.gov/od/science/regs/hrpp/researchDefinition.htm
II. PROGRAM IMPLEMENTATION
Recipient Activities:
Develop and maintain the EHDI-IS to accurately identify, match, and collect data that is unduplicated, and individually identifiable (not estimated or aggregated) through the three components of the EHDI process (screening, diagnosis, and early intervention).
For jurisdictions that have implemented a fully developed EHDI-IS (i.e. able to collect unduplicated individual identifiable data on every occurrent birth) enhance the current EHDI-IS by:
Leveraging IT innovations and public health informatics solutions with proven potential to better serve EHDI stakeholders (i.e. hospitals, audiologists, physicians/medical homes, families, public health partners etc).
Increasing the EHDI program’s operational capacity to exchange data accurately, effectively, securely, and consistently between EHDI-IS and Electronic Health Record Systems (EHR-S) with a specific focus on reducing the duplicate data entry burden and a reduction in loss to recommended follow-up services (screening, diagnosis, and intervention).
Collect and report individualized demographic and age specific data for every occurrent birth about the child’s status and progress through the three components of the EHDI process (screening, diagnosis, and early intervention).
Develop and implement a process for potential reporting sources to monitor the quality and completeness of individualized demographic and age specific EHDI data.
Collaborate with potential reporting sources to develop data collection and sharing agreements on individual unduplicated EHDI data. Examples of reporting sources include hospitals, audiologists, physicians/medical homes, families, public health partners, and early intervention services, including Part C of the Individuals with Disabilities Act (IDEA).
Develop and implement plans for monitoring progress and evaluating success and accomplishment of the funded activities in terms of completeness, effectiveness, and data quality. Plans should include process, performance, and quality assurance measures.
During the first budget year, successful applicants will be expected to coordinate with CDC to refine and improve the evaluation designs/methods.
Analyze and disseminate EHDI data and related analyses to shape the ongoing development of EHDI-IS and inform partners and stakeholders of the program’s successes and challenges.
Contribute data annually to the National CDC EHDI Hearing Screening and Follow-up Survey (OMB No. 0920-0733 -see Appendix E) providing aggregated data derived from unduplicated identifiable data. Data should address:
Hearing screening
Diagnosis
Early intervention services
Type and severity of diagnosed hearing losses and
Demographics
Attendance by two EHDI program staff at the annual National EHDI conference in order to share the latest information and collaborate with other experts in the field of early hearing loss on best practices in early hearing detection and surveillance.
In a cooperative agreement, CDC staff is substantially involved in the program activities, above and beyond routine grant monitoring.
CDC Activities:
Provide technical and operational support to ensure award recipients make timely progress in meeting the goals and objective of this FOA;
Provide technical assistance as needed related to the collection and analysis of data;
Facilitate collaborative efforts to compile and disseminate program results through presentations and publications;
Review the evaluation plans developed by applicants and provide technical assistance in refining and improving the evaluation methods and design.
Provide technical assistance on the development, use, and testing of nationally recognized standards, implementation specifications, and certification criteria for EHDI-IS/EHR-S interoperability.
Work with selected EHDI awardees, national experts, and organizations, including representatives from recognized certification bodies, selected EHR-S vendors, and informaticians in providing technical assistance related to national data standards, implementation specifications, and certification criteria.
III. AWARD INFORMATION AND REQUIREMENTS
Type of Award: Cooperative Agreement.
CDC substantial involvement in this program appears in the Activities Section above
Award Mechanism: UR3 - Health Investigations and Assessments of Control, Prevention, and Testing Methods
Fiscal Year Funds: FY-2011
Approximate Current Fiscal Year Funding: $8,400,000
Approximate Total Project Period Funding: $42,000,000 (This amount is an estimate, and is subject to availability of funds.) This amount includes direct and indirect costs.)
Approximate Number of Awards: 56
Approximate Average Award: $150,000 (This amount is for the first 12-month budget period, and includes both direct and indirect costs.)
Floor of Individual Award Range: None.
Ceiling of Individual Award Range: $175,000 (This ceiling is for the first 12-month budget period.) This amount includes both direct and indirect costs.
Anticipated Award Date: July 1, 2011
Budget Period Length: 12 months
Project Period Length: 5 years
Throughout the project period, CDC’s commitment to continuation of awards will be conditioned on the availability of funds, evidence of satisfactory progress by the recipient (as documented in required reports), and the determination that continued funding is in the best interest of the Federal government.
IV. ELIGIBILITY
Eligible applicants that can apply for this funding opportunity are listed below:
State and local governments or their Bona Fide Agents (this includes the District of Columbia, the Commonwealth of Puerto Rico, the Virgin Islands, the Commonwealth of the Northern Marianna Islands, American Samoa, Guam, the Federated States of Micronesia, the Republic of the Marshall Islands, and the Republic of Palau)
A Bona Fide Agent is an agency/organization identified by the state as eligible to submit an application under the state eligibility in lieu of a state application. If applying as a bona fide agent of a state or local government, a legal, binding agreement from the state or local government as documentation of the status is required. Attach with “Other Attachment Forms” when submitting via www.grants.gov.
Eligibility is limited to State/Territorial agencies or their Bona Fide agents because they have the public health authority and/or legislative mandate to conduct newborn hearing screening activities that include monitoring and tracking the disposition of every occurrent birth in the state. This authority allows them to work collaboratively with multiple reporting sources including but not limited to vital records, birthing facilities, diagnostic centers, audiologists, physicians/medical homes, early intervention services, birth defect registries, immunization registries and bloodspot programs to ensure accurate monitoring and tracking of all births statewide. They must also have the experience, capacity, expertise, and resources to complete the comprehensive activities for this statewide surveillance program.
SPECIAL ELIGIBILITY CRITERIA: Licensing/Credential/Permits
Cost sharing or matching funds are not required for this program.
Note: Title 2 of the United States Code Section 1611 states that an organization described in Section 501(c)(4) of the Internal Revenue Code that engages in lobbying activities is not eligible to receive Federal funds constituting a grant, loan, or an award.
Intergovernmental Review of Applications
The application is subject to Intergovernmental Review of Federal Programs, as governed by Executive Order (EO) 12372. This order sets up a system for state and local governmental review of proposed federal assistance applications. Contact the state single point of contact (SPOC) as early as possible to alert the SPOC to prospective applications and to receive instructions on the State’s process. Visit the following Web address to get the current SPOC list: http://www.whitehouse.gov/omb/grants_spoc/.
V. Application Content
CDC Assurances and Certifications can be found on the CDC Web site at the following Internet address: http://www.cdc.gov/od/pgo/funding/grants/foamain.shtm
Other Requirements
Letter of Intent (LOI):
Prospective applicants are requested to submit a letter of intent that includes the following information:
Descriptive title of proposed project.
Name, address, and telephone number of the Principal Investigator/Project Director.
Names of other key personnel.
Participating institutions.
Number and title of this funding opportunity.
LOI Submission Address: Submit the LOI by express mail, delivery service, fax, or E-mail to:
Deidra Green
CDC, NCBDDD, EHDI
1600 Clifton Road, NE MS E-88
Atlanta, Georgia 30333
Telephone: (404) 498-3034
Fax: (404) 498-3060
E-mail: [email protected]
Although a letter of intent is not required, is not binding, and does not enter into the review of a subsequent application, the information that it contains allows CDC Program staff to estimate and plan the review of submitted applications.
Requested LOIs should be provided not later than by the date indicated in the Section I entitled “Authorization and Intent”.
A Project Abstract must be completed in the Grants.gov application forms. The Project Abstract must contain a summary of the proposed activity suitable for dissemination to the public. It should be a self-contained description of the project and should contain a statement of objectives and methods to be employed. It should be informative to other persons working in the same or related fields and insofar as possible understandable to a technically literate lay reader. This abstract must not include any proprietary/confidential information.
A Project Narrative must be submitted with the application forms. The project narrative must be uploaded in a PDF file format when submitting via Grants.gov. The narrative must be submitted in the following format:
Maximum number of pages: 25 If your narrative exceeds the page limit, only the first pages which are within the page limit will be reviewed.
Font size: 12 point unreduced, Times New Roman
Double spaced
Page margin size: One inch
Number all narrative pages; not to exceed the maximum number of pages.
The narrative should address activities to be conducted over the entire project period and must include the following items in the order listed:
The Project Narrative will be composed of the following sections:
Background and Need
Provide a detailed description of the current EHDI program with a focus on data reporting protocols, EHDI-IS and any other child health data systems that are linked or integrated with the EHDI data systems, collaborative relationships, and any state/territorial legislation and/or rules regarding newborn hearing screening and follow-up.
The detailed description of the background should include information about the current EHDI program under Category and Questions in Appendix G. An EHDI State Profile can be included as an attachment as well as a discussion of the background in the Project Narrative.
The baseline performance metrics (calendar year 2009) listed in Appendix C must be included as an EHDI Performance Metrics attachment. A brief explanation in the narrative should be included for any performance metrics that are currently unavailable.
Summarize recent accomplishments and challenges of the program.
Project Work plan
The Project Work Plan should include a detailed plan describing program goals as well as, annual objectives that are specific, measurable, attainable, realistic, and time phased (SMART), for accomplishing recipient activities over the 5 year project period. The activities addressed in the Work Plan should describe a process to establish, or improve methods to identify, match, collect and report standardized unduplicated individually identifiable data.
A Work Plan Table must be included as an attachment (see Appendix A: Sample Work Plan Table)
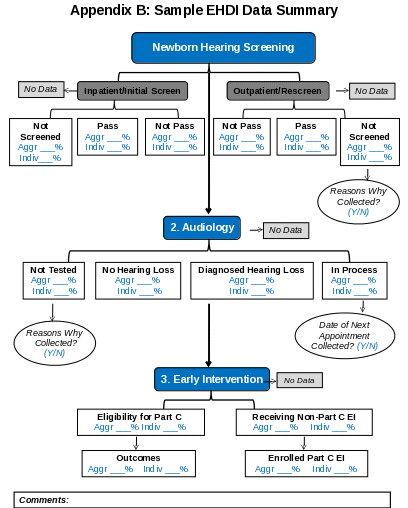
Information requested in Appendix B must be included as an EHDI Data Summary attachment. The Work Plan should describe how any data gaps (i.e., aggregate or missing data) will be addressed by providing the following information:
Reason(s) why data are not collected or only collected at the aggregate level.
Specific actions/steps that will be taken to collect these data at the individual level. Note that in such cases, the proposed goals and objectives should be framed to minimize the collection of aggregate data and support the collection of additional individualized data that is not currently captured.
Describe how any missing data in the EHDI Performance Metrics (See Appendix C) will be addressed.
Include the timeframes for assessing progress both in short term (funding period) and long term (project period)
Describe the program staff responsible for conducting the activities and the amount of time in terms of FTE program staff. The total of FTE time for the activities of each program staff should total the amount funded under this cooperative agreement.
Clearly describe how any collaborative efforts by stakeholders and roles of partners contribute to elements of the work plan.
Detail any other sources of funding for these activities.
Indicate if aggregated data will be contributed annually to the National CDC EHDI Hearing Screening and Follow-up Survey.
If data are not expected to be contributed to this survey during the project period, an explanation should be included.
Indicate if data are managed by a non-state agency or contractor; if so the following should be included:
Name of the agency/contractor
Statement indicating whether any data are aggregated by the contracted data manager prior to being made available to the EHDI program
Collaborative Efforts
Describe past, current, and proposed collaboration with potential reporting sources within the program’s service area that provide data, resources, or other support to address EHDI related services. For support anticipated in the future, provide documentation such as letters of support, memoranda of understanding (MOU) or memoranda of agreement (MOA) dated within the past three months. Ongoing working relationships should specify current collaborative activities.
While letters of general support are acceptable, the strongest documents will list specific, commitments and activities that:
Clearly contribute to the elements of the work plan in this proposal, and
Can be measured or demonstrated as evidence of success.
Program Capacity
Describe state and local resources, program infrastructure, and current and prior experience in tracking and monitoring EHDI surveillance activities. As an attachment to this application provide a job description and experience/background for each key personnel who will implement and carry out the activities of this surveillance and tracking program, regardless of whether or not funded by this cooperative agreement.
Evaluation Plan
Describe a plan for monitoring progress and evaluating success and accomplishment of the funded activities. Success should be described in terms of completeness, effectiveness, and data quality. The evaluation plan should:
Be consistent with the objectives being proposed during the project period
Include descriptions of process measures, performance measures, and quality assurance measures
For all measures defined in the evaluation plan, identify potential sources and methods/tools for data collection.
Specify a list of activities that will be conducted for evaluating process, performance, and data quality using the measures defined in the evaluation plan
For activities that span multiple funding years, include progress milestone for each budget year
Describe staff responsible and the timeline estimated for conducting the planned evaluation activities
See Appendix D for guidance in developing an evaluation plan. An Evaluation Table can be included as an attachment as well as a discussion of the evaluation plan in the narrative.
Budget
Provide a budget by line item, and a detailed line-item justification for all operating expenses for the first 12-month budget period. This budget should be consistent with the stated objectives and planned activities of the workplan. Also submit one year budgets for each of the subsequent project period years.
Contracts and Consultants: Provide the following information for each contract and/or consultant: (a) name of contractor or consultant, (b) method of selection, (c) period of performance, (d) scope of work, (e) method of accountability, and (f) separate itemized budget with justification. Go to http://www.cdc.gov/od/pgo/funding/budgetguide.htm Appendix A and Appendix B for detailed description of each category.
Additional information may be included in the application appendices. The appendices will not be counted toward the narrative page limit. This additional information includes:
EHDI-IS Enhancement Activities
Examples of enhancement related activities could include but are not limited to:
Instituting system modifications to improve EHDI-IS/EHR-S interoperability (See Appendix F)
Updating the state or territory’s current EHDI-IS with new and emerging computerized decision support tools that support various EHDI stakeholders. Tools may also include individualized information for parents or care providers.
Updating the state or territory’s current EHDI-IS with detailed electronic processes to report and disseminate information on progress towards programmatic, jurisdictional, and national goals.
Other activities that leverage emerging IT innovations and technologies to support the EHDI program generally and the EHDI IS specifically. As examples:
enhance the capacity of EHDI-IS to increase data sharing, integration and linkage with other child health reporting systems within the jurisdiction.
expand EHDI-IS in order to collect developmental outcome data from Early Intervention programs or to automate electronic referrals directly to Part-C. see The Value of Health IT in Improving Population Health and Transforming Public Health Practice for additional information: http://www.phii.org/resources/doc/eHealth-strategy%20FINAL.pdf
Curriculum Vitae or Resumes for Key Personnel (limit 2 pages per person)
Job Descriptions for Key Personnel
Evaluation Table
Letters of support
Current Legislation
Organizational chart
Additional information submitted via Grants.gov should be uploaded in a PDF file format, and should be named:
(2 state letter abbreviation) <plus> document name
No more than 25 electronic attachments should be uploaded per application.
Additional requirements for additional documentation with the application are listed in Section VII. Award Administration Information, subsection entitled “Administrative and National Policy Requirements.”
APPLICATION SUBMISSION
Registering your organization through www.Grants.gov, the official agency-wide E-grant website, is the first step in submitting an application online. Registration information is located on the “Get Registered” screen of www.Grants.gov. Please visit www.Grants.gov at least 30 days prior to submitting your application to familiarize yourself with the registration and submission processes. The “one-time” registration process will take three to five days to complete. However, the Grants.gov registration process also requires that you register your organization with the Central Contractor Registry (CCR) annually. The CCR registration can require an additional one to two days to complete.
Submit the application electronically by using the forms and instructions posted for this funding opportunity on www.Grants.gov. If access to the Internet is not available or if the applicant encounters difficulty in accessing the forms on-line, contact the HHS/CDC Procurement and Grant Office Technical Information Management Section (PGO TIMS) staff at (770) 488-2700 for further instruction.
Note: Application submission is not concluded until successful completion of the validation process.
After submission of your application package, applicants will receive a “submission receipt” email generated by Grants.gov. Grants.gov will then generate a second e-mail message to applicants which will either validate or reject their submitted application package. This validation process may take as long as two (2) business days. Applicants are strongly encouraged check the status of their application to ensure submission of their application package is complete and no submission errors exists. To guarantee that you comply with the application deadline published in the Funding Opportunity Announcement, applicants are also strongly encouraged to allocate additional days prior to the published deadline to file their application. Non-validated applications will not be accepted after the published application deadline date.
In the event that you do not receive a “validation” email within two (2) business days of application submission, please contact Grants.gov. Refer to the email message generated at the time of application submission for instructions on how to track your application or the Application User Guide, Version 3.0 page 57.
Dun and Bradstreet Universal Number (DUNS)
The applicant is required to have a Dun and Bradstreet Data Universal Numbering System (DUNS) identifier to apply for grants or cooperative agreements from the Federal government. The DUNS is a nine-digit number which uniquely identifies business entities. There is no charge associated with obtaining a DUNS number. Applicants may obtain a DUNS number by accessing the Dun and Bradstreet website or by calling 1-866-705-5711. International registrants can confirm by sending an e-mail to [email protected], including Company Name, D-U-N-S Number, and Physical Address, and Country.
Electronic Submission of Application:
Applications must be submitted electronically at www.Grants.gov. Electronic applications will be considered as having met the deadline if the application has been successfully made available to CDC for processing from Grants.gov on the deadline date. The application package can be downloaded from www.Grants.gov. Applicants can complete the application package off-line, and then upload and submit the application via the Grants.gov Web site. The applicant must submit all application attachments using a PDF file format when submitting via Grants.gov. Directions for creating PDF files can be found on the Grants.gov Web site. Use of file formats other than PDF may result in the file being unreadable by staff.
Applications submitted through Grants.gov (http://www.grants.gov), are electronically time/date stamped and assigned a tracking number. The AOR will receive an e-mail notice of receipt when Grants.gov receives the application. The tracking number serves to document submission and initiate the electronic validation process before the application is made available to CDC for processing.
If the applicant encounters technical difficulties with Grants.gov, the applicant should contact Grants.gov Customer Service. The Grants.gov Contact Center is available 24 hours a day, 7 days a week, with the exception of all Federal Holidays. The Contact Center provides customer service to the applicant community. The extended hours will provide applicants support around the clock, ensuring the best possible customer service is received any time it’s needed. You can reach the Grants.gov Support Center at 1-800-518-4726 or by email at [email protected]. Submissions sent by e-mail, fax, CD’s or thumb drives of applications will not be accepted.
Organizations that encounter technical difficulties in using www.Grants.gov to submit their application must attempt to overcome those difficulties by contacting the Grants.gov Support Center (1-800-518-4726, [email protected]). After consulting with the Grants.gov Support Center, if the technical difficulties remain unresolved and electronic submission is not possible to meet the established deadline, organizations may submit a request prior to the application deadline by email to GMO/GMS for permission to submit a paper application. An organization's request for permission must: (a) include the Grants.gov case number assigned to the inquiry, (b) describe the difficulties that prevent electronic submission and the efforts taken with the Grants.gov Support Center (c) be submitted to the GMO/GMS at least 3 calendar days prior to the application deadline. Paper applications submitted without prior approval will not be considered.
If a paper application is authorized, the applicant will receive instructions from PGO TIMS to submit the original and two hard copies of the application by mail or express delivery service.
Submission Dates and Times
This announcement is the definitive guide on LOI and application content, submission, and deadline. It supersedes information provided in the application instructions. If the application submission does not meet the deadline published herein, it will not be eligible for review and the applicant will be notified the application did not meet the submission requirements.
Letter of Intent (LOI) Deadline Date: February 17, 2011
Application Deadline Date: March 21, 2011, 5:00pm Eastern Standard Time.
VI. Application Review Information
Eligible applicants are required to provide measures of effectiveness that will demonstrate the accomplishment of the various identified objectives of the CDC-RFA-DD11-1101. Measures of effectiveness must relate to the performance goals stated in the “Purpose” section of this announcement. Measures of effectiveness must be objective, quantitative and measure the intended outcome of the proposed program. The measures of effectiveness must be included in the application and will be an element of the evaluation of the submitted application.
Evaluation Criteria
Eligible applications will be evaluated against the following criteria:

Work plan (40 Points)
Does the plan describe an effective process to establish or improve methods to identify, match, collect and report standardized data that is unduplicated and individually identifiable for every occurrent birth through the three components of the EHDI process? (screening, diagnosis and intervention)
Does the applicant clearly describe the methods to be used to develop or improve reporting systems in order to ensure accurate tracking and surveillance of unduplicated individual identifiable data?
Does the applicant clearly describe the methods to be used to develop or improve reporting systems from multiple sources? (For example: hospitals, audiologists, physicians/medical homes, families, public health partners, early intervention services)
Is there an effective and realistic plan to address the challenges, barriers, and problems related to enhancing the state or territory’s tracking and surveillance system to minimize the loss to follow up?
Has the EHDI Data Summary been submitted?
Does the Work Plan include activities that address each gap identified in the EHDI Data Summary?
Has adequate staffing been assigned to each activity within the work plan?
Does the applicant list objectives that are specific, measurable, attainable, realistic, and time phased (SMART)?
Are the goals and objectives likely to be met within the timeframe described by the applicant?
For each objective, does the plan provide activities, timeline, responsible staff, and measures of effectiveness?
Evaluation Plan (25 points)
Is the evaluation plan consistent with the objectives being proposed during the grant cycle? Does the evaluation plan state the specific purposes of the evaluation? Are they clearly aligned with the program activities proposed in the application?
Does the evaluation plan include a description of process measures which entail assessment of the EHDI surveillance process and coverage/acceptability of the EHDI surveillance system and activities?
Does the evaluation plan include a description of performance measures for assessing the effectiveness of the EHDI surveillance system and activities? Are ALL performance metrics in Appendix C included?
Does the evaluation plan include a description of quality assurance measures for assessing the accuracy, validity, and completeness of data reported to the state/territorial EHDI programs and to CDC?
Does the evaluation plan specify a list of activities that will be conducted for evaluating process, performance, and data quality?
Program Capacity (15 points)
Does the applicant describe the capacity and infrastructure of the program that would enable them to conduct the proposed activities?
Is the applicant’s proposed staffing plan sufficient to accomplish the program goals?
Are staff roles and responsibilities clearly defined?
Do key personnel have skills and experience to carry out the proposed activities and program evaluation?
Is there sufficient dedicated staff time to carry out the proposed activities and program evaluation?
Background (10 points)
Does the applicant describe the existing capacity of the state’s EHDI program including descriptions of state legislation (if applicable), data systems, reporting protocols, collaborative relationships, etc.?
Does the applicant adequately describe the current program gaps and needs?
Has the applicant provided calendar year 2009 baseline data for each stage of screening, diagnosis, and intervention, listed as performance metrics in Appendix C and a brief explanation in the narrative for any performance metrics that were unavailable?
Collaborative Efforts (10 points)
Does the applicant describe methods for collaborating with multiple data sources such birthing facilities, and linking with other state or territorial screening, tracking and surveillance programs?
Are these methods documented with written assurances?
Does the applicant provide documentation from future partners related to tracking and surveillance efforts, such as letters of support, memoranda of understanding (MOU) or memoranda of agreement (MOA) that are dated within three months prior to the submission of the application? Do they describe a specific role and a specific, verifiable commitment of the partner?
Does the applicant describe plans to work collaboratively with others on effective mechanisms for obtaining screening, evaluation, and early intervention data?
These partners may include hospitals, audiologists, physicians/medical homes, families, public health partners, early intervention services, stakeholder or advisory boards/councils, other state and territorial EHDI programs, CDC, and other federal and national agencies (e.g. HRSA/MCHB).
Do the roles of the partners clearly contribute to elements of the work plan and can they be measured or demonstrated as evidence of success?
Budget (SF 424A) and Budget Narrative (Reviewed, but not scored) Although the budget is not scored applicants should consider the following in development of their budget. Is the itemized budget for conducting the project, and justification reasonable and consistent with stated objectives and planned program activities?
If the applicants requests indirect costs in the budget, a copy of the indirect cost rate agreement is required. If the indirect cost rate is a provisional rate, the agreement should be less than 12 months of age. The indirect cost rate agreement should be uploaded as a PDF file with “Other Attachment Forms” when submitting via Grants.gov.
Funding Restrictions
Restrictions, which must be taken into account while writing the budget, are as follows:
Recipients may not use funds for research.
Recipients may not use funds for clinical care.
Recipients may only expend funds for reasonable program purposes, including personnel, travel, supplies, and services, such as contractual.
Awardees may not generally use HHS/CDC/ATSDR funding for the purchase of furniture or equipment. Any such proposed spending must be identified in the budget.
The direct and primary recipient in a cooperative agreement program must perform a substantial role in carrying out project objectives and not merely serve as a conduit for an award to another party or provider who is ineligible.
Reimbursement of pre-award costs is not allowed.
Recipients may not use funds to purchase screening or diagnostic equipment.
Recipient may not use funds to supplant funds available for screening, diagnosis, early intervention, or tracking for hearing lost or other disorders detected by newborn screening.
Recipient may not use funds to purchase promotional items.
http://pgo.cdc.gov/pgo/webcache/SOP/revised_use_of_funds_8.13.07.doc
The applicant can obtain guidance for completing a detailed justified budget on the CDC website, at the following Internet address:
http://www.cdc.gov/od/pgo/funding/budgetguide.htm.
Application Review Process
All eligible applications will be initially reviewed for completeness by the Procurement and Grants Office (PGO) staff. In addition, eligible applications will be jointly reviewed for responsiveness by NCBDDD and PGO. Incomplete applications and applications that are non-responsive to the eligibility criteria will not advance through the review process. Applicants will be notified the application did not meet eligibility and/or published submission requirements.
An objective review panel will evaluate complete and responsive applications according to the criteria listed in Section VI. Application Review Information, subsection entitled “Evaluation Criteria”. The objective review process will follow the policy requirements as stated in the GPD 2.04 at http://198.102.218.46/doc/gpd204.doc. The objective review panel will consist of HHS employees with 100 % being from outside the funding Division and at least 50% being from outside the funding Center who will be randomly assigned application to review and score. Based on the score the panel will make recommendations to approve, disapprove or defer applications.
Applications Selection Process
Applications will be funded in order by score and rank determined by the review panel.
VII. Award Administration Information
Award Notices
Successful applicants will receive a Notice of Award (NoA) from the CDC Procurement and Grants Office. The NoA shall be the only binding, authorizing document between the recipient and CDC. The NoA will be signed by an authorized Grants Management Officer and e-mailed to the program director. A hard copy of the NoA will be mailed to the recipient fiscal officer identified in the application.
Unsuccessful applicants will receive notification of the results of the application review by mail.
Administrative and National Policy Requirements
Successful applicants must comply with the administrative requirements outlined in 45 Code of Federal Regulations (CFR) Part 74 or Part 92, as appropriate. The following additional requirements apply to this project:
AR-7 Executive Order 12372
AR-10 Smoke-Free Workplace Requirements
AR-11 Healthy People 2020
AR-12 Lobbying Restrictions
AR-14 Accounting System Requirements
AR-20 Conference Support
AR-21 Small, Minority, and Women-Owned Business
AR-24 Health Insurance Portability and Accountability Act Requirements
AR-25 Release and Sharing of Data
AR-26 National Historic Preservation Act of 1966
(Public Law 89-665, 80 Stat. 915)
AR-27 Conference Disclaimer and Use of Logos
AR-29 Compliance with E.O. 13513 Federal Leadership on Reducing
Text Messaging While Driving, October 1, 2009.
AR-30 Compliance with Section 508 of the Rehab Act of 1973
Additional information on the requirements can be found on the CDC Web site at the following Internet address: http://www.cdc.gov/od/pgo/funding/Addtl_Reqmnts.htm.
For more information on the Code of Federal Regulations, see the National Archives and Records Administration at the following Internet address: http://www.access.gpo.gov/nara/cfr/cfr-table-search.html
TERMS AND CONDITIONS
Reporting Requirements
Each funded applicant must provide CDC with an annual Interim Progress Report submitted via www.grants.gov:
The interim progress report is due no less than 90 days before the end of the budget period. The Interim Progress Report will serve as the non-competing continuation application, and must contain the following elements:
Standard Form (“SF”) 424S Form.
SF-424A Budget Information-Non-Construction Programs.
Budget Narrative.
Indirect Cost Rate Agreement.
Project Narrative.
Additionally, funded applicants must provide CDC with an original, plus two hard copies of the following reports:
Annual progress report, due 90 days after the end of the budget period.
Current budget period progress
Current budget period financial progress
New budget proposed objectives and activities;
Detailed line item budge and justification
Financial Status Report (SF 269) and annual progress report, no more than 90 days after the end of the budget period.
Final performance and Financial Status Reports, no more than 90 days after the end of the project period.
These reports must be submitted to the attention of the Grants Management Specialist listed in the Section VIII below entitled “Agency Contacts”.
VIII. Agency Contacts
CDC encourages inquiries concerning this announcement.
For programmatic technical assistance, contact:
Deidra Green
CDC, NCBDDD, EHDI
1600 Clifton Road, NE MS E-88
Atlanta, Georgia 30333
Telephone: (404) 498-3034
Fax: (404) 498-3060
E-mail: [email protected]
For financial, grants management, or budget assistance, contact:
Stephanie Lankford, Grants Management Specialist
Department of Health and Human Services
CDC Procurement and Grants Office
2920 Brandywine Road, MS E-09
Atlanta, GA 30341
Telephone: (770)488-2936
E-mail: [email protected]
For assistance with submission difficulties (also see page 20), contact:
Grants.gov Contact Center Phone: 1-800-518-4726.
Hours of Operation: 24 hours a day, 7 days a week. Closed on federal holidays.
For submission questions, contact:
Technical Information Management Section
Department of Health and Human Services
CDC Procurement and Grants Office
2920 Brandywine Road, MS E-14
Atlanta, GA 30341
Telephone: 770-488-2700
Email: [email protected]
CDC Telecommunications for the hearing impaired or disabled is available at:
TTY 1-888-232-6348
Other Information
Other CDC funding opportunity announcements can be found at www.grants.gov.
Goals are general, big-picture statements of outcomes a program intends to accomplish to fulfill its mission. |
Measures of Success are standards that a program sets for itself to measure progress in achieving program goals. Measures of success should be significant and truly gauge success in attaining the goal. They should contain a numeric value or observable behavior. |
||||||
Objectives |
Activities/Steps |
Data/Evaluation |
Timeframe for Assessing Progress |
Program Staff Responsible |
% of FTE (CDC EHDI) |
Other Sources of Funding for This Activity |
|
State the big-steps a program will take to attain its goal. These can be used to determine a program’s status at any given point in time, and can be measured during the project period. Objectives should be: S.M.A.R.T. that is, specific (can identify who, what, and where) measurable (can identify how many by when) achievable (can be attained) realistic (can be attained given time and resources available) time framed (can identify when) They should not include more than one expectation.
|
These are what a program does or the specific tasks to meet its objectives and ultimately fulfill its goal. Examples include educating the public about the importance of dental sealants for prevention of tooth decay through distributing printed materials, using outreach workers to enroll children for oral screenings, and training health professionals about screening technology. |
These are pieces of information that can be used to access program activities or outcomes. This information can be obtained from: immunizations, birth defects registries, vital records, blood spot programs and other surveillance programs that identify children with special health needs.
Assessment data is more focused and typically answers the question did the activity contribute significantly to the desired outcome? Provide the evidence for the conclusion. Determine which components of the activity contributed to the desired outcome and which did not. |
Examples:
1st Quarter - Fiscal Year 1
4th Quarter - Fiscal Year 2
|
Examples:
EHDI Coordinator
EHDI Data Manager |
e.g.:
5% FTE
10% FTE
All personnel funded under this agreement should be accounted for as specified in the budget |
Examples:
HRSA MCHB UNHS
HRSA MCHB Block Grant
State Funds |
|
Appendix A: Sample Project Work Plan Table
Appendix C
EHDI Performance Metrics
Data for each of the below performance metrics should be based on data for calendar year 2009 (January 1st – December 31st).
If data are not available for one or more of the metrics, indicate “N/A” and include a brief explanation of why it is not available.

Total number of live occurrent births
Total number and percent of infants documented as being screened for hearing loss (based on the final or most recent hearing screening)
Percentage Formula: number of infants screened for hearing loss / total number of live occurrent births x 100%
Total number and percent of infants screened for hearing loss, who were documented as being screened for hearing loss before 1 month of age
Percentage Formula: number of infants screened for hearing loss, who were screened before 1 month of age / total number of infants screened for hearing loss x 100%
Percent of all newborns that did not pass the initial screening and any subsequent re-screening before hospital discharge
Percentage Formula: number of infants who did not pass the initial screening and any subsequent re-screening before hospital discharge / total number of live occurrent births x 100%
Total number and percent of infants that did not pass the final or most hearing screening, who were documented as being diagnosed with a permanent hearing loss
Percentage Formula: number of infants that did not pass the final or most recent hearing screen, who were diagnosed with a permanent hearing loss / total number not passing the final or most recent hearing screening x 100%
Total number and percent of infants with a diagnosis of permanent hearing loss, who were documented as being diagnosed before 3 months of age
Percentage Formula: number of infants with a documented diagnosis of permanent hearing loss, who were diagnosed before 3 months of age / total number diagnosed with normal hearing and permanent hearing loss x 100%
Total number and percent of infants not passing the final or most recent hearing screening that that are “In Process” for a diagnosis. Infants reported as being “In Process” must have been seen by audiologist (or other qualified provider) at least one time and have a follow-up appointment already scheduled
Percentage Formula: number of infants not passing the final or most recent hearing screening that are “In Process” for a diagnosis / total number not passing the final or most recent hearing screening x 100%
Total number and percent of infants not passing the final or most recent hearing screening that did not receive a documented diagnosis i.e.., loss to follow-up / loss to documentation (please include the definition and criteria used to determine when follow-up by the program is discontinued and/or cases are closed)
Note: Do not include cases where the infant 1) died, 2) was a non-resident or moved 3) parents refused, or 4) were in process). Cases that were “closed” or for which follow-up was discontinued prior to a diagnosis being received should be included.
Percentage Formula: number of infants not passing the final or most recent hearing screening that did not receive a documented diagnosis / number not passing the final or most recent hearing screening x 100%
Total number and percent of infants diagnosed with a permanent hearing loss documented as being enrolled in early intervention (including both Part C and Non-Part C services)
Percentage Formula: number of infants with a permanent hearing loss that are enrolled in early intervention / total number of cases with a permanent hearing loss x 100%
Total number and percent of infants with a permanent hearing loss receiving early intervention (including both Part C and Non-Part C services), who were documented as being enrolled in early intervention before 6 months of age
Percentage Formula: number of infants with a permanent hearing loss receiving early intervention, who were enrolled in early intervention before 6 months of age / total number of infants with a permanent hearing loss enrolled in early intervention x 100%
Total number and percent of infants with a permanent hearing loss not documented to be receiving any early intervention (excluding cases where the infant 1) died, 2) was a non-resident or moved or the 3) parents refused)
Percentage Formula: number of infants with a permanent hearing loss not documented to be receiving any early intervention / total number of cases with permanent hearing loss x 100%
Appendix D: Evaluation Guidance
Guide to developing evaluation plans
The evaluation plan should be consistent with the objectives being proposed during the grant cycle. The evaluation plan must state the specific purposes of the evaluation, which should be clearly aligned with the program activities proposed in the application.
The evaluation plan includes a description of process measures. This entails assessment of the EHDI surveillance process and coverage/acceptability of the surveillance system and activities.
Process: Briefly describe the essential features of the EHDI surveillance process (e.g. using a logic model) and define measures that focus on examining the implementation of the program and determining whether activities are being implemented as planned and whether inputs and resources are being used effectively.
Coverage/Acceptability: Define measures for evaluation of the coverage and acceptability of the proposed activities to determine whether these activities serve and meet the needs of the target clients/population they are intended to serve.
The evaluation plan should include a description of performance measures that will be used to assess effectiveness of the EHDI surveillance system and activities. The performance measures MUST include ALL performance metrics as described in Appendix C, plus other key indicators that the program will use to measure the success and accomplishment of the EHDI surveillance system and activities.
The evaluation plan should include a description of quality assurance measures that will be used to assess the accuracy, validity, and completeness of data reported to the state/territorial EHDI programs and to CDC. The evaluation plan should include a brief description of the protocol in place or planned for quality assurance/control, including: data validation at the individual level, de-duplication, and linkage (if applicable). The measures should include an assessment of the rate of missing/incomplete data for demographics, screening, diagnosis, and follow-up service, and how the EHDI-IS performs in terms of eliminating/minimizing inconsistent/incorrect data.
For all measures defined in the evaluation plan, identify potential sources and methods/tools for data collection.
The evaluation plan should specify a list of activities that will be conducted for evaluating process, performance, and data quality using the measures defined in the plan. Staff responsible and the timeline estimated for conducting the activities should also be included. For activities that span multiple funding years, include progress milestone for each budget year.
A template for developing an evaluation plan using this guide is provided on the next page.
Evaluation Goals: Be specific and aligned with the program activities proposed in the application. |
Evaluation Design and Implementation: Define the measures that will be used to assess the process, performance and data quality of the program and activities. Specify the source and method for data/evidence collection, and timeline and person responsible for a particular evaluation activity. When applicable, indicate whether an evaluation activity is also listed in the project work-plan. (You should still list the activity in the evaluation plan even if it has been included in the project work plan) |
||||||
Design |
Implementation |
||||||
Measures Define relevant, understandable, and useful measures for the evaluation. The measures can be either quantitative ( e.g. percentage of infant screened for hearing loss) or qualitative (e.g. what is the user acceptance level toward the EHDI-IS) |
Data sources More than one data sources can be used toward each measure. Examples of data sources include: the EHDI-IS and other public health database/information systems, hospital/provider EHR systems, records or charts, participant observations, interviews or surveys |
Methods/Tools Depending on the data sources, use proper and efficient method and/or tools for data collection. (e.g. review the raw data from the EHDI-IS, conduct a focus group study) |
Activities/Steps List specific tasks that the program plans to implement or already implemented for gathering evidence regarding the measure(s). An activity may cover multiple measures (e.g. a comprehensive study on data quality) , and a measure can be addressed using data drawn from several activities (e.g. use both a hospital staff survey and data from the EHDI-IS to assess the effectiveness of a newly implemented reporting protocol) |
Timeline For activities span multiple years, list milestones for each budget year |
Person responsible |
Part of the work-plan? Yes if the applicant choose to use a work plan to describe the project activities and the evaluation activities have also been included in the work plan. Also specify a reference to the activity in the work plan here |
|
Process: Briefly describe the EHDI process, attach the program logic model if available, and list the process measures below |
|||||||
Performance: List the performance measures below, including ALL performance metrics as described in Appendix C, plus any additional ones that the program consider as key for assessing success and accomplishment of the project |
|||||||
Data Quality: Briefly describe the quality assurance/control protocol, and list the quality measures below |
|||||||
Sample
Evaluation Plan
2009 CDC EHDI
Hearing Screening and Follow-up Survey (HSFS)
Directions
Please complete the following survey with the requested data for infants born between January 1, 2009 and December 31, 2009. The survey is divided into several parts and sections, which include Hearing Screening data, Diagnostic data, Early Intervention data, Type/Severity data, and Demographic data. Please enter any comments and/or caveats about the data reported in the Comments section at the end of the survey. Please note that only documented, non-estimated data should be reported on this survey.
Note: Data cannot be manually entered into fields highlighted in yellow. Data for these yellow fields will automatically be calculated based on the data entered into the non-highlighted fields. These calculated values will appear in the yellow boxes when you select the "Calculate Totals" button near the top of each page.
If you have any questions please refer to the 2009 survey explanations document or contact Marcus Gaffney at: [email protected] / (404) 498-3031.
The public reporting burden of this collection of information is estimated to average 4 hours per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to - CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS D-74, Atlanta, Georgia 30333 ATTN: PRA (0920-0733)
Part 1: Screening, Diagnostic, and Intervention Data
Hearing
Screening Diagnostic Intervention Type/Severity Finalize
2009 Documented Hearing Screening Data |
|
Total Occurrent Births |
|
Total Occurrent Births According to Vital Records |
|
Optional: Total Occurrent Births at Military Facilities According to Vital Records |
|
Optional: Total Occurrent Births at Military Facilities with Hearing Screening Results Reported to the EHDI Program |
|
Hearing Screening |
|
Total Documented as Screened |
(automatically calculated) |
Total Documented as Not Screened |
(automatically calculated) |
Infant Died |
|
Parents / Family Declined Services |
|
Missed |
|
Unknown |
|
Passed (final screen) |
|
Total Pass |
(automatically calculated) |
Pass Before 1 Month of Age |
|
Pass After 1 month but Before 3 Months of Age |
|
Pass After 3 Months of Age |
|
Pass: Age Unknown |
|
Not Passed (final screen) |
|
Total Not Pass |
(automatically calculated) |
Not Pass Before 1 Month of Age |
|
Not Pass After 1 month but Before 3 Months of Age |
|
Not Pass After 3 Months of Age |
|
Not Pass: Age Unknown |
|
Optional: Inpatient (IP) /Outpatient (OP) Screening Protocol Only |
|
Not Pass IP screen and did not Receive an OP Screen |
|
|
|
Total Occurrent Births (automatically calculated)* |
(automatically calculated) |
Calculate Totals (yellow fields)
Notes*
“Total Occurrent Births (automatically calculated)” is based on the sum of the values for Total Pass + Total Not Pass + Infants Died + Parents/Family Declined Services + Missed + Unknown. The field “Not Pass IP screen and did not Receive an OP Screen” is not included in the calculation of “Total Occurrent Births (automatically calculated)”
The value calculated for “Total Occurrent Births (automatically calculated)” should match the value entered for “Total Occurrent Births” at the top of this page. If there is any difference you will receive an error message.
Hearing
Screening Diagnostic Intervention Type/Severity Finalize
Calculate Totals (yellow fields)
2009 Documented Diagnostic Data |
|
Total Not Pass Screening |
(from Screening section) |
No Documented Hearing Loss |
|
Total with No Hearing Loss |
|
No Hearing Loss Before 3 Months of Age |
|
No Hearing Loss After 3 Months but Before 6 Months of Age |
|
No Hearing Loss After 6 Months of Age |
|
No Hearing Loss Documented: Age Unknown |
|
Documented Permanent Identified (ID) Hearing Loss |
|
Total Hearing Loss |
(automatically calculated)* |
Hearing Loss ID: Before 3 Months of Age |
|
Hearing Loss ID After 3 Months but Before 6 Months of Age |
|
Hearing Loss ID After 6 Months of Age |
|
Hearing Loss ID: Age Unknown |
|
No Documented Diagnosis / Undetermined |
|
Total with No Diagnosis |
(automatically calculated)* |
Audiologic Diagnosis in Process (Awaiting Diagnosis) |
|
Non-resident or Moved Out of Jurisdiction |
|
Infant Died |
|
Parents / Family Declined Services |
|
Parent / Family Contacted but Unresponsive |
|
Unable to Contact |
|
Unknown |
|
|
|
Total Not Pass |
(automatically calculated)* |
Optional: Documented Cases of Non-Permanent ID Hearing Loss |
|
Cases of non-permanent, transient hearing loss ID |
|
Note*: Only cases of hearing loss not reported in the above Diagnostics Section (e.g., cases of late-onset hearing loss) should be reported in the below “Hearing Loss not included in above Permanent Identified (ID) Hearing Loss” section.
Hearing Loss Cases not included in above “Permanent Identified (ID) Hearing Loss” (e.g., Cases of permanent late onset hearing loss)* |
|
Hearing Loss ID: Before 3 Months of Age |
|
Hearing Loss ID After 3 Months but Before 6 Months of Age |
|
Hearing Loss ID After 6 Months of Age |
|
Hearing Loss ID: Age Unknown |
|
Total Cases of Hearing Loss (not included above) |
(automatically calculated)* |
Hearing
Screening Diagnostic Intervention Type/Severity Finalize
Calculate Totals (yellow fields)
2009 Documented Intervention Data |
|
Total Cases Hearing Loss |
(from Diagnostic section) |
Referrals to Part C Early Intervention (EI) |
|
Total Referrals to Part C EI |
(automatically calculated)* |
Referred and Eligible for Part C EI |
|
Referred and Not Eligible for Part C EI |
|
Referred but Eligibility Unknown |
|
Not Referred to Part C EI and Unknown |
|
|
|
Total Referred, Not Referred, and Unknown |
(automatically calculated)* |
Enrolled in Part C Early Intervention (EI) |
|
Total Enrolled in Part C EI |
(automatically calculated)* |
Enrolled Before 6 Months of Age |
|
Enrolled After 6 Months but Before 12 Months of Age |
|
Enrolled After 12 Months of Age |
|
Enrolled: Age Unknown |
|
Monitoring Services |
|
Receiving Only Monitoring Services |
|
Receiving ONLY Intervention Services from Non-Part C EI |
|
Total from Non-Part C EI Services Only |
(automatically calculated)* |
Services Before 6 Months of Age |
|
Services After 6 Months but Before 12 Months of Age |
|
Services After 12 Months of Age |
|
Services: Age unknown |
|
No Intervention Services |
|
Total No Services |
(automatically calculated)* |
Not Eligible for Part C Services |
|
Infant Died |
|
Parents / Family Declined Services |
|
Non-resident or Moved Out of Jurisdiction |
|
Parent / Family Contacted but Unresponsive |
|
Unable to Contact |
|
Unknown |
|
Total Intervention & No Services |
(automatically calculated)* |
Cases of Hearing Loss not included in the above “Intervention” Section (e.g., Cases of late onset hearing loss) |
|
Hearing Loss Not included in above “Total Hearing Loss” |
(From Diagnostics Section) |
Total Enrolled in Part C EI |
|
Total Services from Non-Part C EI services |
|
No Intervention: Monitoring Only |
|
No Intervention: Unknown |
|
Hearing
Screening Diagnostic Intervention Type/Severity Finalize
|
Calculate Totals (yellow fields)
|
||||||||||
|
|
BILATERAL |
UNILATERAL |
LATERALITY UNKNOWN (for CASES where it is unknown if the loss is unilateral or bilateral) |
|||||||
|
|
|
|
||||||||
|
|
RIGHT EAR |
LEFT EAR |
UNKNOWN EAR (Note: record degree of loss for each ear) |
RIGHT EAR |
LEFT EAR |
UNKNOWN EAR |
|
|||
Sensorineural |
Mild |
|
|
|
|
|
|
|
|
||
Moderate |
|
|
|
|
|
|
|
|
|||
Severe |
|
|
|
|
|
|
|
|
|||
Profound |
|
|
|
|
|
|
|
|
|||
Unknown Severity |
|
|
|
|
|
|
|
|
|||
Conductive |
Mild |
|
|
|
|
|
|
|
|
||
Moderate |
|
|
|
|
|
|
|
|
|||
Severe |
|
|
|
|
|
|
|
|
|||
Unknown Severity |
|
|
|
|
|
|
|
|
|||
Mixed |
Mild |
|
|
|
|
|
|
|
|
||
Moderate |
|
|
|
|
|
|
|
|
|||
Severe |
|
|
|
|
|
|
|
|
|||
Profound |
|
|
|
|
|
|
|
|
|||
Unknown Severity |
|
|
|
|
|
|
|
|
|||
Type Unknown |
Mild |
|
|
|
|
|
|
|
|
||
Moderate |
|
|
|
|
|
|
|
|
|||
Severe |
|
|
|
|
|
|
|
|
|||
Profound |
|
|
|
|
|
|
|
|
|||
Unknown Severity |
|
|
|
|
|
|
|
|
|||
Auditory Neuropathy |
Mild |
|
|
|
|
|
|
|
|
||
Moderate |
|
|
|
|
|
|
|
|
|||
Severe |
|
|
|
|
|
|
|
|
|||
Profound |
|
|
|
|
|
|
|
|
|||
Unknown Severity |
|
|
|
|
|
|
|
|
|||
|
Totals by Ear* |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
||
Totals by Child |
|
|
|
|
|
|
|||||
|
|||||||||||
Note: The total of all the “Totals by Child” fields must be the same as number of the “Total Cases of Permanent Hearing Loss” reported in Part 1. If there is any difference between these values you will receive an error message. |
|||||||||||
Screening
Demographics Diagnostics Demographics Intervention
Demographics Finalize
|
Screening |
Diagnostics |
Intervention |
||||||||||
|
Total Occurrent Births |
Total Pass |
Total Pass Before 1 Month |
Total Not Pass |
Total Not Pass Before 1 Month |
Normal Hearing |
Normal Hearing Before 3 Months |
Hearing Loss |
Hearing Loss Before 3 Months |
Total Enrolled in Part C EI |
Total Enrolled in Part C EI Before 6 Months |
Total Services Non-Part C EI |
Total Services Non-Part C EI Before 3 Months |
Totals (from Part 1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
Male |
|
|
|
|
|
|
|
|
|
|
|
|
|
Female |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unknown |
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals (auto calculated) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Maternal Age |
|
|
|
|
|
|
|
|
|
|
|
|
|
<15 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
15-19 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
20 – 24 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
25-34 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
35 – 50 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
> 50 years |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unknown |
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals (auto calculated) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Mothers Education |
|
|
|
|
|
|
|
|
|
|
|
|
|
Less than High School |
|
|
|
|
|
|
|
|
|
|
|
|
|
High School Graduate or GED |
|
|
|
|
|
|
|
|
|
|
|
|
|
Some College or AA/AS degree |
|
|
|
|
|
|
|
|
|
|
|
|
|
College Graduate or above |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unknown |
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals (auto calculated) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Maternal Ethnicity |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hispanic or Latino |
|
|
|
|
|
|
|
|
|
|
|
|
|
Not Hispanic or Latino |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unknown |
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals (auto calculated) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Maternal Race |
|
|
|
|
|
|
|
|
|
|
|
|
|
White (Not Hispanic) |
|
|
|
|
|
|
|
|
|
|
|
|
|
White (Hispanic) |
|
|
|
|
|
|
|
|
|
|
|
|
|
White (Ethnicity Unknown) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Black or African American (Not Hispanic) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Black or African American (Hispanic) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Black or African American (Ethnicity Unknown) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Asian |
|
|
|
|
|
|
|
|
|
|
|
|
|
Native Hawaiians & other Pacific Islanders |
|
|
|
|
|
|
|
|
|
|
|
|
|
American Indian & Alaska Natives |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unknown |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
|
|
|
|
|
|
|
|
|
Totals (auto calculated) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hearing
Screening Diagnostic Intervention Type/Severity Finalize
Dear Respondent:
Thank you for completing this survey Please enter any comments and/or caveats about the data reported in the below Comments section. To submit the survey click the red “Submit Survey” button located below the Comments box.
Comments |
|
Submit
Survey
Appendix F: Enhancements for EHDI-IS/EHR Interoperability
As an EHDI programs upgrades their information system, it shall utilize, where available, health information technology systems and products that meet recognized interoperability standards. EHDI-IS enhancements can employ an incremental approach to adopting standards, implementation specifications, and certification criteria to enhance the interoperability, functionality, utility, and security of health information technology and to support its meaningful use. Funding may be used to plan, enhance, adopt, and apply nationally endorsed health information technology standards, implementation specifications, and certification criteria for direct health care system interoperability.
The purpose of these enhancement activities is to increase the EHDI program’s operational capacity to exchange data accurately, effectively, securely, and consistently between EHDI-IS and EHRs with a specific focus on reducing the duplicate data entry burden on providers and a reduction in loss to recommended follow-up services (screening, diagnosis, and intervention). Enhanced EHDI-IS/EHR interoperability will improve the completeness of meaningful health information available to clinicians and public health, the timeliness of EHDI data submission to an EHDI-IS, and the quality of EHDI-IS data. Improved interoperability may also reduce the number of infants lost to recommended follow-up services, thereby saving time and resources.
To conduct these enhancement activities, the awardees should consider proposing a phased approach such as the following:
PHASE ONE: Environmental Scan and Gap Analysis
Review the current EHDI-IS software application to identify gaps and compatibility with nationally recognized standards, implementation specifications, and certification criteria for EHDI-IS/EHR interoperability during the funding period
Submit an EHDI-IS/EHR Interoperability Assessment Report at the end of Phase One
PHASE TWO: Implementation Plan
Develop a plan to replace or modify the EHDI-IS software application for compatibility with nationally recognized standards, implementation specifications, and certification criteria for EHDI-IS/EHR interoperability
The Implementation Plan describes how EHDI-IS/EHR interoperability improvements will be deployed, installed and transitioned into the health care provider environment. The plan shall contain an overview of the EHDI-IS and EHRs, a description of the major tasks involved in the implementation, the overall resources needed to support the implementation effort (such as hardware, software. facilities, materials, and personnel), and any provider site-specific implementation requirements. The plan should include, at a minimum:
a. Description of the implementation
b. Description of an evaluation plan to assess the effectiveness of EHDI-IS interoperability
Include objectives and benchmarks that are specific, measurable, achievable, realistic and can be achieved within the specified funding period
Describe the method of evaluating success or accomplishment of each objective
c. Major tasks. Examples of major tasks are the following:
i. Providing overall planning and coordination for the implementation
ii. Providing appropriate training for personnel
iii. Providing all needed technical assistance
iv. Performing site surveys before implementation
v. Ensuring that all prerequisites have been fulfilled before the implementation date
vi. Providing personnel and training for the implementation team
vii. Acquiring special hardware or software
viii. Preparing site facilities for implementation
d. Implementation schedule (timeline) for reaching each objective
f. Staffing Plan to include key person(s) responsible for the task. Identify staff members by name and title
g. Resources and budget required to accomplish the task
Submit an EHDI-IS/EHR Interoperability Implementation Plan at the end of the Phase Two
PHASE THREE: Implementation
Conduct pre-interoperability enhancement benchmarking of all measureable outcomes included in the Interoperability Implementation Plan, including the number of EHRs electronically exchanging data with EHDI-IS
Conduct and test modifications to enhance the EHDI-IS software application for compatibility with nationally recognized standards, implementation specifications, and certification criteria for EHDI-IS/EHR interoperability
Implement enhancement modifications to the EHDI-IS software application for compatibility with nationally recognized standards, implementation specifications, and certification criteria for EHDI-IS/EHR interoperability
Work with existing certified hospital-based vendors to implement developed specifications, install software enhancements, and provide training at the number of EHR sites identified in the Interoperability Implementation Plan
Conduct post-interoperability enhancement of all measureable outcomes included in the Interoperability Implementation Plan, including the number of EHRs electronically exchanging data with EHDI-IS
Submit an EHDI-IS/EHR Interoperability Implementation Report at the end of the Phase Three
PHASE FOUR: Sustainability Plan
The Sustainability Plan describes how EHDI-IS-EHR interoperability improvements will continue to be deployed, installed and transitioned into the health care provider environment after the period of performance for this project ends.
Submit an EHDI-IS/EHR Interoperability Sustainability Plan at the end of the Phase Four
Additional Resources:
The CDC Unified Process (CDC UP) provides guidance in the form of a best practice systematic approach to managing projects. It includes a set of recommended adaptable templates designed to be to meet the needs of each project. The templates include instructions and boilerplate language to make them readily useful to project teams.

Appendix G: EHDI State and Territorial Profiles
<Jurisdiction>
This profile includes information about a state or territorial EHDI program related to general program information, hearing screening, re-screening and diagnostic evaluations, early intervention, and the EHDI data system.
Category and Questions |
Information
|
General Program Information |
|
Official name of the state/territory Early Hearing Detection and Intervention (EHDI) program |
|
Contact for the state/territory EHDI program. Name: Adress: Phone: Email: |
|
Legislation regarding newborn hearing screening? If yes, does the legislation mandate or provide funds for newborn hearing screening and follow-up services? |
|
State/territory website related to infant/child hearing loss? |
|
State/territory CDC/EHDI Cooperative Agreement related to hearing screening? |
|
State/territory have a Maternal and Child Health Bureau Grant related to hearing screening? |
|
Participate in a CDC funded research project? |
|
Hearing Screening Information |
|
State/territory written guidelines and/or protocols for performing hearing screenings? |
|
Primarily responsible in most hospitals for conducting in-hospital hearing screenings? |
|
Estimated percentage of newborns that are initially screened with OAE or AABR. (Of the newborns that failed the initial screen, estimated percentage that are re-screened in the hospital with OAE or AABR.) |
|
State/territory requires parental consent for hearing screening(s) to be done at the time of birth? |
|
What happens if a baby does not pass the initial hearing screening(s)? |
|
Birthing hospitals/facilities/providers required to report hearing screening results to the state? |
|
How birthing hospitals/facilities report hearing screening information to the state/territory |
|
Hearing screening results reported to the infant’s physician? [If yes, are all results reported or only failed screening?] |
|
Rescreening and Diagnostic Evaluations |
|
State/territory written guidelines and/or protocols for performing hearing re-screenings? |
|
State’s program guidelines for helping families with third party preauthorization of re-screening or diagnostic evaluations? |
. |
State/territory written guidelines and/or protocols for performing diagnostic evaluations? |
|
State/territory EHDI program list of audiologic diagnostic centers and audiologists skilled in providing pediatric services to infants? |
|
Number of pediatric audiologists and/or diagnostic centers on the list |
|
Who is responsible for scheduling appointments for outpatient hearing re-screenings? |
|
Who is responsible for scheduling appointments for diagnostic audiologic evaluations? |
|
How audiologists report diagnostic audiological evaluation results to the state/territory |
. |
Guidelines and/or protocols for audiologists to report diagnostic audiological evaluation results to the state/territory? |
. |
One or more persons on the EHDI staff who are responsible for the follow-up of families who need to return for re-screen, diagnostics, or referral to intervention? |
|
Early Intervention |
|
Lead agency for the Part C Early Intervention Program |
|
State/territory written guidelines, and/or protocols for providing intervention services for children with hearing loss? |
|
Eligibility criteria for Part C services for infants and toddlers with hearing loss |
|
Eligibility criteria for Part B services for preschool children with hearing loss |
|
Children with mild or unilateral hearing loss eligible for services under Part C or Part B? |
|
Other public or private programs(s) and services (other than Part C or Part B) that provide intervention services to children with hearing loss |
|
Services such as family support, transportation to follow-up appointments, parent to parent available to families with children with hearing loss? List. |
|
Program receives information regarding whether or not a referred child is receiving Part C services or other intervention services? |
. |
Intervention programs report other information about children, such as type of intervention, developmental test scores, use of assistive devices? |
|
EHDI Data System |
|
State/territory written guidelines and/or protocols related to the EHDI tracking system? |
|
Type of system program uses to track hearing screening and follow-up information. . |
|
State EHDI tracking system includes data items to identify infants and children with risk factors for hearing loss? |
|
Unique identifier is used to identify infants/children in the state/territory EHDI tracking system |
|
How program addresses de-duplication of screening and diagnostic evaluation data
|
|
EHDI system linked to or integrated with any of the following: -Blood spot card -EBC -Audiology -Early Intervention -Immunizations -Other |
|
Other EHDI Questions |
|
State materials/ brochures/ protocols for parents and professionals about the EHDI program. (Link to matrix).
|
|
Agencies, foundations, organizations, or other programs that provide funding for the purchase of any of the assistive devices for children with hearing loss |
|
Statewide hearing aid loaner program for infants, toddlers, and children with hearing loss? |
|
Resources, other than those from the state or territory, available to help families with the costs of caring for an infant, toddler, or child with a hearing loss? |
. |
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Centers for Disease Control & Prevention |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy