1 Clinician survey
HIV Clinician Workforce Study
HIV Clinician Workforce Study - Final Design Report (To HRSA)
HIV Clinician Survey
OMB: 0915-0349
Mathematica Policy Research
Ellen Bouchery
Boyd Gilman
Julie Ingels
Margaret Hargreaves
Cicely Thomas
The Lewin Group
Paul Hogan
Namrata Sen
Rita Furst Seifert
Rod Hooker




Improving
public well-being by conducting high-quality, objective research and
surveys
Princeton,
NJ ■
Ann Arbor, MI ■
Cambridge, MA ■
Chicago, IL ■
Oakland, CA ■
Washington, DC
Mathematica®
is a registered trademark of Mathematica Policy Research
www.mathematica-mpr.com



CONTENTS
I Introduction
A. Objectives of the Model
B. Overview of Approach
1. Primary Data and Information Sources
2. Components of the Model
II Model design
A. Baseline Estimates of Supply and Demand
1. Baseline Supply of HIV Clinicians
2. Baseline Demand for HIV Clinicians
B. Estimating Excess Demand
1. Needs-Based Estimate of Demand
2. Market-Based Estimate of Demand
C. Projecting Supply and Demand
1. Projecting Supply of HIV Clinicians
2. Projecting Demand for HIV Clinicians
references
APPENDIX
A: methodology for identifying hiv clinicians
from claims
data
APPENDIX
b: icd-9-cm and cpt codes for identifying TREATED
patients
with HIV infection
APPENDIX
c: national drug codes for identifying TREATED
patients with
HIV infection
This page has been left blank for double-sided copying.
TABLES
II.1 Number of FTE Clinicians in Total and HIV Care
II.2 Open
Vacancies for Funded HIV Clinicians and Length of Time
to Fill
Position
II.3 Summary of Supply-Side Factors
II.4 Summary of Demand-Side Factors
This page has been left blank for double-sided copying.
FIGURES
II.1 Approach to Estimating FTE Physician Supply
II.2 Approach to Projecting Total FTE Supply of HIV Clinicians
The purpose of this report is to provide a comprehensive description of our methodology for developing, estimating, and projecting the HIV clinician supply and demand model. We have tailored the approach to the specific characteristics of the HIV clinician workforce, and have incorporated comments made by participants at the expert consultation meeting held on February 23-24 in Washington, DC. In the next section, we outline the objectives of the model. Then we provide an overview of the primary data and information sources that will be used to develop the model and the basic components of the model.
The objective of the study is to provide the HIV/AIDS Bureau (HAB) in the Health Resources and Services Administration (HRSA) with national- and regional-level estimates of the number of clinicians providing HIV-related medical care and projections of the magnitude of expected HIV clinician shortages or surpluses in the future.
The primary research questions related to the HIV clinician supply and demand model are as follows:
How many clinicians currently provide HIV-related medical care in Ryan White- and non-Ryan White-funded settings and what are their characteristics?
What is the current market demand and need for HIV-related clinicians? What will be the market demand and need for HIV-related clinicians in the future?
What specific factors will influence the market demand and need for HIV-related clinicians in the future?
What specific factors will influence the supply of HIV-related clinicians in the future?
Will the projected supply of HIV-related clinicians in the future be sufficient to meet the demand and need for HIV-related care?
How does HIV workforce capacity vary by type of clinician, practice setting, and geographic region?
We designed the HIV clinician supply and demand model to address these research questions. We model baseline and projected supply and demand by geographic location so that regional variation in workforce capacity issues can be addressed.
In this section we provide an overview of our approach to the model. First, we highlight the primary data and information sources that will provide the basis for our estimates. Then we provide a brief overview of our approach to developing estimates for each component of the model.
1. Primary Data and Information Sources
In this section we provide an overview of the primary data and information sources that will provide the basis for the model. The primary data sources include:
Expert Consultation Meeting. The project team convened and facilitated an expert consultation meeting on February 23-24, 2011. The participating experts and staff from HRSA reviewed the proposed model design and provided feedback. We revised the model design based on this feedback.
Clinical Consultants. The project team includes several clinical consultants with experience providing HIV medical care in a range of settings and geographic locations. On an ad hoc basis, the project team will consult with these individuals to obtain information on clinical topics.
National HIV Clinic and Clinician Workforce Surveys. We will conduct two nationally representative surveys, one of HIV clinicians who bill independently for their services and the other of the clinics in which they practice as part of this study. The clinician (individual-level) survey will include questions on provider demographic and professional characteristics, hours worked in total and HIV patient care, practice setting characteristics, and strategies for increasing HIV clinician workforce capacity. The clinic (organization-level) survey will include questions on facility characteristics, workforce capacity characteristics, organizational characteristics, patient characteristics, staffing and patient management practices, and output measures.
Claims Data. We will use ambulatory medical and pharmacy claims data representing Medicare and Medicaid fee-for-service beneficiaries and the commercially insured population to identify physicians, nurse practitioners, and physician assistants who bill for services and provide a minimum level of HIV care. We will also use these data to understand utilization and practice patterns to support our model assumptions.
Centers for Disease Control and Prevention (CDC) Surveillance System. The CDC surveillance system provides counts of the number of individuals living with HIV and AIDS by demographic and clinical characteristics including age, gender, geographic location, and AIDS diagnosis. They also provide estimates of undiagnosed cases. These data will form the basis of our demand assumptions.
Other Existing Data Sources. We will use other existing sources of utilization and provider characteristics data to support the study. In particular, the National Center for Health Statistics (NCHS) health care utilization surveys will help inform the demand analysis, and data from the Surveys of Physicians Over/Under 50 conducted jointly by the American Association of Medical College (AAMC) and the American Medical Association (AMA) will provide information on physician retirement and hours worked by age and gender. In addition, we will supplement our counts of HIV providers with membership and certification data from the HIV Medicine Association (HIVMA) and American Academy of HIV Medicine (AAHIVM), as well with lists of attendees at the 2010 national HIV/AIDS Clinical Conference and participations in regional AIDS Education and Training Center (AETC) training programs.
In the next section, we provide information on how these data and information sources will be used to support the model.
2. Components of the Model
We divide the model into three components: (1) baseline or the current stock of supply and demand, (2) estimates of excess demand, and (3) projected supply and demand. We address each of these components below.
Baseline Supply and Demand
Our baseline estimates will be developed for 2010, the most recent year for which claims data are available for our analysis. We provide an overview of our approach to measuring baseline supply and demand here.
Baseline Supply. Because no specific credential or specialty exists that is common across physicians providing HIV care, we will identify physicians who appear to focus on HIV care based on the services they provide as reported in ambulatory medical and pharmacy claims data. Because nurse practitioners and physician assistants often do not bill directly for their services, we will supplement our estimate of the number of nurse practitioners and physician assistants currently providing HIV services based on data reported in this study’s clinician survey, using the average number of nonphysician clinicians providing HIV care per physician providing HIV care.
Baseline Demand. We will develop two estimates of baseline demand: market-based and needs-based demand. Market-based demand, as defined in this study, is the effective demand for services observed in the healthcare market today. We will estimate market-based demand based on observed utilization of HIV services nationally. The foundation for our needs-based demand estimate will be the HRSA clinical guidelines for the treatment of HIV/AIDS. We will review these guidelines and current utilization levels with clinical experts to obtain their input on how the guidelines translate into clinician time and how observed utilization patterns today would need to shift to achieve the optimal standards reflected in the guidelines.
Excess Demand
We will also develop two estimates of current excess demand: market-based and needs-based. Excess demand is the amount of demand for care that cannot be met by current supply. The market-based estimate will rely primarily on findings from the clinic survey as measured by (1) the difficulty of hiring clinicians, (2) the difference between the number of open positions and the number of new entrants, and (3) measures of patient access to care. The needs-based estimate of excess demand will be calculated as the difference between the baseline estimate of needs-based demand and the baseline supply of care.
Projected Supply and Demand
We will project supply and demand from 2010 through 2015. To capture the effect of retirement among the second half of the baby boom generation, we will also discuss with HAB the value of projecting HIV clinician supply through 2020. We provide a brief overview of our approach to projecting supply and demand here.
Projected Supply. We will estimate active clinician supply in the next year as clinician supply in the current year plus new entrants minus attrition. We will calculate our estimate of new entrants as a share of recent graduates. We will base our estimate of attrition on retirements and mortality. During the expert consultation meeting, the majority view was that mid-career entrance into and exit from HIV clinical care was rare; participants explained that most clinicians enter HIV care early in their career and remain in HIV care until they retire. Thus, we do not plan for these mid-career shifts to be a significant component of entry and exit in the model. Nevertheless, we will test this conclusion in the clinician survey. Supply projections will also allow for simulation of the impact of changes in productivity and substitution of supply across provider type and medical specialty on the capacity of the HIV clinician workforce.
Projected Demand. We will develop two distinct estimates of projected demand: market-based and needs-based. We will base both estimates on the same set of factors. However, the baseline assumptions for the two projections will be distinct. We will derive market-based demand estimates from observed utilization patterns in the market. We will derive needs-based demand estimates from normative assumptions and recommended treatment guidelines about the optimal use of services among people with HIV and AIDS. We will adjust both estimates for changes in population size, prevalence of HIV, and service use per individual with HIV over time relative to their baseline estimate. For both demand projections, the model will allow for simulating the impact of changes in diagnosis rates, economic growth rates, the distribution of insurance coverage, and advances in clinical treatments for HIV.
In the next section, we further detail our approach to each of these components.
This page has been left blank for double-sided copying.
This section discusses our approach to model development. We first discuss our plan for estimating baseline supply and demand. Then, we discuss how we will estimate whether current supply and demand are in equilibrium. Finally, we discuss our methodology for projecting HIV clinician supply and demand from 2010 to 2015.
A. Baseline Estimates of Supply and Demand
In this section, we present our approach to estimating the baseline supply of and demand for HIV clinicians. To estimate this baseline, we use observed data on the number of clinicians currently providing HIV-related health care services and the number of services they currently provide. Because there is a lag between the provision of health care services and the availability of data for research on these services, the most recent period of observed data available for analysis from most of the sources for this study will be 2010.1 Therefore, baseline estimates will be for 2010. We will project supply and demand from 2010 through 2015.
1. Baseline Supply of HIV Clinicians
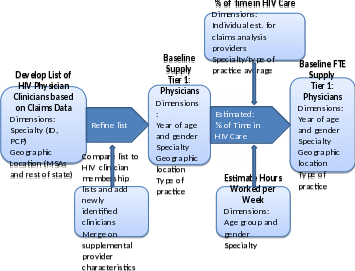
Within the baseline HIV clinician supply model, we will develop baseline counts (or stock) of currently practicing clinicians with the following dimensions:
Age/Gender. Within the model, counts of physicians will be available by year of age and gender. Counts of nonphysician clinicians may not available by age/gender.
Provider Specialty/Type. We will organize the clinician workforce into four types/specialties. These will be (1) physicians specializing in infectious disease; (2) primary care physician (including internal medicine, family/general medicine, pediatrics, and geriatrics); (3) nurse practitioners, and (4) physician assistants.
Geographic Location. We will develop clinician counts for the eligible metropolitan areas (EMAs) and transitional grant areas (TGAs) defined under the Ryan White program, as well as other metropolitan statistical areas (MSAs) defined by the United States census bureau. For each state, we will group rural areas not otherwise included in these metropolitan jurisdictions and analyze them separately.
Type of Practice. Because individual clinicians may organize their time across care settings differently, we will develop estimates of the total number of clinician hours and the share of total hours dedicated to HIV patient care by type of primary practice. We will define the practice type categories based on the survey data, but they may include (1) community health centers, (2) hospital-based ambulatory care clinics, (3) community-based organizations, (4) health department clinics, and (5) private physician practices. We will also consider developing separate supply estimates for Ryan White-funded clinics versus non-Ryan White-funded primary and specialty ambulatory care settings.
Including this level of detail within the model will enable us to profile the clinician workforce by age, gender, provider type and specialty, geographic location, and type of practice. We can also use details on clinician characteristics, such as age and gender, to develop a foundation for projection assumptions, such as retirement rates.
We will measure the baseline count of HIV clinicians in two ways. First, we will present the “active supply.” This count is simply the number of active clinicians in each year providing a minimum threshold of HIV care. The second measure is the full-time equivalent (FTE) supply. This measure normalizes the count of clinicians by average weekly hours worked in HIV care per clinician relative to the average hours worked by all HIV-related clinicians in all types of patient care in 2010. For example, if a given HIV clinician works 30 hours per week in HIV-related care and the average number of patient care hours worked across all HIV clinicians is 40 hours per week, the FTE supply of that given HIV clinician would be 0.75 or 75 percent of the active supply. The FTE supply provides a more precise measure of the supply of HIV services that each active clinician can be expected to produce. If many HIV clinicians work part-time or devote a substantial share of their time to general primary care or other infectious disease care, for example, then the level of HIV services each active clinician can be expected to provide will be reduced accordingly. Next, we describe our approach to estimating the baseline active supply. Then, we discuss our approach to estimating the baseline FTE supply.
Estimating Baseline Active Supply
One of the most difficult challenges in the model development is identifying how many physicians, nurse practitioners, and physician assistants provide HIV-specific services nationally. The master file of the AMA is often used to provide an estimate of the current number of physicians of a given medical specialty. Information on board certification, fellowship/residency training, or self-reported specialty in the AMA file is used to infer medical specialty. However, there is no explicit credential or self-reported specialty for those who provide, focus on, or specialize in the provision of services to HIV patients. Moreover, many of those who focus on providing HIV-related health care services do not do so exclusively. A primary care physician might, in addition to providing care to a significant number of HIV patients, provide primary care services to a general patient population, and an infectious disease specialist who focuses on HIV might also treat patients with other infectious diseases. Although the HIVMA and AAHIVM offer credentialing in HIV medicine, many physicians providing HIV care do not have this certification. This is also true for physician assistants and nurse practitioners providing care to HIV patients. There is no specific required credential or list of professionals that we can use to estimate the baseline supply of HIV clinicians for this study.
As a result, we propose using a two-tiered approach to identifying the baseline supply or stock of HIV clinicians. The first tier will focus on physicians and mid-level clinicians who independently bill for their services. We will organize physicians into two groups: primary care and infectious disease specialists. The second tier of clinicians will include physician assistants and nurse practitioners, some of whom may not be able to bill independently or do not bill under their own name. We will base our approach to identifying the number of clinicians in the first tier primarily on prescription drug and other HIV-related ambulatory medical claims data. These data enable us to link the delivery of a particular type of service—medical treatment of HIV patients—with the billing clinician to discern which clinicians focus on the provision of services to patients with HIV. Physicians and mid-level providers who bill independently are identifiable in claims data. Because clinicians in the second tier often do not bill for their services independently, we will estimate the number of each of these clinician types currently providing HIV services based on data reported in the HIV clinic workforce survey to be conducted as part of this project.
Tier One: Baseline Count or Stock of HIV Physicians.2 Given the lack of an established credential for physicians and other nonphysician clinicians providing HIV services, we propose to define HIV clinicians based on the services they provide and for which they bill. Using prescription drug and other ambulatory medical claims data, we will identify HIV services based on HIV-related diagnosis, procedure, and drug codes. Then, for each clinician providing and billing for HIV care, we will determine the total number of visits and/or prescriptions and the percentage of the visits and/or prescriptions provided that are for HIV care. We will include clinicians exceeding a minimum threshold in the number of visits and/or prescriptions or the share of visits and/or prescriptions that are for HIV care in our list of HIV clinicians. Alternatively, we can use the number and share of patients treated for HIV to identify HIV clinicians. We will determine this minimum threshold empirically (in consultation with HAB and clinical experts) based on an analysis of the claims data; it could vary by provider type. We will establish this threshold high enough to filter-out episodic providers (such as emergency department physicians or medical residents), but low enough to capture a substantial majority of HIV care. We will also test various combinations of thresholds based on pharmacy and medical claims and assess the effect of each algorithm on the selected list of clinicians before making the final determination.3
The detail on clinician characteristics will enable us to profile the HIV clinician workforce by age, gender, provider type and specialty, geographic location, and type of practice. We will also be able to also use the details on clinician characteristics to develop a foundation for projection assumptions, such as differences in retirement rates by age category or differences in number of hours worked between men and women. We are currently reviewing these sources for this information.
The services included in the claims data that we will use to identify HIV clinicians do not comprehensively reflect HIV services provided nationally. As a result, by using claims, we might inadvertently exclude subsets of clinicians who provide services to individuals not represented in these data, such as the uninsured. To address this limitation, representatives of HIVMA and AAHIVM have agreed to provide data from their membership lists for this study. We will compare their membership lists with the list of clinicians providing HIV services derived from the claims analysis. If substantial numbers of physicians in the membership lists are not included on the list derived from the claims analysis, we will work with the medical associations to understand these gaps. We will supplement the clinicians identified through the claims analysis with those who appear on the organization membership lists only. We will also match and supplement the claims-based list of clinicians with individuals who attended the 2010 national HIV/AIDS clinical conference and/or participated in the regional AETC training sessions.
This list of physicians and nonphysician clinicians who independently billing for HIV services will give us our baseline count or stock of active supply of Tier One HIV clinicians.
Tier Two: Baseline Count of Nonphysician HIV Clinicians. The claims analysis will identify some nonphysician clinicians who can bill independently. We will include these clinicians in our list of tier one HIV clinicians. However, we expect representation of these nonphysician clinicians to be limited and potentially biased. We will address this limitation through the HIV clinic survey. This survey will be sent to a sample of the clinics in which the clinicians identified in tier one practice. To estimate the number of nonphysician clinicians providing HIV services who are not billing independently, we will include the following question on the survey (see Table II.1):
We are interested in the number of clinician FTEs in this clinic and the share of these FTEs that is allocated to caring for patients with HIV or AIDS. In column A, please indicate the number of clinician FTEs in this clinic providing patient care in general. In column B, please indicate the number of clinician FTEs devoted to HIV patient care.
Table II.1. Number of FTE Clinicians in Total and HIV Care
Type of Clinician |
Column
A |
Column
B |
Infectious disease specialists |
|
|
Primary care physicians |
|
|
Physician assistants |
|
|
Nurse practitioners |
|
|
Note: Primary care physicians include internal medicine, family/general medicine, pediatrics, and geriatrics.
We will use responses to this survey question to estimate the ratio of nonphysician HIV clinicians to physicians providing HIV services. We will assess the variation in this ratio across practice settings and geographic areas (for example, regions and urban versus rural areas) and incorporate it into the baseline estimate of nonphysician clinician supply. Then, the number of nonphysician clinicians nationally will be calculated for each geographic areas and practice settings and nationally, based on the number of physicians identified in tier one and this ratio.
Estimating Baseline FTE Supply
The clinic survey questions related to nonphysician clinicians will ask for FTE clinicians only. That is, if an administrator respondent has two part-time nonphysician clinicians in his or her practice each working 20 hours per week, the respondent will be asked to report this as one FTE clinician assuming full-time is 40 hours per week.4 Thus, estimates of nonphysician clinicians (tier two) will be produced only as FTE supply. To translate the active supply of HIV physicians (tier one) into FTE supply, we will need to apply two supply-intensity adjustments for each HIV physician identified in our baseline count of active HIV physicians. These adjustments will be
Percentage of total time spent in HIV-related patient care5
Expected hours worked per week relative to the average HIV physician
Percentage of Time in HIV Patient Care. We will estimate the percentage of time a clinician spends in HIV patient care based on the claims analysis. For each physician specialty in the study, we will establish a threshold share of visits, prescriptions, or patients with HIV diagnoses or services above which the clinician will be considered to be fully engaged in HIV care. Then, for each active clinician identified in the claims analysis, we will calculate the share of visits, prescriptions, or patients for HIV patient care relative to this threshold to yield the clinician’s percentage of time in HIV care. For example, if the threshold number of visits per year is 100, then we will consider a clinician with 50 visits reported on the claims data to be 0.5 FTE. Alternatively, we can determine a patient threshold (say, 20 patients in care) to identify full-time HIV physicians. If we set the patient threshold at 20 patients, then we would consider a physician with only 10 unique patients observable from the claims data to be 0.5 FTE. We will use findings from the national HIV clinician workforce survey to refine this calculation. For physicians identified in the claims analysis we will estimate this measure empirically. For physicians identified through other potential sources—such as HIV provider association membership—we will assign the average share of time in HIV care for physicians with the same personal and practice characteristics.
Expected Hours Worked Relative to the Average HIV Physician. The expected relative hours worked for each active physician will be assigned based on the estimated average hours worked for physicians of the same age and gender. We will develop estimates of hours worked specific to HIV clinicians based on our study’s HIV clinician survey by including questions on age, gender, and mean weekly hours spent in all patient care generally and HIV patient care specifically across all practice locations. The benefit of using our study’s survey is that the results will be specific to physicians providing HIV care. We will compare the results from our survey to national norms for physician hours worked for primary care physicians and medical specialties from existing surveys. Existing surveys include a survey sponsored by the Bureau of Health Professions (BHPr) during 2002 and 2003 that collected information on patient care hours worked and the AMA/AAMC’s Survey of Physicians Over/Under 50.
We will use Equation (1) to calculate FTE clinician supply based on these two adjustments. The FTE supply for each physician equals the share of time in HIV-related patient care multiplied by the ratio of expected hours spent in HIV-related care relative to the average hours worked for all HIV physicians. We will add FTE supply across physician HIV providers (i) to yield the estimate of total FTE supply of HIV physicians.
Eq. (1)
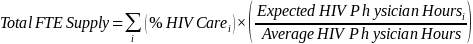
Figure II.1 below illustrates our approach to estimating the baseline supply or stock of FTE physicians.
Figure II.1. Approach to Estimating FTE Physician Supply
2. Baseline Demand for HIV Clinicians
Within the model, we will develop baseline estimates of demand based on counts of patients with HIV or AIDS. We will disaggregate patients into cells based on the following dimensions:
Patient Age and Gender. Within the model, we will develop estimates of demand by patient age group and gender.
Geographic Location. We will develop HIV patient counts by geographic location at multiple levels. Geographic areas modeled will include EMA and TGA jurisdictions funded under the Ryan White program, MSAs, and nonmetropolitan areas within each state. Using Part A and MSA designations, which can cross state boundaries, will help us develop estimates based on actual patient flow patterns. We will also develop demand estimates for all non-metropolitan areas within each state.
Insurance Coverage. We will estimate the distribution of HIV patients by insurance status and type of insurance, including privately insured, Medicaid only, Medicare, and uninsured.
AIDS Status. We will organize HIV patients into two groups, one based on diagnosis of HIV infection only and one based on having an AIDS-defining condition.
For each of these cells or subgroups, we will estimate the level of HIV care demanded and how this care is currently distributed by provider type.
We will estimate baseline market demand for clinical services per individual with HIV infection or AIDS diagnosis based on two separate components. These components are
Population. This measure includes the number of individuals living with HIV infection only or with an AIDS-defining diagnosis.
Utilization of HIV Services. This measure can be based on counts of visits or prescriptions or on medical expenditures. All utilization measures will be for HIV care only and will vary by patient subgroup.
We will disaggregate both of these components (prevalence and service use) into cells defined by patients’ age group, gender, geographic location, health insurance coverage, and AIDS status. We will divide the estimate of service utilization for each of these cells by the estimate of the United States population living with HIV infection only or an AIDS-defining diagnosis for the respective cell to calculate the demand per individual living with HIV infection or AIDS diagnosis in each cell. Baseline estimates of demand will reflect only those individuals who are currently diagnosed and in care. In the projections of future demand, we will consider how increased screening and diagnosis and improvements in retention in care will affect demand. Next, we describe in more detail the data sources for developing population and utilization of service estimates.
Population
We will obtain counts of the number of individuals living with HIV infection and AIDS from the Centers for Disease Control and Prevention (CDC). These counts are available by age group, gender, geographic location, and AIDS status. The CDC does not provide information on the insurance status of individuals with HIV. We will review the literature to determine whether another source for estimating this dimension exists.
Service Utilization
We will consider three potential sources for estimating market utilization of services among individuals living with HIV infection or an AIDS diagnosis:
National Center for Health Statistics (NCHS) National Health Care Utilization Surveys. NCHS offers three nationally representative provider surveys of health care utilization, each representing a different type of care. These are the National Ambulatory Medicare Care Survey (NAMCS), National Hospital Ambulatory Medicare Care Survey (NHAMCS), and the National Hospital Discharge Survey (NHDS). We will use information on patient diagnoses in these surveys to identify HIV-related care. These surveys also provide information on patient demographics, such as age, gender, urban/rural location, and health insurance coverage. Physician specialty is included so that utilization can be disaggregated by type of clinician. The strength of these surveys is that they are nationally representative and they include uninsured patients who would receive services through Ryan White clinics.
Medicaid, Medicare, and Private Health Insurance Claims Data. We can also use the claims to identify service utilization per unique HIV or AIDS patient represented in these data. The claims data include patient demographic information, such as age, gender, and location. The claims also include physician specialty, so we can disaggregate utilization by physician specialty. However, the population included in claims data may not be nationally representative; these data sources would not include information on services provided to uninsured patients and medical services not covered by health plans.
Ryan White HIV/AIDS Program Services Reports. Ryan White HIV/AIDS Program Services Reports (RSRs) include client-level data on client characteristics and services provided to clients of Ryan White-funded clinics. These data reflect only services provided through the clinics. Despite that limitation, this is an important segment of the current care system and may provide a useful source of information for estimating demand for medical care.
The NCHS surveys are likely to provide a good source of data for the market demand analysis because they are nationally representative and include a variety of insurers and care settings. However, the data in the NCHS surveys provide less detail on providers and services than is likely to be available through the other sources. We believe these data comprehensively represent HIV services. Pooling multiple years of the NCHS survey data might be necessary to get a sufficient sample of HIV services.
The NCHS surveys provide estimates of market utilization nationally. The Medicaid, Medicare, and private health insurance claims data also provide a promising source for the demand analysis. However, we would have to adjust utilization estimates derived from these data based on the subset of the national population represented. For example, the claims analysis will not represent services provided to the uninsured (including those covered under the Ryan White program), but the NCHS survey data will include these services. Similarly the claims analysis includes services for only a subset of the commercially insured population. Given the limitations of each of the three data sources, no single data source is likely to be able to provide a reliable estimate of the national average level of service utilization per individual living with HIV. Thus, the sources will be used in combination to develop nationally representative estimates of market-based service utilization per individual living with AIDS.
Mounting evidence suggests that HIV clinician supply might not be keeping pace with the growth in demand for HIV-related health care services. In the general literature, studies in the 1990s predicted shortages of primary care physicians and surpluses of specialists by the end of the 1990s (see Greenberg and Cultice [1997] for an example of this research). However, by the early 2000s, new approaches to studying supply and demand of health care clinicians predicted shortages of all types of physicians (Cooper et al. 2002). The more recent literature emphasizes that physician work effort might be declining because the workforce is aging, more likely to be employees (rather than self-employed or in partnerships), experiencing greater pressure on personal time, facing greater complexity in treatment, and retiring. These and other pressures on clinician supply are believed to be particularly true for HIV-related care (HRSA CARE Action April 2010).
In a letter to Congress requesting greater support, HIVMA concluded
Both the increase in patient load and the demands of HIV medicine are exacerbated by retirement and burnout among the first generation of HIV clinicians. Many of us from the first generation of HIV care clinicians will be retiring during the next decade, and there is not a sufficient and qualified pool of HIV medical clinicians to take our places (HIVMA 2008).
Mathematica’s study for HAB on the effect of state health reforms on access to care found that HIV care clinicians across the country face increasing administrative pressures associated with credentialing, prior approvals, referrals, and billing (Gilman et al. 2008). Clinicians also reported spending more time helping patients manage their treatment protocols when limitations on the use of pharmaceuticals and other medical services are imposed, and helping clients navigate the increasingly stringent requirements for Supplemental Security Income (SSI) and Medicaid eligibility. In addition, clinicians reported that Medicaid reimbursement rates are insufficient to cover the cost of treating people with HIV. The study concluded that low Medicaid payment rates, combined with increased administrative responsibilities, contribute to a lack of qualified clinicians—particularly medical specialists and clinicians in rural areas—willing to treat people with HIV. Mathematica’s qualitative assessment of clinician workforce capacity issues in Ryan White program care settings for HAB echoed many of these findings (Gilman et al. 2009).
Excess Demand or Supply
We suggest using two approaches to estimating baseline excess demand or supply. The first approach will use observed data, clinical guidelines, and expert opinion from clinicians to estimate the level of care that would be minimally adequate to meet the needs of those currently living with HIV or AIDS and compare this level of services with the level currently provided. The second approach would look at market-based indicators of excess demand or supply. Many health care workforce studies focus on market-based measures of supply and demand. However, because this study is motivated by a public health concern about the adequacy of treatment for HIV patients, we believe it is appropriate to estimate a needs-based model as well as to develop a market-based estimate. We describe these two approaches in turn.
1. Needs-Based Estimate of Demand
A needs-based approach would use a combination of observed data, clinical guidelines, and expert opinion to estimate demand per individual with HIV infection or AIDS, as well as to estimate the need for AIDS treatment for currently undiagnosed individuals. They will differ from market demand because of two components. First, undiagnosed patients or patients who have been diagnosed but are not currently in treatment will be the major component of needs-based demand that is not included in observed market demand. The second component will be the implications on needs based demand of treating HIV patients according to accepted guidelines or protocols of appropriate standards of care. Clinical guidelines published by HRSA will be the foundation of the needs-based estimates developed under this study. However, the guidelines only provide guidance on the frequency of appointments and specify the treatment goals for certain clinical phases. The guidelines do not specify a particular level of clinician effort or a volume of clinical services recommended. As a result, they cannot be used directly to an estimate of needs-based demand, but rather need to be translated into an estimate of clinician time.
As a starting point for the needs-based estimates, we will examine observed data on the current volume of treatment being provided in Ryan White-funded clinics separately for patients diagnosed with HIV only and those diagnosed with AIDS. With input from clinical experts, we will compare this observed level of treatment to the HRSA treatment guidelines and the HAB performance and HIV Quality Improvement (HIVQUAL) measures. We will ask the clinical experts to assess how the level of care currently being provided deviates from the HRSA treatment guidelines and how the level of clinician time per person diagnosed with HIV only or those diagnosed with AIDS would need to change to meet the guidelines. Based on this input, we will develop a range of estimates for needs-based demand under alternative assumptions as recommended by the clinical experts.
The second and larger source of needs-based demand is to include those patients who are currently undiagnosed or diagnosed but not in treatment. We will use CDC estimates of the undiagnosed population, as well as the targets set forth in the national HIV/AIDS strategy, to measure unmet need among the undiagnosed. We rely on use estimates from the literature, as well as the targets from the national HIV/AIDS strategy, to capture the demand that would occur if those who are diagnosed but not in regular care were to receive the appropriate levels and duration of treatment.
2. Market-Based Estimate of Demand
We will use data from this study’s clinic survey to develop our market-based estimate of excess demand for HIV providers. Our clinic survey will include questions to collect the following information, which will help us to develop this estimate:6
Assuming no change in current resource levels, such as funding or HIV medical clinician FTE, what is the total number of new HIV-positive patients that your clinic would be able to absorb?
Is your clinic currently accepting new commercially insured patients with HIV? Medicaid patients? Medicare patients? Uninsured patients?
What is the average waiting time (in weeks) for scheduling appointments for each of the following types of patient: newly diagnosed patients, patients new to your clinic but not newly diagnosed, and established patients?
What is the average length of the typical visit for each of the following types of patient: newly diagnosed patients, patients new to your clinic but not newly diagnosed, and established patients?
How difficult is it to recruit HIV primary care clinicians (physicians, nurse practitioners, and physician assistants) or infectious disease specialists? How easy is it to retain HIV primary care clinicians or infectious disease specialists?
Please indicate in column A the number of HIV-care related clinical vacancies (by FTE) in your clinic that are the result of retirement or staff expansion (as opposed to turnover), and in column B the average length of time these positions have been vacant. Please limit vacancies to only to positions for which funding exists.
Table II.2. Open Vacancies for Funded HIV Clinicians and Length of Time to Fill Position
Type of Clinician |
Column
A |
Column
B |
Infectious disease specialists |
|
|
Primary care physicians |
|
|
Physician assistants |
|
|
Nurse practitioners |
|
|
We will regard as excess demand the difference between the number of new positions or positions available as a result of retirement and the number of individuals expected to complete training and enter HIV care in the current year. For example if we find that clinics and physician offices have 75 open positions that are new or related to retirement (not related to staff turnover) for primary care physicians providing HIV care, but we estimate that only 50 of the primary care physicians completing their training in the given year will enter HIV care, we would estimate a shortage of 25 primary care physicians in the baseline year. The results from this analysis will be validated against information reported in other survey questions such as questions related to patient access to care, appointment waiting times, and difficulty hiring new clinicians.
C. Projecting Supply and Demand
In this section, we discuss our proposed approach to projecting supply and demand from the baseline year 2010 to 2015.
1. Projecting Supply of HIV Clinicians
We will project the supply of HIV clinicians from 2010 through 2015, and will discuss with HRSA the benefit of projecting supply through 2020 to capture the retirement rates associated with the second half of the baby boom generation. Similar to baseline supply, we will include measures of both active and FTE supply. We first discuss how we will project active supply. Then, we discuss how we will adjust the active supply estimate to produce an estimate of FTE supply.
Mathematically, active supply in the next year (t + 1) is a function of supply in the current year (t) plus new entrants minus attrition:
Eq. (2)
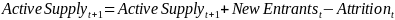
New entrants are physicians completing fellowship training who chose to enter HIV care, as well as currently practicing physicians who shift into HIV medicine. Attrition is physicians who have retired, changed careers, shifted out of HIV medicine into another medical specialty, or died. Since mid-career shifts in clinical specialty are atypical, our projections will focus on new entrants who have just completed clinical training, mortality, and retirement as these are likely to cause the most substantial shifts in supply.
New Entrants into HIV Care
Similar to our method of estimating baseline supply, we will use a two-tiered approach to estimate new entrants, with different approaches for estimating new entrants for physicians and nonphysician clinicians.
Tier One: Estimating New Physician Entrants. We will base our estimate of the number of new physician entrants to HIV care in each year between 2010 and 2015 on the following components:
Share of Physicians Completing Training and Entering HIV Care Between 2000 and 2010. For primary care physicians and infectious disease specialists in the claims analysis we will empirically identify the cohorts of new entrants into HIV care for the past 10 years based on the age of the physicians. For example if the youngest age observed with a substantial number of physicians is 32, we would assume all 32-year-old physicians entered in the most recent year, all 33-year-old physicians entered in the prior year, and so on. We will compare the counts of physicians in these cohorts with the number of clinicians completing training in the respective specialty in the particular year to estimate the share of primary care physicians completing residency and the share of infectious disease specialists completing fellowship training who entered HIV care over the past 10 years. For example, if 100 infectious disease specialists reported that they entered HIV care in 2005 and we know that there were a total of 300 infectious disease specialists who graduated in 2005, then the share of infectious disease specialists entering HIV care is 33 percent. We will assess whether a trend exists in this share and whether we expect the factors that might influence this trend to continue between 2010 and 2015. Based on this analysis, we will project the share of primary care and infectious disease physicians completing training over the next 5 years who will begin providing HIV care.
Number of Physicians Completing Training by Specialty Between 2000 and 2010. We will also assess recent trends in the number of primary care physicians completing residency and infectious disease specialists completing fellowship training annually. Based on this analysis we will develop projections for the number of physicians completing training in these specialties in each year between 2010 and 2015.
We will multiply our projections of the number of specialists completing training each year by the share of each of these specialties projected to enter HIV care to project the number of physicians completing training who will become new entrants in HIV care in each year between 2010 and 2015. In addition to estimating the number of physicians entering HIV medicine, we will develop estimates of the mean number of hours these clinicians will work and the share of their hours that will be devoted to HIV care. The primary source for these estimates will be the clinician survey. Based on this survey, we will estimate the age and gender distribution of new entrants, the number of hours worked, and the share of these hours devoted to HIV care.
Tier Two: Estimating New Nonphysician Clinician Entrants. We will project the number of new nonphysician clinician entrants to HIV care based on data collected in the clinic survey, as well as on national policy changes, such as the change in the community health center physician to PA/NP staffing ratio from a current ratio of 1:1 or 1:2 to a ratio of 1:4 as documented in the Access Transformed report by the National Association of Community Health Centers (NACHC).
We will ask clinic survey respondents to answer the following questions about the nonphysician workforce in their clinic:
How many clinicians of the following types providing HIV care have been added (not replacing another staff member) to the staff in your unit within the past 12 months:
Physician assistants
Nurse practitioners
Based on these survey responses, known policy changes, and the number of new mid-level clinicians entering the workforce annually, we will develop a range for the number of new physician assistant and nurse practitioner entrants annually between 2010 and 2015.
Attrition: Retirement and Mortality
We model attrition from HIV care provision related to two primary sources: retirement and mortality. For each of these types of attrition we address attrition for tier one and two types of clinicians.
Tier One: Estimating Physician Retirement. We will develop baseline estimates of retirement rates for physicians providing HIV care from two sources. The first source identifies recently observed retirement rates among physicians generally. The second source will be specific to physicians providing HIV services. It will reflect anticipated age of retirement in most cases rather than observed behavior, because we will be surveying active clinicians. Because the latter source is based on anticipated age of retirement, it may be less accurate than a source based on observed retirement rates. Prior analysis conducted by The Lewin Group (2009) compared the distribution of observed and anticipated ages of retirement. The analysis found that physicians intend to retire earlier than predicted by historical observed retirement rates. Thus, we expect that the estimates of anticipated age of retirement will project higher retirement rates than will likely occur. In contrast, applying average retirement rates for all physicians to HIV clinicians may result in lower retirement rate projections than will likely be observed because of the aging of the HIV clinician workforce and the relatively high burn-out rate among HIV clinicians (Gilman et al. 2009).
We plan to use the following two sources:
AMA/AAMC Surveys of Physicians 50 and Over. Respondents to a survey of retired physicians older than 50 conducted by the AMA and AAMC were asked to report the age at which they retired. All other respondents were asked to report the age at which they expected to retire. For those physicians ages 70 or older, we will use the reported retirement age or the reported anticipated age of retirement if the physician is still active to estimate the observed distribution of physicians by age of retirement. We will assume that all physicians not yet retired will retire at age 75.
HIV Clinician Survey. In our survey of HIV clinicians, clinicians will be asked how likely they are to reduce the number of HIV patients they serve in the next five years. If they respond that they are somewhat or very likely to reduce their HIV patient load, they will be asked if this is due to retirement. We will also ask them how likely they are to retire from the health profession entirely within the next five years. We will use these responses to estimate the number of HIV physicians expected to retire in the next five years.
Tier Two: Estimating Nonphysician Clinician Retirement and Career Change. As part of the clinic survey, we will ask respondents to provide the age distribution of the physician assistants and nurse practitioners who work in their clinic. We will use this information to project rates of retirement for these mid-level clinicians. We will develop estimates for nonphysician provider attrition associated with retirement from the following sources:
Bureau of Labor Statistics Data on Retirement Rates. We will apply overall rates of retirement across all professions by age to the nonphysician HIV clinicians to determine attrition related to retirement.
Parameter Estimates from the Nursing Supply Literature. A rich literature on nursing supply associates factors such as economic conditions with nursing supply. We will review this literature and adapt parameter estimates developed in this literature for use in our model.
Attrition: Mortality
We will estimate separate mortality rates based on CDC estimates by age and for men and women. We will apply average mortality rates to estimates for tier-two providers based on their age at baseline. To adjust for lower occupational risk of mortality for physicians, their greater access to quality health care services, and their generally better health associated with affluence, we will adjust the average mortality rates for the physician providers in tier-one to 80 percent of the national average for each age group. This adjustment is based on work by Johnson et al. (1999), which found that mortality rates among people ages 25 to 64 are lower for physicians and other professional and technical occupations compared with mortality rates in most nonprofessional occupations. For white males, age-adjusted mortality rates for professional and technical occupations are approximately 75 percent as high as the rates across all occupations. For white females, the mortality rates for professional and technical occupations are about 85 percent as high as rates across all occupations. Mortality rates for women are lower than those for men.
Attrition: Overall
We will apply losses related to mortality to the baseline supply of physicians by age and gender in 2010. Then, we will apply retirement and change-of-profession rates to the remaining supply of physicians to calculate the number of physicians remaining in the workforce in 2011. These adjustments will again be applied to the 2011 projection to obtain the remaining workforce in 2012. We will repeat this process until we can calculate the workforce remaining in 2015.
FTE Supply
After projecting an estimate of active supply for each year between 2010 and 2015, we can translate this measure into FTE supply. Under the previous discussion of baseline supply, we discussed the potential data sources for estimating hours worked by the age and gender of the provider. We will start by multiplying the number of physicians in each age and gender category in each projection year by the estimated hours worked for their respective age and gender groups. We will then sum the products for each age and gender group across all the groups. Finally, we will divide this total by the average hours worked across all physicians in the baseline year to estimate the FTE supply in each year of the projection. Thus, the FTE supply in each future year (t) is equal to multiplying the active supply in year (t) by the adjustment for changes in average patient care hours worked in the baseline year.
Eq. (3)

For example, if clinicians increasingly work part-time in HIV care, we might find that the ratio of patient care hours per clinician in 2011 is 90 percent of that for the base year 2010. Then, if the active supply of clinicians in 2011 is 1,100, the effective FTE supply will be only 990 (90 percent of 1,100).
We will review the general literature on physician supply and incorporate any findings on generation shifts in hours worked into our model. We will also test the sensitivity of our findings to potential shifts in hours worked among clinicians.
Productivity Change and Substitution Across Provider Types
Based on information collected on this study’s clinic survey, we will estimate a production function for HIV care. Gilman and Green (2008) estimated a similar model to identify the determinants of cost variation among programs that offer early intervention services to people living with HIV and AIDS in the United States. Their model found that practice setting and patient characteristics had a significant impact on average costs, measured in terms of both costs per visit and costs per client. Hogan and Bouchery (2009) estimated a production function for cardiology services. This model provided estimates of the marginal productivity of cardiologists, nurse practitioners, and physician assistants in the practice setting.
For this study, we will estimate a production function for HIV care at the clinic level. We will test the variability of the model results based on alternative measures of level of HIV care produced. These measures may include number of HIV care visits, total revenue, and/or total relative value units (RVUs), as feasible given the data. The level of HIV care produced will be a function of
Physician hours worked in HIV care
Nonphysician clinician hours worked in HIV care
Practice setting characteristics (for example, primary/specialty care, practice size, Ryan White clinic, share of patients non-English speakers, and hospital- versus community-based)
Practice location characteristics (for example, urban/rural, and local HIV prevalence)
Patient mix (proportion new to care, AIDS diagnosis, and other comorbidity)
Meaningful use of health information technology (such as electronic medical records and telemedicine)
Implementation of streamlined scheduling procedures (such as open booking)
Use of improved workflow strategies (such as task shifting and task sharing)
Use of care coordination and management models (such as medical homes and patient-centered navigation)
Parameters estimated in the model will indicate whether each factor has a significant impact on HIV workforce productivity. We will use the size of these parameter estimates to model the impact of improvements in efficiency or shifts in a practice or patient characteristics between 2010 and 2015 on clinician supply.
Summary
In this section, we summarize our approach to the supply side of the model. Table II.3 indicates the factors that we will include in the model with the associated data sources and methods.
Table II.3. Summary of Supply-Side Factors
Factor |
Measure |
Data Sources |
Baseline supply of physicians |
|
|
Baseline supply of nonphysician clinicians |
|
|
Base supply of physicians completing training |
|
|
Share of physicians completing training and providing HIV services |
|
|
Retirement rates |
|
|
Professional entry/exit |
|
|
Hours worked |
|
|
Note: HIVMA = HIV Medical Association, AAHIVM = American Academy of HIV Medicine, AAMC = American Association of Medical Colleges.
Figure II.2 illustrates the steps we will take to project the supply of HIV clinicians through 2015.
Figure II.2. Approach to Projecting Total FTE Supply of HIV Clinicians
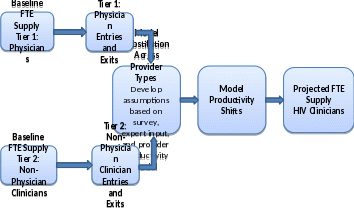
2. Projecting Demand for HIV Clinicians
We will base our HIV-related health care demand projections on the following components:
Demographic Trends. We will use United States census population projections to estimate the size of the United States population by age and gender in each year between 2010 and 2015. To project future demand, we will use population projections stratified by age, gender, and region.
HIV Prevalence Rates. We will multiply CDC estimates of HIV/AIDS prevalence rates in each age and gender group by the United States census population projections in each age and gender group to produce estimates of the number of people living with HIV infection or AIDS in each year from 2010 to 2015.
Service Use per Individual with HIV. We will multiply estimates of service use per individual by age group, gender, urban/rural location, health insurance coverage, type and specialty of clinician (primary care physician, infectious disease specialist, and mid-level clinician), and HIV infection versus AIDS. We will derive the baseline demand estimates based on currently observed levels of demand observed in Medicare, Medicaid, and private insurance claims data, in NCHS survey data, and in RSR reports, by the projected number of individuals in these cells to yield the total level of services that those individuals will demand. We will translate the total level of services demanded into an estimate of the number of FTE physicians demanded using the estimate of FTE services provided by each physician also developed as part of the baseline demand estimate.
We will use these three components to produce the baseline demand projections. We will also consider alternative scenarios incorporating the following additional demand-side factors:
Trends in HIV Diagnosis. CDC estimates that approximately one-fifth of people living with HIV in the United States are unaware of their serostatus; it recommends implementing routine opt-out testing in nonprimary care settings. Prevalence estimates might increase if more people are tested and diagnosed with HIV. We will develop estimates of the likely impact of increased testing on prevalence from the literature. We will also review estimates of the impact of testing and outreach initiatives on the cost of serving the out-of-care population being developed by NIH, the CDC Medical Monitoring Project, and the AHRQ HIV research network to develop a needs-based estimate of the cost of treating individuals currently not in care. Finally, we will refer to the targets put forth in the national HIV/AIDS strategy to develop scenarios for the number of people who might enter care within the next five years.
Economic Growth. Continued income growth in the United States will result in increased demand for all types of medical care, including HIV care. In general, as income increases, the demand for goods and services that people value also rises.
Insurance Status. The Patient Protection and Affordable Care Act of 2010 (ACA) will increase insurance coverage for many individuals living with HIV. This increase in insurance coverage is likely to result in increased diagnosis and demand for services. In the baseline demand calculations, we will estimate demand by type of insurance coverage. We will use these estimates of the variation in demand by type of insurance coverage to develop an estimate of the impact of the expected insurance coverage changes under the ACA.
Technological Advances. As new procedures are developed and prove efficacious, demand for care could increase above the projected increases related to demographic trends. Alternatively, new ARV treatments might require fewer doses and offer more resistance, reducing demand for care.
Based on this list of epidemiological and clinical factors, we will develop various scenarios for the demand for the HIV workforce. Next, we summarize our approach to the demand-side factors of the model and the associated data sources and methods.
Table II.4 provides a summary of the demand-side factors that we will use to forecast the demand for HIV clinical services, along with the data sources we will use to measure each factor.
Table II.4. Summary of Demand-Side Factors
Factor |
Measure |
Data Sources |
Demographic trends |
|
|
HIV prevalence rates |
|
|
Service use per HIV patient |
|
|
Change in insurance status |
|
|
Impact of increased testing |
|
|
Change in treatment |
|
|
Notes: ARV = antiretroviral; RSR = Ryan White HIV/AIDS Program Services Report; NCHS = National Center for Health Statistics.
This page has been left blank for double-sided copying.
Cooper, RA; Getzen, TE; McKee, HJ; and Prakash, L. “Economic and Demographic Trends Signal an Impending Physician Shortage.” Health Affairs. 21(1) 2002:140-153.
Gilman B, Hargreaves M, Au M, Kim J, Mathematica Policy Research, Inc. Factors impacting the retention of clinical providers and other key personnel in Ryan White HIV/AIDS Program care settings. [unpublished]; March 6, 2009.
Gilman, Boyd H., and Jeremy C. Green. “Understanding the
Variation in Costs Among HIV Primary Care Providers.” AIDS
Care: Psychological and Socio-Medical Aspects of AIDS/HIV, vol.
20, no. 9, 2008, pp. 1050–1056. Retrieved from
http://www.informaworld.com
/10.1080/09540120701854626 on
January 10, 2011.
Greenberg, L. and J. M. Cultice. “Forecasting the need for physicians in the United States: the Health Resources and Services Administration's physician requirements model.” Health Services Research 31(6) 1997:723-37.
Greenberg, Leonard; Cultice, James M. "Forecasting the need for physicians in the United States: the Health Resources and Services Administration's physician requirements model" Health Services Research , Feb 1, 1997.
HRSA CareAction. “Workforce Capacity in HIV” US Department of Health and Human Services, Health Resources and Services Administration, HIV/AIDS Bureau, Rockville, MD, April 2010.
Johnson, N.J., P. D. Sorlie, and E. Backlund. The Impact of Specific Occupation on Mortality in the U.S. National Longitudinal Mortality Study.” Demography, vol. 36, no. 3, 1999, pp. 355–367.
The Lewin Group and the American Association of Medical Colleges. “Cardiovascular Workforce Assessment: Final Report.” Falls Church, VA: March 2009.
This page has been left blank for double-sided copying.
Methodology
for identifying hiv clinicians from claims data
This page has been left blank for double-sided copying.
Objective of the Claims Analysis
This analysis aims to identify providers (physicians and nonphysicians) who have a critical volume of HIV-related visits, prescriptions and/or HIV-related patients. To meet this objective, we will undertake a two-pronged approach using SDI claims data representative of those insured by Medicare, Medicaid, and commercial insurance
Approach 1: Using the Physician Claims Database
Step 1: Identify HIV-Related Clinical Events at the Provider Level
We will create a national extract of ambulatory medical claims from the DX database.
Using International Classification of Diseases, 9th Edition, diagnosis codes and current procedural terminology codes (see Appendix B), we will identify claims associated with HIV-related clinical events.7
We will perform a quality check on the extracted claims and remove duplicate claims.
We will merge information on medical specialty using the national provider identifier code of the provider.
We will create a summary-level file (File 1) at the provider level with provider information (for example, medical specialty) and total count of HIV-related visits (N). We will arrange File 1 by health profession (physician, nurse practitioner, and physician assistant) and medical specialty (internal medicine, general/family medicine, infectious disease, pediatrics, and geriatrics).
Step 2: Identify Total Number of Services Provided at the Provider Level
We will create a finder file of providers and extract claims for all visits provided by the providers.
We will create a variable that sums all the visits (D) provided by the providers and merge it to File 1 using the national provider identifier.
The proportion of provider visits (P) that is dedicated to HIV-related events at the provider level is N divided by D.
We will repeat these steps to calculate the number and proportion of patients who are treated for HIV for each provider.
Step 3: Define HIV Providers
Issues associated with defining HIV providers include the following:
Should the threshold used to define an HIV provider be based on the absolute volume of visits or patients (N) or the proportion of visits or patients related to HIV (P)?
Should the threshold vary by health profession, medical specialty, and/or geography?
Should the threshold be based on the distribution of N (for example, will providers with N above the 25th percentile be defined as HIV providers)?
Approach 2: Using Pharmacy Claims Database
We will create an extract of pharmacy claims from the RX database as Approach 1.
We will identify pharmacy claims for HIV medications (see Appendix C) using the national drug codes (NDCs).8
Using the prescribing date as a marker, we will summarize the pharmacy claims for HIV medications by prescribing date, patient ID, and prescribing clinician.
We will derive the total count of prescribing visits related to HIV by counting the prescribing dates for HIV medications. This assumes that HIV medications were prescribed during an HIV-related visit. The goal is to count the number of prescribing visits for each provider.
We will link the file with provider information using the national provider identifier of the prescribing clinician.
The resulting file (File 2) will include the number of visits related to HIV clinical events at the provider level. In addition, it will include information on health profession and medical specialty.
We will establish a minimum volume threshold requirement for inclusion in the study, based on the number of visits, scripts, or patients and apply this rule to File 2 to define our baseline HIV clinician population.
icd-9-cm and
cpt codes for identifying
Treated patients with HIV infection
This page has been left blank for double-sided copying.
ICD-9-CM Codes (Diagnosis) |
|
042x to 044x |
HIV disease, with codes for the HIV-related manifestations or conditions, if the results are positive and the patient exhibits symptoms |
V08 |
Asymptomatic HIV infection status if the results are positive but the patient is asymptomatic |
V01.79 |
Exposure to HIV virus |
795.71 |
Nonspecific serologic evidence of HIV |
V65.44 |
HIV counseling (if counseling is provided during the encounter for the test or after the results are available) |
CPT Codes (Laboratory tests) |
|
86701 |
antibody HIV-1 test |
86702 |
antibody HIV-2 test |
86703 |
antibody HIV-1 and HIV-2 single assay |
86689 |
Antibody; HTLV or HIV antibody, confirmatory test (for example, Western Blot) |
87534 |
Infectious agent detection by nucleic acid (DNA or RNA); HIV-1, direct probe technique |
87535 |
Infectious agent detection by nucleic acid (DNA or RNA); HIV-1, amplified probe technique |
87536 |
Infectious agent detection by nucleic acid (DNA or RNA); HIV-1, quantification |
87390 |
Infectious agent antigen detection by enzyme immunoassay technique, qualitative or semi-quantitative, multiple step method; HIV-1 |
99211–99215 |
HIV counseling for patients with positive test results; office or other outpatient visit for the evaluation and management of an established patient |
87536 |
HIV viral load test |
86359 |
T-cells, total count |
86360 |
Absolute CD4/CD8 count with ratio |
After February 2010 (Medicare HCPCS) (Laboratory tests) |
|
G0432 |
Infectious agent antigen detection by enzyme immunoassay (EIA) technique, qualitative or semi-quantitative, multiple-step method, HIV-1 or HIV-2, screening (conventional test) |
G0433 |
Infectious agent antigen detection by enzyme-linked immunosorbent assay (ELISA) technique, antibody, HIV-1 or HIV-2, screening |
G0435 |
Infectious agent antigen detection by rapid antibody test of oral mucosa transudate, HIV-1 or HIV-2, screening |
Source: http://www.nachc.org/client/2010HIVTestingandICD-9CodingGuideUpdatedFrom2008.pdf
Notes: CD4/CD8 = cluster of differentiation 4/8; CPT = current procedural terminology; DNA = deoxyribonucleic acid; HCPCS = Health Care Procedural Coding System; HTLV = human T-lymphotropic virus; ICD-9-CM = International Classification of Diseases, 9th Edition, Clinical Modification; RNA = ribonucleic acid.
This page has been left blank for double-sided copying.
national drug codes for identifying
treated patients with hIV infection
This page has been left blank for double-sided copying.
NDC Code |
NDC Description |
00003196401 |
ZERIT 15 MG CAPSULE |
00003196501 |
ZERIT 20 MG CAPSULE |
00003196601 |
ZERIT 30 MG CAPSULE |
00003196701 |
ZERIT 40 MG CAPSULE |
00003196801 |
ZERIT 1 MG/ML SOLN RECON |
00003362212 |
REYATAZ 300 MG CAPSULE |
00003362312 |
REYATAZ 100 MG CAPSULE |
00003362412 |
REYATAZ 150 MG CAPSULE |
00003363112 |
REYATAZ 200 MG CAPSULE |
00004024451 |
INVIRASE 500 MG TABLET |
00004024515 |
INVIRASE 200 MG CAPSULE |
00004038039 |
FUZEON 90 MG KIT |
00006022761 |
ISENTRESS 400 MG TABLET |
00006057062 |
CRIXIVAN 100 MG CAPSULE |
00006057143 |
CRIXIVAN 200 MG CAPSULE |
00006057301 |
CRIXIVAN 400 MG CAPSULE |
00006057318 |
CRIXIVAN 400 MG CAPSULE |
00006057340 |
CRIXIVAN 400 MG CAPSULE |
00006057342 |
CRIXIVAN 400 MG CAPSULE |
00006057354 |
CRIXIVAN 400 MG CAPSULE |
00006057362 |
CRIXIVAN 400 MG CAPSULE |
00006057465 |
CRIXIVAN 333 MG CAPSULE |
00054005221 |
ZIDOVUDINE 300 MG TABLET |
00056047030 |
SUSTIVA 50 MG CAPSULE |
00056047330 |
SUSTIVA 100 MG CAPSULE |
00056047492 |
SUSTIVA 200 MG CAPSULE |
00056051030 |
SUSTIVA 600 MG TABLET |
00069080760 |
SELZENTRY 150 MG TABLET |
00069080860 |
SELZENTRY 300 MG TABLET |
00074052260 |
KALETRA 100MG-25MG TABLET |
00074194063 |
NORVIR 80 MG/ML SOLUTION |
00074333330 |
NORVIR 100 MG TABLET |
00074395646 |
KALETRA 400-100/5 SOLUTION |
00074395977 |
KALETRA 133.3-33.3 CAPSULE |
00074663322 |
NORVIR 100 MG CAPSULE |
00074663330 |
NORVIR 100 MG CAPSULE |
00074679922 |
KALETRA 200MG-50MG TABLET |
00087663241 |
VIDEX FNL10MG/ML SOLN RECON |
00087663341 |
VIDEX FNL10MG/ML SOLN RECON |
00087667117 |
VIDEX EC 125 MG CAPSULE DR |
00087667217 |
VIDEX EC 200 MG CAPSULE DR |
00087667317 |
VIDEX EC 250 MG CAPSULE DR |
00087667417 |
VIDEX EC 400 MG CAPSULE DR |
00173010793 |
RETROVIR 10 MG/ML VIAL |
00173010855 |
RETROVIR 100 MG CAPSULE |
00173010856 |
RETROVIR 100 MG CAPSULE |
00173011318 |
RETROVIR 10 MG/ML SYRUP |
00173047001 |
EPIVIR 150 MG TABLET |
00173047100 |
EPIVIR 10 MG/ML SOLUTION |
00173050100 |
RETROVIR 300 MG TABLET |
00173059500 |
COMBIVIR 150-300MG TABLET |
00173059502 |
COMBIVIR 150-300MG TABLET |
00173066100 |
ZIAGEN 300 MG TABLET |
00173066101 |
ZIAGEN 300 MG TABLET |
00173066400 |
ZIAGEN 20 MG/ML SOLUTION |
00173067900 |
AGENERASE 50 MG CAPSULE |
00173068700 |
AGENERASE 15 MG/ML SOLUTION |
00173069100 |
TRIZIVIR 150-300MG TABLET |
00173071400 |
EPIVIR 300 MG TABLET |
00173072100 |
LEXIVA 700 MG TABLET |
00173072700 |
LEXIVA 50 MG/ML ORAL SUSP |
00173074200 |
EPZICOM 600-300MG TABLET |
00378504091 |
STAVUDINE 15 MG CAPSULE |
00378504191 |
STAVUDINE 20 MG CAPSULE |
00378504291 |
STAVUDINE 30 MG CAPSULE |
00378504391 |
STAVUDINE 40 MG CAPSULE |
00378610691 |
ZIDOVUDINE 300 MG TABLET |
00378888693 |
DIDANOSINE 125 MG CAPSULE DR |
00378888793 |
DIDANOSINE 200 MG CAPSULE DR |
00378888893 |
DIDANOSINE 250 MG CAPSULE DR |
00378888993 |
DIDANOSINE 400 MG CAPSULE DR |
00555058801 |
DIDANOSINE 200 MG CAPSULE DR |
00555058901 |
DIDANOSINE 250 MG CAPSULE DR |
00555059001 |
DIDANOSINE 400 MG CAPSULE DR |
00597000201 |
APTIVUS 100 MG/ML SOLUTION |
00597000302 |
APTIVUS 250 MG CAPSULE |
00597004660 |
VIRAMUNE 200 MG TABLET |
00597004724 |
VIRAMUNE 50 MG/5 ML ORAL SUSP |
15584010101 |
ATRIPLA 600-200MG TABLET |
16590006106 |
COMBIVIR 150-300MG TABLET |
16590006110 |
COMBIVIR 150-300MG TABLET |
16590006418 |
CRIXIVAN 400 MG CAPSULE |
16590006430 |
CRIXIVAN 400 MG CAPSULE |
16590006460 |
CRIXIVAN 400 MG CAPSULE |
16590006490 |
CRIXIVAN 400 MG CAPSULE |
173010793 |
RETROVIR 10 MG/ML VIAL |
173010855 |
RETROVIR 100 MG CAPSULE |
173010856 |
RETROVIR 100 MG CAPSULE |
173011318 |
RETROVIR 10 MG/ML SYRUP |
173047001 |
EPIVIR 150 MG TABLET |
173047100 |
EPIVIR 10 MG/ML SOLUTION |
173050100 |
RETROVIR 300 MG TABLET |
173059500 |
COMBIVIR 150-300MG TABLET |
173059502 |
COMBIVIR 150-300MG TABLET |
173066100 |
ZIAGEN 300 MG TABLET |
173066101 |
ZIAGEN 300 MG TABLET |
173066400 |
ZIAGEN 20 MG/ML SOLUTION |
173067200 |
AGENERASE 150MG CAPSULE |
173067900 |
AGENERASE 50 MG CAPSULE |
173068700 |
AGENERASE 15 MG/ML SOLUTION |
173069100 |
TRIZIVIR 150-300MG TABLET |
173069120 |
TRIZIVIR 150-300MG TABLET |
173071400 |
EPIVIR 300 MG TABLET |
173072100 |
LEXIVA 700 MG TABLET |
173072700 |
LEXIVA 50 MG/ML ORAL SUSP |
173074200 |
EPZICOM 600-300MG TABLET |
21695036212 |
KALETRA 200MG-50MG TABLET |
21695036618 |
CRIXIVAN 400 MG CAPSULE |
21695036706 |
EPIVIR 150 MG TABLET |
21695036918 |
ZIDOVUDINE 300 MG TABLET |
21695084606 |
COMBIVIR 150-300MG TABLET |
23490708706 |
COMBIVIR 150-300MG TABLET |
31722050960 |
ZIDOVUDINE 300 MG TABLET |
31722051560 |
STAVUDINE 15 MG CAPSULE |
31722051660 |
STAVUDINE 20 MG CAPSULE |
31722051760 |
STAVUDINE 30 MG CAPSULE |
31722051860 |
STAVUDINE 40 MG CAPSULE |
3196401 |
ZERIT 15 MG CAPSULE |
3196501 |
ZERIT 20 MG CAPSULE |
3196601 |
ZERIT 30 MG CAPSULE |
3196701 |
ZERIT 40 MG CAPSULE |
3196801 |
ZERIT 1 MG/ML SOLN RECON |
3362212 |
REYATAZ 300 MG CAPSULE |
3362312 |
REYATAZ 100 MG CAPSULE |
3362412 |
REYATAZ 150 MG CAPSULE |
3363112 |
REYATAZ 200 MG CAPSULE |
35356006406 |
ATRIPLA 600-200MG TABLET |
35356006430 |
ATRIPLA 600-200MG TABLET |
35356006530 |
EPIVIR 300 MG TABLET |
35356006624 |
EPIVIR 10 MG/ML SOLUTION |
35356006706 |
LEXIVA 700 MG TABLET |
35356006760 |
LEXIVA 700 MG TABLET |
35356006806 |
REYATAZ 150 MG CAPSULE |
35356006860 |
REYATAZ 150 MG CAPSULE |
35356006990 |
SUSTIVA 200 MG CAPSULE |
35356007006 |
TRUVADA 200-300MG TABLET |
35356007030 |
TRUVADA 200-300MG TABLET |
35356007106 |
VIRAMUNE 200 MG TABLET |
35356007160 |
VIRAMUNE 200 MG TABLET |
35356007224 |
VIRAMUNE 50 MG/5 ML ORAL SUSP |
35356007306 |
VIREAD 300 MG TABLET |
35356007330 |
VIREAD 300 MG TABLET |
35356007460 |
ZERIT 40 MG CAPSULE |
35356007506 |
ZIAGEN 300 MG TABLET |
35356007560 |
ZIAGEN 300 MG TABLET |
35356010906 |
EPZICOM 600-300MG TABLET |
35356010930 |
EPZICOM 600-300MG TABLET |
35356011006 |
ISENTRESS 400 MG TABLET |
35356011060 |
ISENTRESS 400 MG TABLET |
35356011160 |
KALETRA 100MG-25MG TABLET |
35356011201 |
KALETRA 200MG-50MG TABLET |
35356011230 |
KALETRA 200MG-50MG TABLET |
35356011301 |
PREZISTA 300 MG TABLET |
35356011330 |
PREZISTA 300 MG TABLET |
35356011406 |
REYATAZ 300 MG CAPSULE |
35356011430 |
REYATAZ 300 MG CAPSULE |
35356011506 |
SUSTIVA 600 MG TABLET |
35356011530 |
SUSTIVA 600 MG TABLET |
35356011606 |
TRIZIVIR 150-300MG TABLET |
35356011660 |
TRIZIVIR 150-300MG TABLET |
35356011701 |
VIRACEPT 625 MG TABLET |
35356013830 |
NORVIR 100 MG CAPSULE |
35356013918 |
CRIXIVAN 400 MG CAPSULE |
35356013960 |
CRIXIVAN 400 MG CAPSULE |
35356018630 |
VIDEX EC 400 MG CAPSULE DR |
35356020530 |
EMTRIVA 200 MG CAPSULE |
35356020660 |
FUZEON 90 MG KIT |
35356020760 |
REYATAZ 200 MG CAPSULE |
35356020860 |
SELZENTRY 150 MG TABLET |
35356020960 |
SELZENTRY 300 MG TABLET |
35356025930 |
DIDANOSINE 400 MG CAPSULE DR |
35356028460 |
PREZISTA 600 MG TABLET |
35356028560 |
ZERIT 30 MG CAPSULE |
378504091 |
STAVUDINE 15 MG CAPSULE |
378504191 |
STAVUDINE 20 MG CAPSULE |
378504291 |
STAVUDINE 30 MG CAPSULE |
378504391 |
STAVUDINE 40 MG CAPSULE |
378610691 |
ZIDOVUDINE 300 MG TABLET |
4022001 |
HIVID 0.375MG TABLET |
4022101 |
HIVID 0.750MG TABLET |
4024451 |
INVIRASE 500 MG TABLET |
4024515 |
INVIRASE 200 MG CAPSULE |
4024648 |
FORTOVASE 200MG CAPSULE |
4038039 |
FUZEON 90 MG KIT |
49999006206 |
COMBIVIR 150-300 MG TABLET |
49999006210 |
COMBIVIR 150-300MG TABLET |
49999006260 |
COMBIVIR 150-300MG TABLET |
49999011906 |
EPIVIR 150 MG TABLET |
49999011960 |
EPIVIR 150 MG TABLET |
49999038618 |
RETROVIR 100 MG CAPSULE |
49999043103 |
VIRACEPT 250 MG TABLET |
50962045010 |
RETROVIR 10MG/ML SYRUP |
50962045205 |
UNKNOWN |
51129299902 |
DIDANOSINE 400 MG CAPSULE DR |
52959028930 |
VIRACEPT 250 MG TABLET |
52959038706 |
RETROVIR 300 MG TABLET |
52959050712 |
CRIXIVAN 400 MG CAPSULE |
52959050718 |
CRIXIVAN 400 MG CAPSULE |
52959050724 |
CRIXIVAN 400 MG CAPSULE |
52959050730 |
CRIXIVAN 400 MG CAPSULE |
52959050802 |
EPIVIR 150 MG TABLET |
52959050804 |
EPIVIR 150 MG TABLET |
52959050806 |
EPIVIR 150 MG TABLET |
52959050808 |
EPIVIR 150 MG TABLET |
52959050814 |
EPIVIR 150 MG TABLET |
52959050815 |
EPIVIR 150 MG TABLET |
52959050860 |
EPIVIR 150 MG TABLET |
52959050906 |
RETROVIR 100 MG CAPSULE |
52959050912 |
RETROVIR 100 MG CAPSULE |
52959050918 |
RETROVIR 100 MG CAPSULE |
52959050920 |
RETROVIR 100 MG CAPSULE |
52959050924 |
RETROVIR 100 MG CAPSULE |
52959050928 |
RETROVIR 100 MG CAPSULE |
52959050930 |
RETROVIR 100 MG CAPSULE |
52959054602 |
COMBIVIR 150-300MG TABLET |
52959054603 |
COMBIVIR 150-300MG TABLET |
52959054604 |
COMBIVIR 150-300MG TABLET |
52959054606 |
COMBIVIR 150-300MG TABLET |
52959054608 |
COMBIVIR 150-300MG TABLET |
52959054610 |
COMBIVIR 150-300MG TABLET |
52959054614 |
COMBIVIR 150-300MG TABLET |
52959054615 |
COMBIVIR 150-300MG TABLET |
52959054620 |
COMBIVIR 150-300MG TABLET |
52959054628 |
COMBIVIR 150-300MG TABLET |
52959096812 |
KALETRA 100MG-25MG TABLET |
52959096903 |
TRUVADA 200-300MG TABLET |
54005221 |
ZIDOVUDINE 300 MG TABLET |
54390558 |
VIRAMUNE 50MG/5ML ORAL SUSP |
54464721 |
VIRAMUNE 200MG TABLET |
54464725 |
VIRAMUNE 200MG TABLET |
54569177200 |
RETROVIR 100MG CAPSULE |
54569177201 |
RETROVIR 100MG CAPSULE |
54569177202 |
RETROVIR 100MG CAPSULE |
54569177203 |
RETROVIR 100MG CAPSULE |
54569177204 |
RETROVIR 100MG CAPSULE |
54569177205 |
RETROVIR 100MG CAPSULE |
54569365700 |
VIDEX 100MG TAB CHEW |
54569387700 |
HIVID 0.750MG TABLET |
54569387701 |
HIVID 0.750MG TABLET |
54569397100 |
VIDEX 150MG TAB CHEW |
54569405300 |
ZERIT 30 MG CAPSULE |
54569405400 |
ZERIT 40 MG CAPSULE |
54569405401 |
ZERIT 40MG CAPSULE |
54569422100 |
EPIVIR 150 MG TABLET |
54569422101 |
EPIVIR 150MG TABLET |
54569422102 |
EPIVIR 150MG TABLET |
54569424200 |
INVIRASE 200MG CAPSULE |
54569424201 |
INVIRASE 200MG CAPSULE |
54569424202 |
INVIRASE 200MG CAPSULE |
54569424203 |
INVIRASE 200MG CAPSULE |
54569431300 |
VIDEX 100MG TAB CHEW |
54569431301 |
VIDEX 100MG TAB CHEW |
54569433300 |
EPIVIR 10 MG/ML SOLUTION |
54569433400 |
RETROVIR 10MG/ML SYRUP |
54569433500 |
NORVIR 100MG CAPSULE |
54569448500 |
HIVID 0.375MG TABLET |
54569451400 |
VIDEX FNL10MG/ML SOLN RECON |
54569452400 |
COMBIVIR 150-300MG TABLET |
54569452401 |
COMBIVIR 150-300MG TABLET |
54569452402 |
COMBIVIR 150-300MG TABLET |
54569452403 |
COMBIVIR 150-300MG TABLET |
54569453800 |
RETROVIR 300 MG TABLET |
54569454300 |
VIRACEPT 250MG TABLET |
54569454301 |
VIRACEPT 250MG TABLET |
54569454302 |
VIRACEPT 250MG TABLET |
54569454303 |
VIRACEPT 250 MG TABLET |
54569454304 |
VIRACEPT 250MG TABLET |
54569454305 |
VIRACEPT 250MG TABLET |
54569454306 |
VIRACEPT 250MG TABLET |
54569456100 |
VIRAMUNE 200 MG TABLET |
54569456101 |
VIRAMUNE 200MG TABLET |
54569456200 |
RESCRIPTOR 100MG TAB DISPER |
54569456300 |
FORTOVASE 200MG CAPSULE |
54569456301 |
FORTOVASE 200MG CAPSULE |
54569461100 |
SUSTIVA 200 MG CAPSULE |
54569461300 |
NORVIR 80MG/ML SOLUTION |
54569479200 |
NORVIR 100MG CAPSULE |
54569481300 |
AGENERASE 150MG CAPSULE |
54569488300 |
ZIAGEN 300 MG TABLET |
54569490500 |
VIDEX 200MG TAB CHEW |
54569512200 |
RESCRIPTOR 200MG TABLET |
54569514200 |
KALETRA 133.3-33.3 CAPSULE |
54569517600 |
VIDEX EC 400 MG CAPSULE DR |
54569519100 |
TRIZIVIR 150-300MG TABLET |
54569533400 |
VIREAD 300 MG TABLET |
54569537400 |
SUSTIVA 600 MG TABLET |
54569538700 |
ZERIT 1MG/ML SOLN RECON |
54569539000 |
ZIAGEN 20 MG/ML SOLUTION |
54569541200 |
ZERIT 15MG CAPSULE |
54569548000 |
ZERIT 20 MG CAPSULE |
54569550100 |
EPIVIR 300 MG TABLET |
54569550400 |
VIDEX EC 250 MG CAPSULE DR |
54569552100 |
EMTRIVA 200 MG CAPSULE |
54569552500 |
KALETRA 100-400/5 SOLUTION |
54569553000 |
REYATAZ 150 MG CAPSULE |
54569553200 |
REYATAZ 200 MG CAPSULE |
54569555000 |
LEXIVA 700 MG TABLET |
54569558800 |
TRUVADA 200-300MG TABLET |
54569559400 |
EPZICOM 600-300MG TABLET |
54569560200 |
RESCRIPTOR 200 MG TABLET |
54569564200 |
DIDANOSINE 250 MG CAPSULE DR |
54569564300 |
DIDANOSINE 400 MG CAPSULE DR |
54569565600 |
NORVIR 100 MG CAPSULE |
54569566400 |
INVIRASE 500 MG TABLET |
54569575200 |
KALETRA 200MG-50MG TABLET |
54569578100 |
FUZEON 90 MG KIT |
54569580500 |
ATRIPLA 600-200MG TABLET |
54569581400 |
PREZISTA 300 MG TABLET |
54569603400 |
ISENTRESS 400 MG TABLET |
54569614300 |
SELZENTRY 150 MG TABLET |
54569615900 |
PREZISTA 400 MG TABLET |
54569617000 |
NORVIR 100 MG TABLET |
54569617100 |
ZIDOVUDINE 300 MG TABLET |
54569862000 |
CRIXIVAN 400 MG CAPSULE |
54569862001 |
CRIXIVAN 400MG CAPSULE |
54864725 |
VIRAMUNE 200MG TABLET |
54868011700 |
ISENTRESS 400 MG TABLET |
54868197400 |
RETROVIR 100 MG CAPSULE |
54868197402 |
RETROVIR 100 MG CAPSULE |
54868197403 |
RETROVIR 100 MG CAPSULE |
54868249901 |
HIVID 0.375MG TABLET |
54868250001 |
HIVID 0.750MG TABLET |
54868250002 |
HIVID 0.750MG TABLET |
54868250200 |
VIDEX 100 MG TAB CHEW |
54868250401 |
RETROVIR 10 MG/ML SYRUP |
54868335200 |
ZERIT 40 MG CAPSULE |
54868335201 |
ZERIT 40 MG CAPSULE |
54868335300 |
ZERIT 20 MG CAPSULE |
54868336000 |
ZERIT 15 MG CAPSULE |
54868344800 |
ZERIT 30 MG CAPSULE |
54868369300 |
EPIVIR 150 MG TABLET |
54868369302 |
EPIVIR 150 MG TABLET |
54868369900 |
INVIRASE 200 MG CAPSULE |
54868369901 |
INVIRASE 200 MG CAPSULE |
54868369902 |
INVIRASE 200 MG CAPSULE |
54868378200 |
NORVIR 100MG CAPSULE |
54868378201 |
NORVIR 100 MG CAPSULE |
54868378202 |
NORVIR 100 MG CAPSULE |
54868378203 |
NORVIR 100 MG CAPSULE |
54868384400 |
VIRAMUNE 200 MG TABLET |
54868384401 |
VIRAMUNE 200 MG TABLET |
54868394700 |
VIRACEPT 250 MG TABLET |
54868411000 |
FORTOVASE 200MG CAPSULE |
54868411300 |
CRIXIVAN 400 MG CAPSULE |
54868411400 |
COMBIVIR 150-300MG TABLET |
54868411406 |
COMBIVIR 150-300MG TABLET |
54868452000 |
RESCRIPTOR 200 MG TABLET |
54868452200 |
ZIAGEN 300 MG TABLET |
54868452201 |
ZIAGEN 300 MG TABLET |
54868452400 |
KALETRA 133.3-33.3 CAPSULE |
54868466600 |
VIDEX EC 400 MG CAPSULE DR |
54868466800 |
SUSTIVA 600 MG TABLET |
54868466900 |
VIREAD 300 MG TABLET |
54868485300 |
EMTRIVA 200 MG CAPSULE |
54868485400 |
REYATAZ 200 MG CAPSULE |
54868485700 |
REYATAZ 150 MG CAPSULE |
54868495400 |
LEXIVA 700 MG TABLET |
54868506100 |
VIRACEPT 625 MG TABLET |
54868514100 |
TRUVADA 200-300MG TABLET |
54868541600 |
EPIVIR 300 MG TABLET |
54868546400 |
DIDANOSINE 250 MG CAPSULE DR |
54868556600 |
KALETRA 200MG-50MG TABLET |
54868559500 |
VIDEX EC 250 MG CAPSULE DR |
54868560000 |
EPZICOM 600-300MG TABLET |
54868563100 |
PREZISTA 300 MG TABLET |
54868580900 |
SELZENTRY 300 MG TABLET |
54868583800 |
REYATAZ 300 MG CAPSULE |
54868586400 |
INTELENCE 100 MG TABLET |
54868596900 |
PREZISTA 400 MG TABLET |
55045220701 |
HIVID 0.750MG TABLET |
55045348103 |
TRUVADA 200-300MG TABLET |
55045348201 |
KALETRA 200MG-50MG TABLET |
55045354901 |
ZIDOVUDINE 300 MG TABLET |
55175449401 |
RETROVIR 100MG CAPSULE |
55175520706 |
COMBIVIR 150-300MG TABLET |
55175520807 |
VIRACEPT 250MG TABLET |
55175520901 |
CRIXIVAN 400MG CAPSULE |
55289038904 |
COMBIVIR 150-300MG TABLET |
55289038906 |
COMBIVIR 150-300MG TABLET |
55289038914 |
COMBIVIR 150-300MG TABLET |
55289038920 |
COMBIVIR 150-300MG TABLET |
55289039203 |
VIRAMUNE 200 MG TABLET |
55289047727 |
VIRACEPT 250 MG TABLET |
55289093118 |
KALETRA 133.3-33.3 CAPSULE |
55289094712 |
KALETRA 200MG-50MG TABLET |
555058801 |
DIDANOSINE 200 MG CAPSULE DR |
555058901 |
DIDANOSINE 250 MG CAPSULE DR |
555059001 |
DIDANOSINE 400 MG CAPSULE DR |
55887023030 |
CRIXIVAN 400 MG CAPSULE |
55887023060 |
CRIXIVAN 400 MG CAPSULE |
55887023090 |
CRIXIVAN 400 MG CAPSULE |
55887023130 |
COMBIVIR 150-300MG TABLET |
55887023160 |
COMBIVIR 150-300MG TABLET |
55887023190 |
COMBIVIR 150-300MG TABLET |
56047030 |
SUSTIVA 50 MG CAPSULE |
56047330 |
SUSTIVA 100 MG CAPSULE |
56047492 |
SUSTIVA 200 MG CAPSULE |
56051030 |
SUSTIVA 600 MG TABLET |
58016068900 |
EPIVIR 150 MG TABLET |
58016068930 |
EPIVIR 150 MG TABLET |
58016068960 |
EPIVIR 150 MG TABLET |
58016068990 |
EPIVIR 150 MG TABLET |
58016069000 |
RETROVIR 100 MG CAPSULE |
58016069018 |
RETROVIR 100 MG CAPSULE |
58016069030 |
RETROVIR 100 MG CAPSULE |
58016069060 |
RETROVIR 100 MG CAPSULE |
58016069090 |
RETROVIR 100 MG CAPSULE |
58016069800 |
COMBIVIR 150-300MG TABLET |
58016069830 |
COMBIVIR 150-300MG TABLET |
58016069860 |
COMBIVIR 150-300MG TABLET |
58016069890 |
COMBIVIR 150-300MG TABLET |
58016069900 |
CRIXIVAN 400 MG CAPSULE |
58016069930 |
CRIXIVAN 400 MG CAPSULE |
58016069960 |
CRIXIVAN 400 MG CAPSULE |
58016069990 |
CRIXIVAN 400 MG CAPSULE |
58016079500 |
EPIVIR 300 MG TABLET |
58016079530 |
EPIVIR 300 MG TABLET |
58016079560 |
EPIVIR 300 MG TABLET |
58016079590 |
EPIVIR 300 MG TABLET |
58016086400 |
RETROVIR 300 MG TABLET |
58016086430 |
RETROVIR 300 MG TABLET |
58016086460 |
RETROVIR 300 MG TABLET |
58016086490 |
RETROVIR 300 MG TABLET |
58864046230 |
RETROVIR 100MG CAPSULE |
58864046260 |
RETROVIR 100MG CAPSULE |
58864046293 |
RETROVIR 100MG CAPSULE |
59676056001 |
PREZISTA 300 MG TABLET |
59676056101 |
PREZISTA 400 MG TABLET |
59676056201 |
PREZISTA 600 MG TABLET |
59676056301 |
PREZISTA 75 MG TABLET |
59676056401 |
PREZISTA 150 MG TABLET |
59676057001 |
INTELENCE 100 MG TABLET |
597000201 |
APTIVUS 100 MG/ML SOLUTION |
597000302 |
APTIVUS 250 MG CAPSULE |
597004601 |
VIRAMUNE 200MG TABLET |
597004660 |
VIRAMUNE 200 MG TABLET |
597004661 |
VIRAMUNE 200MG TABLET |
597004724 |
VIRAMUNE 50 MG/5 ML ORAL SUSP |
59762119001 |
STAVUDINE 15 MG CAPSULE |
59762119101 |
STAVUDINE 20 MG CAPSULE |
59762119201 |
STAVUDINE 30 MG CAPSULE |
59762119301 |
STAVUDINE 40 MG CAPSULE |
59762365001 |
ZIDOVUDINE 300 MG TABLET |
6022761 |
ISENTRESS 400 MG TABLET |
6057062 |
CRIXIVAN 100 MG CAPSULE |
6057142 |
CRIXIVAN 200MG CAPSULE |
6057143 |
CRIXIVAN 200 MG CAPSULE |
6057301 |
CRIXIVAN 400 MG CAPSULE |
6057318 |
CRIXIVAN 400 MG CAPSULE |
6057340 |
CRIXIVAN 400 MG CAPSULE |
6057342 |
CRIXIVAN 400 MG CAPSULE |
6057354 |
CRIXIVAN 400 MG CAPSULE |
6057362 |
CRIXIVAN 400 MG CAPSULE |
6057465 |
CRIXIVAN 333 MG CAPSULE |
60760001018 |
VIRACEPT 250 MG TABLET |
60760001063 |
VIRACEPT 250 MG TABLET |
60760059504 |
COMBIVIR 150-300MG TABLET |
60760059514 |
COMBIVIR 150-300MG TABLET |
61958040101 |
VIREAD 300 MG TABLET |
61958060101 |
EMTRIVA 200 MG CAPSULE |
61958060201 |
EMTRIVA 10 MG/ML SOLUTION |
61958070101 |
TRUVADA 200-300MG TABLET |
62584004611 |
DIDANOSINE 250 MG CAPSULE DR |
62584004621 |
DIDANOSINE 250 MG CAPSULE DR |
62584004811 |
DIDANOSINE 400 MG CAPSULE DR |
62584004821 |
DIDANOSINE 400 MG CAPSULE DR |
62682104801 |
COMBIVIR 150-300MG TABLET |
63010001027 |
VIRACEPT 250MG TABLET |
63010001030 |
VIRACEPT 250 MG TABLET |
63010001190 |
VIRACEPT 50 MG/G POWDER |
63010002036 |
RESCRIPTOR 100 MG TAB DISPER |
63010002118 |
RESCRIPTOR 200 MG TABLET |
63010002770 |
VIRACEPT 625 MG TABLET |
63304092060 |
ZIDOVUDINE 300 MG TABLET |
65862002460 |
ZIDOVUDINE 300 MG TABLET |
65862004660 |
STAVUDINE 30 MG CAPSULE |
65862004760 |
STAVUDINE 40 MG CAPSULE |
65862004824 |
ZIDOVUDINE 10 MG/ML SYRUP |
65862010701 |
ZIDOVUDINE 100 MG CAPSULE |
65862011160 |
STAVUDINE 15 MG CAPSULE |
65862011260 |
STAVUDINE 20 MG CAPSULE |
65862031030 |
DIDANOSINE 125 MG CAPSULE DR |
65862031130 |
DIDANOSINE 200 MG CAPSULE DR |
65862031230 |
DIDANOSINE 250 MG CAPSULE DR |
65862031330 |
DIDANOSINE 400 MG CAPSULE DR |
66267050906 |
COMBIVIR 150-300 MG TABLET |
66267051418 |
VIRACEPT 250 MG TABLET |
66267051463 |
VIRACEPT 250 MG TABLET |
67253010910 |
ZIDOVUDINE 100 MG CAPSULE |
67253076120 |
STAVUDINE 1 MG/ML SOLN RECON |
67253096124 |
ZIDOVUDINE 10 MG/ML SYRUP |
67263023060 |
REYATAZ 150 MG CAPSULE |
67263023212 |
KALETRA 200 MG-50 MG TABLET |
67263025860 |
EPIVIR 150 MG TABLET |
67263026030 |
TRUVADA 200-300 MG TABLET |
67263038760 |
LEXIVA 700 MG TABLET |
67263040260 |
SELZENTRY 150 MG TABLET |
67263043460 |
VIRAMUNE 200 MG TABLET |
67263045530 |
VIREAD 300 MG TABLET |
67263045836 |
RESCRIPTOR 100 MG TAB DISPER |
67263051401 |
ZIDOVUDINE 100 MG CAPSULE |
67263056830 |
SUSTIVA 600 MG TABLET |
67263059060 |
PREZISTA 600 MG TABLET |
68030605901 |
RETROVIR 100 MG CAPSULE |
68030606001 |
EPIVIR 150 MG TABLET |
68030606401 |
EPIVIR 150 MG TABLET |
68030606501 |
RETROVIR 100 MG CAPSULE |
68030728301 |
COMBIVIR 150-300 MG TABLET |
68030728401 |
VIRACEPT 250 MG TABLET |
68115009006 |
COMBIVIR 150-300 MG TABLET |
68258900301 |
VIREAD 300 MG TABLET |
68258902001 |
SUSTIVA 600 MG TABLET |
68258902101 |
SUSTIVA 200 MG CAPSULE |
68258910801 |
EPIVIR 150 MG TABLET |
68258912601 |
ZERIT 20 MG CAPSULE |
68258914201 |
REYATAZ 150 MG CAPSULE |
68258915801 |
TRIZIVIR 150-300 MG TABLET |
69080760 |
SELZENTRY 150 MG TABLET |
69080860 |
SELZENTRY 300 MG TABLET |
74052260 |
KALETRA 100 MG-25 MG TABLET |
74194063 |
NORVIR 80 MG/ML SOLUTION |
74333330 |
NORVIR 100 MG TABLET |
74395646 |
KALETRA 400-100/5 SOLUTION |
74395977 |
KALETRA 133.3-33.3 CAPSULE |
74663322 |
NORVIR 100 MG CAPSULE |
74663330 |
NORVIR 100 MG CAPSULE |
74679922 |
KALETRA 200 MG-50 MG TABLET |
74949202 |
NORVIR 100 MG CAPSULE |
74949254 |
NORVIR 100 MG CAPSULE |
81010793 |
RETROVIR IV 10 MG/ML VIAL |
81010855 |
RETROVIR 100 MG CAPSULE |
81010856 |
RETROVIR 100 MG CAPSULE |
81011318 |
RETROVIR 10 MG/ML SYRUP |
87661443 |
VIDEX 100MG PACKET |
87661543 |
VIDEX 167MG PACKET |
87661643 |
VIDEX 250MG PACKET |
87661743 |
UNKNOWN |
87662443 |
VIDEX 50MG TAB CHEW |
87662643 |
VIDEX 150MG TAB CHEW |
87662743 |
VIDEX 100MG TAB CHEW |
87662843 |
VIDEX 25MG TAB CHEW |
87663241 |
VIDEX FNL10 MG/ML SOLN RECON |
87663341 |
VIDEX FNL10 MG/ML SOLN RECON |
87665001 |
VIDEX 25MG TAB CHEW |
87665101 |
VIDEX 50MG TAB CHEW |
87665201 |
VIDEX 100MG TAB CHEW |
87665301 |
VIDEX 150MG TAB CHEW |
87666515 |
VIDEX 200MG TAB CHEW |
87667117 |
VIDEX EC 125 MG CAPSULE DR |
87667217 |
VIDEX EC 200 MG CAPSULE DR |
87667317 |
VIDEX EC 250 MG CAPSULE DR |
87667417 |
VIDEX EC 400 MG CAPSULE DR |
93553006 |
ZIDOVUDINE 300 MG TABLET |
9376103 |
RESCRIPTOR 100 MG TABLET |
9757601 |
RESCRIPTOR 200 MG TABLET |
1 This assumes we will use a proprietary national all-payer claims database developed by SDI for this study. Because of the greater lag in availability of Medicare and Medicaid claims data directly from the Centers for Medicare & Medicaid Services, the most recent public claims data for this study would be 2009.
2 The baseline supply identified in tier one will include a limited number of physician assistants and nurse practitioners who can bill independently. These clinicians will be deduplicated from those identified in tier two.
3 Appendix A includes a detailed description of the analytic approach for identifying HIV clinicians in claims data. Appendices B and C contain, respectively, a comprehensive list of diagnosis/procedures codes and drug codes for HIV disease.
4 The number of hours worked by a full-time clinician is likely to be in excess of 40 hours per week. We will base our estimate of the average hours worked by a full-time clinician on the observed number of hours worked reported in the clinician survey.
5 Our previous work with specialists and subspecialists indicates that some provide “nonspecialty” care (that is, primary care) to round out the time they have available. As greater opportunity for providing care in their specialty arises, they reduce the amount of nonspecialty care and provide more specialty care. We do not know if this type of relationship holds for infectious disease specialists who focus on HIV or for erstwhile primary care physicians who focus on HIV. We will address this issue in the survey, but it is also useful to interview some physician clinicians in HIV to understand their perspective on this issue, as it will affect the implied supply of HIV clinicians.
6 The HIVMA Workforce Survey conducted in 2009 included the first four of these questions.
7 Guides from the Centers for Disease Control and Prevention (CDC) and the American Academy of HIV Medicine (AAHIVM).
8 “Guidelines by the DHHS panel on Antiretroviral Guidelines for Adults and Adolescents – A working Group of the Office of AIDS Research Advisory Council (OARAC).” Department of Health and Human Services, December 1, 2009.
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Margaret Hallisey |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy