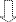57.112 Streamlined Ventilator-Associated Pneumonia
The National Healthcare Safety Network (NHSN)
57.112_SVAP_BLANK
57.112 Streamlined Ventilator-Associated Pneumonia
OMB: 0920-0666

OMB No. XXXX-XXXX
Exp. Date: XX-XX-XXXX
www.cdc.gov/nhsn
Streamlined Ventilator-Associated Pneumonia (SVAP)
Page 1 of 4 |
|||||||||||
*required for saving **required for completion |
|||||||||||
Facility ID: |
Event #: |
||||||||||
*Patient ID: |
Social Security #: |
||||||||||
Secondary ID: |
Medicare #: |
||||||||||
Patient Name, Last: |
First: |
Middle: |
|||||||||
*Gender: F M Other |
*Date of Birth: |
||||||||||
Ethnicity (Specify): |
Race (Specify): |
||||||||||
*Event Type: SVAP |
*Date of Event: |
||||||||||
Post-procedure SVAP: Yes No |
Date of Procedure: |
||||||||||
NHSN Procedure Code: |
ICD-9-CM Procedure Code: |
||||||||||
*MDRO Infection Surveillance: |
|||||||||||
□ Yes, this infection’s pathogen & location are in-plan for Infection Surveillance in the MDRO/CDI Module |
|||||||||||
□ No, this infection’s pathogen & location are not in-plan for Infection Surveillance in the MDRO/CDI Module |
|||||||||||
*Date Admitted to Facility: |
*Location: |
||||||||||
Risk Factors |
|||||||||||
*Endotracheal tube/ventilator status at time of event onset: |
|||||||||||
□ In place |
□ Removed within 2 calendar days prior |
□ Not in place nor within 2 calendar days prior |
|||||||||
*Location of Endotracheal tube/Ventilator Insertion: ______________ |
*Date of Insertion: ___ /___ /_______ |
||||||||||
Event Details |
|||||||||||
*Specify Criteria Used: (check all that apply) |
|||||||||||
STEP 1: Respiratory (≥ 1 REQUIRED) |
|
STEP 2: Infection/Inflammation (≥ 1 REQUIRED)‡ |
|||||||||
□ Increase in daily minimum FiO2 of ≥ 0.15 (15 points) for ≥ 2 days† |
|
□ Fever (> 38°C) |
|||||||||
|
□ Hypothermia (< 36°C) |
||||||||||
□ |
AND |
□ Leukocytosis (WBC count > 12,000 cells/mm3 |
|||||||||
□ Increase in daily minimum mean airway pressure (MAP) ≥ 4 cm H2O for ≥ 2 days† |
|
□ Leukopenia (WBC count < 4,000 cells/mm3 |
|||||||||
†after 2 days of stable or decreasing daily minimum values |
|
□ Purulent respiratory secretions (≥ 25 neutrophils and ≤ 10 epithelial cells [or equivalent] per lpf |
|||||||||
OPTIONAL |
|||||||||||
|
STEP 3: Confirmation (OPTIONAL)‡ |
|
|||||||||
|
□ Pathogen isolated by culture or other specified microbiology test performed on an acceptable respiratory specimen‡ |
|
|||||||||
|
□ Antimicrobial agent(s) is/are started‡ and continued for ≥ 4 days |
|
|||||||||
|
□ ≥ 2 chest imaging studies‡ (chest radiograph, chest CT scan) that each show the presence of ≥ 1 of the following: infiltrate, opacity, density, consolidation, cavitation, air space disease |
|
|||||||||
|
‡ Must be collected or performed within +/- 2 days of onset of increase in FiO2 or PEEP or MAP and after 2 days of intubation/mechanical ventilation |
|
|||||||||
*Secondary Bloodstream Infection: Yes No |
|||||||||||
**Died: Yes No |
SVAP Contributed to Death: Yes No |
||||||||||
Discharge Date: |
*Pathogens Identified: Yes No *If Yes, specify on pages 2-3. |
||||||||||
Assurance of Confidentiality: The voluntarily provided information obtained in this surveillance system that would permit identification of any individual or institution is collected with a guarantee that it will be held in strict confidence, will be used only for the purposes stated, and will not otherwise be disclosed or released without the consent of the individual, or the institution in accordance with Sections 304, 306 and 308(d) of the Public Health Service Act (42 USC 242b, 242k, and 242m(d)). Public reporting burden of this collection of information is estimated to average 25 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to CDC, Reports Clearance Officer, 1600 Clifton Rd., MS D-74, Atlanta, GA 30333, ATTN: PRA (0920-0666). CDC 57.112 (Front), v6.8 |
|||||||||||
Streamlined Ventilator-Associated Pneumonia (SVAP)
Page 2 of 4 |
|||||||||||
Pathogen # |
Gram-positive Organisms |
||||||||||
_______ |
Staphylococcus coagulase-negative |
VANC S I R N |
|||||||||
(specify): ________________________ |
|||||||||||
_______ |
Enterococcus spp.( specify): |
AMP S I R N |
CIPRO/LEVO/MOXI S I R N |
DAPTO S NS N |
DOXY/MINO S I R N |
GENTHL§ S R N |
LNZ S I R N |
||||
___________ |
STREPHL§ S R N
|
TETRA S I R N
|
TIG S NS N
|
VANC S I R N
|
|||||||
_______
|
Enterococcus faecium |
AMP S I R N |
CIPRO/LEVO/MOXI S I R N |
DAPTO S NS N |
DOXY/MINO S I R N |
GENTHL§ S R N |
LNZ S I R N |
||||
|
QUIDAL S I R N
|
STREPHL§ S R N
|
TETRA S I R N
|
TIG S NS N
|
VANC S I R N
|
||||||
_______ |
Staphylococcus aureus |
CHLOR S I R N |
CIPRO/LEVO/MOXI S I R N |
CLIND S I R N |
DAPTO S NS N |
DOXY/MINO S I R N |
ERYTH S I R N |
GENT S I R N |
|||
|
LNZ S R N
|
OX/CEFOX/METH S I R N |
QUIDAL S I R N |
RIF S I R N |
TETRA S I R N |
TIG S NS N |
TMZ S I R N |
VANC S I R N |
|||
Pathogen # |
Gram-negative Organisms |
||||||||||
_______ |
Acinetobacter spp. (specify): |
AMK S I R N |
AMPSUL S I R N |
AZT S I R N |
CEFEP S I R N |
CEFTAZ S I R N |
CIPRO/LEVO S I R N |
COL/PB S I R N |
|||
____________ |
GENT S I R N |
IMI S I R N
|
MERO/DORI S I R N |
PIP/PIPTAZ S I R N |
TETRA/DOXY/MINO S I R N |
||||||
|
|
TMZ S I R N |
TOBRA S I R N |
|
|||||||
_______ |
Escherichia coli |
AMK S I R N |
AMP S I R N |
AMPSUL/AMXCLV S I R N |
AZT S I R N |
CEFAZ S I R N |
CEFEP S I R N |
CEFOT/CEFTRX S I R N |
|||
|
CEFTAZ S I R N |
CEFUR S I R N |
CEFOX/CETET S I R N |
CHLOR S I R N |
CIPRO/LEVO/MOXI S I R N |
COL/PB S I R N |
|||||
|
ERTA S I R N |
GENT S I R N |
IMI S I R N |
MERO/DORI S I R N |
PIPTAZ S I R N |
TETRA/DOXY/MINO S I R N |
|||||
|
|
TIG S I R N |
TMZ S I R N |
TOBRA S I R N |
|
||||||
_______ |
Enterobacter spp. (specify): |
AMK S I R N |
AMP S I R N |
AMPSUL/AMXCLV S I R N |
AZT S I R N |
CEFAZ S I R N |
CEFEP S I R N |
CEFOT/CEFTRX S I R N |
|||
____________ |
CEFTAZ S I R N |
CEFUR S I R N |
CEFOX/CETET S I R N |
CHLOR S I R N |
CIPRO/LEVO/MOXI S I R N |
COL/PB S I R N |
|||||
|
ERTA S I R N |
GENT S I R N |
IMI S I R N |
MERO/DORI S I R N |
PIPTAZ S I R N |
TETRA/DOXY/MINO S I R N |
|||||
|
|
TIG S I R N |
TMZ S I R N |
TOBRA S I R N |
|
||||||
_______ |
Klebsiella spp. (specify): |
AMK S I R N |
AMP S I R N |
AMPSUL/AMXCLV S I R N |
AZT S I R N |
CEFAZ S I R N |
CEFEP S I R N |
CEFOT/CEFTRX S I R N |
|||
____________ |
CEFTAZ S I R N |
CEFUR S I R N |
CEFOX/CETET S I R N |
CHLOR S I R N |
CIPRO/LEVO/MOXI S I R N |
COL/PB S I R N |
|||||
|
ERTA S I R N |
GENT S I R N |
IMI S I R N |
MERO/DORI S I R N |
PIPTAZ S I R N |
TETRA/DOXY/MINO S I R N |
|||||
|
TIG S I R N |
TMZ S I R N |
TOBRA S I R N |
|
|||||||
Streamlined Ventilator-Associated Pneumonia (SVAP) |
|||||||||||||||||||
Page 3 of 4 |
|||||||||||||||||||
Pathogen # |
Gram-negative Organisms (continued) |
||||||||||||||||||
_______ |
Serratia marcescens |
AMK S I R N |
AMP S I R N |
AMPSUL/AMXCLV S I R N |
AZT S I R N |
CEFAZ S I R N |
CEFEP S I R N |
CEFOT/CEFTRX S I R N |
|||||||||||
|
CEFTAZ S I R N |
CEFUR S I R N |
CEFOX/CETET S I R N |
CHLOR S I R N |
CIPRO/LEVO/MOXI S I R N |
COL/PB S I R N |
|||||||||||||
|
ERTA S I R N |
GENT S I R N |
IMI S I R N |
MERO/DORI S I R N |
PIPTAZ S I R N |
TETRA/DOXY/MINO S I R N |
|||||||||||||
|
|
TIG S I R N |
TMZ S I R N |
TOBRA S I R N |
|
||||||||||||||
_______ |
Pseudomonas aeruginosa |
AMK S I R N |
AZT S I R N |
CEFEP S I R N |
CEFTAZ S I R N |
CIPRO/LEVO S I R N |
COL/PB S I R N |
GENT S I R N |
|||||||||||
|
IMI S I R N
|
MERO/DORI S I R N |
PIP/PIPTAZ S I R N |
TOBRA S I R N |
|||||||||||||||
_______ |
Stenotrophomonas maltophilia |
LEVO S I R N |
TETRA/MINO S I R N |
TICLAV S I R N |
TMZ S I R N |
||||||||||||||
Pathogen # |
Fungal Organisms |
||||||||||||||||||
_______ |
Candida spp. (specify): ____________
|
ANID S I R N |
CASPO S NS N |
FLUCO S S-DD R N |
FLUCY S I R N |
ITRA S S-DD R N |
MICA S NS N |
VORI S S-DD R N |
|||||||||||
Pathogen # |
Other Organisms |
||||||||||||||||||
_______ |
Organism 1 (specify) ____________
|
_______Drug 1 S I R N |
_______ Drug 2 S I R N |
______ Drug 3 S I R N |
_______ Drug 4 S I R N |
_______Drug 5 S I R N |
______ Drug 6 S I R N |
______ Drug 7 S I R N |
______ Drug 8 S I R N |
______ Drug 9 S I R N |
|||||||||
_______ |
Organism 1 (specify) ____________
|
_______Drug 1 S I R N |
_______ Drug 2 S I R N |
______ Drug 3 S I R N |
_______ Drug 4 S I R N |
_______Drug 5 S I R N |
______ Drug 6 S I R N |
______ Drug 7 S I R N |
______ Drug 8 S I R N |
______ Drug 9 S I R N |
|||||||||
_______ |
Organism 1 (specify) ____________
|
_______Drug 1 S I R N |
_______ Drug 2 S I R N |
______ Drug 3 S I R N |
_______ Drug 4 S I R N |
_______Drug 5 S I R N |
______ Drug 6 S I R N |
______ Drug 7 S I R N |
______ Drug 8 S I R N |
______ Drug 9 S I R N |
|||||||||
Result Codes
S = Susceptible I = Intermediate R = Resistant NS = Non-susceptible S-DD = Susceptible-dose dependent N = Not tested
§ GENTHL and STREPHL results: S = Susceptible/Synergistic and R = Resistant/Not Synergistic
Drug Codes: |
|
|
|
|
AMK = amikacin |
CEFTRX = ceftriaxone |
ERYTH = erythromycin |
MICA = micafungin |
STREPHL = streptomycin – high level test |
AMP = ampicillin |
CEFUR= cefuroxime |
FLUCO = fluconazole |
MINO = minocycline |
TETRA = tetracycline |
AMPSUL = ampicillin/sulbactam |
CETET= cefotetan |
FLUCY = flucytosine |
MOXI = moxifloxacin |
TICLAV = ticarcillin/clavulanic acid |
AMXCLV = amoxicillin/clavulanic acid |
CHLOR= chloramphenicol |
GENT = gentamicin |
OX = oxacillin |
TIG = tigecycline |
ANID = anidulafungin |
CIPRO = ciprofloxacin |
GENTHL = gentamicin –high level test |
PB = polymyxin B |
TMZ = trimethoprim/sulfamethoxazole |
AZT = aztreonam |
CLIND = clindamycin |
IMI = imipenem |
PIP = piperacillin |
TOBRA = tobramycin |
CASPO = caspofungin |
COL = colistin |
ITRA = itraconazole |
PIPTAZ = piperacillin/tazobactam |
VANC = vancomycin |
CEFAZ= cefazolin |
DAPTO = daptomycin |
LEVO = levofloxacin |
QUIDAL = quinupristin/dalfopristin |
VORI = voriconazole |
CEFEP = cefepime |
DORI = doripenem |
LNZ = linezolid |
RIF = rifampin |
|
CEFOT = cefotaxime |
DOXY = doxycycline |
MERO = meropenem |
|
|
CEFOX= cefoxitin |
ERTA = ertapenem |
METH = methicillin |
|
|
CEFTAZ = ceftazidime |
|
|
|
|
Streamlined Ventilator-Associated Pneumonia (SVAP)
Page 4 of 4 |
|||
Custom Fields |
|||
Label |
Label |
||
______________________ |
____/____/____ |
_______________________ |
____/____/_____ |
_______________________ |
_____________ |
_______________________ |
______________ |
_______________________ |
_____________ |
_______________________ |
______________ |
_________________________ |
______________ |
_______________________ |
______________ |
_________________________ |
______________ |
_______________________ |
______________ |
_________________________ |
______________ |
_______________________ |
______________ |
_________________________ |
______________ |
_______________________ |
______________
|
Comments |
|||
|
|||
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| Author | Amy Schneider |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy
 Increase
in daily minimum PEEP of ≥ 2 cm H2O
for ≥ 2 days†
Increase
in daily minimum PEEP of ≥ 2 cm H2O
for ≥ 2 days†