Survey of "Health Care Providers' Responses to Medical Device Labeling Content
Survey of "Health Care Providers' Responses to Medical Device Labeling"
Attachment A - Questionnaire and Confidentiality Assurance
Survey of "Health Care Providers' Responses to Medical Device Labeling Content
OMB: 0910-0715
Web Questionnaire
FDA Device Labeling
HCP Questionnaire Final Version--093011
Text for Project Homepage:
Welcome to the FDA Medical Device Labeling Study website! We appreciate you taking the time to help us with this important project by providing your valued opinion on abbreviated medical device libeling’s. In order to participate in the study, you will need your username and password, which we sent to you either by U.S. mail or email. Please enter this information below.
USERNAME: ______________
PASSWORD: ______________
Please enter the Case ID and password that you received. Passwords are CASE SENSITIVE, so please type carefully.
<PLACE AT BOTTOM OF SCREEN, SEPARATE FROM TEXT, IN DIFFERENT FONT:>
OMB Approved No. XXXXXXXXXXX Expires xx/xx/xxxx
SURVEY SCREEN 1
Welcome to the Food and Drug Administration’s (FDA’s) Medical Device Labeling Survey! The purpose of this project is to examine what information needs to be provided with medical devices, and how that information should be organized. We are asking health care professionals to provide their opinion about an example medical device label created for this survey. The project findings will provide evidence to inform the FDA’s regulatory approach to standardizing device labeling.
The survey will take approximately 30 minutes. To thank you for your time, you will be paid [FILL] for completing the survey. Your participation in this research will be kept private to the fullest extent allowed by law. Your participation in this research is also voluntary. Refusal to participate will involve no penalty, and you may discontinue participation at any time. There are no direct benefits to you for completing the survey.
If you have any questions about the study, please contact 1-800-334-8571, x26902. If you have any questions about your rights as a study participant, you can call RTI's Office of Research Protection at 1-866-214-2043 (a toll-free number).
Thank you for participating in this important study!
SURVEY SCREEN 2
Please be sure to review and print the example medical device abbreviated labeling prior to beginning the questionnaire. To access the example abbreviated labeling, please click <here>.
When you are ready to begin taking your survey, please click on the other box. This will bring you to the FDA survey.
Always use the 'Logoff' button to exit the survey. You may stop the survey at any time and resume where you left off.
To resume the survey you will need to re-enter your username and password. If you do not complete your survey in one session, the responses that you have previously entered can be viewed by someone else if they gain access to your username and password, so please keep this information secure.
This survey is best viewed with Google Chrome. It may also be viewed in Firefox or Internet Explorer V. 8.0 or higher.
NAVIGATION INSTRUCTIONS
Do NOT use your browser’s ‘Back’ and ‘Forward’ buttons to navigate through the survey questions.
After you enter or select your answer, click on the ‘Next’ button below the question to continue to the next question.
To go back to the previous question, click on the ‘Previous’ button below the question.
You may stop the survey at any time and resume where you left off. To re-enter and resume the survey you will need to navigate to the web site address provided to you by RTI and re-enter your login credentials.
Once you submit your survey you will not be able to access it again.
Please review the example medical device abbreviated labeling. You will be unable to provide informed answers without reviewing the example abbreviated labeling. Have you reviewed the example abbreviated labeling?
1 YES
2 NO
[IF 2, DISPLAY SOFT EDIT TEXT: Please review the example medical device abbreviated labeling. You will be unable to provide informed answers without reviewing the example abbreviated labeling. The abbreviated labeling can be accessed from the study’s main webpage.]
First, we’d like to ask some questions about you. For the following questions please choose the best answer.
Which of the following best describes the place where you perform most of your work?
2 Clinic or doctor’s office
3 Home health
4 In-patient care facility
5 Outpatient diagnostic facility (lab or medical imaging center)
6 Outpatient treatment facility (dialysis, infusion therapy, cancer)
7 Other, specify:
During a typical week of practice how many hours of direct patient care do you provide? By direct patient care we mean seeing patients, reviewing tests, preparing for and performing procedures, or providing other related patient care services.
1 None
2 1–15 hours
3 16–25 hours
4 26–40 hours
5 More than 40 hours
6 Don’t know
Which of the following best describes your occupation?
1 Physician
2 Nurse practitioner or physician assistant
3 Registered nurse
4 Licensed practical nurse
5 Therapist
6 Lab technologist or technician
7 Other, specify:
If Q3 = 1 (Physician) go to Q5, otherwise continue.
Which of the following best describes your main area of practice?
1 Acute care facility
2 Clinic
3 Education/academic
4 Home care
5 Rehabilitation or chronic care
6 Surgical
7 Other, specify:
Go to Q6
Which of the following best describes your main area of practice?
1 Academic
2 Acute care facility
3 Public health
4 Private or group practice
5 Rehabilitation or chronic care
6 Surgical
7 Other, specify:
Go to Q8
How many years have you been practicing health care since completing your training?
[PLEASE ENTER ONLY WHOLE NUMBERS. IF NECESSARY, ROUND UP TO THE NEAREST WHOLE NUMBER.]
___________ YEARS <ALLOW UP TO TWO DIGITS; SOFT EDIT CHECK IF 60+>
What is your gender?
1 Male
2 Female
Go to Q10
How many years have you been practicing since completing your residency or fellowship?
[PLEASE ENTER ONLY WHOLE NUMBERS. IF NECESSARY, ROUND UP TO THE NEAREST WHOLE NUMBER.]
___________ YEARS <ALLOW UP TO TWO DIGITS; SOFT EDIT CHECK IF 60+>
What is your gender?
1 Male
2 Female
If Q3 = 1 or 2 (Physician, Nurse Practitioner, or Physician Assistant) continue, else go to Q11.
In the last month, about how many times did you prescribe the use of an infusion pump?
1 0
2 1–2 times
3 3–5 times
4 6–10 times
5 11 or more times
In the last month, about how many times did you use an infusion pump with a patient?
1 0
2 1–2 times
3 3–5 times
4 6–10 times
5 11 or more times
Defining the Document and Device
In the remainder of the survey we are going to ask questions about the “Tohamadi Large Volume Infusion Pump” document. Please click here [INSERT LINK] to see this document. Please note this short type of document is NOT intended to include all the information needed for the use of a medical device. We are using an infusion pump as an example of a commonly used medical device.
[RESPONDENT WILL RANDOMLY RECEIVE ONE OF THE THREE FOLLOWING RESPONSE OPTION ARRANGEMENTS:]
In your opinion, which of the following best describes the document?
1 Device or instrument labeling
2 User or operator guide
3 Instructions for use
4 Quick guide
5 Reference guide
6 Something else, specify: _____________________________________.
In your opinion, which of the following best describes the document?
1 Quick guide
2 Reference guide
3 Device or instrument labeling
4 User or operator guide
5 Instructions for use
6 Something else, specify: _____________________________________.
In your opinion, which of the following best describes the document?
1 Instructions for use
2 Reference guide
3 Quick guide
4 Device or instrument labeling
5 User or operator guide
6 Something else, specify: _____________________________________.
How often would you refer to a document such as our Tohamadi Infusion Pump example when…
|
All of the Time |
Most of the Time |
Some of the Time |
Rarely |
Never |
Not Applicable |
a. Unpacking the device? |
1 |
2 |
3 |
4 |
5 |
6 |
b. Setting up the device? |
1 |
2 |
3 |
4 |
5 |
6 |
c. Using the device? |
1 |
2 |
3 |
4 |
5 |
6 |
d. Prescribing the use of the device? |
1 |
2 |
3 |
4 |
5 |
6 |
e. Selecting the proper device for a patient? |
1 |
2 |
3 |
4 |
5 |
6 |
f. Storing the device? |
1 |
2 |
3 |
4 |
5 |
6 |
g. Disposing of products used with the device? |
1 |
2 |
3 |
4 |
5 |
6 |
h. A patient has an adverse event? |
1 |
2 |
3 |
4 |
5 |
6 |
i. A warning or error message appears? |
1 |
2 |
3 |
4 |
5 |
6 |
j. An alarm on the device sounds? |
1 |
2 |
3 |
4 |
5 |
6 |
k. Troubleshooting? |
1 |
2 |
3 |
4 |
5 |
6 |
l. Looking for risks? |
1 |
2 |
3 |
4 |
5 |
6 |
How likely is it that you would refer to a diagram of a medical device, such as the one on the front page of the Tohamadi Infusion Pump document, when…
|
Very Likely |
Likely |
Unlikely |
Very Unlikely |
Not Applicable |
a. Unpacking the device? |
1 |
2 |
3 |
4 |
5 |
b. Setting up the device? |
1 |
2 |
3 |
4 |
5 |
c. Using the device? |
1 |
2 |
3 |
4 |
5 |
d. Prescribing the use of the device? |
1 |
2 |
3 |
4 |
5 |
e. Selecting the proper device for the patient? |
1 |
2 |
3 |
4 |
5 |
f. A warning or error message appears? |
1 |
2 |
3 |
4 |
5 |
g. An alarm on the device sounds? |
1 |
2 |
3 |
4 |
5 |
The following series of questions is about how the document is organized.
Please look at the headings in the <link>document</link, for example Description and Uses. How easy or difficult are these headings to understand?
1 Very easy
2 Somewhat easy
3 Somewhat difficult
4 Very difficult
QUESTION 16 HAS BEEN DELETED AND IS INTENTIONALLY MISSING.
Are you satisfied with the way the information is ordered?
1 Yes
2 No
3 Don’t know
If Q17 = 1 or DK Go to Q18, otherwise continue
17a. Please indicate the order in which the information should appear. Please order from 1 (first) to 10 (last).
___ Description
___ Uses
___ Contraindications
___ Risks
___ Instructions for use
___ Warnings and precautions
___ Cleaning the pump
___ Compatible accessories
___ Common alarms
___ Troubleshooting
If you believe there is a better term for any of the current headings in the document please indicate below.
1 No change to headings needed.
[DO NOT ALLOW SELECTION OF 1- NO CHANGE AND ANY OTHER RESPONSE.]
-
The heading in this list should be
Replaced with (please specify)
2 Description
3 Uses
4 Contraindications
5 Risks
6 Instructions For Use
7 Warnings and Precautions
8 Cleaning the Pump
9 Compatible Accessories
10 Common Alarms
11 Troubleshooting
Evaluating the Content
The next series of questions is about the content of the document.
Which of the following sections should be added? Please keep in mind that this type of document is to be used for quick reference only.
1 Fine as is. No additions are needed.
[DO NOT ALLOW THE SELECTION OF 1- FINE AS IS AND ANY OTHER RESPONSE.]
|
Add to Document |
a. Adverse Effects |
1 |
b. Battery Type |
1 |
c. Boxed Warnings |
1 |
d. Compatibility |
1 |
e. Additional Diagram |
1 |
f. Disposal of Accessories |
1 |
g. Electronic Interference |
1 |
h. Expiration Date |
1 |
i. Frequently Asked Questions (FAQs) |
1 |
j. How Supplied |
1 |
k. Maintenance |
1 |
l. Patient/Special Populations |
1 |
m. Storage |
1 |
n. Table of Contents |
1 |
o. Use Environment |
1 |
p. Other, specify: |
1 |
Thinking about medical devices in general, how useful is it to have the following sections included in an abbreviated document?
|
Very Useful |
Useful |
Somewhat Useful |
Not Useful at All |
a. Description? |
1 |
2 |
3 |
4 |
b. Uses? |
1 |
2 |
3 |
4 |
c. Contraindications? |
1 |
2 |
3 |
4 |
d. Risks? |
1 |
2 |
3 |
4 |
e. Instructions for Use? |
1 |
2 |
3 |
4 |
f. Warnings and Precautions? |
1 |
2 |
3 |
4 |
g. Cleaning a Device? |
1 |
2 |
3 |
4 |
h. Compatible Accessories? |
1 |
2 |
3 |
4 |
i. Common Alarms? |
1 |
2 |
3 |
4 |
j. Troubleshooting? |
1 |
2 |
3 |
4 |
Format of the Document
The next series of questions is about the format of the document.
In this type of abbreviated document do you think that words such as “not” or “only” should appear in bold-face type in some instances, all instances, or are the instructions easy enough to understand without special emphasis? Choose one.
1 In some instances
2 In all instances
3 Special emphasis is not necessary
If Q21 = 2, 3go to Q22, otherwise continue.
21a. In what instances should words such as “not” or “only” appear in bold-face type?
How would you like the information under each heading presented: in a bulleted list, numbered list, in paragraph form, or some other way? Please review the examples of the four formats and choose one format for each heading. If you prefer the information be provided in some other way please specify.
22a Instructions for Use?
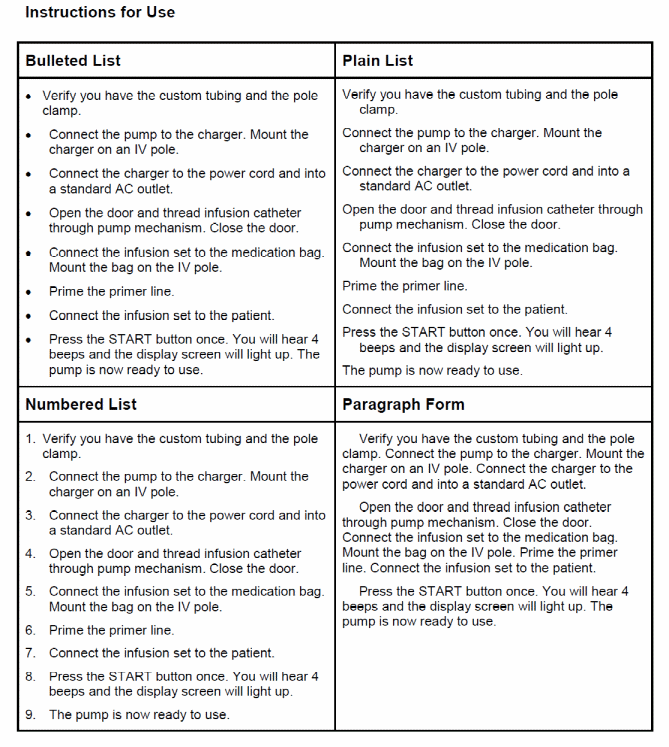
1 Bulleted List
2 Plain List
3 Numbered List
4 Paragraph Form
5 Some Other Way
[IF 22a= 5, CONTINUE; ELSE, SKIP TO 22b]
22aSpec
Please specify: ______________________________
22b Warnings and Precautions?
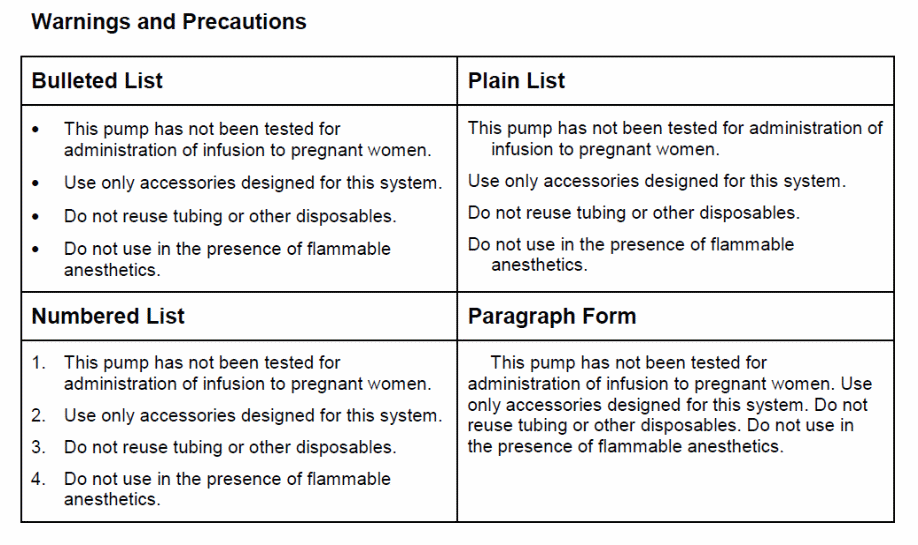
1 Bulleted List
2 Plain List
3 Numbered List
4 Paragraph Form
5 Some Other Way
[IF 22b= 5, CONTINUE; ELSE, SKIP TO 23]
22bSpec
Please specify: ______________________________
In this example we use several symbols, such as
 and
and
 .
In general, how useful are symbols?
.
In general, how useful are symbols?
1 Very useful
2 Somewhat useful
3 Not very useful
4 Not useful at all
Where do you think you would typically look for information on how to contact the manufacturer’s customer service?
1 On the first page
2 On the last page
3 In the troubleshooting section
4 By the device name and model number
An abbreviated document such as this would include the following disclaimer:
“These instructions do not include all the information needed for the safe and effective use of this pump. See the full device labeling for more complete information on the use of this device.”
Where do you think is the best location for the disclaimer?
1 Top of the first page
2 Bottom of the first page
3 Top of the last page
4 Bottom of the last page
[RESPONDENT WILL RANDOMLY RECEIVE ONE OF THE TWO FOLLOWING RESPONSE OPTION ARRANGEMENTS:]
26_1 A table of contents should be included in a document like this…
1 Always
2 If 5 or more pages
3 If 3 or more pages
4 Never
5 Don’t know
26_2. A table of contents should be included in a document like this…
1 Never
2 If 3 or more pages
3 If 5 or more pages
4 Always
5 Don’t know
Completeness of the Information
The next series of questions focuses on the completeness of the information.
Based on this example document, how confident are you that you would be able to respond to the following situations…?
|
Very Confident |
Somewhat Confident |
Not Very Confident |
Not At All Confident |
a. The infusion pump’s low-battery alarm sounded? |
1 |
2 |
3 |
4 |
b. An accessory didn’t fit properly? |
1 |
2 |
3 |
4 |
c. The screen went blank? |
1 |
2 |
3 |
4 |
d. You wanted to dispose of the accessories? |
1 |
2 |
3 |
4 |
e. You needed to contact the pump’s manufacturer? |
1 |
2 |
3 |
4 |
f. You needed to set up the pump? |
1 |
2 |
3 |
4 |
Do you think an abbreviated document such as our example should indicate whether or not you can…
|
Yes |
No |
Don’t Know |
a. Dispose of it in your regular trash? |
1 |
2 |
3 |
b. Disable features designed to facilitate safe operation? |
1 |
2 |
3 |
c. Clean it with a disinfectant? |
1 |
2 |
3 |
d. Replace parts of this pump yourself? |
1 |
2 |
3 |
29. An abbreviated document such as this includes a variety of information. Thinking about medical devices in general, do you think an abbreviated document should specifically include information about whether a medical device can be used…
|
Yes |
No |
Don’t Know |
REFUSE |
a. With rechargeable batteries? |
1 |
2 |
3 |
-7 |
b. With over-the-counter batteries? |
1 |
2 |
3 |
-7 |
c. With replacement parts from a similar device? |
1 |
2 |
3 |
-7 |
d. Near equipment that emits high-energy radio frequencies? |
1 |
2 |
3 |
-7 |
e. With any size tubing? |
1 |
2 |
3 |
-7 |
f. Near flammable gasses? |
1 |
2 |
3 |
-7 |
g. Without a prescription? |
1 |
2 |
3 |
-7 |
h. In the presence of an MRI? |
1 |
2 |
3 |
-7 |
i. In the home? |
1 |
2 |
3 |
-7 |
j. With patients under the age of 12? |
1 |
2 |
3 |
-7 |
k. With patients who have specific health conditions? |
1 |
2 |
3 |
-7 |
l. With pregnant patients? |
1 |
2 |
3 |
-7 |
The last question focuses on where you would want to find this type of information when using a medical device.
If you were caring for a patient and you needed to access this document where would you want to obtain it? (Select up to 3 options from the lists below.)
In Hard Copy Form
1 With or on the device
2 In a central location in your office or hospital
In Electronic Form
3 On the device’s computer memory, displayed on its screen
4 On the manufacturer’s or distributor’s website
5 On the FDA’s website
6 On your hospital’s or office’s internal website
7 On a mobile device like a smart phone
8 Other
9 No preference for either hard copy or electronic formats
[ALLOW UP TO 3 RESPONSES. EDIT CHECK: DO NOT ALLOW A COMBINATION OF 9-NO PREFERENCE AND ANY OTHER]
[IF 8-OTHER WAS SELECTED, CONTINUE; ELSE, SKIP TO 31.]
30Spec:
Please specify where else you would want to obtain this information:
_______________________________________________________________
_______________________________________________________________
Thank you for completing the survey! As outlined earlier, your participation in this research will be kept private to the extent provided by law. Your name and contact information will be separated from the data so it will not be linked with the responses you provided.
Please provide us with your name and the mailing address where you would like to receive your check.
a. Name:
b. Address:
c. Address1:
d. City:
e. State:
f. Zip:
g. Phone number: ____________________________________________________
PROGRAMMER: ADD SOFT EDIT CHECK IF ANY FIELDS ARE BLANK: “You have left your contact information blank. If you do not provide complete contact information, we cannot guarantee that your incentive payment will reach you.”
You should receive your check at the address you specified in approximately 1-2 weeks. Thank you again for your participation in this important study. If you have any further questions, you may contact us at 1-800-334-8571 x26902.
[END]
| File Type | application/vnd.openxmlformats-officedocument.wordprocessingml.document |
| File Title | Instructions |
| File Modified | 0000-00-00 |
| File Created | 2021-01-31 |
© 2026 OMB.report | Privacy Policy